Abstract
Objective
Adolescents with Attention Deficit/Hyperactivity Disorder (ADHD) are at an increased risk for substance use but the pathways through which this risk emerges are insufficiently understood.
Method
Tobacco, alcohol, and marijuana outcomes were compared between adolescents diagnosed with ADHD in early childhood (N=113) and demographically similar controls (N=65). Participants were assessed from age 5 until age 18. A comprehensive history of adolescent substance use was compiled for each participant and growth in ADHD and Conduct Disorder (CD) were modeled as they related to substance use outcomes.
Results
Results indicated that when compared to controls, adolescents with ADHD were more likely to try cigarettes, initiate alcohol use at early ages, and smoke marijuana more frequently. Furthermore, adolescents with ADHD were four to five times more likely than controls to escalate to heavy cigarette and marijuana use after trying these substances once. Adolescents with ADHD who escalated to heavy use patterns were more likely to display early cigarette use and marked problems with family members, but displayed fewer peer problems. There was evidence of baseline effects (latent intercept, measured at age five) for both ADHD and CD on substance use outcomes. Furthermore, growth in ADHD symptoms accounted for much of the growth in CD symptoms, and consequently, escalating CD symptoms in childhood (latent slope) were viewed as a mediator of the relationship between ADHD and cigarette and marijuana use. Maternal drinking in early childhood was the strongest predictor of early adolescent alcohol use.
Conclusions
These findings are discussed with respect to the role of ADHD in the development of adolescent risk outcomes.
Keywords: ADHD, Adolescent Substance Use, Longitudinal Outcome Study
Children diagnosed with ADHD are at risk for a number of adverse academic and behavioral impairments in adolescence (Barkley, Fischer, Edelbrock, & Smallish, 1990; Kent et al., 2011; Molina & Pelham, 2003; Molina et al, 2009; Sibley et al., 2011). Among these poor outcomes, one of the most dangerous and concerning is adolescent substance abuse because it is associated with serious drug and alcohol addictions in adulthood (Kandel, 2002; Palmer et al., 2009). Longitudinal follow-up studies of children diagnosed with ADHD often find that these youth display elevated rates of substance use and abuse during adolescence (Barkley et al., 1990; Burke, Loeber, & Lahey, 2001; Hartsough & Lambert, 1987; Mannuzza et al., 1991; Milberger, Biederman, Faraone, Wilens, & Chu, 1997; Molina & Pelham, 2003; Molina, Pelham, Gnagy, Thompson, & Marshal, 2007), although exceptions to this finding exist (Biederman et al., 1997; Bussing, Mason, Bell, Porter, & Garvan, 2010). Additionally, ADHD is more prevalent in adolescent substance abusers than non-users (Kuperman et al., 2001; Szobot et al., 2007). However, the mechanisms and longitudinal pathways through which ADHD increases risk for adolescent substance abuse are insufficiently understood (Charach, Yeung, Climans, & Lillie, 2011; Flory & Lynam, 2003; Lee, Humphreys, Flory, Liu, & Glass, 2011; Molina et al., 2013), necessitating further research in this area (Molina & Pelham, in press).
Although studies between ADHD and comparison adolescents typically report at least one significant group difference in substance use, substantial variability in outcome exists across studies (Charach et al, 2011). Most research examines multiple substances separately (i.e., tobacco, alcohol, marijuana), with some studies finding significant group differences for all substances (e.g., Elkins, McGue, & Iacono, 2007; Wilens et al., 2007) and other studies finding differences for some substances, but not others (e.g., Barkley et al., 1990; Hartsough et al., 1987; Molina & Pelham, 2003). For example, a recent meta-analysis found a significant relationship between ADHD and both tobacco use and Alcohol Use Disorders (aggregating adolescent and young adult studies; Charach et al., 2011). However, the authors were unable to obtain a stable effect for the relationship between ADHD and Cannabis Use Disorders because the six included studies reported inconsistent results (Charach et al., 2011). These results highlight: (1) the possibility of a distinct relationship between ADHD and each substance and (2) substantial outcome heterogeneity within substance.
In addition to outcome variability across studies, children with ADHD also display substantial heterogeneity in substance use within samples. Though children with ADHD likely share a common biological risk for substance use, environmental factors at home, at school, and in peer settings appear to moderate outcome (Zucker, 2006). Within the home setting, family conflict and parenting practices, such as monitoring, may moderate an adolescent with ADHD’s risk for deviant behavior (Molina et al, 2012; Patterson, DeGarmo, & Knutson, 2000). Furthermore, parental drinking is repeatedly implicated as a predictor of adolescent alcohol use, both in ADHD (Biederman, Faraone, Monteaux, & Feighner, 2000) and non-ADHD samples (Chassin, Pillow, Curran, Molina, & Barrera, 1993; Sartor, Lynskey, Heath, Jacob, & True, 2007; Sher, Walitzer, Wood, & Brent, 1991). When a child has ADHD, parents may use alcohol to reduce parenting stress (Pelham et al., 1997, 1998), which contributes to more prevalent drinking in these families (Knopik et al., 2006). In turn, this alcohol use likely incurs a deleterious effect on parenting practices (Pelham & Lang, 1993) and increases youth access to alcohol (Komro, Maldonado-Molina, Tobler, Bonds, & Muller, 2007).
Beyond parent variables, additional situational factors may play a key role in the development of substance use among adolescents with ADHD (Moffit & Caspi, 2001). For example, there is evidence that adolescents who experience academic and social failure during early adolescence may be more prone to heavy substance use in late adolescence (Marshal, Molina, & Pelham, 2003; Townsend, Flisher, & King, 2007). Finally, although there is yet to be evidence that ADHD treatments produce long-term therapeutic benefit or influence adolescent outcome (Molina et al., 2009), ADHD medications (e.g., methylphenidate, atomoxetine) and behavior therapies may acutely reduce concurrent adolescent substance use (Riggs et al., 2011; Thurstone Riggs, Salomonsen-Sautel, & Mikulich-Gilbertson, 2010). Thus, it is possible that an adolescent with ADHD’s treatment history influences his/her substance use trajectory. In sum, efforts to understand the substance use of adolescents with ADHD should include careful consideration of family, academic, and social factors that may increase situational risk.
Another major question is whether the association between ADHD and substance use primarily is driven by a shared association with comorbid conduct problems (Flory et al., 2003). Some studies suggest that both ADHD and CD bestow unique additive risks to the development of adolescent substance use (Abrantes, Strong, Ramsey, Lewinsohn, & Brown, 2005; Burke et al., 2001; Burke, Loeber, White, Stouthamer-Loeber, & Pardini, 2007; Kuperman et al., 2001; Malone, Van Eck, Flory, & Lamis, 2010; Szobot et al., 2007; Wilens, Biederman, Mick, Faraone, & Spencer, 1997). Others find that the influence of ADHD is reduced when comorbid conduct problems are simultaneously modeled (Biederman et al., 1997; Bussing, Mason, Bell, Porter, & Garvan, 2010; Disney, Elkins, McGue, & Iacono, 1999; Lynskey & Fergusson, 1995; Molina & Pelham, 2003). However, there is clear evidence that ADHD precedes CD developmentally (Patterson et al., 2000), and that analysis of the contributions of CD and ADHD to substance use needs to consider their relationship over time. CD may mediate the pathway between ADHD and adolescent substance use either partially (Elkins et al., 2007) or fully (Fergusson, Horwood, & Ridder, 2007). Alternatively, the relationship between ADHD and adolescent substance use may be moderated by comorbid CD (August et al., 2006; Molina, Smith & Pelham, 1999). However, without a longitudinal perspective, these effects are difficult to disentangle statistically because individuals with childhood CD but without ADHD are uncommon, and the shared variance between ADHD and CD is therefore very large (Derefinko & Pelham, in press; Plizska, Carlson, & Swanson, 1999; Waschbusch, 2002). These above-mentioned studies suggest that ADHD and substance use relate in a complicated manner, perhaps involving comorbid CD under some circumstances but not others (Zucker, 2006).
Thus, a major issue in examining the influences of ADHD and CD on later substance use is when the two disorders are measured and whether the proximal relationship between CD and substance use masks the influence of ADHD, which may occur prior to a study’s measurement period (Lahey, Molina, Pelham, Applegate, & Rathouz, under review). For example, in the Collaborative Study on the Genetics of Alcoholism, onset of ADHD occurred before the development of CD, and CD before Alcohol Use Disorder (Kuperman et al., 2001). This interpretation is consistent with developmental models of deviance proneness (e.g., Iacono, Malone, & McGue, 2008; Sher, Grekin, & Williams, 2005; Zucker, 2006) and findings that characteristics of ADHD, such as poor inhibitory control and impulsivity, are important risk factors for many forms of delinquent and antisocial behaviors in adolescence and adulthood, including substance use and disorders (Loeber et al., 1993; Moffit, 1993; Molina et al., 2013; Newman & Wallace, 1993; White et al., 1994). In fact, several investigations find that childhood measures of conduct problems were not reliable predictors of later SUD in an ADHD sample, but concurrent measures in adolescence and adulthood were strongly associated with substance use (Molina & Pelham, 2003; Molina et al., 2007). Accordingly, developmental trajectories may be a key to understanding relations between ADHD, CD, and substance use. For example, Malone and colleagues (2010) reported that escalating ADHD symptoms in early adolescence are highly predictive of adolescent substance use, even in cases of subclinical childhood ADHD severity. In sum, these findings underscore the importance of concurrently measuring ADHD and CD from an early age until the onset of substance abusing behaviors.
Independent of longitudinal course issues, studies of substance use among adolescents with ADHD possess substantial methodological variability (i.e., indices of ADHD and CD; measures of substance use; sample characteristics; periods of follow-up), which may contribute to the heterogeneous results discussed above. Categorical examinations of ADHD and CD may obscure dimensional influences above or below the diagnostic threshold (Burke et al., 2001), just as examining presence of a Substance Use Disorder may hide sub-clinical use (Kuperman et al., 2001; Molina & Pelham, 2003, in press). Individuals first diagnosed with ADHD in adolescence may possess a different clinical profile than samples that were well diagnosed in childhood (Wolraich et al., 2005; Flory et al., 2003). With regard to assessment, most studies rely on retrospective report or a snapshot of dolescent substance use, rather than collecting annual prospective data. These methods are limiting because adolescents, especially those with ADHD, may not provide consistent or valid retrospective report of their behavior (Shillington, Woodruff, Clapp, Reed, & Lemus, 2013; Sibley et al., 2010, 2012) and snapshots may fail to accurately capture development of use (Molina et al., 2007). In sum, the existing literature tells a fractured story about the relationship between ADHD, CD, and substance use. Careful consideration of the above-noted methodological issues, longer follow-up studies within prospective longitudinal samples, and quantitatively sophisticated examinations of the interplay between ADHD, CD, and substance use are needed to disentangle how these behaviors relate (Barkley et al., 1990; Biederman et al., 1997; Charach et al., 2011; Flory et al., 2003; Lee et al., 2011; Molina et al, 1999). Most literature addresses the presence and cross-sectional patterns of substance use in adolescents, but there are fewer longitudinal investigations of the how ADHD and CD may be instrumental in the escalation of use (Molina et al., 2012; Molina & Pelham, in press).
The present study seeks to address these recommendations with a prospective longitudinal study of young children diagnosed with ADHD and demographically similar controls (Lahey et al., 1998). Participants provided almost yearly reports of childhood ADHD and CD symptoms and adolescent substance use until age 18, affording us the ability to examine growth in ADHD and CD as related to substance use. First, we examined ADHD vs. comparison group differences in prevalence, frequency, and age of initiation for tobacco, alcohol, and marijuana use. Second, we examined whether ADHD status increased risk for escalating use amongst adolescents who tried cigarettes, alcohol, and marijuana. Considering a gateway hypothesis, we also examined whether early cigarette use played a mediational role in the relationship between escalated alcohol and marijuana use and childhood ADHD. We also examined proximal predictors of escalated use within the ADHD group, to detect factors that differentiated heavy substance users with ADHD from light/non-users. Finally, we investigated the effect of baseline levels and growth in ADHD and CD symptoms as they inter-relate and predict adolescent substance use patterns. Overall, we hypothesized significant group differences across indices of substance use and expected that heavy use would be associated with greater impairment in functioning during early adolescence. We further hypothesized that early childhood ADHD, but not CD, would uniquely predict adolescent use patterns across substances and that growth in CD would partially mediate the relationship between childhood ADHD and adolescent substance use.
Method
All procedures described herein were approved by the appropriate Institutional Review Boards at both sites.
Participants
Two cohorts of 3.8- to 7.0-year-old children were recruited in consecutive years in both Chicago and Pittsburgh (N=259). Participants lived with their biological mothers, and 133 initially met Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM–IV) diagnostic criteria for ADHD. In Chicago, children with ADHD were recruited from a university child psychiatry clinic. In Pittsburgh, 42% of the children with ADHD were recruited from a university child psychiatry clinic, and 58% were recruited through advertisements, schools, and other community sources, but no differences were found between recruitment methods in any demographic variable or measure of functional impairment in year 1 (Lahey et al., 1998). Comparison children (N=126) were recruited from similar schools and neighborhoods as probands and approximately matched probands on sex, ethnicity, and age. They had never been referred for mental health problems but were not excluded if they met criteria for a disorder other than ADHD. Informed parental consent and oral child assent was obtained for all participants. The comparison group was reduced during years 12 through 14 because of funding limitations. All comparison females were retained, but 50% of other comparison youth were randomly dropped within each year of age and race-ethnic group. Retention was high, with 79.8% to 91.5% of eligible cases assessed in years 6 through 14.
Diagnosis of ADHD was made in year 1 using the standard recommended practice in the field (Pelham, Fabiano, & Massetti, 2005). Symptoms of ADHD were considered to be present if reported by either the parent on the Diagnostic Interview Schedule for Children (DISC; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000) or the child’s teacher on the Disruptive Behavior Disorder (DBD) Rating Scale (Pelham, Gnagy, Greenslade, & Milich, 1992). Impairment was assessed using the DISC and the Impairment Rating Scale completed by parents and teachers (IRS; Fabiano et al., 2006). Children were given the diagnosis of ADHD in year 1 if they met DSM-IV symptom criteria and exhibited impairment in at least one setting (home, peer environments, or school). Because of their young age, all met the age of onset criterion, but cross-situational impairment was not required because not all children with ADHD had (yet) exhibited impairment in school. During the next 7 assessments, 96.0% of children with ADHD exhibited impairment in both home/peer environments and school (Lahey et al., 2004).
The present analyses were based on the 67.4% of the original sample (N=178; 113 with ADHD in Year 1 and 65 comparison children without ADHD) who continued study enrollment in adolescence and completed at least one annual assessment during years 12 through 14. Table 1 displays demographic characteristics of the subsample at Year 1. For both the ADHD and the control groups, there were no significant differences on Table 1 baseline demographic variables with the exception of a higher proportion of females retained (discussed above). The control subsample contained significantly more females and possessed a slightly higher IQ than the ADHD subsample. Subsequent analyses revealed that gender was unrelated to all indices of substance use examined in the current study. IQ was significantly correlated with several indices of substance use. There was a non-significant trend for individuals in the comparison group to possess slightly higher family incomes than the ADHD group and family income was significantly related to several substance use variables in our sample. As a result, IQ and family income were included as a covariate in all models.
Table 1. Characteristics of the Subsample at Year 1 and Follow-up.
| ADHD (N=113) | Control (N=65) | |
|---|---|---|
| Year 1 Demographic and Diagnostic Information | ||
| Gender (% male)* | 84.1 | 67.7 |
| Age M(SD) | 5.19(.74) | 5.20(.80) |
| Family Income M(SD) | $37,942($33,267) | $44,881($34,312) |
| Ethnicity (%) | ||
| White/Caucasian | 63.7 | 60.0 |
| Black/African-American | 30.1 | 36.9 |
| Other | 6.2 | 3.1 |
| IQ M(SD)* | 90.51(14.12) | 100.83(15.12) |
| Diagnosis | ||
| ADHD-PI (%) | 14.5 | -- |
| ADHD-H/I (%) | 5.6 | -- |
| ADHD-C (%) | 43.0 | -- |
| ODD (%) | 59.3 | 4.6 |
| CD (%) | 11.5 | 1.5 |
| Follow-up Variables | ||
| Age at final substance use assessment | 17.55 (.74) | 17.73 (.76) |
| ADHD Symptom Count M(SD) | ||
| Year 1 | 14.78(2.86) | 1.62(2.11) |
| Year 9 | 10.02(5.52) | 3.60(5.20) |
| CD Symptom Count M(SD) | ||
| Year 1 | 1.03(1.30) | .13(.47) |
| Year 9 | .92(1.61) | .23(.79) |
p<.10
Procedure
Participants underwent approximately annual assessments during years 1 (age 5), 2 (age 6), 3 (age 7), 4 (age eight), 6 (age ten), 7 (age 11), 8 (age 12), 9 (age 13), 12 (age 16), 13 (age 17), and 14 (age 18). Visits did not occur in in years 5, 10, and 11 due to funding limitations. During clinic visits, parents and children were assessed by trained interviewers who held at least a bachelor’s degree in psychology or a related field.
Measures
Symptoms of ADHD and CD
Symptoms of ADHD and CD were assessed using the Diagnostic Interview Schedule for Children (DISC; Shaffer, Fisher, Piacentini, Schwab-Stone, & Wicks, 1993), which was administered to mothers at each assessment. Additionally, at each annual assessment, the child’s main teacher completed the DSM–IV version of the Disruptive Behavior Disorders Rating Scale (DBD; Pelham et al., 1992) by mail. Following standard procedures (Pelham et al., 1992), teacher-reported ADHD symptoms rated “pretty much” or “very much” were scored as present. Parent and teacher symptom reports were combined and ADHD symptoms were considered to be present if each symptom was reported by either teacher or parent (Piacentini, Cohen, & Cohen, 1992). For the present study, ADHD and CD symptom counts at years 1-9 were calculated and modeled in order to provide a stable estimate of the trajectory of these symptoms across childhood (see Table 1).
Substance Use
Substance use data were collected during Years 9, 12, 13, and 14 using an adolescent-reported substance use questionnaire (Molina & Pelham, 2003; see Table 2) and compiled to create a history of adolescent substance use for each participant. The measure included items for lifetime and current (past 12 months) use of licit substances (alcohol, cigarettes, chewing tobacco) and illicit drugs (marijuana and other street drugs). The measure was modeled after similar substance use measures in longitudinal or national survey studies of alcohol and other drug use (Donovan, 1994; Jessor, Donovan, & Costa, 1989; National-Household-Survey-on-Drug-Abuse-1992) that also rely on confidential youth self-report as the best source of such data (Winters & Fahnhorst, 2005) and has been employed in a number of published studies (Molina & Pelham, 2003; Molina et al. 2007; 2012; 2013). Reports of past and current cigarette, alcohol, and marijuana use were combined across assessments to provide an estimate of cumulative adolescent use. First, dichotomous variables indicating adolescent initiation (no/yes) of cigarettes (smoked a cigarette, not just a puff), alcohol (had a drink, not just a sip), and marijuana (tried marijuana) were calculated. Age of initiation was also computed for each type of substance by comparing multiple reports and selecting the earliest age at which use was reported (e.g., how old were you the first time you smoked a cigarette?). A frequency of use variable was computed for each substance by selecting the highest frequency of current use (past 12 months) from 12 options (0-11: ranging from 0 = not at all to 11 = several times a day) reported by a participant during all available assessments. For cigarettes, heavy use was defined as daily smoking. For alcohol and marijuana, heavy use was defined as using these substances at least weekly. For the gateway hypothesis models, early cigarette use was defined as age 14 or younger and late cigarette use was defined as age 15 or older, based previous research on the initiation of smoking in youth with ADHD (e.g., Milberger et al., 1997). Substance use was assessed annually until year 14 for a majority of participants (52.8%) with final assessment occurring for 9.6% at year 12 and 37.0% at year 13. Average age at provision of final report was approximately 18 for both groups (17.55 for probands and 17.74 for controls).
Table 2. Initiation and Frequency of Cigarette, Alcohol, and Marijuana Use.
| ADHD % | Control % | X2 | p | OR 95% CI | |
|---|---|---|---|---|---|
| Ever smoked a cigarette | 46.9 | 27.7 | 6.35 | .012 | 1.20-4.45 |
| Frequency of cigarette usea | 15.01 | .059 | ----- | ||
| Not at all | 59.3 | 78.5 | --- | ||
| Less than 1x per month | 11.5 | 9.2 | --- | ||
| 1-3x per month | 0.9 | 3.1 | --- | ||
| 1-6x per week | 1.8 | 3.1 | --- | ||
| Daily | 26.5 | 6.1 | --- | ||
| Ever had a drink | 73.5 | 80.0 | .96 | .326 | .33-1.45 |
| Frequency of alcohol usea | 7.58 | .577 | ----- | ||
| Not at all | 37.2 | 33.8 | |||
| Less than 1x per month | 28.3 | 35.4 | |||
| 1-3x per month | 15.9 | 18.5 | |||
| 1-6x per week | 16.8 | 12.3 | |||
| Daily | 1.8 | 0.0 | |||
| Ever tried marijuana | 53.1 | 50.8 | .09 | .765 | .60-2.02 |
| Frequency of marijuana usea | 20.17 | .043 | ----- | ||
| Not at all | 54.9 | 61.5 | |||
| Less than 1x per month | 18.5 | 21.6 | |||
| 1-3x per month | 3.6 | 9.3 | |||
| 1-6x per week | 12.4 | 1.5 | |||
| Daily | 10.6 | 6.1 |
Significance tests conducted on 12-category response set; however, responses were collapsed into fewer categories for ease of presentation. Frequency variable represents the highest frequency of substance use reported during year 9, 12, 13, and 14 visits.
Functional Impairment
To measure impairment in early adolescence, the IRS (Fabiano et al., 2006) was administered to parents and teachers at year 9. Parents and teachers indicated the adolescent’s impairment severity in seven domains by marking an X on a line representing the continuum from “no problem” to “extreme problem.” Responses were coded 0 (no impairment) to 6 (extreme impairment). Parent and teacher symptom reports were combined at the item level and when disagreement occurred, the more severe rating was retained. The academic (“how the child’s problems affect academic progress at school”), social (“how the child’s problems affect relationships with peers”), and family (administered only to parents; “how the child’s problems affect your family in general”) impairment items were utilized to assess cross-situational impairment. The IRS demonstrates strong psychometrics and accurately identifies impairment in children and adolescents with ADHD across settings and informants (Evans et al., 2013; Fabiano et al., 2006).
Parental Monitoring
Parental monitoring was measured at year 9 using a reliable and well-validated structured interview of the mother (Patterson & Stouthamer-Loeber, 1984). Six items queried the frequency with which adolescents participate in unmonitored activities with peers and the parents’ degree of knowledge about the adolescent’s social activities. Parents indicated their degree of monitoring on 5-point scale (1=Never, 5=Very Often). A total parental monitoring score was computed as the mean of the six item responses.
Growth Curve Model Covariates
Year 1 maternal drinking (alcohol use model only), income, and IQ were used as covariates in the latent growth models. Maternal drinking was obtained for mothers using the Drinking History Questionnaire, which is a measure of drinking behavior that provides information regarding drinking frequency and quantity. As is typically done, a frequency/quantity quotient was calculated as a measure of typical alcohol consumption by multiplying average quantity and frequency of use in the past 12 months (Armor & Polich, 1982; Marlatt, 1973). At year 1, IQ was obtained using the Stanford-Binet Intelligence Scale, Fourth Edition (Thorndike, Hagen, & Sattler, 1996) and family income was reported by the parent.
Results
Initiation of cigarette, alcohol, and marijuana use
To investigate group differences in the initiation of substance use, four 2 (ever tried: yes v. no) × 2 (group: ADHD v. control) chi-square analyses were conducted for each type of substance use (i.e., cigarettes, alcohol, marijuana). Results (see Table 2) indicated that by age 18, adolescents with ADHD were significantly more likely than controls to have tried a cigarette.
Frequency of cigarette, alcohol, and marijuana use
To examine group differences in the frequency of substance use, three 12 (level of substance use: not at all-to several times a day) × 2 (group: ADHD v. control) chi-square analyses were conducted. Results (see Table 2) indicated that adolescents with ADHD used cigarettes and marijuana at higher frequencies than controls, although the effect for cigarettes was only marginally significant (p=.059). Across substances, group differences were particularly pronounced at the higher levels of use (e.g.. 26.5% of ADHD group reporting daily cigarette use v. 6.1% of controls; 23.0% of ADHD group reporting at least weekly marijuana use compared to 7.6% of controls).
Age of cigarette, alcohol, and marijuana initiation
To investigate group differences in age of substance use initiation, three one-way ANOVAs were conducted within subsets of participants who initiated each type of substance use by age 18. Prior to analyses, all data were found to meet the assumptions of the General Linear Model (i.e., linearity, normality, and homogeneity of variance). Results (see Table 3) indicated that adolescents with ADHD initiated cigarette, alcohol, and marijuana use at slightly earlier ages than controls; however, this effect was only statistically significant for alcohol (p=.008).
Table 3. Group differences in age of initiation amongst reported users.
| ADHD M(SD) | Control M(SD) | F | p | d | |
|---|---|---|---|---|---|
| Cigarette Use | 13.35(2.93) | 14.56(2.20) | 2.52 | .117 | .43 |
| Alcohol Use | 14.05(2.02) | 15.02(1.75) | 7.25 | .008 | .50 |
| Marijuana Use | 14.33(2.00) | 15.00(2.07) | 2.18 | .144 | .33 |
Note. Age of initiation data was provided by 69 cigarette users (ADHD N =51; Control N=18), 120 alcohol users (ADHD N=73; Control N=47), and 88 marijuana users (ADHD N=57, Control N=31).
Escalation of Use
To examine whether ADHD status promotes escalation to heavy substance use amongst those adolescents who tried cigarettes, alcohol, and marijuana, we conducted 2 (group: ADHD v. control) × 2 (heavy use: yes v. no) chi-square analyses for each substance. Results indicated that adolescents with ADHD were more likely than controls to escalate to daily cigarette use after smoking their first cigarette (ADHD=56.6%; Control=22.2%; χ2(1) = 6.37, p=.012, OR=4.57, 95% CI=1.33-15.72) and to weekly marijuana use after trying marijuana (ADHD= 43.3%; Control=15.2%; χ2(1) = 7.61, p=.006, OR=4.28, 95% CI=1.45-12.61). Results were in the same direction for alcohol use, but the difference was not statistically significant (ADHD=25.3%; Control=15.4%; χ2(1) = 1.86, p=.172, OR=1.86, 95% CI=.76-4.59).
To examine whether cigarette use served as a gateway to alcohol and marijuana use, we conducted two path models using MPlus 6.12 (Múthen & Múthen, 1998-2010). Analyses were performed using a robust Maximum Likelihood estimator (Huber/White; Huber, 1967; White, 1980) and a logit link function, given the categorical nature of the endogenous mediator. In these models, onset of cigarette use in adolescence (early, late, never) was modeled as a categorical mediator of the relationship between group (ADHD vs. control) and alcohol age of onset and marijuana frequency of use. For the alcohol age of initiation model, there was evidence for partial mediation whereas group marginally predicted early smoking (b=-.61, SE=.33, p=.06, OR=1.83), which in turn significantly predicted early alcohol use (b=.76, SE=.19, p=.01). The direct path from group to age of alcohol initiation was also marginally predictive (b=-.65, SE=.34, p=.06). For the marijuana frequency model, there was evidence for full mediation whereas group marginally predicted early smoking (b=-.61, SE=.33, p=.06, OR=1.83), which in turn significantly predicted frequency of marijuana use (b=-1.85, SE=.32, p<.01). The direct path from group to age of alcohol initiation was nonsignificant (b=.34, SE=.43, p=43). These results should be interpreted with caution given the marginal (p=.06) significance of some paths.
To detect factors that differentiate youth with ADHD who escalated to heavy substance from those who did not, a binomial logistic regression analysis was conducted. Heavy user (yes/no) was the dependent variable, defined as meeting the criteria for heavy use in any of the three substance use categories (i.e., daily cigarette smoking, weekly alcohol or marijuana use). Predictors measured at year 9 (first year of adolescence) included ADHD and CD symptoms, academic, social, and family impairment, parent monitoring. Childhood histories of stimulant medication and psychosocial treatment were also included as predictors, represented as the percentage of childhood assessments at which current treatment utilization was reported by parents. The overall model was significant [χ2(8) = 16.69, p=.034] and results are displayed in Table 4. Adolescents with ADHD were more likely to display heavy substance use (41.3%) if they had less severe problems with peers and more severe problems with family members. There was a marginal effect suggesting that heavy users may possess more severe academic problems.
Table 4. Early Adolescent Predictors of Subsequent Heavy Substance Use within the ADHD Group.
| b | SE | Wald | p | OR | |
|---|---|---|---|---|---|
| ADHD Symptoms | -.04 | .07 | .30 | .59 | .96 |
| CD Symptoms | .34 | .23 | 2.16 | .14 | 1.40 |
| Social Impairment | -.37 | .18 | 4.29* | .04 | .69 |
| Academic Impairment | .33 | .17 | 3.55† | .06 | 1.39 |
| Family Impairment | .36 | .16 | 5.12* | .02 | 1.44 |
| Parent Monitoring | .43 | .62 | .46 | .50 | 1.53 |
| Stimulant Treatment | 1.00 | 1.32 | .58 | .45 | 2.73 |
| Psychosocial Treatment | .03 | 1.23 | .00 | .98 | 1.03 |
Childhood ADHD/CD Symptoms and Adolescent Substance Use
To investigate the relative contributions of ADHD and CD symptoms to the significant group differences noted above, three parallel process models were evaluated for cigarette, alcohol, and marijuana outcomes. Latent Growth Analyses (LGA; Duncan, Duncan, Strycker, Li, & Alpert, 1999) were conducted using MPlus 6.12 (Múthen & Múthen, 1998-2010). Univariate and multivariate outliers were detected; however, the decision was made to retain these cases, as they were believed to represent the population of study. Univariate and multivariate tests of normality indicated that the data did not conform to this assumption. As a result, a Full Information Maximum Likelihood approach using a robust estimator (Huber/White; Huber, 1967; White, 1980) was used to address non-normality in the data.
Model Specification
One hypothesized model was evaluated for each of three separate outcome variables in which there were significant differences between ADHD and controls (cigarette frequency, marijuana frequency, and alcohol age of initiation; see Figure 1). Latent growth variables for ADHD and CD symptoms were first modeled separately to confirm that the latent variables possessed good model fit. For both ADHD and CD, we formulated latent growth factors for intercept and slope with eight time indicators at years 1-9. Factor loadings on the latent slope variables were fixed to 0 for year 1, 1 for year 2, 2 for year 3, 3 for year 4, 5 for year 6, 6 for year 7, 7 for year 8, and 8 for year 9 in order to accurately model the passage of time. Chi-square analyses and examination of model fit indices indicated that both the ADHD [X2 (28)=58.92.; CFI=.97; RMSEA=.07; SRMR=.04] and the CD [X2 (31)=69.66; CFI=.89; RMSEA=.08; SRMR=.10] latent variables possessed acceptable fit for inclusion in the full model. Means and variances for the latent intercept factors were significant for ADHD (MI=10.16, p<.001; DI =37.48, p<.001) and for CD (MI=.86, p<.001; DI =1.18, p<.001). Means and variances for the latent slope factors were significant for ADHD (MS=-.31, p<.001; DS =.41, p<.001). For the CD latent slope factor (MS =-.02, p=.09; DS =.02, p=.01), the mean effect was marginal while the variance was significant.
Figure 1. Hypothesized Model of Associations between ADHD and CD and Adolescent Substance Use.
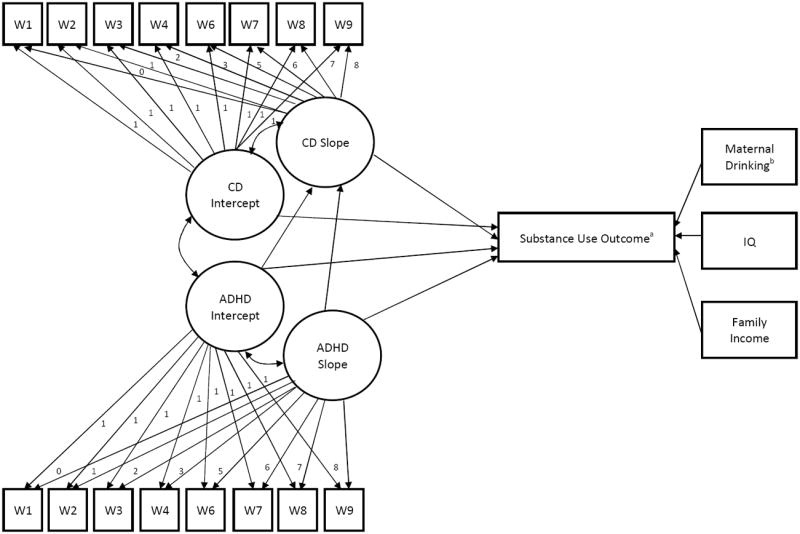
Note. Path diagram represents hypothesized model for the effect of ADHD and CD on substance use. W: Year 1- Year 9 of data collection. Residual terms, means, and variances are estimated but not shown in the model. aModel was tested for each of three statistically significant substance use outcomes: cigarette frequency, marijuana frequency, and age of first drink. bMaternal drinking included in the alcohol initiation model as a covariate.
Evaluating Parallel Process Models
In each of the three parallel process models (see Figure 1), direct paths from the ADHD latent intercept and slope factors to the CD latent slope factor were modeled to examine whether baseline ADHD severity and growth in ADHD severity over time predicted growth in CD severity over time. Direct paths from the latent ADHD and CD intercept and slope factors to substance use outcome were modeled to examine the extent to which absolute levels of ADHD and CD in early childhood (latent intercepts), as well as escalating ADHD and CD across the childhood years (latent slopes), predicted adolescent substance use.
For the cigarette frequency model (N=176), chi-square analyses and examination of model fit indices indicated that the model possessed adequate fit [X2(166)=357.89; CFI=.89; RMSEA=.08; SRMR=.12]. Results (see Table 5) of the path model suggested that after controlling for covariates, the ADHD latent slope factor significantly predicted the CD latent slope factor. Furthermore, the CD latent intercept factor was the only significant predictor of cigarette use frequency in adolescence. The direct path from the CD latent slope factor to cigarette use frequency was marginally predictive. For the marijuana frequency model (N=176), chi-square analyses and examination of model fit indices indicated that the model possessed adequate fit [X2(166)=359.06; CFI=.89; RMSEA=.08; SRMR=.12]. Results (see Table 5) of the path model suggested that after controlling for covariates, the ADHD latent slope factor significantly predicted the CD latent slope factor. The CD latent slope factor significantly predicted marijuana use frequency in adolescence. There were also marginal effects for the ADHD and CD latent intercept factors, as well as IQ, predicting frequency of marijuana use. Finally, for the alcohol age of initiation model (N=146), chi-square analyses and examination of model fit indices indicated that the model possessed adequate fit [X2(182)=399.82; CFI=.87; RMSEA=.09; SRMR=.13]. Results (see Table 5) of the path model suggested that the ADHD latent slope factor significantly predicted the CD latent slope factor. In addition, maternal drinking observed at baseline was significantly predictive of the age at which adolescents had their first drink. There were also marginal effects for the latent ADHD intercept and slope factors predicting alcohol age of initiation.
Table 5. Path Estimates for Latent Growth Models.
| b | SE | t | B | |
|---|---|---|---|---|
| Cigarette Frequency | ||||
| ADHD Intercept → CD Slope | .00 | .00 | .19 | .02 |
| ADHD Slope → CD Slope | .09 | .03 | 3.40** | .45 |
| ADHD Intercept → Cigarette Frequency | .14 | .08 | 1.63 | .19 |
| ADHD Slope → Cigarette Frequency | .55 | .95 | .58 | .08 |
| CD Intercept → Cigarette Frequency | 1.12 | .44 | 2.56* | .28 |
| CD Slope → Cigarette Frequency | 8.50 | 4.73 | 1.80† | .25 |
| IQ →Cigarette Frequency | .01 | .02 | .44 | .03 |
| Income → Cigarette Frequency | .29 | .30 | .97 | .08 |
| Marijuana Frequency | ||||
| ADHD Intercept → CD Slope | .00 | .00 | .18 | .02 |
| ADHD Slope → CD Slope | .09 | .03 | 3.35** | .44 |
| ADHD Intercept → Marijuana Frequency | .12 | .07 | 1.75† | .21 |
| ADHD Slope → Marijuana Frequency | .82 | .62 | 1.32 | .15 |
| CD Intercept → Marijuana Frequency | .58 | .34 | 1.69† | .18 |
| CD Slope → Marijuana Frequency | 8.53 | 3.11 | 2.74* | .31 |
| IQ → Marijuana Frequency* | .03 | .02 | 1.72† | .14 |
| Income → Marijuana Frequency | .04 | .24 | .18 | .01 |
| Alcohol Age of Initiation | ||||
| ADHD Intercept → CD Slope | .00 | .00 | -.53 | -.06 |
| ADHD Slope → CD Slope | .08 | .03 | 3.05** | .45 |
| ADHD Intercept → Alcohol Initiation | -.11 | .06 | -1.66† | -.33 |
| ADHD Slope → Alcohol Initiation | -.73 | .42 | -1.74† | -.24 |
| CD Intercept → Alcohol Initiation | -.16 | .24 | -.66 | -.09 |
| CD Slope → Alcohol Initiation | .00 | 2.25 | .00 | .00 |
| IQ → Alcohol Initiation | -.02 | .02 | -1.16 | -.17 |
| Income → Alcohol Initiation | .17 | .18 | .94 | .10 |
| Maternal Drinking → Alcohol Initiation | -.08 | .04 | -2.42* | -.17 |
Note: b=unstandardized estimate, B=standardized coefficient
=p<.10,
p<.05,
p<.01
Discussion
This study investigated the initiation and escalation of cigarette, alcohol, and marijuana use among adolescents who were diagnosed with ADHD in early childhood and followed regularly until they were approximately 18 years old. Compared to controls, adolescents with ADHD: (1) tried cigarettes at higher rates and smoked cigarettes more frequently, (2) initiated drinking at earlier ages, and (3) smoked marijuana more frequently. Furthermore, for cigarettes and marijuana, adolescents with ADHD who initiated use were four to five times more likely than controls to escalate to heavy use. Among adolescents with ADHD, heavy use by late adolescence was also associated with early adolescent risks that included cigarette smoking, less severe peer problems, and more severe problems with family members. Regarding developmental predictors of adolescent substance use, our findings were that: (1) growth in ADHD symptoms across childhood (latent slope) was the most robust predictor of subsequent growth in CD symptoms, (2) maternal drinking was the most robust predictor of age at first alcohol use, and (3) adolescent substance use was influenced both by ADHD and CD through indirect and direct paths. Below, we discuss these findings.
Consistent with the literature (Charach et al., 2011; Lee et al., 2011; Molina et al, 2013), almost half of adolescents with ADHD (46.9%) reported smoking a cigarette and over a quarter (26.9%) reported daily smoking. Previously reported rates of cigarette initiation for these youth range from 35.2%-57% (Barkley et al., 1990; Burke et al., 2001; Elkins et al., 2007; Hartsough et al., 1987) and daily smoking is also reported to be similarly elevated (29%; Wilens et al., 2007). In our study, youth with ADHD also smoked their first cigarette about a year before controls; however, this effect was not statistically significant. Most remarkably, after smoking their first cigarette, adolescents with ADHD were approximately five times more likely than controls to escalate to heavy (daily) smoking. These data suggest that deficits associated with ADHD may uniquely influence both the tendency to try smoking and the escalation of smoking to daily use (Kollins, McLernan, & Fuemmeler, 2005). Elevated cigarette use among adolescents with ADHD is particularly concerning as youth who smoke cigarettes are at risk for developing later substance use disorders (Palmer et al., 2009) and smokers with ADHD report difficulty quitting (Pomerleau, Downey, Stelson, & Pomerleau, 1995). In the current sample, youth who initiated smoking at early ages were at specific risk for initiating early alcohol use and engaging in frequent marijuana use.
Youth with ADHD began drinking at earlier ages than their peers (see Table 3), which is consistent with some previous research (Milberger et al., 1997). Early initiation of alcohol use is associated with higher levels of problematic drinking behaviors in adulthood such as alcohol dependence, driving while intoxicated, and unintentional injury (Hingson & Zha, 2009; McGue, Iacono, Legrand, Malone, & Elkins, 2001). Not surprisingly, some of these behaviors occur at higher rates in adults with ADHD (Barkley, Murphy, & Fischer, 2008). It is important to note that we did not detect group differences in the initiation, frequency, or escalation of alcohol use. Although some previous studies also report no significant group differences in adolescent alcohol use (Barkley et al., 1990; Biederman et al., 1997; Hartsough et al., 1987), others report that youth with ADHD initiate and use alcohol more frequently than their peers (Elkins et al., 2007; Milberger et al., 1997; Wilens et al., 1997, 2007). Molina and colleagues (2007) point out that elevated alcohol use by adolescents with ADHD may be most evident in middle adolescence, before controls initiate normative heavy drinking. Our age of initiation finding supports this contention- had we ceased substance use assessment at age 14, group differences in initiation and frequency would have emerged because controls did not initiate drinking, on average, until age 15 (see Table 3). There is also evidence that modeling moderators of substance use may be necessary to detect group differences between older ADHD and non-ADHD teens (e.g., Molina et al., 2012).
Although adolescents with ADHD did not try marijuana at higher rates or earlier ages than peers, they used marijuana more frequently (see Table 2). This finding appears consistently in the small literature on ADHD and marijuana use (e.g., Molina et al, 2013). ADHD and control adolescents appear equally likely to try marijuana, but adolescents with ADHD seem to engage in heavier overall use (Bussing et al., 2010; Hartsough et al., 1987; Wilens et al, 2007). By our estimate, 23% of adolescents with ADHD smoked marijuana at least weekly, which is similar to an estimate (27%) by Wilens and colleagues (2007). Further, our analysis of escalation suggests that adolescents with ADHD were approximately four times more likely than controls to escalate to heavy use after trying marijuana once. Unfortunately, relatively little is known about the relationship between ADHD and marijuana use. This is in part because many studies aggregate marijuana with other illicit drugs due to marijuana’s relatively low rates of use, especially among younger teenagers (e.g., Lynskey et al., 1995; Milberger et al., 1997; Molina et al., 1999). As a result, future work is needed to better understand patterns and mechanisms that are specific to marijuana use among adolescents with ADHD. Arguably, the factors influencing marijuana use may be distinct from those involved with other drugs because marijuana differs in its subjective and neuropharmacological effects (Gonzalez, Vassileva, & Scott, 2009).
The findings above suggest that cigarettes and marijuana, but not alcohol, may share a unique escalation pattern within adolescents with ADHD. About half of teens with ADHD tried smoking cigarettes and marijuana, at an average age of approximately 13 to 14 years (see Tables 2 and 3). This pattern of initiation is generally shared by non-ADHD teens. However, after trying cigarettes and marijuana once, teens with ADHD are four to five times more likely than their peers to escalate to heavy use. These data are consistent with previous work documenting that adolescent cigarette and marijuana use may often occur in tandem (Amos, Wiltshire, Bostock, Haw, & McNeill, 2004) and that some youth may use cigarettes to counteract the negative neuropsychological effects of marijuana (Schuster, Crane, Mermelstein, & Gonzalez, 2013). Broad escalation of substance use in adolescence is associated with factors that are specific to the neurocognitive deficits of youth with ADHD (Stice, Myers, & Brown, 1998). Namely, when compared to peers, adolescents with ADHD display higher levels of poor self-control and impulsivity (Barkley, 1997; Sibley et al., 2012)--traits associated with heavy substance use, rapid substance use escalation, and subsequent addiction (Elkins et al., 2007; Fuemmeler, Kollins, & McClernon, 2007; Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Wills & Stoolmiller, 2002). As mentioned, this pattern was not present for alcohol use. Further work is needed to better understand how the pharmacological properties and social roles of various substances may differentiate the use patterns of teens with and without ADHD.
Given heterogeneity in substance use outcome within the ADHD group, we examined early adolescent predictors of subsequent substance use. Our data indicated that impairment levels, but not symptom variables, were significant predictors of use. Specifically, results (see Table 4) suggested that young adolescents with more severe problems with family members, but few problems with peers, were most likely to engage in heavy substance use later in adolescence. These findings are consistent with a recent study (Molina et al., 2012) suggesting a similar paradoxical effect for peer impairment—it may be the case that for adolescents with ADHD, possessing few friends may impede social access to substances. The relationship between problems with family members and heavy substance use echoes developmental findings that high conflict with parents during the teenage years may play a direct role in the escalation of risk behavior (Patterson et al., 2000). Diverging from past research, however, inconsistent parental monitoring in early adolescence was not related to eventual heavy use amongst ADHD teens (Chilcoat & Breslau, 1999; Molina et al., 2012). It is possible that this null finding related to the timing of our measurement—had we measured late adolescent parent monitoring, perhaps an effect would have emerged. Similar to previous studies longitudinal follow-up studies (e.g., Molina et al., 2009), it was also the case that childhood treatment history did not influence adolescent substance use.
With respect to the contribution of ADHD and CD to substance use development, our findings suggest unique influences of both early childhood and later developing ADHD and CD symptoms in predicting cigarette and marijuana frequency (see Table 5). Specifically, there was evidence that early childhood CD, and in the case of marijuana, ADHD (latent intercepts) contributed somewhat to adolescent frequency of use. However, in both models, growth in ADHD symptoms over childhood (latent slope) was the strongest predictor of growth in CD symptoms (latent slope), which in turn was the most consistent predictor of adolescent use (i.e., growth in CD largely mediated the relationship between ADHD and substance use). These findings suggest that measurement timing may contribute to existing discrepant accounts of the relationship between ADHD, CD, and substance use (Flory et al., 2003). Our earliest measures of ADHD and CD symptoms were obtained at a younger age than most other longitudinal studies of ADHD--at an age when CD symptoms are rare. Subsequently, our models captured the predictive role of ADHD symptoms in the development of conduct problems and substance use (see Table 5). Therefore, it may be the case that measuring ADHD symptoms at a very early age removes a statistical artifact that commonly obscures the initial influence of ADHD on eventual substance use development. As others have argued (Burke, Loeber, & Birmaher, 2002; Patterson et al., 2000) these data suggest that intensifying ADHD symptoms predict the emergence of CD symptoms. However, when examining the full chain of childhood and adolescent symptom development, CD symptoms appeared to be a proximal outcome of ADHD that always preceded, but in only some cases directly spurred, adolescent substance use (see Table 5).
For the age of alcohol initiation outcome, our growth curve model results were slightly different from those for cigarettes and marijuana (see Table 5). Although the ADHD latent intercept and slope factors were marginally predictive of outcome, the most robust predictor of early alcohol use was the observed maternal drinking quantity/frequency variable measured in early childhood. This finding was not surprising given the documented link between ADHD and problematic parental substance use that in turn affects parenting practices (Knopik et al., 2006; Pelham & Lang, 1993). It is also likely the case that adolescents with alcohol abusing parents possessed the strongest genetic risks for early alcohol use (Sher et al, 2005, Zucker, 2006), and the easiest access to substances. Thus, our data suggest that the earlier age of alcohol initiation among adolescents with ADHD may at least partially stem from a combination of factors related to maternal alcohol use. Unlike for cigarettes and marijuana, there was no relationship between CD and the alcohol use variable (see Table 5).
In considering the results of this study, limitations are important to acknowledge. First, our sample size prevented us from examining a more complex growth model that included additional covariates, mediators, and moderators of substance use outcome. In addition, this study may have possessed insufficient power to detect some small effects (see Table 5). Additionally, all participants were accompanied to assessments by their mothers. As a result, we did not have a measure of paternal drinking to include in our analyses. Finally, because of their young age, participants were not required to display cross-situational impairment at baseline.
In conclusion, this study suggests that adolescents with ADHD engage in more prevalent substance use across several indices and that these differences may be largely, but not entirely, attributable to very early ADHD symptoms. These findings highlight the need for longitudinal studies that carefully examine the development and interaction of ADHD and CD symptoms as they relate to adolescent substance use, with particular attention to developmental moderators. Our findings further indicate a need to increase the availability of effective treatments for children and adolescents with ADHD (Pelham & Fabiano, 2008; Sibley et al., in press) to reduce risk for developing CD and substance use. These prevention efforts may be most effective when they target family problems and parental drinking. Furthermore, our results suggest that substance use prevention programs may be especially critical for young adolescents with ADHD who have tried gateway substances, such as cigarettes, but have not escalated to frequent use (Winters & Leitten, 2007).
References
- Abrantes AM, Strong DR, Ramsey SE, Lewinsohn PM, Brown RA. Substance use disorder characteristics and externalizing problems among inpatient adolescent smokers. Journal of Psychoactive Drugs. 2005;37:391–399. doi: 10.1080/02791072.2005.10399812. [DOI] [PubMed] [Google Scholar]
- Armor DJ, Polich JM. Measurement of alcohol consumption. In: Pattison E, Kaufman E, editors. Encyclopedic Handbook of Alcoholism. New York: Gardner; 1982. pp. 73–80. [Google Scholar]
- Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. ‘You can’t go without a fag... you need it for your hash’—a qualitative exploration of smoking, cannabis and young people. Addiction. 2004;99:77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- August GJ, Winters KC, Realmuto GM, Fahnhorst T, Botzet A, Lee S. Prospective study of adolescent drug use among community samples of ADHD and Non-ADHD participants. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(7):824–832. doi: 10.1097/01.chi.0000219831.16226.f8. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in adults: What the science says. New York, NY: Guilford Press; 2008. [Google Scholar]
- Biederman J, Faraone SV, Monuteaux MC, Feighner JA. Patterns of alcohol and drug use in adolescents can be predicted by parental substance use disorders. Pediatrics. 2000;106:792–797. doi: 10.1542/peds.106.4.792. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, et al. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Birmaher B. Oppositional defiant disorder and conduct disorder: a review of the past 10 years, part II. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1275–1293. doi: 10.1097/00004583-200211000-00009. [DOI] [PubMed] [Google Scholar]
- Burke JD, Loeber R, White HR, Stouthamer-Loeber M, Pardini DA. Inattention as a key predictor of tobacco use in adolescence. Journal of Abnormal Psychology. 2007;116(2):249–259. doi: 10.1037/0021-843X.116.2.249. [DOI] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? Journal of Child Psychology and Psychiatry. 2001;42(4):493–502. [PubMed] [Google Scholar]
- Bussing R, Mason DM, Bell L, Porter P, Garvan C. Adolescent outcomes of childhood attention-deficit/hyperactivity disorder in a diverse community sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(6):595–605. doi: 10.1016/j.jaac.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Chassin L, Pillow DR, Curran PJ, Molina BS, Barrera M., Jr Relation of parental alcoholism to early adolescent substance use: a test of three mediating mechanisms. Journal of Abnormal Psychology. 1993;102:3–19. doi: 10.1037//0021-843x.102.1.3. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Pathways from ADHD to early drug use. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1347–1354. doi: 10.1097/00004583-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Derefinko KD, Pelham WE. ADHD and Substance Use. In: Sher K, editor. Oxford Handbook of Substance Use and Substance Dependence. in press. [Google Scholar]
- Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. American Journal of Psychiatry. 1999;156:1515–1521. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- Donovan JE. The Teen Drinking Questionnaire. Pittsburgh: Pittsburgh Adolescent Alcohol Research Center, University of Pittsburgh; 1994. [Google Scholar]
- Duncan TE, Duncan SC, Strycker LA, Li F, Alpert A. An Introduction to Latent Variable Growth Curve Models. Mahwah NJ: Lawrence Erlbaum Associates, Inc; 1999. [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Evans SW, Brady CE, Harrison JR, Bunford N, Kern L, State T, Andrews C. Measuring ADHD and ODD Symptoms and Impairment Using High School Teachers’ Ratings. Journal of Clinical Child & Adolescent Psychology. 2013;42:197–207. doi: 10.1080/15374416.2012.738456. [DOI] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, et al. A practical measure of impairment: psychometric properties of the Impairment Rating Scale in samples of children with attention deficit hyperactivity disorder and two schoolbased samples. Journal of Clinical Child & Adolescent Psycholology. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM. Conduct and attentional problems in children and adolescence and later substance use. Drug and Alcohol Dependence. 2007;88:S14–S26. doi: 10.1016/j.drugalcdep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Flory K, Lynam DR. The relation between attention deficit hyperactivity disorder and substance abuse: What role does conduct disorder play? Clinical Child and Family Psychology Review. 2003;6(1):1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. Journal of pediatric psychology. 2007;32:1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Scott JC. Neuropsychological consequences of drug abuse. In: Grant I, Adams K, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. New York: Oxford University Press; 2009. pp. 455–479. [Google Scholar]
- Hartsough CS, Lambert NM. Pattern and progression of drug use among hyperactives and controls: A prospective short-term longitudinal study. Journal of Child Psychology and Psychiatry. 1987;28(4):543–553. doi: 10.1111/j.1469-7610.1987.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W. Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics. 2009;123:1477–1484. doi: 10.1542/peds.2008-2176. [DOI] [PubMed] [Google Scholar]
- Huber PJ. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Vol. 1. Berkeley, CA: University of California Press; 1967. The behavior of maximum likelihood estimates under nonstandard conditions; pp. 221–233. [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Jessor R, Donovan JE, Costa FM. Health Behavior Questionnaire. Institute of Behavioral Science, University of Colorado; Boulder, CO: 1989. [Google Scholar]
- Kandel DB. Stages and pathways of drug involvement: Examining the gateway hypothesis. New York, NY: Cambridge University Press; 2002. [Google Scholar]
- Kent KM, Pelham WE, Molina BSG, Sibley MH, Waschbusch DA, Yu J, et al. The academic experience of male high school students with ADHD. Journal of Abnormal Child Psychology. 2011;39:451–462. doi: 10.1007/s10802-010-9472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Martin NG, et al. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychological medicine. 2006;36:1461–1472. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernan FJ, Fuemmeler BF. Association between smoking and attention-deficity/hyperactivyt disorder symptoms in a population-based sample of young adults. Archives of General Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Komro KA, Maldonado-Molina MM, Tobler AL, Bonds JR, Muller KE. Effects of home access and availability of alcohol on young adolescents’ alcohol use. Addiction. 2007;102:1597–1608. doi: 10.1111/j.1360-0443.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, et al. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. The American Journal of Psychiatry. 2001;158(12):2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Quay HC, Applegate B, Shaffer D, Waldman I, et al. Validity of DSM-IV subtypes of conduct disorder based on age of onset. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:435–442. doi: 10.1097/00004583-199804000-00022. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Molina BSG, Pelham WE, Applegate B, Rathouz PJ. Dynamic covariation of ADHD symptoms with symptoms of other common forms of psychopathology across childhood and adolescence. (under review). Paper submitted for publication. [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Kipp H, Ehrhardt A, Lee SS, et al. Three-year predictive validity of DSM-IV attention deficit hyperactivity disorder in children diagnosed at 4-6 years of age. American Journal of Psychiatry. 2004;161:2014–2020. doi: 10.1176/appi.ajp.161.11.2014. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Wung P, Keenen K, Giroux B, Stouthamer-Loeber M, Van Kammen WB, Maughan B. Developmental pathways in disruptive child behavior. Development and Psychopathology. 1993;3:103–134. [Google Scholar]
- Lynskey MT, Fergusson DM. Childhood conduct problems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. Journal of Abnormal Child Psychology. 1995;23:281–302. doi: 10.1007/BF01447558. [DOI] [PubMed] [Google Scholar]
- Malone PS, Van Eck K, Flory K, Lamis DA. A mixture-model approach to linking ADHD to adolescent onset of illicit drug use. Developmental psychology. 2010;46:1543. doi: 10.1037/a0020549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA. Hyperactive boys almost grown up: V. Replication of psychiatric status. Archives of General Psychiatry. 1991;48:77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. The Drinking Profile: A Questionnaire for the Behavioral Assessment of Alcoholism. University of Washington; 1973. available from Dr Marlatt at marlatt@uwashington.edu. [Google Scholar]
- Marshal GA, Molina BS, Pelham WE., Jr Childhood ADHD and adolescent substance use: an examination of deviant peer group affiliation as a risk factor. Psychology of Addictive Behaviors. 2003;17:293. doi: 10.1037/0893-164X.17.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and Consequences of Age at First Drink. I. Associations With Substance-Use Disorders, Disinhibitory Behavior and Psychopathology, and P3 Amplitude. Alcoholism: Clinical and Experimental Research. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Wilens T, Chu MP. Associations between ADHD and psychoactive substance use disorders: Findings from a longitudinal study of high-risk siblings of ADHD children. The American Journal on Addictions. 1997;6:318–329. [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Development and psychopathology. 2001;13:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Arnold LE, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent Substance Use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a Function of Childhood ADHD, Random Assignment to Childhood Treatments, and Subsequent Medication. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 Years: Prospective follow-Up of children treated for combined type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE. Attention-Deficit/Hyperactivity Disorder and Risk of Substance Use Disorder: Developmental Considerations, Potential Pathways, and Opportunities for Research. Annual Review of Clinical Psychology. doi: 10.1146/annurev-clinpsy-032813-153722. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Cheong J, Marshal MP, Gnagy EM, Curran PJ. Childhood ADHD and growth in adolescent alcohol use: The roles of functional impairments, ADHD symptom persistence, and parental knowledge. Journal of Abnormal Psychology. 2012;121:922–935. doi: 10.1037/a0028260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Smith BH, Pelham WE. Interactive effects of attention deficit hyperactivity disorder and conduct disorder on early adolescent substance use. Psychology of Addictive Behaviors. 1999;13:348–358. [Google Scholar]
- Molina BSG, Pelham WE, Gnagy EM, Thompson AL, Marshal MP. Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcoholism: Clinical and Experimental Research. 2007;31(4):643–654. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Múthen LK, Múthen BO. Mplus: Statistical Analysis with Latent Variables. Los Angeles CA: Múthen & Múthen; 1998-2004. [Google Scholar]
- Newman JP, Wallace JF. Diverse pathways to deficient self-regulation: Implications for disinhibitory psychopathology in children. Clinical Psychology Review, Special Issue: Disinhibition disorders in childhood. 1993;13:699–720. [Google Scholar]
- NHSDA. National Household Survey on Drug Abuse. U.S Department of Health and Human Services, Public Health Service Alcohol, Drug Abuse and Mental Health Administration. National Institute on Drug Abuse; Rockville, MD: 1992. [Google Scholar]
- Palmer RC, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug and Alcohol Dependence. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GR, DeGarmo DS, Knutson N. Hyperactive and antisocial behaviors: Comorbid or two points in the same process? Development and Psychopathology. 2000;12:91–106. doi: 10.1017/s0954579400001061. [DOI] [PubMed] [Google Scholar]
- Patterson GR, Stouthamer-Loeber M. The correlation of family management practices and delinquency. Child development. 1984:1299–1307. [PubMed] [Google Scholar]
- Pelham W, Fabiano G. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2008;37:184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34(3):449–76. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr, Gnagy E, Greenslade KE, Milich R. Teacher ratings of DSM–III–R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Lang AR. Parental alcohol consumption and deviant child behavior: Laboratory studies of reciprocal effects. Clinical Psychology Review. 1993;13:763–784. [Google Scholar]
- Pelham WE, Lang AR, Atkeson B, Murphy DA, Gnagy EM, Greiner AR, et al. Effects of deviant child behavior on parental distress and alcohol consumption in laboratory interactions. Journal of Abnormal Child Psychology. 1997;25:413–424. doi: 10.1023/a:1025789108958. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Lang AR, Atkeson B, Murphy DA, Gnagy EM, Greiner AR, et al. Effects of Deviant Child Behavior on Parental Alcohol Consumption: Stress-Induced Drinking in Parents of ADHD Children. The American Journal on Addictions. 1998;7:103–114. [PubMed] [Google Scholar]
- Piacentini JC, Cohen P, Cohen J. Combining discrepant diagnostic information from multiple sources: are complex algorithms better than simple ones? Journal of Abnormal Child Psychology. 1992;20:51–63. doi: 10.1007/BF00927116. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Carlson CL, Swanson JM. ADHD with comorbid disorders: Clinical assessment and management. New York: Guilford; 1999. [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. Journal of substance abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Riggs PD, Winhusen T, Davies RD, Leimberger JD, Mikulich-Gilbertson S, Klein C, Liu D. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:903–914. doi: 10.1016/j.jaac.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102:216–225. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Schuster RM, Mermelstein R, Gonzalez R. Compensatory effects of tobacco on declarative memory among cannabis users. (under review). Paper submitted for publication. [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Piacentini J, Schwab-Stone M, Wicks J. Diagnostic Interview Schedule for Children. New York, NY: Columbia University; 1993. [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Review of Clinical Psychology. 2005;1(1):493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Shillington AM, Woodruff SI, Clapp JD, Reed MB, Lemus H. Self-reported age of onset and telescoping for cigarettes, alcohol, and marijuana: Across eight years of the National Longitudinal Survey of Youth. Journal of Child and Adolescent Substance Abuse. 2012;21:333–348. doi: 10.1080/1067828X.2012.710026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, Smith BH. Pharmacological and psychosocial treatments for ADHD in adolescents: An updated systematic review of the literature. Paper submitted for publication. doi: 10.1016/j.cpr.2014.02.001. under review. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Waschbusch DA, Gnagy E, Babinski DE, et al. Inconsistent self-report of delinquency by adolescents and young adults with ADHD. Journal of Abnormal Child Psychology. 2010;38:645–656. doi: 10.1007/s10802-010-9404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, Biswas A, et al. The delinquency outcomes of boys with ADHD with and without comorbidity. Journal of Abnormal Child Psychology. 2011;39:21–32. doi: 10.1007/s10802-010-9443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, Garefino A, Kuriyan AB, Babinski DE, Karch KM. Diagnosing ADHD in Adolescence. Journal of Consulting and Clinical Psychology. 2012;80:139–150. doi: 10.1037/a0026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Myers MG, Brown SA. A longitudinal grouping analysis of adolescent substance use escalation and de-escalation. Psychology of Addictive Behaviors. 1998;12:14. [Google Scholar]
- Szobot CM, Rohde LA, Bukstein O, Molina BSG, Martins C, Ruaro P, et al. Is attention-deficit/hyperactivity disorder associated with illicit substance use disorders in male adolescents? A community-based case–control study. Addiction. 2007;102:1122–1130. doi: 10.1111/j.1360-0443.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet Intelligence Scale. Chicago, IL: Riverside Press; 1986. [Google Scholar]
- Thurstone C, Riggs PD, Salomonsen-Sautel S, Mikulich-Gilbertson SK. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:573–582. doi: 10.1016/j.jaac.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L, Flisher AJ, King G. A systematic review of the relationship between high school dropout and substance use. Clinical Child and Family Psychology Review. 2007;10:295–317. doi: 10.1007/s10567-007-0023-7. [DOI] [PubMed] [Google Scholar]
- Waschbusch DA. A meta-analytic examination of comorbid hyperactive-impulsive-attention problems and conduct problems. Psychological Bulletin. 2002;128(1):118–150. doi: 10.1037/0033-2909.128.1.118. [DOI] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. [Google Scholar]
- White JL, Moffitt TE, Caspi A, Bartusch DJ, Needles DJ, Stouthamer-Loeber M. Measuring impulsivity and examining its relationship to delinquency. Journal of Abnormal Psychology. 1994;103:192–205. doi: 10.1037//0021-843x.103.2.192. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Mick ME, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset of substance use disorders. Journal of Nervous and Mental Disease. 1997;185:475–482. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adamson J, Sgambati S, Whitley J, Santry A, Monuteaux MC, et al. Do individuals with ADHD self-medicate with cigarettes and substances of abuse? Results from a controlled family study of ADHD. American Journal on Addictions. 2007;16(Suppl 1):14–21. doi: 10.1080/10550490601082742. [DOI] [PubMed] [Google Scholar]
- Wills TA, Stoolmiller M. The role of self-control in early escalation of substance use: a time-varying analysis. Journal of Consulting and Clinical Psychology. 2002;70:986. doi: 10.1037//0022-006x.70.4.986. [DOI] [PubMed] [Google Scholar]
- Winters KC, Fahnhorst T. Assessment issues in adolescent drug abuse treatment research. In: Galanter M, editor. Recent Developments in Alcoholism, Alcohol Problems in Adolescents and Young Adults. Vol. 17. New York: Kluwer Academic/Plenum; 2005. pp. 409–425. [DOI] [PubMed] [Google Scholar]
- Winters KC, Leitten W. Brief interventions for moderate drug abusing adolescents. Psychology of Addictive Behaviors. 2007;21:151–156. doi: 10.1037/0893-164X.21.2.249. [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Wibbelsman CJ, Brown TE, Evans SW, Gotlieb EM, Knight JR, Wilens T, et al. Attention-deficit/hyperactivity disorder among adolescents: a review of the diagnosis, treatment, and clinical implications. Pediatrics. 2005;115:1734–1746. doi: 10.1542/peds.2004-1959. [DOI] [PubMed] [Google Scholar]
- Zucker RA. The developmental behavior genetics of drug involvement: Overview and comments. Behavioral Genetics. 2006;36:616–625. doi: 10.1007/s10519-006-9070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


