Summary
Intranasal (IN) administration is a widely used method for examining the effect of oxytocin (OT) on social behavior and cognition in healthy subjects and psychiatric populations. IN-OT in humans enhances trust, emotional perception, and empathetic behavior and is under investigation as a potential pharmacotherapy to enhance social functioning in a variety of neuropsychiatric disorders, including autism spectrum disorders (ASD). Nonhuman primates (NHP) are an important model for understanding the effect of OT on social cognition, its neural mechanisms, and the development of IN-OT as a pharmacotherapy for treating social deficits in humans. However, nonhuman primates and even some human populations, such as very young infants and children, cannot easily follow the detailed self-administration protocol used in the majority of human IN-OT studies. Therefore, we evaluated the efficacy of several OT-administration routes for elevating central OT concentrations in rhesus macaques. First, we examined the effect of IN and intravenous (IV) routes of OT administration on concentrations of OT and vasopressin (AVP) in plasma and lumbar CSF. Second, we examined these same measures in monkeys after an aerosolized (AE) OT delivery route. All three administration routes significantly increased plasma OT concentrations, but only the AE-OT route significantly increased concentrations of CSF OT. No route affected concentrations of AVP in plasma or CSF. This study confirms that the AE route is the most effective method for increasing central OT concentrations in monkeys, and may also be an effective route, alternative to IN, for administering OT to some human populations.
Keywords: Oxytocin, vasopressin, social cognition, intranasal, autism, rhesus monkey
Introduction
The neuropeptide oxytocin (OT) has been shown to modulate social cognition and social behavior in a wide range of mammalian species, including humans. For example, in animals OT enhances social information processing, social recognition, maternal care, and social attachment through actions on central OT receptors (Ross and Young, 2009). Because of its enhancing effect on prosocial behavior and social cognition, research on the potential for OT’s use as a pharmacotherapy to treat the social deficits associated with a number of psychiatric disorders, most notably autism, has dramatically increased (Young and Flanagan-Cato, 2012). However, OT is a large peptide molecule and resultantly fails to cross the blood brain barrier efficiently. In fact, peripheral administration of OT results in the entry of less than 1% of the exogenous neuropeptide into brain areas protected by the blood-brain barrier (BBB; Ermisch et al., 1985). This impermeability of the BBB endogenously allows for independent regulation of central and peripheral neuropeptide concentrations, resulting in its discrete behavioral and physiological functions (Landgraf and Neumann, 2004). In the periphery, for example, OT is essential for regulating a variety of female reproductive functions including uterine contraction during labor and milk stimulation during nursing, but it is thought that it is its central function in the brain that leads to the reported prosocial effects (Churchland and Winkielman, 2011). Thus, the failure of peripheral OT to readily penetrate the BBB has necessitated the development of alternative methods to effectively administer OT to the brain and further characterize its prosocial behavioral effects.
One such method involves a nasal administration of peptides, which is presumed to enable these compounds to pass across the BBB at the level of the nasal epithelium. It is hypothesized that the rapid turnover of cells in the nasal epithelium leads to the loosening of tight junctions facilitating the transport of large molecules into the lamina propria (Altner and Altner-Kolnberger, 1974). Once in the lamina propria the molecules can either be absorbed into blood vessels and enter peripheral circulation or diffuse into perineural or perivascular spaces and gain access to the CNS (Lochhead and Thorne, 2011). In 2002, Born and colleagues reported that an intranasally sprayed administration of arginine vasopressin (AVP), a peptide that is structurally-related to oxytocin, effectively elevated levels of AVP in the CSF of humans (Born et al., 2002). Based on these encouraging findings, researchers have now almost exclusively adopted the intranasal (IN) spray administration method to examine the effects of OT on human social cognition (Guastella and MacLeod, 2012). These studies report numerous effects of IN-OT on human social cognition, including enhanced eye gaze, increased trust, better recognition of facial emotions, improved face memory, and empathy (Domes et al., 2010; Guastella and MacLeod, 2012; Guastella et al., 2008; Kosfeld et al., 2005; Rimmel et al., 2009; Savaskan et al., 2008). Moreover, several independent studies have now demonstrated the ability of IN-OT to enhance social congition in ASD (Anagnostou et al., 2012; Andari et al., 2010; Bartz and Hollander, 2008; Guastella et al., 2010; Tachibana et al., 2013). Given the promising therapeutic potential of IN-OT, and other drugs that target the oxytocinergic system, in promoting social functioning (Modi and Young, 2012), it is critical to develop a clear understanding of mechanisms by which OT influences social cognition including its site of action.
Preliminary evidence in humans supports the assertion that IN administration provides some access to the central compartment. Nasal delivery of several peptides in humans, including insulin, α-melanocyte stimulating hormone, and AVP results in their increased concentrations in cerebrospinal fluid (CSF) over baseline levels (Born et al., 2002). Similarly, CSF OT concentrations are higher in humans who received IN-OT spray compared to those who received a placebo/saline treatment (Striepens et al., 2013). A more thorough characterization of the pharmacokinetic/pharmacodynamic profile of OT after intranasal administration, though, is limited due to the invasiveness of measuring central peptide concentrations in humans. Nor would humans be ideally suited for further mechanistic studies using novel primate selective OT agonists/antagonists, microdialysis or in vivo electrophysiology to investigate the role of the oxytocinergic system in modulating social cognition. Animal models are critically needed to more fully understand the precise neural mechanisms by which OT modulates social cognition and behavior, for developing appropriate profiles of therapeutic doses, and exploring the effects of repeated administration on social behavior and brain function over longer time scales. Additionally, neither animals, such as nonhuman primates, nor some populations of humans, such as young children and infants, are able to conform to the detailed self-administration protocol used in the majority of human IN-OT studies.
Both rodents and nonhuman primates have been employed to assess the efficacy of IN administration in permeating the BBB and the behavioral consequences. In rat, nasally administered OT has been shown to increase extracellular concentrations of OT in limbic brain regions using microdialysis (Neumann et al., 2013). Non-human primates, however, present several advantages over rodent models for evaluating the efficacy of administration routes with humans, including similar nasal architecture, greater homology in the OT receptor system, and more complex social behavioral repertoires (Donaldson and Young, 2008; Harkema, 1990; Klein et al., 2009). Moreover, the neuroanatomical distribution of OT receptors in rhesus macaques and the coppery titi monkey have recently been described, providing insights into the neural mechanisms of OT action in the brain in these NHP model organisms (Freeman et al, submitted; Freeman et al, submitted). Here, we performed two separate studies to evaluate the efficacy of several administration routes for delivering OT to the central nervous system in rhesus monkeys. The first study compared the two primary routes utilized in the majority of human studies, intranasal spray (IN) and intravenous (IV) injection. Six rhesus monkeys were administered OT using these two methods under anesthesia and the resulting concentrations of OT and AVP in plasma and lumbar cerebrospinal fluid (CSF) were measured. Since OT in the hypothalamus can modulate central AVP release, AVP concentrations were also measured as an indicator of potential feed-forward modulation of the neurohypophyseal system (Neumann et al., 2006).
In the second study, a passive administration route using aerosolized OT (AE) was evaluated. This route has been shown to elicit effects on social behavior and perception in monkeys in three separate studies (Chang et al., 2013; Ebitz et al., 2013; Parr et al., 2013). Chang and colleagues (2013) were the first to show preliminary evidence that the AE route could be effective in elevating central concentrations of OT in two rhesus monkeys. The results of the present study will be extremely important not only in identifying effective administration routes for delivering OT to nonhuman primates, but also in establishing some alternative routes for administering OT to human populations, such as infants and children, or some psychiatric populations who may, like monkeys, be unable to easily follow the detailed self-administration protocols used in the majority of IN-OT studies in adults. This study also represents the first detailed report of the impact of IN, IV and AE-OT administration on both plasma and CSF concentrations of OT and AVP in primates.
Methods
Subjects and OT Administration
All OT administration and cerebrospinal fluid/blood collection procedures were performed in adult male rhesus monkeys (Macaca mulatta; 9–14kg) that were anesthetized via ketamine induction (5–10 mg/kg) and then maintained with intravenous propofol (10 mg/kg/min) under veterinary supervision. An intravenous anesthetic was used, despite its greater effect on the cerebrovasculature, to facilitate normal respiration during nasal drug administration. No other respiratory interventions were undertaken to allow for the persistence of normal breathing patterns. After a sufficient depth of anesthesia was achieved, baseline blood and CSF samples were collected. Animals were then dosed with the synthetic OT analogue, Syntocinon (Novartis, Basel, Switzerland), or its placebo (Novartis) using one of three administration routes, described below. Within each experiment, the order of treatment condition was counterbalanced across subjects. Within-subject treatment sessions were separated by a six-week period to maximize recovery of CSF levels, and minimize stress on the individual subjects.
Experiment 1: IN spray and IV-OT Administration
A single cohort of animals was used for Syntocinon (OT) and placebo (X) treatments via both IN spray (IN-OT) and IV injection (IV-OT) routes, resulting in a within-subject experimental design. For IN-OT administration, each animal (N=6) was positioned in an inclined upright position, and 3 100 μl sprays of Syntocinon were delivered directly into each nostril using the Novartis nasal actuator to achieve a dose of 24 IU of Syntocinon (0.6 ml), or an equivalent volume of placebo in an identical delivery device, consistent with most human studies. If substantial amounts of the treatment were observed to run out of the nostril, the actuator was adjusted an additional squirt was administered. For IV administration, the same 6 subjects received a bolus 1.2ml injection of 48 IU of Syntocinon or an equivalent volume of placebo into an indwelling venous catheter.
Experiment 2: AE-OT Administration
At the time of this second experiment, which took place approximately six months after the conclusion of the first experiment, the original 6 monkeys were no longer available, so the AE-OT treatment was performed on a separate group of 8 adult male rhesus monkeys using a between-subjects design (N=4 Syntocinon; N=4 placebo). The AE-OT study in Experiment 2 was undertaken in response to the results of Experiment one, in which we were unable to detect increases in CSF OT concentrations following IN- and IV-OT delivery (see Results below), and to extend the preliminary results reported by Chang and colleagues (2013). This was achieved by measuring OT CSF in a larger sample size and including a within-subject baseline measurement by which AE-OT induced changes in CSF OT could be more accurately assessed. A between-subjects design was used to maximize the samples that could be collected from the subjects, while minimizing the total length of the experiment given the physiological constraints of repeated CSF collection. To facilitate aerosolized administration, 24 IU of Syntocinon, or an equivalent volume of placebo, was diluted into sterile saline to a final volume of 2ml. The subjects were then positioned in a lateral recumbent posture to facilitate normal breathing during aerosolized administration. The aerosolized OT was administered via an oro-nasal aerosol mask attached to a nebulizer (PARI Respiratory Equipment, Richmond, VA). The nebulizer aerosolized saline at a rate of 0.5 ml/min, therefore 4 minutes of breathing were required to deliver the 2-ml dose volume.
Sample Collection
Serial CSF samples were collected within each subject from the lumbar region at baseline (prior to drug administration) and then 60 and 120 min after administration. CSF samples were collected by passive drip through a 22 G needle inserted into the lumbar subarachnoid space for each sample. Any sample with a pink tinge (indicating the presence of red blood cells) was discarded. Three attempts to obtain a clean sample were made for each time point, if a clean sample could not be obtained, that time sample was not collected during that session and the treatment was re-administered at a later date (with at least six weeks intervening) to obtain any missed samples for each subject. Plasma samples were collected at baseline and then 60 and 120 min after IN-OT administration and at 5, 15, 60 and 120 minutes after IV and AE-OT administration. Plasma samples were collected through independent venipuncture of the femoral vein for each sample. Time points were chosen to maximize the likelihood of identifying a potential OT peak within the ethical considerations limiting the collection of serial CSF samples (Born et al., 2002). The additional 5 and 15 minute plasma sampling time points after IV administration were included to capture any rapid changes in OT concentrations due to the short half-life of OT in the blood stream. Each blood sample was 3ml and each CSF sample was 300μl in volume. Blood was collected into two 2 ml Eppendorf tubes containing 10mg of EDTA and stored on ice until the end of the session to prevent coagulation and limit protease activity. CSF was placed immediately on dry ice after collection. At the conclusion of each session, the blood samples were spun at 3000 rpm at 4°C for 15 minutes after which the plasma fraction was collected. All samples were stored at −80°C until analysis. All procedures were approved by the Institutional Animal Care and Use Committee of Emory University and were in compliance with regulations governing the ethical treatment of animals.
Radioimmunoassay
All concomitantly collected plasma and CSF samples to be compared directly were treated identically, i.e. transported, extracted and assayed in the same batch at the same time. In brief, samples were extracted using a heat activated (700°C for 3 hrs.) LiChroprep Si60 (Merck, Whitehouse Station, NJ). The lyophilized extract was then divided to assay both neuropeptides from the same sample. Both the OT and AVP radioimmunoassays (RIAgnosis, Munich, Germany) are highly sensitive and specific and were strictly standardized and validated in many rodent and human studies using a wide variety of stimuli (hypertonicity, parturition, lactation, stress, etc.) to reliably detect bioavailable neuropeptides in both the central (microdialysates, CSF) and peripheral (plasma) compartments (Landgraf, 1985; Neumann et al., 1993; Neumann and Landgraf, 2012). Samples were incubated with anti-OT and anti-AVP antibodies (raised in rabbits) and 125I-labeled neuropeptide tracers (after a one hour pre-incubation period; PerkinElmer, Salem, MA) for three days at 4°C as previously described (Neumann et al., 2013). Assay sensitivities are in the 0.1–0.5pg/sample range, cross-reactivity with related peptides and degraded fragments is <0.7% and intra- and inter-assay variability is <10%. Data are presented as pg/ml in most cases. Unfortunately the plasma samples for the AE-OT group thawed, resulting in a shift towards lower OT values for the plasma samples, which contain high levels of peptidases unlike the CSF samples.
Statistical Analysis
The OT and AVP concentrations sampled from the intranasal spray (IN-OT) and the intravenous (IV-OT) conditions (Experiment 1) were compared in a 2-way repeated measures ANOVA with time point (0, 60 and 120 minutes or 0, 5 15, 60, 120 minutes) and drug condition (OT vs. placebo) as within-subject factors. Separate analyses were performed on the plasma and CSF samples. Significant effects were followed up by a post-hoc comparison using Bonferroni correction procedure. Peptide levels from the intranasal aerosolized (AE-OT) condition (Experiment 2) were compared independently using a 2-way mixed model ANOVA with time point (0, 60 and 120 minutes) as the repeated factor and drug condition (OT and placebo) as a between-subject factor. Significant effects of time were followed up with a post hoc comparison using Bonferroni correction procedure, adjusting alpha levels based on the number of comparisons.
Results
Experiment 1
Effect of Intravenous OT Administration on Central and Peripheral OT Levels
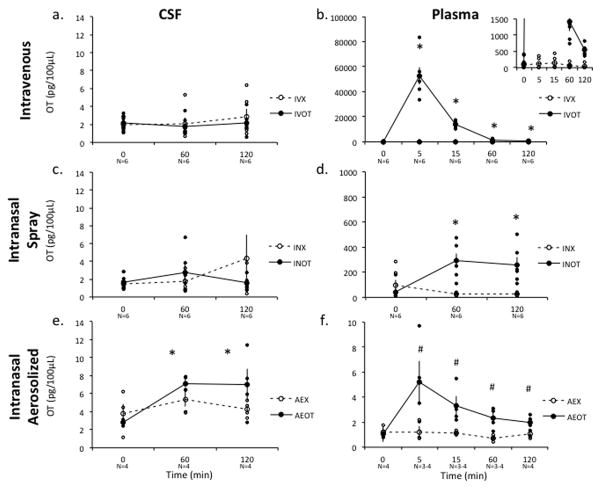
IV-OT resulted in a significant, 1,000-fold increase in plasma OT after 5 min which was rapidly cleared, but nonetheless remained significantly elevated over the placebo condition for the full 120 minute sampling session (Figure 1b). Specifically, a 2-way repeated measure ANOVA revealed a significant main effect of treatment [F(1,5)=2, p<0.001] and time [F(4,20)=53.079, p<0.001] and a significant interaction between these variables [F(4,20)=52.957, p<0.001]. A post-hoc analysis Student’s T-test with a Bonferroni correction revealed an elevation in plasma level over baseline at 5 (IV-X vs. IV-OT, p=0.0006), 15 (IV-X vs. IV-OT, p=0.0002), 60 (IV-X vs. IV-OT, p=0.006) and 120 (IV-X vs. IV-OT, p=0.0008) minutes after administration IV-OT. had no impact on lumbar CSF concentrations across treatment [F(1,5)=0.387, p=0.561] or time [F(2,10)=0.663, p=0.537] conditions and there was no significant interaction [F(2,4)=0.408, p=0.69] (Figure 1a).
Figure 1. Effect of AE, IN and IV OT on Central and Peripheral Oxytocin (OT) Levels.
AE-OT administration resulted in significant accumulation of OT in the lumbar CSF of rhesus monkeys, however neither IN-OT nor IV-OT resulted increases in CSF OT concentrations compared to either baseline (Time= 0) or placebo (X), as measured in lumbar CSF samples (a,c,e). However, IN, AE and IV routes of administration did increase plasma levels of OT compared to placebo treatments and over the pre-administration time points (b,d,f). Plasma concentrations of OT dramatically increased 100 fold after IN-OT administration and 1000 fold after IV-OT administration at both the 60 and 120 minute time points (b; inserted graph better depicts the elevated levels at later time points for the IV-OT with a narrowed scale relative to the larger graph). Raw data points for the treatment condition are depicted as closed circles and raw data points for the placebo condition are depicted as open circles. Bars indicate S.E.M. * Indicates a significant difference in OT levels in post-hoc comparisons with a probability of error <0.01. # Indicates a significant difference in OT levels in a post-hoc comparisons with a probability error of <0.05, which does not satisfy a Bonferroni correction. OT-Syntocinon condition; X-Placebo condition.
Effect of Intranasal Spray OT Administration on Central and Peripheral OT Levels
IN-OT administration significantly increased plasma levels of OT but not CSF levels of OT compared to placebo administration (Figure 1c–d). OT levels in the plasma were elevated 100-fold over baseline for 120 minutes after IN-OT administration. In a 2-way repeated measures ANOVA of plasma levels, there was a significant main effect of treatment condition on plasma OT levels after IN-OT compared to placebo administration [F(1,5)=10.072, p=0.005], but not time [F(2,4)=2.182, p=0.229]. There was a near significant interaction between treatment condition and time [F(2,4)=4.995, p=0.08]. A post hoc Student’s T-test with a Bonferroni correction showed all post OT administration times points to be elevated above placebo treatment (60 min p=0.004; 120 min p=0.01). No significant effect of either treatment [F(1,5)=0.248, p=0.64] or time [F(2,10)=0.997, p=0.403] or interaction [F(2,4)=0.777, p=0.519] was detected in a repeated measure 2-way ANOVA of OT CSF levels.
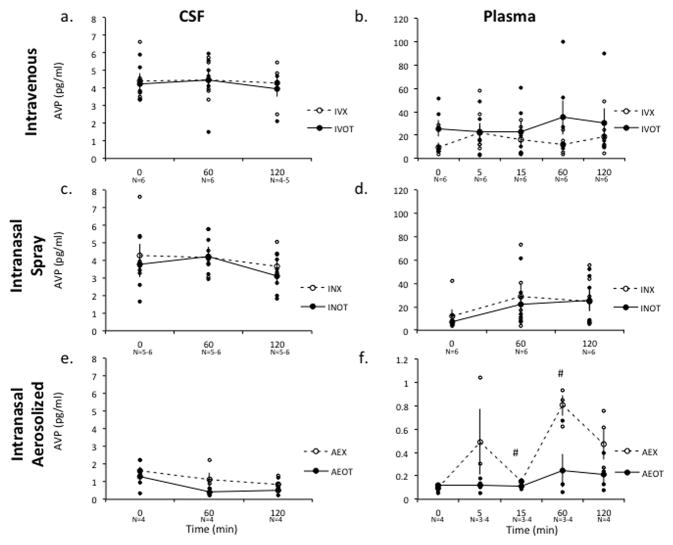
Effect of IN and IV OT Administration on Central and Peripheral Vasopressin Levels
Neither treatment nor route of administration had a significant effect on either CSF or plasma levels of AVP (Figure 2a–f). A 2-way repeated measures ANOVA comparing AVP levels in CSF revealed no significant main effect of treatment or time with IV administration [F(1,2)=0.033, p=0.872; F(2,4)=0.036, p=0.966, respectively] or IN administration [F(1,5)=1.56, p=0.267; [F(2,10)=0.979, p=0.451], respectively]. Likewise, there was no significant change in plasma AVP levels relative to treatment or time with IV administration [F(1,5)=2.11, p=0.206; F(4,20)=0.726, p=0.649, respectively] or IN administration [F(1,5)=0.44, p=0.536; F(2,10)=1.953, p=0.256, respectively].
Figure 2. Effect of IN and IV OT on Central and Peripheral Vasopressin (AVP) Levels.
Due to the cross affinity of the OT and AVP peptides for both OT and AVP receptors in the brain and periphery, the effect of OT on AVP levels was measured to assess for potential feed forward activation of the AVP system. Administration of neither AE-OT, IN-OT nor IV-OT resulted in significant measurable increases in central (a,c,e) or peripheral (b,d,f) AVP levels compared to either baseline (Time = 0) or placebo (X), as measured in lumbar CSF or plasma samples. Raw data points for the treatment condition are depicted as closed circles and raw data points for the placebo condition are depicted as open circles. Bars indicate S.E.M. * Indicates a significant difference in OT levels in post-hoc comparisons with a probability of error <0.01. # Indicates a significant difference in OT levels in a post-hoc comparisons with a probability error of <0.05, which does not satisfy a Bonferroni correction. OT-Syntocinon condition; X-Placebo condition.
Experiment 2
Effect of Aerosolized Intranasal OT Administration on Central and Peripheral OT Levels
AE-OT significantly increased OT concentrations in the lumbar CSF of rhesus monkeys compared to pretreatment baseline or placebo (Figure 1e). A 2-way mixed model ANOVA revealed a significant main effect of the repeated measure of time [F(2,5)=31.965, p=0.001] and a near significant interaction between time and treatment condition [F(2,5)=5.355, p=0.057] for CSF OT concentrations. A Student’s T-test with a Bonferroni correction post hoc analysis revealed that in the AE-OT group, CSF OT was significantly elevated over baseline concentrations at the 60 minute time point (p<0.001), while no time points were elevated over baseline in the placebo condition (0 vs. 60 minute p= 0.28 and 0 vs. 120 minute p=0.66). This is in contrast to IN-OT experiment in which no post-hoc comparison showed a significant increase over baseline (Student’s T-test; IN-OT 0 vs. 60 minute p=0.27 and 0 vs. 120 minute p=0.97; IN-X 0 vs. 60 minute p=0.54 and 0 vs. 120 minute p=0.33).
AE-OT resulted in significant increases in plasma OT levels over baseline for two hours after administration (Figure 1f). In the AE-OT condition, there was a significant main effect of time [F(2,10)=19.212, p=0.05] and a significant interaction between time and treatment condition [F(2,10)=28.291, p=0.034]. A post-hoc comparison revealed that in the AE-OT treatment condition, OT levels were elevated in the plasma at 5, 15, 60 and 120 (p=0.046; p=0.026; p=0.026; p=0.019 respectively) minutes after administration relative to baseline, however this increase did not survive a Bonferroni correction. It should be noted that the plasma samples from this experiment thawed, and the absolute values for OT in these samples were significantly lower than for the other groups. Nevertheless, OT remained elevated in the AE-OT compared to placebo plasma samples, which were all handled in a uniformed fashion.
Effect of AE OT Administration on Central and Peripheral Vasopressin Levels
Similarly, there was no effect of time on AVP levels under the AE condition in a two-way mixed model ANOVA (time within subjects and treatment between subjects) in CSF [F(2,12)=2.589, p=0.169] or plasma [F(4,20)=3.266 p=0.248], although for several time points AVP concentrations appeared lower in the AE-OT group compared to placebo. Post hoc comparisons indicated that AVP in plasma was significantly higher after vehicle administration at 15 (p=0.03) minutes and 60 (p=0.03) minutes relative to OT administration, which may indicate an inhibition of vasopressinergic activity by OT. These findings suggest that an increase in CSF OT may alter other neuropeptide systems, whereas peripheral elevation does not (Freund-Mercier and Stoeckel, 1995; Neumann et al., 1994).
Discussion
Our results confirm that aerosolized (AE-OT) OT, but not IN or IV-OT, significantly increased OT concentrations measured from the CSF of anesthetized rhesus monkeys. After AE-OT administration, CSF OT concentration were elevated 2–3 times over baseline levels, which confirms the level of increase seen in a previous study (Chang et al., 2011) after a similar aerosolized administration procedure. The corroboration of these independent assessments confirms the simple conclusion that some routes of nasal delivery facilitate an increase in CSF OT in rhesus monkeys, while other methods do not. Neither IN nor IV-OT administration produced increases in central OT or AVP levels. The IV-OT route could have increased central levels of OT through a low rate of passive diffusion (~1%) of peptide from the plasma to the CSF. However, despite a greater than ten thousand fold increase in plasma OT levels 5 minutes after IV-administration, there was no accompanying increase CSF OT predicted by passive diffusion. Likely, this is due to the extremely short half-life, approximately 2 minutes, of OT in the blood (Mens et al., 1983). One may speculate that prolonged, dramatic elevations of peripheral OT levels, through passive diffusion, may have been necessary to achieve the behavioral effects seen in some studies (Hollander et al., 2003; 2007), although it is unknown the mechanism by which these effects could have been achieved. In our study, the plasma levels of OT fell to a thousand fold increase after 15 minutes and then a hundred fold increase after 60 minutes, indicating rapid degradation of the peripherally injected peptide.
In contrast, based on the findings from human studies, the IN spray administration route was hypothesized to elevate levels of CSF OT based on its ability to access to the central compartment relative to other administration routes. However, similar to our findings from the IV route, this method also failed to produce significantly elevated levels of OT in the CSF of rhesus monkeys. The privileged access of the intranasal method is presumed to result from direct connections between the environment and the central nervous system (CNS) afforded by the nasal mucosa (Guastella et al., 2013). However, the mechanism and efficacy of penetration from the nose to either the CSF or the extracellular fluid (ECF) is dependent on the distribution of the compound along the nasal epithelium. We speculate that the discordance in effect on CSF OT concentrations between the two routes of nasal OT administration, AE and IN, suggests the two methods of application may have different dynamics within the nasal passage of rhesus monkeys and humans. Optimal penetration of the CNS via neuronal pathways requires targeting of the drug to the upper third of nasal cavity in the olfactory region. In human IN-OT studies, this specificity is maximized by providing the participants with detailed instructions for self-administering the IN-spray, which includes holding one nostril closed while deeply ‘sniffing’ as they pump the required number of sprays into the other nostril (Dhuria et al., 2009; Guastella et al., 2013; Lochhead and Thorne, 2011; Striepens et al., 2013). Using these methods, a recent study reported elevated CSF OT levels in humans (Striepens et al., 2013). In our study, several factors could have affected the deposition pattern of droplets within the nasal epithelium of the rhesus monkeys (compared to humans) after the IN procedure, including their head position, the presence of hairs within the nasal cavity that would block the penetration of the spray, and perhaps most importantly the failure of the monkeys to actively ‘sniff’ the compound at the precise moment the spray pump is depressed. In contrast, we propose that the aerosolized delivery method overcomes many of these limitations by increasing the deposition of small aerosolized particles to the posterior area of the nasal cavity and covering a broad surface of the nasal epithelium (Frank et al., 2011). Therefore, we propose that the aerosolized administration route is an extremely effective method for increasing central OT levels in primates. Because this is a passive method of administration that does not require detailed self-administration protocols, it is not only an ideal, effective method for use in nonhuman primate studies, but it would be a recommended route to administer OT to select human populations, like young children and infants, and some psychiatric populations, and thua this route should be evaluated further in these populations.
All three routes, IN, IV and AE, resulted in dramatic increases in plasma OT concentrations. The increase over baseline, though, differed by several orders of magnitude between the three different routes of administration. This difference likely reflects the degree of penetration into the vasculature achieved by the administration routes and the increased dose given in the IV condition to compensate for enzymatic degradation. The dramatic difference in plasma levels between IN and IV administration is consistent with a previous study in humans, in which a four-fold lower intramuscularly dosed OT resulted in a 10 fold higher plasma OT concentration than nasal administration (Landgraf, 1985). The kinetics of the decline from peak OT concentrations also differed between the administration routes. Plasma OT after IV administration rapidly declined after dosing, however, due to the extremely high levels of plasma OT achieved, OT concentrations are still 100 times above baseline 120 minutes after administration. Plasma OT levels after IN administration were maintained at a consistently elevated level throughout sampling, suggesting regulatory mechanisms other than proteolysis potentially including differential absorption or central control (Burri et al., 2008; Landgraf, 1985). As both nasal and peripheral routes of administration result in a sustained increase in plasma OT concentration, one must also consider the possibility that some of the behavioral effects of IN OT reported in human studies are mediated by peripheral OT receptor stimulation.
Finally, there are several important issues that should be considered in the interpretation and implications of these findings. First, although studies of the behavioral effects of IN-OT in humans do not necessarily implicate specific mechanisms by which OT is exerting its effects, it is commonly presumed that they are, at least in part, centrally-mediated due to the reported efficacy of IN administration in increasing CSF OT levels (Born et al., 2002; Striepens et al., 2013). However, given the data reported here, it does not appear that an IN-OT route in monkeys produces the same central penetrance as this method in humans (Striepens et al., 2013). Therefore, these results have important implications for the methodology to be used in future studies that aim to explore the effects of OT on primate behavior and neurophysiology. While it may be desirable to measure these effects using comparable methods as human studies, e.g., IN-spray administration, this method may not achieve the similar dosing effects in monkeys as it does in humans. Second, regardless of the method used to show elevated levels of CSF OT, IN in humans or AE in monkeys, these results may not be achieved through BBB penetration alone. The endogenous central release of peptides stimulated by the nasaly administered peptide cannot be ruled out. However, based on the lack of a barrier between the extracellular fluid (ECF) and the CSF, changes in CSF OT concentration are likely to be indicative of changes in OT concentrations in brain and thus its bioavailability for behavioral effects. A recent study in rodents, for example, confirmed the ability of nasally administered OT to increase OT in the ECF of limbic brain areas while not detecting an increase is CSF OT (Neumann et al., 2013). Changes in CSF OT levels after OT administration, therefore, may be an underestimate of the changes occurring in the brain, as only a small portion of peptides reach the CSF by diffusion or bulk flow (Landgraf and Neumann, 2004). Finally, human IN-OT studies often suffer from a high degree of inter-subject variability and small effect sizes. Variability in the self-administration procedures to deliver IN-OT have been implicated as one possible source of this variability, leading authors to propose more standardized guidelines to be followed in human studies (Guastella et al., 2013). Because it eliminates the need for user involvement, the AE method may actually represent a more consistent method for dosing participants in behavioral studies, particularly in certain populations such as young children and infants or some psychiatric populations that may not be able to follow detailed self-administration protocols. This could result in larger effect sizes by reducing inter-subject variability in the administration of this peptide.
Acknowledgments
The authors would like to thank the YNPRC veterinary staff for their vital participation in the collection of samples and Dr. Mar Sanchez for her advice and assistance. We would also like to acknowledge funding support from NSF Center for Behavioral Neuroscience Pilot Grant (LAP), Emory Neuroscience Initiative Seed Grant (MEM, LJY, LAP), Center for Translational Social Neuroscience Pilot Grant (LAP, LJY), NIH MH068791 (LAP) and MH064692 (LJY), P50MH100023 (LJY and LAP) and National Center for Research Resources P51RR165 to YNPRC, which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132.
Grants
NSF Center for Behavioral Neuroscience Pilot Grant (LAP), Emory Neuroscience Initiative Seed Grant (MEM, LJY, LAP), Center for Translational Social Neuroscience Pilot Grant (LAP, LJY), National Center for Research Resources P51RR165 to YNPRC, which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132), NIH MH068791 (LAP) and MH064692 (LJY).
Footnotes
Conflicts of Interest
FCS, RL, LJY and LAP have no conflict of interest to declare. MEM is currently employed by Pfizer Inc., though was not so during the time this research was conducted.
Contributors
Authors MEM, LJY and LAP designed the study. Authors MEM, LAP and FCS collected the samples. RL analyzed the samples. MEM carried out the statistical analyses and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altner H, Altner-Kolnberger I. Freeze-fracture and tracer experiments on the permeability of the zonulae occludentes in the olfactory mucosa of vertebrates. Cell Tissue Res. 1974;154:51–59. doi: 10.1007/BF00221071. [DOI] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, Wang AT, Pepa L, Tanel N, Kushki A, Hollander E. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Prog Brain Res. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Burri A, Heinrichs M, Schedlowski M, Kruger TH. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology. 2008;33:591–600. doi: 10.1016/j.psyneuen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci U S A. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm Behav. 2011 doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. J Pharm Sci. 2009;98:2501–2515. doi: 10.1002/jps.21604. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Ebitz RB, Watson KK, Platt ML. Oxytocin blunts social vigilance in the rhesus macaque. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11630–11635. doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermisch A, Barth T, Ruhle HJ, Skopkova J, Hrbas P, Landgraf R. On the blood-brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinology Exp. 1985;19:29–37. [PubMed] [Google Scholar]
- Frank DO, Kimbell JS, Pawar S, Rhee JS. Effects of Anatomy and Particle Size on Nasal Sprays and Nebulizers. Otolaryngol Head Neck Surg. 2011 doi: 10.1177/0194599811427519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman Sara M, Inoue Kiyoshi, Smith Aaron L, Young Larry J. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the rhesus macaque (Macaca mulatta) doi: 10.1016/j.psyneuen.2014.03.023. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman Sara M, Walum Hasse, Inoue Kiyoshi, Smith Aaron L, Bales Karen L, Young Larry J. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) doi: 10.1016/j.neuroscience.2014.04.055. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME. Somatodendritic autoreceptors on oxytocin neurones. Adv Exp Med Biol. 1995;395:185–194. [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA. Genomic and epigenetic evidence for oxytocin receptor defciency in autism. BMC Medicine. 2009:7. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38:612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Harkema JR. Comparative pathology of the nasal mucosa in laboratory animals exposed to inhaled irritants. Environ Health Perspect. 1990;85:231–238. doi: 10.1289/ehp.85-1568334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JT, Shepherd SV, Platt ML. Social attention and the brain. Curr Biol. 2009;19:R958–962. doi: 10.1016/j.cub.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Landgraf R. Plasma oxytocin concentrations in man after different routes of administration of synthetic oxytocin. Exp Clin Endocrinol. 1985;85:245–248. doi: 10.1055/s-0029-1210444. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landis MS, Boyden T, Pegg S. Nasal-to-CNS drug delivery: where are we now and where are we heading? An industrial perspective. Ther Deliv. 2012;3:195–208. doi: 10.4155/tde.11.149. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wilmersma Greidanus TB. Penetration of neurophyophyseal hormones from plasma into cerebrospinal fluid: half-times of disappearance of these neuropeptides from CSF. Brain Research. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biological Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61:340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I, Koehler E, Landgraf R, Summy-Long J. An oxytocin receptor antagonist infused into the supraoptic nucleus attenuates intranuclear and peripheral release of oxytocin during suckling in conscious rats. Endocrinology. 1994;134:141–148. doi: 10.1210/endo.134.1.8275928. [DOI] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Neumann I, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.03.003. In press. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Toschi N, Veenema AH. Oxytocin actions within the supraoptic and paraventricular nuclei: differential effects on peripheral and intranuclear vasopressin release. Am J Physiol Regul Integr Comp Physiol. 2006;291:R29–36. doi: 10.1152/ajpregu.00763.2005. [DOI] [PubMed] [Google Scholar]
- Parr LA, Modi M, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013a doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Modi ME, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013b doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Southall J, Ellis C. Development in nasal drug delivery. Innov Pharm Technol 2000 [Google Scholar]
- Suman JD, Laube BL, Dalby R. Comparison of nasal deposition and clearance of aerosol generated by nebulizer and an aqueous spray pump. Pharm Res. 1999;16:1648–1652. doi: 10.1023/a:1011933410898. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, Oishi M, Kimura T, Onaka T, Ozono K, Taniike M. Long-Term Administration of Intranasal Oxytocin Is a Safe and Promising Therapy for Early Adolescent Boys with Autism Spectrum Disorders. Journal of child and adolescent psychopharmacology. 2013 doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]