Abstract
Implantation of ventricular assist devices (VADs) for treatment of end-stage heart failure (HF) falls decidedly short of clinical demand, which exceeds 100,000 HF patients per year. VAD implantation often requires major surgical intervention with associated risk of adverse events and long recovery periods. To address these limitations, HeartWare, Inc. (Miami Lakes, FL) has developed a platform of miniature ventricular devices with progressively reduced surgical invasiveness and innovative patient peripherals. One surgical implant concept is a transapical version of the miniaturized left ventricular assist device (MVAD). The HeartWare MVAD Pump® is a small, continuous flow, full-support device that has a displacement volume of 22mL. A new cannula configuration has been developed for transapical implantation, where the outflow cannula is positioned across the aortic valve. The two primary objectives for this feasibility study were to evaluate anatomic fit and surgical approach and efficacy of the transapical MVAD configuration. Anatomic fit and surgical approach were demonstrated using human cadavers (n=4). Efficacy was demonstrated in acute (n =2) and chronic (n = 1) bovine model experiments and assessed by improvements in hemodynamics, biocompatibility, flow dynamics, and histopathology. Potential advantages of the MVAD Pump include flow support in the same direction as the native ventricle, elimination of cardiopulmonary bypass, and minimally-invasive implantation.
Keywords: Heart Failure, LVAD, MVAD, Transapical, Cardiac Assist, Minimally Invasive
INTRODUCTION
Heart failure (HF) is one of the largest unsolved problems in cardiac care today with more than five million patients in the United States (US) alone.1 In 2008, the direct and indirect cost of HF in the US was estimated to be $34.8 billion.2 According to the American Heart Association, approximately 670,000 new cases of HF are diagnosed annually.1 Current treatment options for HF include pharmacological therapy, cardiac resynchronization therapy (CRT), mechanical circulatory support (MCS), and heart transplantation (HT). Despite improvements in survival with pharmacological therapy and CRT, the prognosis remains poor, with one-year mortality at 15.0% and 28.0% for NYHA Class III and IV patients, respectively.3 In advanced HF, transplantation offers the best opportunity for long-term survival; however, the number of available donor organs cannot meet the growing demand. The shortage of appropriate donor hearts is worse in other countries, such as Canada (<200 heart donors per year) and Japan (11 heart transplants in 2008).4-5
The large patient population with advanced HF and the limited number of donor organs has stimulated development of MCS devices. Despite years of research and excellent long-term results with MCS, only a small percentage of patients receive MCS therapy annually. Device implantation requires major surgical intervention as well cardiopulmonary bypass (CPB). The invasiveness and complexity of the surgical procedure has associated complications including bleeding, thromboembolism, stroke, and infection. Additionally, CPB increases implantation time, blood loss, and risk of exposure to donor blood products. Currently, the INTERMACS registry reports a 30-day-post left ventricular assist device (LVAD) implant mortality rate of 7%.6
To overcome some of these limitations, the HeartWare MVAD pump with transapical cannulation is being developed, which may enable implantation with less invasive techniques. The transapical intraventricular device design originated from early cardiopulmonary bypass strategies published in the 1960's and 1970's to unload the LV using a single cannula passed through the LV apex as a technique to establish rapid bypass initiation in cardiogenic shock patients7. In 1992, Yamazaki and colleagues 8 published findings on a transapical axial LVAD wherein the pump sits at the apex and LV blood is advanced into the aorta via a cannula resting anterograde across the aortic valve. The authors evaluated the device in mock circulatory loops and in a healthy dog model, demonstrated feasibility of pump placement and assessment of flow characteristics. Following design modifications, the authors additionally evaluated a new purged seal system and reported improved hemocompatibility9. In 2007, Wang et al10, in collaboration with Dr. Kolff 7, published findings on a transapical-to-aorta intraventricular LVAD demonstrating feasibility of device placement, pump flow and hemodynamic characteristics, and the absence of significant blood trauma and thrombosis using a healthy sheep model. Notably, both Yamazaki and Wang demonstrated a pulsatile pump flow pattern as a result of LV contractile contributions to device inflow8,10. The prototype design for the HeartWare MVAD with transapical cannulation has been previously reported11. Based upon early proof-of-concept testing, the MVAD was re-designed to improve anatomical fit, surgical implant and removal procedures, and performance. In this study, we present development and testing of the re-designed MVAD with transapical cannulation in human cadaver and large animal bovine models to demonstrate feasibility.
METHODS
Device Description
The HeartWare MVAD Pump is a miniature, full-support, continuous-flow LVAD. Operational speed ranges from 12,000 RPM to 22,000 RPM and the pump is capable of generating partial and full-support flows. The MVAD Pump is fitted with an outflow cannula that is designed to pass through the aortic valve (transvalvular).11 This allows for intraventricular placement of the pump (via a transapical implant technique) with the outflow cannula passing transvalvular.
The MVAD Pump in this transapical configuration consists of six components: a pedestal, standpipe, pump body, impeller, diffuser, and outflow cannula. The pedestal is sutured to the epicardial surface of the left ventricle (LV) via a gimbaled sewing ring, while the standpipe supports the pump body, vane diffuser, and outflow cannula (Figure 1A). The standpipe is a hollow cylinder (3 mm in diameter) that carries the motor cables that drive pump operation via an external controller. The standpipe also provides adjustability to accommodate different long-axis ventricular lengths. The vane diffuser guides flow through the vanes reducing turbulence and improving hydraulic efficiency. The outflow cannula is a composite of silicone and barium sulfate, which provides radiopacity to allow verification of device placement via echocardiography and fluoroscopy. The cannula tip implements a trilobar diffuser, which provides a radial force component and minimizes movement of the outflow cannula. The tip of the trilobar outflow cannula has been designed for placement immediately distal to the aortic root and proximal to the innominate artery (Figure 1B).
Figure 1.

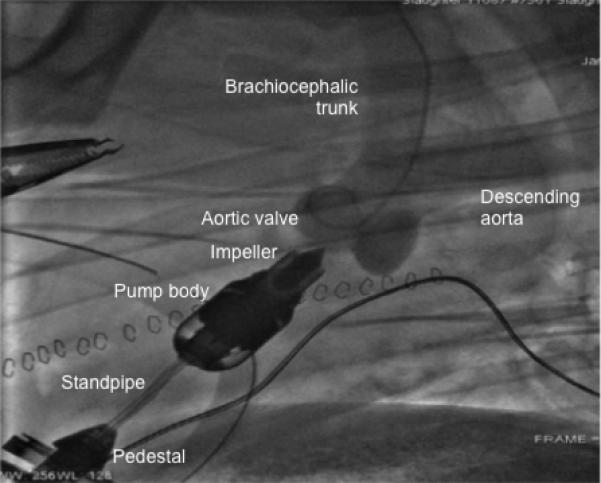
A, Photograph of the transapical miniature ventricular assist (MVAD) device with an adjustable length standpipe to provide better anatomical fit for the majority of adult patients. B, Fluoroscopic image of the device during full support with the outflow cannula positioned distal to the aortic valve and proximal to the bifurcation in a bovine animal model. The anatomical fit studies assessed the surgical access options for the pump implantation in a human cadaver model (n=4). Feasibility was demonstrated in acute (n=2) and 30-day chronic (n=2) bovine animal model experiments.
The MVAD Pump uses a platinum alloy impeller, which is suspended in a ceramic tube and is rotated by sequentially energizing stator coils. Impeller position is maintained via a hybrid suspension system based on both passive magnetic and hydrodynamic forces. The primary flow path through the MVAD Pump is through the impeller channels with a secondary flow path through the radial gap between the impeller and inner pump housing. The hybrid system allows for a wearless design and elimination of support structures.
Study Objectives
The first objective of this study was to evaluate anatomic fit and surgical approach. This was demonstrated using an anatomic fit study conducted in human cadavers (n=4). The second objective of this study was to demonstrate feasibility of MVAD Pump with the integrated outflow cannula in acute (n =2) and chronic (n = 1) bovine model experiments by evaluating hemodynamic metrics (LV pressure-volume (PV) loops), biocompatibility (blood chemistry, complete blood count (CBC)), flow dynamics (fluoroscopy), transthoracic echocardiography (TTE), and histopathology (aortic valve, myocardium, and end-organs).
Cadaver Study
An anatomical fit study of this MVAD Pump configuration was performed in human cadavers (45 – 120 kg, n=4). The MVAD Pump with integrated outflow cannula was implanted via two minimally invasive approaches: subcostal incision (Figure 2, top left) and mini-thoracotomy (Figure 2, bottom left). Factors including surgical access, ease of use, and surgical tools were evaluated and compared to standard LVAD implantation techniques. The heart (including pump) was then excised from the cadaver to assess device fit and placement across the aortic valve (Figure 2, right).
Figure 2.
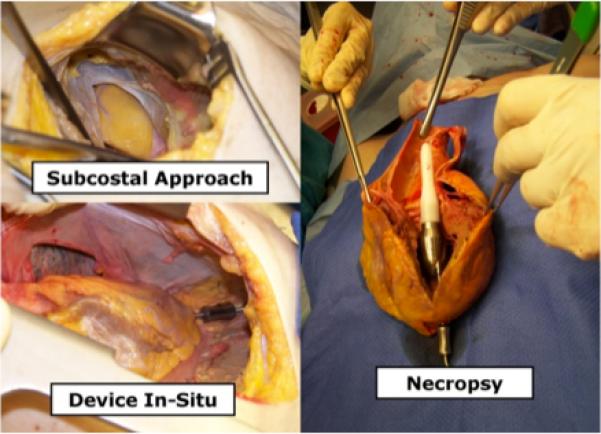
Cadaver study demonstrated that the pump with integrated outflow cannula may be implanted through a subcostal incision (left) or a mini-thoracotomy. The pump is inserted through the left ventricular (LV) apex with the pump residing inside the LV and the outflow cannula self-centered across the aortic valve to deliver antegrade blood flow (right).
Acute and Chronic Bovine Experiments
Acute (n=4) and 30-day chronic (n=1) bovine model (Jersey calves, 60-80 kg) experiments were conducted to evaluate the hemodynamic efficacy, biocompatibility, hemocompatibility, and the aortic valve response to the outflow cannula. Animals were anesthetized with 1-5% isoflurane and 100% oxygen. A left thoracotomy was performed at the 5th intercostal space to provide access and exposure of the pulmonary artery, LV apex, and descending thoracic aorta. Heparin (100-300 units/kg via intravenous (IV) central line) was administered. Eight to twelve pledgeted sutures were placed to secure the gimbal sewing ring to the LV apex. A small incision was made within the confines of the sewing ring and the modified MVAD Pump was inserted and secured. The pump was de-aired through the lumen of a Swan-Ganz catheter, which also provided an arterial pressure waveform facillitating placement of the cannula across the aortic valve. TTE and fluoroscopy were also performed to verify pump positioning and placement of the outflow cannula across the aortic valve.
During acute experiments the animal was instrumented with high-fidelity pressure-volume conductance catheters (Millar Instruments, Houston, TX) to measure left atrial pressure, LV pressure and volume, and aortic pressure. Flow probes (Transonic Systems, Ithaca, NY) were placed for the measurement of pulmonary artery, coronary, and aortic flow. Hemodynamic waveforms were recorded at 400 Hz. The animal's normal hemodynamic condition was tested before pharmacologically inducing the following test conditions: HF (esmolol), hypotension (nitroprusside), hypertension (phenylepherine), and exercise (epinepherine). At each test condition, the pump was run at baseline (0 RPM), partial pump support (16,000 RPM and 19,000 RPM), and full pump support (22,000 RPM). Baseline measurements were repeated between test conditions to ensure state-steady baseline was maintained. During chronic experiments, the animal was instrumented with only a pulmonary artery flow probe and an arterial fluid-filled catheter. For both acute and chronic experiments, hemodynamic parameters were analyzed using Hemodynamic Evaluation and Assessment Research Tool (HEART), custom software developed on a Matlab platform (MathWorks, Natick, MA).12
Echocardiography was performed pre-operatively, after pump insertion, and on a weekly basis post-operatively (for chronic studies) to assess cardiac function, blood flow, and device placement. A Phillips iE 33 TTE system with S8-3 ultrasound probe was used to obtain M-mode and Doppler echocardiographic recordings. LV enddiastolic volume, end-systolic volume, ejection fraction, and aortic valve function were recorded. Color Doppler was used to detect and quantify potential aortic regurgitation resulting from placement of the outflow cannula across the aortic valve (0-4+ severity scale). Fluoroscopy was also performed in the chronic animal model using an angiography catheter placed in the LV and aorta for injection of radioopaque dye to evaluate blood flow, coronary perfusion, and anatomical positioning of the modified MVAD Pump during implant and prior toeuthanasia.
Routine blood samples (15 mL) were collected to assess biocompatibility in the chronic bovine model. A baseline sample was collected pre-implantation and then samples were collected on a daily basis during the first post-operative week and on a weekly basis from post-operative day 7 through 30. Complete blood chemistry (CMP), complete blood count (CBC), liver enzymes, activated clotting time, arterial blood gases, platelet function test, and plasma free hemoglobin (PfHb) were measured. Following animal euthanasia, a complete necropsy and histopathologic analyses were performed, including examination of the aortic valve and root. In the aortic leaflets, tissue blocks were sectioned at 5 μm thick and prepared for staining with hematoxylin and eosin (H&E) for visualization of histopathological changes under light microscopy. Immunohistochemistry was performed to identify potential damage to endothelial cells (stained with endothelin) and smooth muscle cells (stained with actin). The explanted MVAD Pump with integrated outflow cannula was visually inspected for evidence of thrombus, wear or any other defects.
RESULTS
Cadaver Studies
Two surgical access points were evaluated in our cadaver studies (n=4). Results demonstrated surgical feasibility via a minimally invasive subcostal or a thoracotomy incision. The cadaver studies showed that both surgical approaches to implant and explant the modified MVAD Pump were feasible but demonstrated the need for re-engineering of the next generation outflow cannula and standpipe to ensure anatomical fit for multiple sized-hearts in HF patient population.
Acute Bovine Experiments
The MVAD Pump with integrated outflow cannula across the aortic valve was capable of sufficiently unloading the LV as demonstrated through acute bovine experiments under test conditions of normal and pharmacologically induced HF, hypotension, hypertension, and exercise. Increasing pump speed resulted in reduced ventricular workload as evidenced by decreasing LV pressures and volumes (Figure 3). Additionally, there was a leftward shift of LV PV loops and diminishing aortic pressure pulsatility with increasing MVAD Pump support (Figures 4 and 5). Gross necropsy of the calves showed no evidence of embolization in the brain, heart, or end organs.
Figure 3.
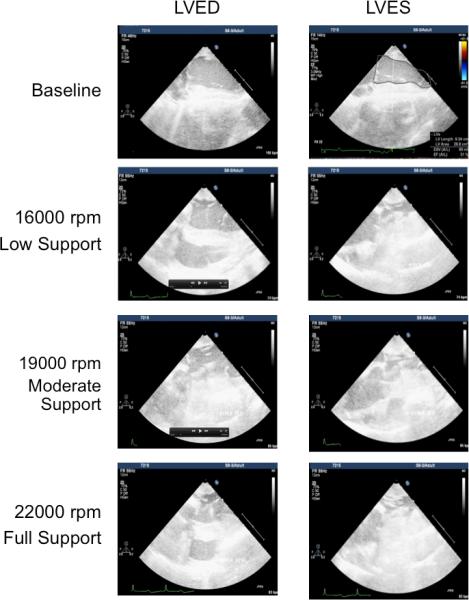
Echocardiography images (3D iE33 Phillips Medical Systems) at end-diastole (LVED, left) and endsystole (LVES, right) and recorded pre-implant (baseline, left) and at post-implant during low (16,000 rpm), moderate (19,000 rpm), and full (22,000 rpm) support demonstrating ability of the MVAD to volume unload the left ventricle (LV).
Figure 4.
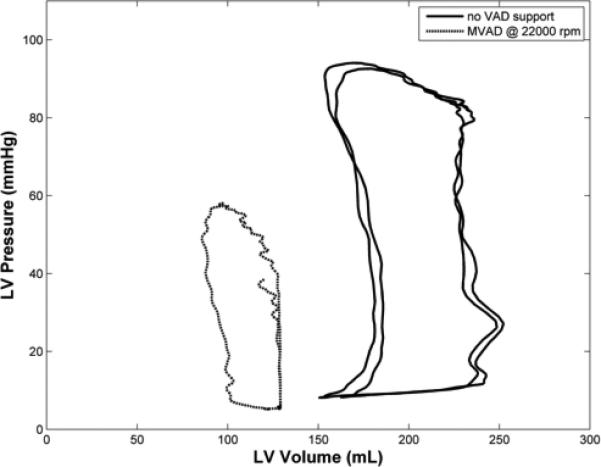
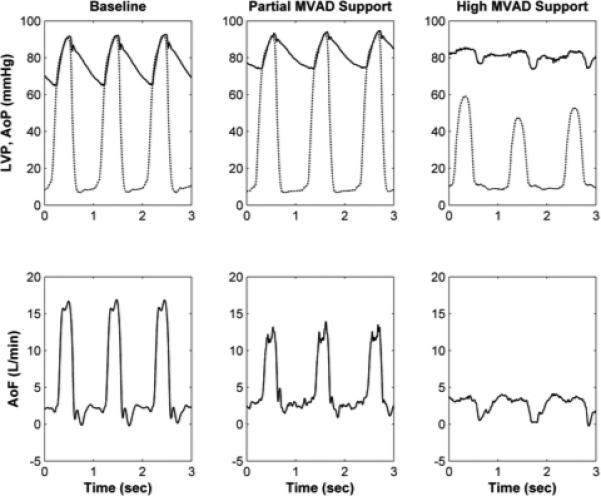
A, Left ventricular (LV) pressure-volume (PV) loops with native heart (no support) and during full volume unloading with MVAD (22,000 rpm). B, Aortic pressure (AoP), left ventricular pressure (LVP, dashed), and aortic flow (AoF) waveforms during baseline (MVAD off), partial low (16,000 rpm), and full (22,000 rpm) MVAD support (right). These data demonstrate that the MVAD volume unloads the native left ventricle (LV).
Figure 5.
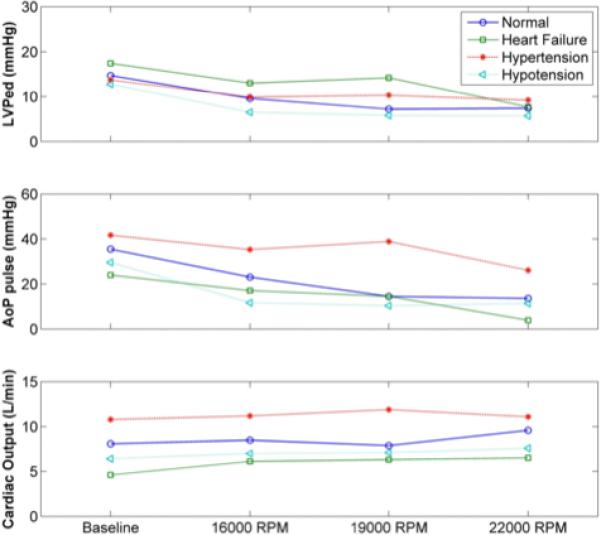
Summary of hemodynamic markers recorded at pre-implant (baseline) and post-implant with the MVAD at partial (16,000 and 19,000 rpm) and full (22,000 rpm) support in acute bovine model (n=2). These data demonstrate that the system is able to reduce left ventricular end-diastolic pressure, diminishing aortic pressure pulsatility, and increasing cardiac output. AoP, aortic blood pressure; LVPed, LV pressure at end-diastole.
Chronic Bovine Experiments
Biocompatibility and long-term impact on aortic valve function were assessed in a 30-day chronic bovine model. Echocardiography and fluoroscopy verified the in vivo position of the device and demonstrated the aortic valve leaflets co-apt around the pump's outflow cannula. One animal (n=1) was electively sacrificed during the first post-operative day due to abnormally high PfHb levels and observed hematuria, while the other animal (n=1) was electively sacrificed at study term. In the animal that was terminated prematurely, a small piece of myocardial tissue was found lodged in an impeller flow channel, causing an obstruction. This myocardial tissue debris was likely dislodged during the ventricular coring process. For the animal supported throughout the study period, there was no indication of aortic insufficiency. CMP evaluation of venous blood samples demonstrated that there were no significant changes in measurements of hepatic function (ALP, ALT) or renal function (creatinine). This suggests the device had no negative effect on end-organ function. PfHb, blood urea nitrogen (BUN), and creatinine were normal at all measured time points (Table 1). One animal was electively sacrificed at study term and a complete necropsy and histopathological analyses were performed, including examination of the device, aortic valve, all major abdominal and thoracic organs, and the brain. An independent pathologist (Mass Histology, Boston MA) examined all end-organ tissues and concluded that there were no notable histopathological changes. In addition, the explanted pump outflow cannula and impeller were free of thrombus.
Table 1.
| Clinical Marker | Pre- implant | 6-hour | 1-week | 2-week | 3-week | 4-week |
|---|---|---|---|---|---|---|
| Hemoglobin, Hbg (g/L) | 4.5 | --- | 10.2 | 10.5 | 10.2 | 9.5 |
| Hematocrit, Hct (%) | 31.1 | 22.0 | 34.1 | 34.6 | 32.1 | 31.9 |
| White Blood Cell, WBC (per 1000) | 7.1 | 7.9 | 7.9 | 7.8 | 7.9 | 8.2 |
| Platelet, PLT (per 1000) | 311 | --- | 351 | 284 | 344 | 326 |
| Blood urea nitrogen, BUN (mg/dl) | 11 | 35 | 3 | 3 | 3 | 3 |
| Creatinine, Cr (mg/dl) | 0.7 | 0.7 | 0.4 | 0.5 | 0.5 | 0.5 |
| Lactate dehydrogenase, LDH (U/L) | 4156 | 7686 | 5835 | 5645 | 4281 | 4124 |
| Plasma Free Hemoglobin, PfHb (mg/dl) | 0 | 10 | 0 | 10 | 10 | 0 |
| International Normalized Ratio, INR | 1.9 | 1.9 | 4.2 | 2.9 | 4.6 | 5.3 |
A considerable portion of this work was focused on determining the long-term effect of the cannula crossing the aortic valve. Histological analysis of the aortic valve cusps revealed minimal to mild, multifocal subendothelial hyperplasia and minimal neutrophilic inflammation (Figure 6). The observed valvular changes were likely due to repeated physical contact between the aortic cusps and outflow cannula in this animal model. The explanted weight of this animal was 90 kg. According to Inman et al the approximate resting cardiac output for this animal model size is 7.3± 0.9 L/min.13 Based on these assumptions we believe that this animal model is not representative of the conditions the aortic valve would encounter in a patient with heart failure implanted with the MVAD Pump and likely represents a worst-case scenario.
Figure 6.
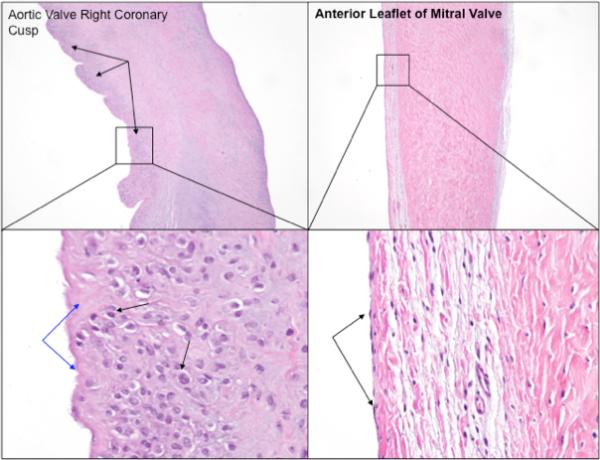
Photographs of the right coronary cusp of the aortic valve, mild subendothelial hyperplasia (black arrows) with higher magnification showing flattened layer of endothelial cells (right), and a normal leaflet of the mitral valve (also with the single layer of flattened endothelial cells with higher magnification demonstrating the absence of proliferating reactive cell layer, which is present in the aortic valve cusps (hematoxylin and eosin (H&E) stain, 40x and 400x magnification)).
DISCUSSION
In this feasibility study, we demonstrated in human cadaver model (n=4) that the MVAD Pump with transaortic outflow cannula can provide proper anatomic fit and may be implanted with minimally invasive surgical approaches (subcostal and mini-thoracotomy). Acute (n =2) and 30-day chronic (n=2) bovine experiments demonstrated ventricular volume unloading without device failure, significant aortic valve damage, thrombosis, or hemolysis. Functional and cell morphology changes to the valve leaflets is nonexistent in the literature in regards to the potential long-term impact of a LVAD being placed across the aortic valve. The Impella 2.5 (Abiomed Inc, Danvers, Massachusetts) is a short-term partial circulatory support device that is designed to sit across the aortic valve. Studies with a primary endpoint of 30 days have shown no aortic valve dysfunction.14-16
Yamazaki and Wang demonstrated advantages of the transapical approach including a compact and accessible design that requires a less-invasive and off-pump surgical approach, minimal blood loss during device placement, the absence of lengthy grafts and cannulae that minimize surface area of blood contact and which eliminate the need for vessel cannulation/anastamoses, and anterograde blood flow comparable to native circulation8,10.. Similarly, the modified MVAD Pump requires a single incision via a subcostal or mini-left thoracotomy. The, surgical procedures can be simplified, thereby shortening patient recovery times and improving patient outcomes. Implantation of this MVAD Pump and integrated outflow cannula may be performed without CPB, which will reduce surgery time as well as the complications associated with CPB. Unlike current LVADs, this MVAD Pump configuration also delivers blood flow in the same direction (i.e. antegrade flow) as native ventricular ejection across the aortic valve. This shortens the flow delivery path and also prevents blood stasis associated with retrograde flow from an outflow graft implanted in the aorta.17-18 Potential limitations of the transapical approach include the following: pump resides in the left ventricle, no direct visibility during pump placement instead requires imaging to verify correct placement, and challenges quantifying pump flow.
Future Development
Future enhancements to this modified MVAD Pump design include a straight standpipe and two cannula lengths (radiopaque) to improve device fit and placement. Additionally, a valved access port for a guidewire has been added to the pedestal to enable implant using the Seldinger technique19 (Figure 7).
Figure 7.
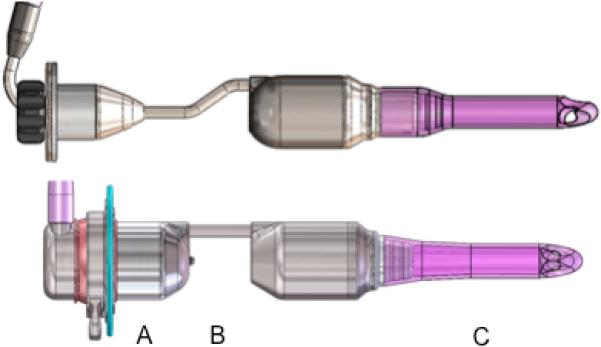
Illustration of engineering challenges identified in phase I design (top) and proposed solutions with redesigned components (bottom): A, add a tunnel port through the pedestal to introduce a guidance catheter for facilitating pump and cannula placement and de-airing; B, straighten the standpipe to improve intraventricular placement; C, increase the barium sulfate concentration by 20% to improve radiopacity.
CONCLUSION
HF is a major health care burden that is expected to double in incidence in the next decade. To address limitations associated with current VAD technology, HeartWare Inc. has developed the MVAD Pump technology, which due to its small size and axial flow configuration, enables multiple cannulation possibilities, including trans-aortic valve placement. This full-support device can be implanted without CPB via a minimally invasive subcostal or mini-thoracotomy surgical approach with minimal or no blood loss.
ACKNOWLEDGEMENTS
The Phase I program (NIH-SBIR phase I grant 1R43HL103014-01A1) began on April 4, 2011 and was completed September 30, 2012. The objective of this proposal was to develop a minimally invasive surgical implant procedure and complete feasibility testing of a transapical miniaturized ventricular assist device for the potential treatment of patients with less advanced HF.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger V. Heart Disease and Stroke Statistics – 2013 Update: A Report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K. Heart Disease and Stroke Statistics – 2008 Update: A Report from the American Heart Association. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Muntwyler J, Abetel G, Gruner C, Follath F. One-year mortality among unselected outpatients with heart failure. Eur Heart J. 2002;23:1861–1866. doi: 10.1053/euhj.2002.3282. [DOI] [PubMed] [Google Scholar]
- 4.e-Statistics Report on Transplant, Waiting List and Donor Statistics, in 2008 Summary Statistics. Canadian Institute for Health Information; Jan 1 31, Dec 1 31, 2008. [June 14, 2013]. Available at: http://www.cihi.ca/cihi-ext-portal/internet/en/document/types+of+care/specialized +services/organ+replacements/report_stats2008. [Google Scholar]
- 5.Donors and Transplants Data. Japan Organ Transplant Network Homepage; Jan 1 31, Dec 1 31, 2008. [June 14, 2013]. Available at: http://www.jotnw.or.jp/english/2008data.html. [Google Scholar]
- 6.Kirklin JK, Naftel DC, Kormos RL. Second INTERMACS annual report: more than 100 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolff WJ. Transapical left ventricular bypass. Arch Surg. 1971;103:656. doi: 10.1001/archsurg.1971.01350110158029. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki K, Umezu M, Koyanagi H. A miniature intraventricular axial flow blood pump that is introduced through the left ventricular apex. ASAIO J. 1992;38:M679–83. doi: 10.1097/00002480-199207000-00124. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki K, Kormos R, Mori T. An intraventricular axial flow blood pump integrated with a bearing purge system. ASAIO J. 1995;41:M327–32. doi: 10.1097/00002480-199507000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Zwischenberger JB, Zhou X. Development of “plug and play” TransApical to aorta VAD. ASAIO J. 2007;53:171–5. doi: 10.1097/01.mat.0000249502.80792.8c. [DOI] [PubMed] [Google Scholar]
- 11.Slaughter MS, Giridharan GA, Tamez D. Transapical miniaturized ventricular assist device: Design and initial testing. Journal of Thoracic and Cardiovascular Surgery. 2011;142:668–74. doi: 10.1016/j.jtcvs.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder MJ, Perrrault B, Ewert DL, Koenig SC. HEART: An automated beat-to-beat cardiovascular analysis package using Matlab™. Computers in Biology and Medicine. 2004;34:371–388. doi: 10.1016/S0010-4825(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 13.Inman RW, Radovancevic B, Conger JL. Measurement of Cardiac Output in Normal Calves. ASAIO J. 2000;46(2):167. doi: 10.1097/00002480-200011000-00033. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill WW, Kleiman NS, Moses J. A Prospective, Randomized Clinical Trial of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention: the PROTECT II Study. Circulation. 2012;126:1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 15.Maini B, Naidu SS, Mulukutla S. Real-World Use of the Impella 2.5 Circulatory Support System in Complex High-Risk Percutaneous Coronary Intervention: The USpella Registry. Catheterization and Cardiovascular Interventions. 2012;80:717–725. doi: 10.1002/ccd.23403. [DOI] [PubMed] [Google Scholar]
- 16.Mahmouid M, Syed AI, Waksman R. The role of percutaneous circulatory assist devices in acute myocardial infarction and high-risk percutaneous coronary intervention in the 21st century. Cardiovascular Revascularization Medicine. 2011;12:237–242. doi: 10.1016/j.carrev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Frazier OH. Unforeseen consequences of therapy with continuous flow pumps. Circulation: Heart Failure. 2010;3:647–649. doi: 10.1161/CIRCHEARTFAILURE.110.959023. [DOI] [PubMed] [Google Scholar]
- 18.Litwak KN, Koenig SC, Giridharan GA, Gillars KJ. Pantalos GM: Ascending aorta outflow graft location and pulsatile ventricular assist provide optimal hemodynamic support in an adult mock circulation. Artificial Organs. 2005;29:629–635. doi: 10.1111/j.1525-1594.2005.29100.x. [DOI] [PubMed] [Google Scholar]
- 19.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiologica. 1953;39(5):368–76. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]


