Abstract
Objective
Botanical compounds and extracts are widely used as nutritional supplements for the promotion of health or prevention of disease. An extract of Artemisia dracunculus (PMI 5011) has been shown to improve insulin action, yet the precise mechanism is not known. We proposed that the mechanism by which PMI 5011 and two related Artimisia extracts improve insulin action is associated with a downregulation of DNL in the liver and an increase in DNL in the adipose tissue.
Materials/Methods
DIO old male mice (C57BL/6J) were divided into 4 groups: (control, 5011,Santa, and Scopa) and fed for 30 days with respective extracts incorporated into the diet at 1%(w/w). Deuterium was administered on day 30 for the measurement of DNL in blood, liver, and white adipose tissue. Individual fatty acids and glycerol levels were also measured.
Results
There was no statistically significant differences in de novo lipogenesis between the control group and the three botanical treatments. Plasma levels of all four long chain fatty acids were significantly lower in all three treatment groups. Glycerol in the plasma was lower in all three groups as compared to the control group, but this did not reach statistical significance in all cases. Tissue levels of the fatty acids and glycerol did not differ between any of the treatment groups.
Conclusions
These results suggest that botanicals may not affect fractional de novo lipogenesis in animals on a high fat diet. However, there were decreases in long chain fatty acids and in glycerol coming from the newly synthesized triglycerides in plasma.
Keywords: Obesity, de novo lipogenesis, fatty acids, glycerol, botanical
1. Introduction
The prevalence of obesity in the United States has risen dramatically in the past two decades. In 2009–2010, over 78 million adults and 12.5 million children and adolescents were obese. [1] Moreover, the prevalence of obesity and overweight among the adult population in the U.S. is approaching 70%. [2] It has been shown that obesity increases the risk of hypertension, type 2 diabetes, and cardiovascular disease.[3–5] The high prevalence of obesity and type 2 diabetes has been associated with abnormalities in carbohydrate and lipid metabolism. When there is an excess of calories in the diet, excess carbohydrate not stored as glycogen or oxidized for energy can be converted to fat by a pathway called de novo lipogenesis (DNL). [6] In DNL, glucose or other carbon precursors use acetyl-CoA and acetyl-CoA carboxylase and fatty acid synthase to generate long chain saturated fatty acids like palmitate. Palmitate can then be modified by desaturase and elongase enzymes to produce additional lipid species. The different species of fatty acids which are produced by DNL are dependent upon the tissue specific concentrations of the desaturase and elongase enzymes. Not only are fatty acids used as storage molecules, but they are also important signaling molecules that are involved in basic physiological processes. DNL can be measured in both plasma and in tissues. When excess calories are consumed, there is a downregulation of DNL in adipose tissue. [7] However, it has not been shown that there is a direct correlation between calorie intake and the quantity of new fatty acids being synthesized from carbohydrate. DNL in the liver is correlated with elevated serum triglycerides and with increasing intrahepatic lipid which can lead to nonalcoholic fatty liver disease. [8] Intrahepatic lipid is also correlated with insulin resistance.[9] Alternatively, DNL in the adipose tissue is associated with improvements in insulin sensitivity.[10] Insulin resistance is associated with both obesity and type 2 diabetes and there is a strong correlation between insulin resistance and lipid accumulation in tissues.
Botanical compounds and extracts are widely used as nutritional supplements for the promotion of health or the prevention of disease. Many of these compounds are specifically targeted towards weight loss and to promoting better glycemic control. There have been a number of reports of plants in the Artemisia family being used as a treatment for diabetes. Specifically, an extract of Artemisia dracunculus (PMI 5011) has been shown to improve insulin action, yet the precise insulin signaling mechanism is not known. [11] We sought to determine whether the mechanism by which PMI 5011 improves insulin action is associated with a downregulation of DNL in the liver and an increase in DNL in the adipose tissue.
2. Materials and Methods
2.1 Animals
All animal experiments were part of a protocol approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center. Twenty 16-week-old male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Upon arrival to Pennington Biomedical Research Center, the mice were housed two per cage and placed into quarantine for one week. After quarantine, mice were singly housed.
2.2 Experimental Design and Diets
Mice were fed Research Diets (New Brunswick, New Jersey) high fat lard diet D12492 (60% kcal as fat) designed to induce dietary obesity from weaning to 16 weeks of age. Mice were randomized by body weight into four dietary groups: Control, Artemisia dracunculus, Artemisia santalinofolia, and Artemisia scoparia. Plants were grown hydoponically under uniform and controlled conditions to ensure standardization of phytochemical content. The growing, quality control, biochemical characterization, and extract preparation have been reported previously. [12] For this study, each diet contained 1% (w/w) of the extract. Once the mice were randomized into the four groups, mice had ad libitum access to assigned diets and water for 28 days. The animals in the control group continued receiving D12492. Each of the three Artemisia sp. diets had 1%(w/w) of an ethanolic extract of the respective plant material incorporated into the D12492 diet. On day 0 of the experimental period, mice were weighed and fasted for 4 hours. After the fast, a submandibular blood collected was performed. On day 28, after a 4 hour fast, another submandibular blood collected was performed. Body weight and body composition were obtained on days 7, 14, 21, and 28. On day 29, food was removed at 1200 hrs. At 1600 hrs, the mice were injected intraperitoneally with 99.9% 2H2O diluted in 0.9% saline (20 uL/g body weight). Mice were returned to their cages and food was returned to the animals at 1745 hrs and lights were turned out at 1800 hrs. At 2000 hrs, animals were sacrificed. Trunk blood, liver, white adipose tissue, brown adipose tissue, and gastrocnemius were collected. Tissues were weighed and snap frozen for later analysis. Blood samples were centrifuged at 3500 rpm for 15 minutes and the serum was separated and aliquotted. Serum was frozen at −80°C until time of analysis.
2.3 Deuterium Measurement
Serum samples were distilled to isolate the water fraction. The distillate was injected into a Thermo Fisher Scientific Inc. Delta V isotope ratio mass spectrometer (Waltham, MA) equipped with an H device for measuring 2H:H ratios. A standard curve was analyzed with the samples and percent deuterium enrichment was calculated.
2.4 Serum Extract for Fatty Acid and Glycerol Measurement
Lipids were extracted according to a modified Folch method [13]. Briefly, serum was ultracentrifuged at 40,000 rpm for 17 hours to isolate the triglyceride rich lipoprotein fraction. After centrifugation, the upper layer was transferred to a glass tube and 100 uL of internal standard was added (Glyceryl-d5 tripentadecanoate). 3 mL of chloroform:methanol (2:1) was added and vortexed. After centrifugation at 2000 rpm for 10 minutes, the top layer (aqueous) was removed and discarded. The extraction procedure was repeated two more times and the chloroform layers were combined. The chloroform layer was dried under nitrogen and then resuspended in 1 mL of chloroform and 1 mL of 3N Methanolic HCl. Tubes were incubated at 37 °C overnight. After incubation 2 mL of 5% NaCl and 3 mL of hexane were added to the tubes and vortexed. The top layer was used for fatty acid analysis and the lower layer was used for glycerol analysis. The non-aqueous layer containing the fatty acids was dried under nitrogen and resuspended in hexane for GC/MS analysis. The aqueous phase containing the glycerol was processed using a series of cation and anion columns and then dried using a Speed Vac. The sample was then derivitized using 100 uL of pyridine:acetic anhydride (1:2). Samples were heated at 60 °C for 30 minutes, dried under nitrogen, and then resuspended in ethyl acetate for analysis by GC/MS.
2.5 Tissue Extract for Fatty Acid and Glycerol Measurement
Tissues were weighed and transferred to a glass tube. Internal standard was added and samples were homogenized with a polytron homogenizer for 1 minute. Lipids were extracted using the procedures describe above.
2.6 GC/MS Parameters for Fatty Acids
The analytical gas chromatography was carried out using an Agilent 7890 gas chromatograph equipped with a DB-5 fused silica column (60 m × 25 mm i.d., film thickness 0.25 uM). Carrier gas was helium with a flow rate of 1 ml/min. Temperature programming was used to improve chromatographic separation and decrease run time. The Agilent 5975 MS was run in SIM mode. Ions that were monitored were C15 group (256.3,257.3), C16 group (268.3,269.3,270.3,271.3,272.3), and C18 group (296.3,297.3,298.3,299.3,300.3). Run time was 12 minutes.
2.7 GC/MS Parameters for Glycerol
The analytical gas chromatography was carried out using an Agilent 7890 gas chromatograph equipped with a or a DX-4 fused silica column (30 m × 0.25 mm i.d., film thicknes 0.25 uM). Carrier gas was helium with a flow rate of 1 ml/min. Temperature programming was used to improve chromatographic separation and decrease run time. The Agilent 5975 MS was run in SIM mode. Ions that were monitored for glycerol were 159.2, 160.2, 164.2. Run time was 8 minutes.
2.7 Statistical Methods
Results were summarized as mean +/− standard error of the mean (SEM). A series of analyses were conducted to investigate effects of 3 botanical extracts on biomarkers in plasma, liver and BAT. Statistical significance was evaluated in terms of a general linear model analysis of variance for a completely randomized design. The general analysis was followed by comparing each of 3 treatments (extracts) to a control with Dunnett’s method for multiple comparisons. The 3 pairwise comparisons were performed testing each of null hypotheses against a two-directional alternative. For each analysis, the global significance level was set at p<0.05. All statistical procedures were performed with SAS 9.3 software (Cary, NC).
3.Results
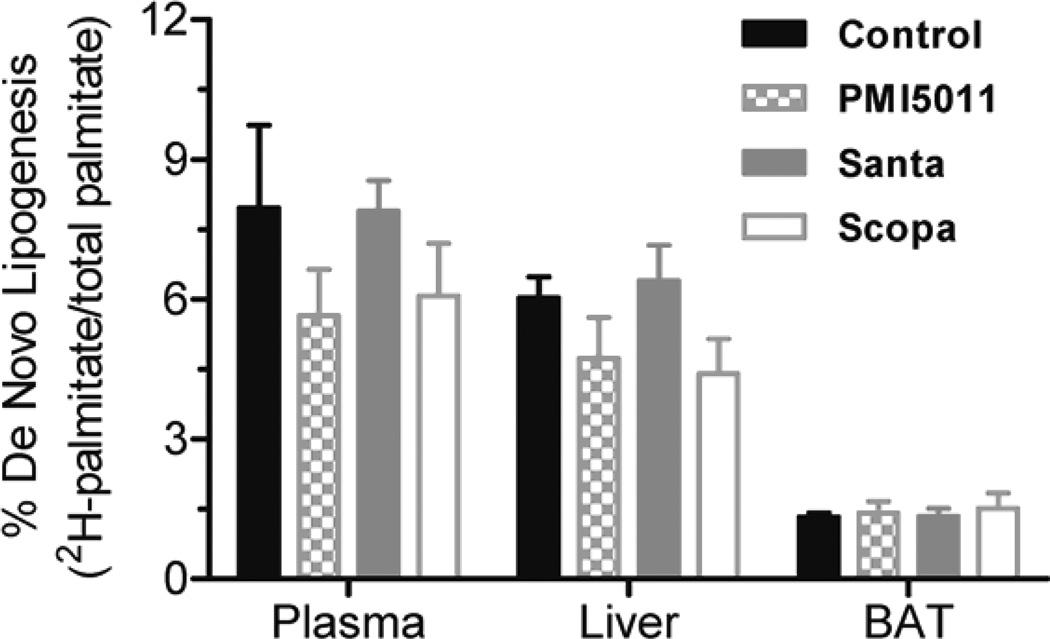
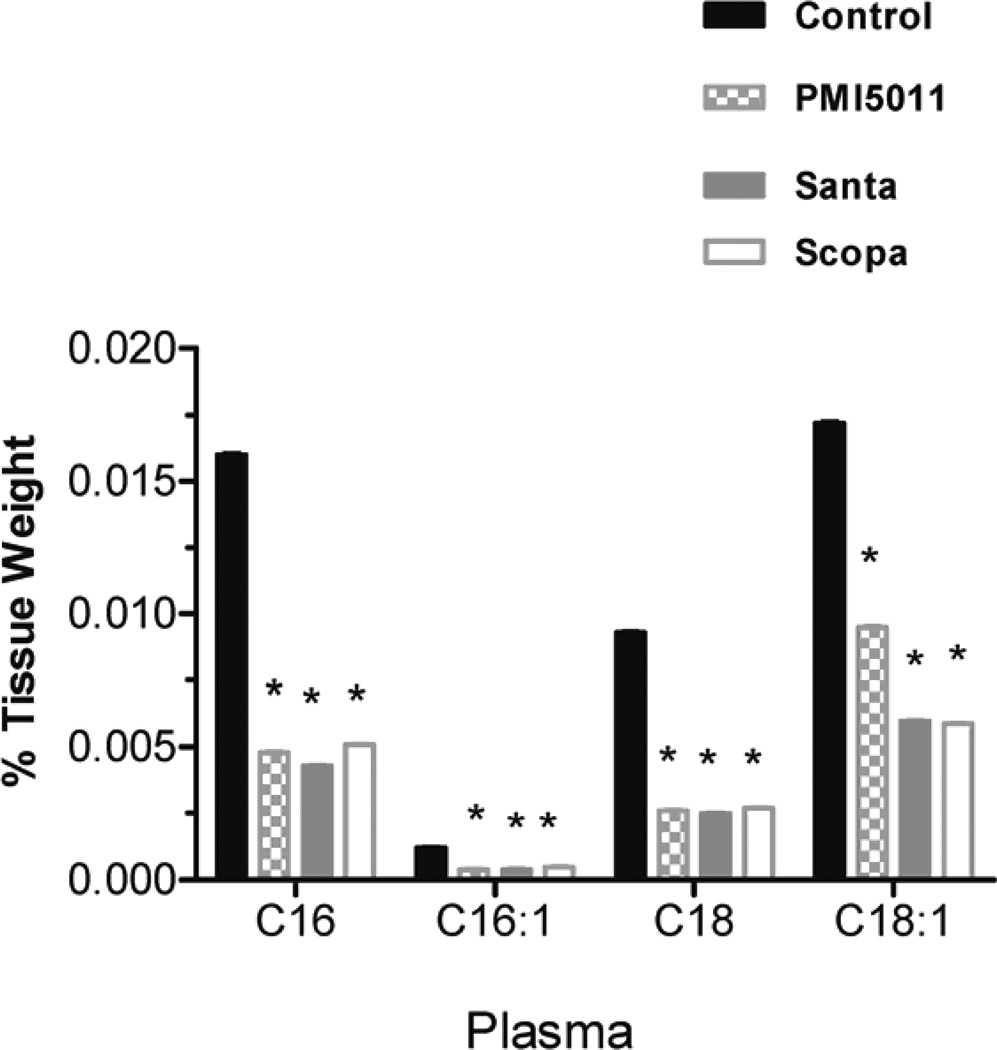
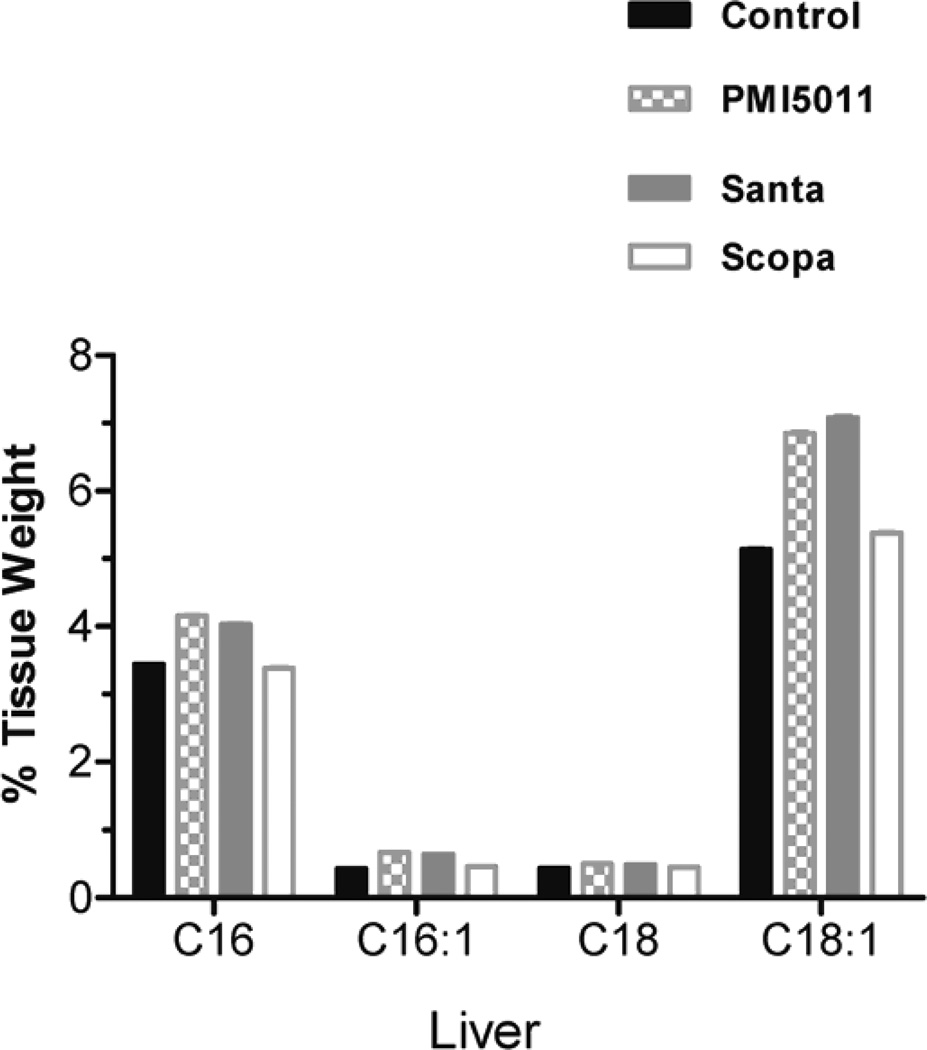
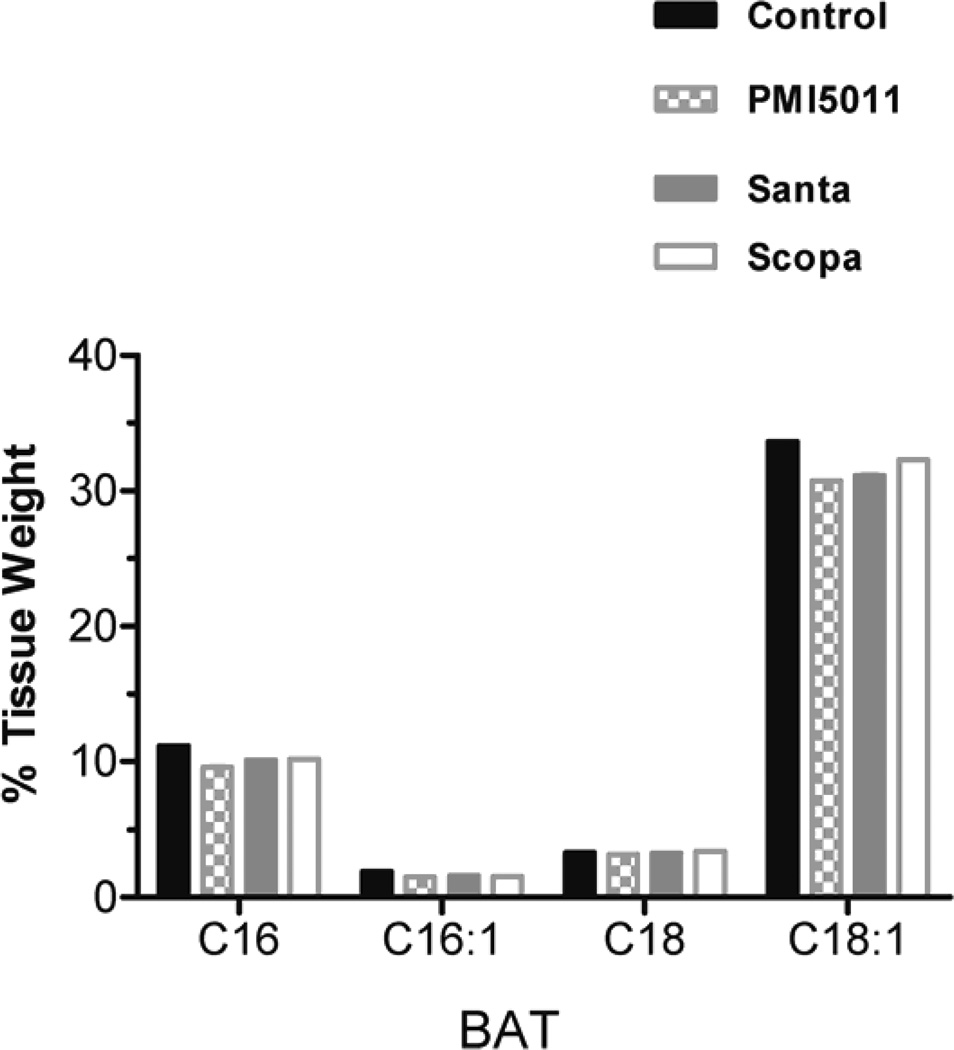
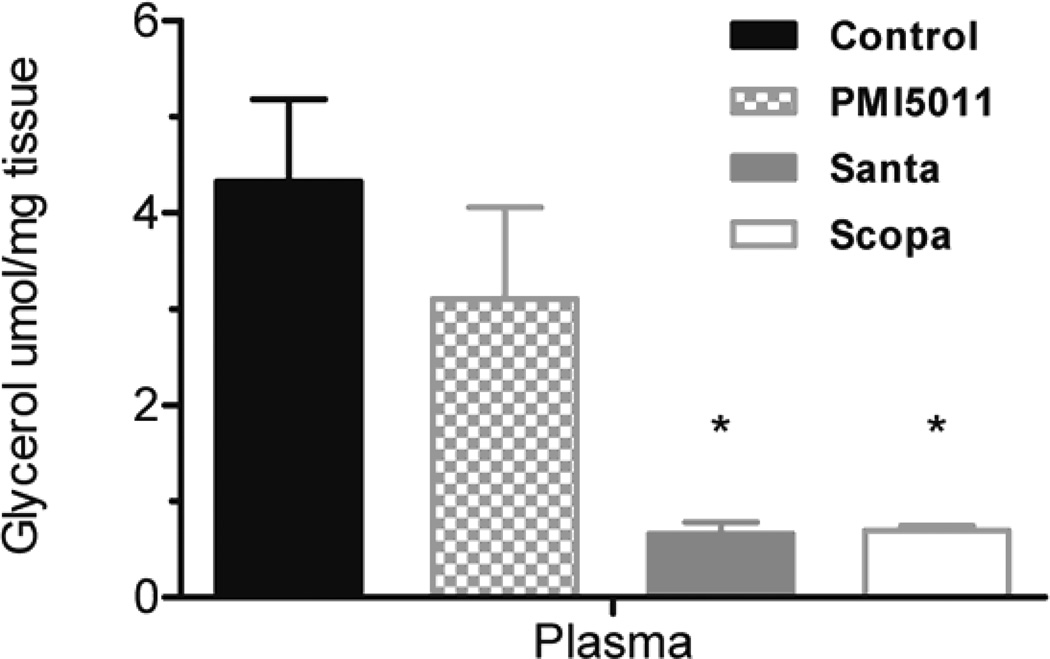
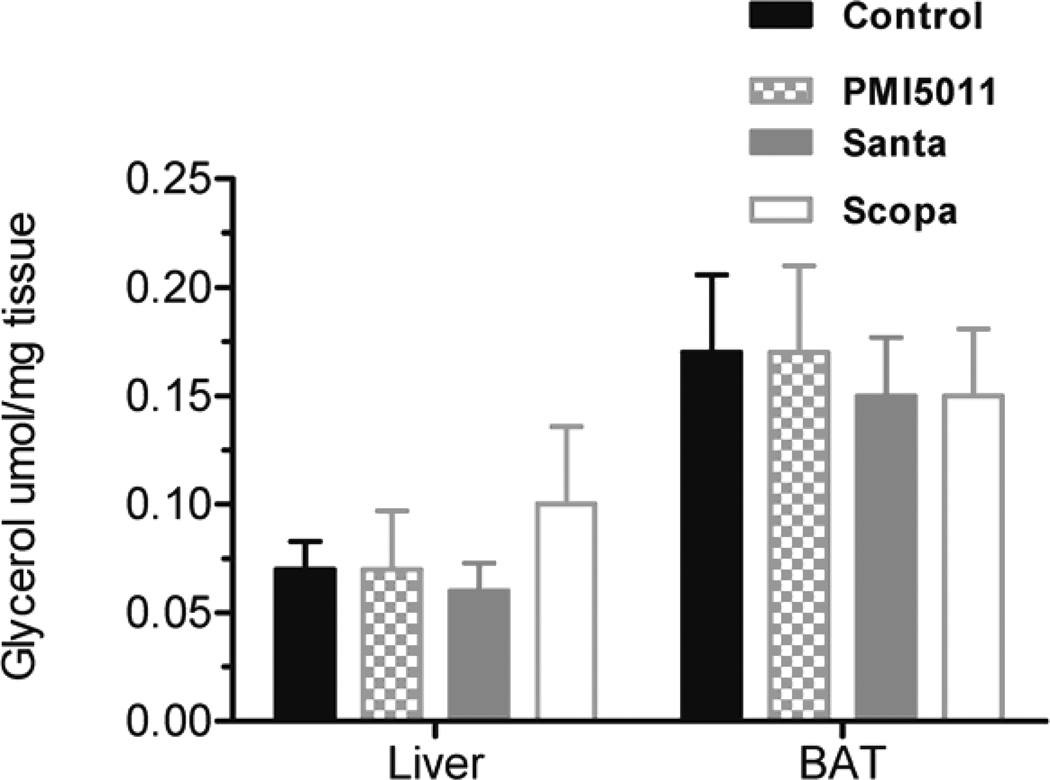
Animals were weighed weekly during the study and individual body weights increased on average by 13% but there were no statistically significant changes between the control and treatment groups. Fractional DNL was calculated as the percentage increase in deuterium labeled palmitate compared to total palmitate. DNL was measured in plasma, liver, and brown adipose tissue. DNL was not calculated in in white adipose tissue and muscle (gastrocnemius) because deuterium enrichments were too low to be measured accurately. There was no change in %DNL in plasma in the Santa group. There was a decrease in %DNL in plasma in the PMI5011 and Scopa groups but these were not statistically significant. (fig 1). Additionally, DNL in liver and brown adipose tissue did not differ between the control group and the three groups treated with botanical extracts. There was a decrease in %DNL in the PMI5011 and Scopa groups in the liver, but it was not statistically significant. Individual fatty acids were measured in both the plasma and the tissues. In all three treatment groups, plasma palmitate(16:0), palmitoleate (C16:1), stearate (18:0) and oleate (C18:1) were decreased when compared to the control animals. There were 3 fold changes in C16 and in C18. The decreases were more modest with C16:1 and C18:1, but they remained statistically significant. (fig 2) However, this association was not seen in the tissues. There were no differences in fatty acid concentrations between the control group and the three treated groups in the liver. (fig 3) Likewise, no changes were observed in the brown adipose tissue. (fig 4). In addition to the fatty acids, samples were also analyzed for glycerol. All three treatment groups showed a decrease in glycerol concentration in the plasma as compared to the control animals. (fig 5) However, only the decreases in the Santa and Scopa group were statistically significant. As with the fatty acids, the effect did not carry over into the tissues. There were no changes observed in glycerol concentration in either the liver or the brown adipose tissue (fig 6).
Figure 1.
Figure 2.
Figure 3.
Figure 4.
Figure 5.
Figure 6.
4.Discussion
Artemisia has been shown to play a role in improving insulin action but the mechanisms remain unknown. The purpose of this study was to determine if the effects on insulin action were mediated with changes in de novo lipogenesis. Our data show with a high fat diet fractional DNL was not effected by Artimisia sp. in the C57/Bl6 mouse on a 60%fat obesogenic diet. It is possible that we did not observe a change in DNL due to the composition of the diet. Most studies that have explored diet and DNL have used diets which are high in carbohydrates. However, there has been data in a human study showing an increase in DNL in hyperinsulemic obese subjects on a high-fat, low-carbohydrate diet. [14]. Despite the fact that no changes in DNL were observed, out data did show that circulating levels of fatty acids were altered by the Artimisia supplementation suggesting a potent inhibition of lipolysis by Artimisia. The composition of long chain fatty acids and the source of the glycerol in the plasma triglyceride-enriched lipoproteins were significantly altered by the botanical treatments. Considered together, the findings support the view that the botanical extracts have altered how triglyceride is assembled and incorporated into lipoproteins.
Acknowledgments
Funding:
Supported by P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS) which funds the Botanical Research Center of Pennington Biomedical Research Center and the Biotech Center of Rutgers University. This work was partially supported by a NORC Center Grant # 2P30DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK.
Abbreviations
- DNL
De novo lipogenesis
- DIO
Dietary induced obesity
- GC/MS
gas chromatography/mass spectroscopy
- SIM
Selective Ion Monitoring
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
The authors have no conflicts of interest to disclose.
Author Contributions:
JCR, JMS, TG, RM AND WC designed the experiments; JCR, JMS, TM, AND WJ processed and analyzed data; JCR AND JMS wrote the manuscript, and all authors contributed to preparation of the final manuscript.
References
- 1.Ogden CL, et al. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012(82):1–8. [PubMed] [Google Scholar]
- 2.CDC. State-specific incidence of diabetes among adults--participating states, 1995–1997 and 2005–2007. MMWR Morb Mortal Wkly Rep. 2008:1169–1173. [PubMed] [Google Scholar]
- 3.Krauss RM, et al. Obesity : impact on cardiovascular disease. Circulation. 1998;98(14):1472–1476. [PubMed] [Google Scholar]
- 4.Stevens J, et al. Sensitivity and specificity of anthropometrics for the prediction of diabetes in a biracial cohort. Obes Res. 2001;9(11):696–705. doi: 10.1038/oby.2001.94. [DOI] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Gaziano JM, Vasan RS. Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Med Clin North Am. 2004;88(5):1273–1294. doi: 10.1016/j.mcna.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–557. doi: 10.1146/annurev.nu.16.070196.002515. [DOI] [PubMed] [Google Scholar]
- 7.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45(3):199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118(3):829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudgins LC, et al. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab. 2011;96(3):861–868. doi: 10.1210/jc.2010-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts R, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52(5):882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- 11.Cefalu WT, Ye J, Wang ZQ. Efficacy of dietary supplementation with botanicals on carbohydrate metabolism in humans. Endocr Metab Immune Disord Drug Targets. 2008;8(2):78–81. doi: 10.2174/187153008784534376. [DOI] [PubMed] [Google Scholar]
- 12.Ribnicky DM, et al. Antihyperglycemic activity of Tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13(8):550–557. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 14.Schwarz JM, et al. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77(1):43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]