Abstract
Purpose
To explore the biomechanical changes induced by repeated cross-linking using scanning acoustic microscopy (SAM).
Methods
Thirty human corneas were divided into three groups. In group A, five corneas were cross-linked once. In group B, five corneas were cross-linked twice, 24 hours apart. In group C, five corneas were cross-linked three times, 24 hours apart. The contralateral controls in all groups had similar treatment but without UV-A. The speed of sound, which is directly proportional to the square root of the tissue’s elastic modulus, was assessed using SAM.
Results
In group A, the speed of sound of the treated corneas was 1677.38 ± 10.70 ms−1 anteriorly and 1603.90 ± 9.82 ms−1 posteriorly, while it was 1595.23 ± 9.66 ms−1 anteriorly and 1577.13 ± 8.16 ms−1 posteriorly in the controls. In group B, the speed of sound of the treated corneas was 1746.33 ± 23.37 ms−1 anteriorly and 1631.60 ± 18.92 ms±1 posteriorly, while it was 1637.57 ± 22.15 ms±1 anteriorly and 1612.30 ± 22.23 ms−1 posteriorly in the controls. In group C, the speed of sound of the treated corneas was 1717.97 ± 18.92 ms−1 anteriorly and 1616.62 ± 17.58 ms−1 posteriorly, while it was 1628.69 ± 9.37 ms−1 anteriorly and 1597.68 ± 11.97 ms−1 posteriorly in the controls. The speed of sound in the anterior (200 × 200 μm) region between the cross-linked and control corneas in groups A, B, and C was increased by a factor of 1.051 (P = 0.005), 1.066 (P = 0.010), and 1.055 (P = 0.005) respectively. However, there was no significant difference among the cross-linked corneas in all groups (P = 0.067).
Conclusions
A significant increase in speed of sound was found in all treated groups compared with the control group; however, the difference among the treated groups is not significant, suggesting no further cross-links are induced when collagen cross-linking treatment is repeated.
Keywords: cornea, collagen cross-linking, repeated-treatment, riboflavin, UV-A, scanning acoustic microscopy (SAM), corneal biomechanics
The biomechanical properties of the cornea after applying collagen cross-linking treatment have been widely investigated in in vitro studies1 and in clinical studies.2 Ultraviolet A irradiation excites the riboflavin to its excited triplet status, producing oxygen radicals that in turn generate new covalent cross-linking of molecules in the stroma,3 including collagens and proteoglycans core proteins such as mimecan and decorin,4 thus increasing the elastic modulus of the cornea. The stiffness of the cornea was found to be significantly increased in vitro, immediately after collagen cross-linking treatment, by a factor of ×1.55 and ×1.056,7 in human corneas and ×1.88 and ×1.39 in porcine corneas. A similar increase in stiffness was reported by Beshtawi et al.7 using scanning acoustic microscopy (SAM) and Schumacher et al.10 using strip extensometry when applying low- and high-intensity collagen cross-linking on human7 and porcine corneas.10 A long-term study reported a persistent increase by ×1.6 in rabbit corneas.11 Additionally, the stiffening effect of UV-A/riboflavin treatment was found to be depth-dependent with 70% absorbed in the anterior (200 μm) cornea and with an additional 20% absorbed in the anterior 400 μm of the stroma.12 Clinically, long-term outcomes of the treatment indicated an improvement and stabilization of the cross-linking effects.13–16
The corneal collagen turnover ranges between 2 and 7 years.17 However, collagen cross-linking is expected to increase the half-life of the stromal collagen to more than 10 years, provided the patient does not experience changes3 such as pregnancy,18 hormonal alterations,19 and neurodermatitis,13 which can trigger the recurrence of keratoconus. It is possible that “renewed” collagen after turnover will not have the same increased strength and other biomechanical properties found in cross-linked collagens.14 Therefore, collagen turnover and unsatisfactory initial results may indicate the need for retreatment.
We have recently used SAM to map the biomechanical properties of human corneas6 after cross-linking and to compare the induced stiffness (which is related to the measured speed of sound) of low- and high-intensity collagen cross-linking on human corneas.7 It has been widely used in industry20 and more recently on stiff, calcified,21,22 and soft biological tissues.6,23 SAM is a novel tool to study the micromechanical properties of biological tissues, with high spatial resolution (around 1 μm at 1 GHz) and in a hydrated environment.23,24 SAM is based on the use of ultrasound waves with high frequency (100 MHz to 1 GHz), and the speed of sound is related to the stiffness of the tested sample.23 The technique is particularly useful for biomechanics research because minimal tissue preparation is needed and the acoustic images obtained with SAM also provide histological information without the need for specific staining.23
The aim of this study is to experimentally investigate the mechanical properties of the cornea after cross-linking treatment and retreatment on eye-banked human corneas using SAM on different depths.
Materials and Methods
Corneas
Thirty human eye-banked corneal buttons with 3-mm scleral rims (15 pairs, from 10 male and 5 female donors) were used in this study and assigned randomly to be treated for one, two, or three times, or to serve as a control. The mean donor age was 75 years (range, 55–88 years). The corneas with the scleral rim were removed from the eyes within 24 hours of death and placed in organ culture media at 34°C.25 During this period, the corneas swell due to changes in the extracellular matrix. After 10 days, the corneas were assessed macroscopically and microscopically. After the cell count, the corneas were placed in fresh media containing 5% dextran and incubated at 34°C for a further 24 hours to allow the corneas to shrink back to their physiological thickness.25 The corneal pairs were divided into three treatment groups.
This study was approved by the Committee on the Ethics of Research on Human Beings of the University of Manchester and followed the tenets of the Declaration of Helsinki. Manchester Eye Bank provided the corneas for this study. These corneas were contraindicated for corneal-transplantation for non-eye related reasons. All the corneas had research permission from the donors’ relatives if transplantation was not possible.
Cross-Linking Procedure
For the cross-linking treatment, the corneal buttons were placed in a Barron artificial anterior chamber (Katena Eye Instruments, Denville, NJ). The protocol used for cross-linking was the same as in Beshtawi et al.7 An 8-mm disc of epithelium at the center of the cornea was debrided by gentle scraping with a blade and exposed to 1 to 2 drops of isotonic riboflavin 0.1% solution (10 mg riboflavin-5-phosphate in 10 mL of 20% dextran-T-500 solution) at 3-minute intervals for 30 minutes. One cornea from each pair was then exposed to UV-A 370 nm, 3 mW/cm2 for 30 minutes while riboflavin was instilled at 5-minute intervals, using a solid-state UV-A illuminator (VEGA CBM X-LINKER; CSO, Florence, Italy). The contralateral cornea was exposed to riboflavin instillation (at 5-minute intervals) for an additional 30 minutes without exposure to UV-A irradiation. The cornea was then rinsed with saline, placed back in a bottle of fresh media containing 5% dextran to limit the corneal swelling, and incubated at 34°C for 24 hours prior to assessment.
Treatment Groups
Group A
In group A, five pairs (n = 10) of corneas were treated once according to the cross-linking procedure described above. The cornea was then rinsed with saline and placed back in a bottle of fresh media containing 5% dextran and incubated at 34°C for 24 hours prior to assessment. Group A corneas were the same as in a previous study (group A).7
Group B
In group B, another five pairs (n = 10) of corneas were treated according to the cross-linking procedure described earlier. The cornea was then rinsed with saline and placed in a bottle of fresh media containing 5% dextran, and incubated at 34°C for 24 hours. On the next day, the corneas were treated again with the same cross-linking protocol. The cornea was then rinsed with saline and placed in a bottle of fresh media containing 5% dextran, and incubated at 34°C for 24 hours prior to assessment.
Group C
The last five corneal pairs (n = 10) were treated according to the cross-linking procedure described above. The cornea was then rinsed with saline and placed in a bottle of fresh media containing 5% dextran, and incubated at 34°C for 24 hours. The next day, the corneas were treated as before. The cornea was then rinsed with saline and placed in a bottle of fresh media containing 5% dextran, and incubated at 34°C for 24 hours. On the following day, the cornea was treated for the third time with the same cross-linking protocol then placed back in a bottle of fresh media containing 5% dextran, and incubated at 34°C for 24 hours prior to assessment.
Tissue Preparation
The corneas were bisected and embedded in optimal cutting temperature (OCT) resin (Tissue-Tek; CellPath Ltd., Powys, UK). The frozen OCT-embedded cornea blocks were secured to a cryostat chunk (Leica CM-3050-S Cryostat; Leica Microsystems, Wetzlar, Germany) and sectioned to a thickness of 7 μm. Both halves were embedded. The sections were then mounted on glass slides, and stored at −20°C until usage. The central corneal sections were evaluated using SAM. Two sections from each cornea were tested by SAM and the average was taken.
Scanning Acoustic Microscopy Evaluation
The main components of SAM (SAM200 PVA; Tepla, Heborn, Germany) are the transducer (sapphire rod with a zinc oxide piezoelectric film) that transmits acoustic waves and receives the reflected waves from the sample, a spherical lens that acts as an acoustic lens, the coupling fluid that is mainly distilled water, and a z-stage that allows a variation in the distance between the lens and the sample down to 0.1 μm.23,26 The sample is set perpendicular to the incident sound beam and the acoustic waves are transmitted longitudinally through the sample from the anterior surface of the cornea toward the posterior surface. SAM testing was carried out in an air-conditioned laboratory with minimal temperature fluctuations. The principles of SAM have been detailed extensively elsewhere.6,7
The SAM images were collected with a custom version of MATSAM software (Julius Wolff Institute & Berlin-Brandenburg School for Regenerative Therapies, Berlin, Germany) and the recently developed phase analysis method, multi-layer phase analysis (MLPA).24 Using this method, the speed of sound data are extracted from the SAM images by utilizing the phase information that occurs at the substrate surface and internal reflections from the lens.
The images were processed off-line with custom software using the phase analysis method developed by Zhao et al.24 Briefly, the gray scale value for every pixel (x, y position) is extracted from all the images at each z position to form a V(z) curve. The V(z) data are then filtered and processed by fast Fourier transformation (FFT).24 A two-dimensional phase array is then obtained for the dataset which is then processed and converted to a speed of sound map.
Variation of the speed of sound values for isotropic materials is proportional to the square root of the Young’s modulus.6 The speed of sound in any selected region was quantitatively measured by averaging the values within a region of the cornea, excluding the artificial gaps (caused by the cryosectioning procedure). The variation of the speed of sound is plotted using a 0 to 255 level gray scale.
Statistical Analysis
All data are reported as mean ± SE. Kolmogrov-Smirnov test was used to determine whether or not the data were normally distributed (P > 0.05). A two sample t-test was used to test whether the mean values of the measurements were significantly different from each other. One-way ANOVA test was used to test whether the differences among the three groups was significant. Data analysis was conducted using statistical software (SPSS 19.0; SPSS, Inc., Chicago, IL) and a P value < 0.05 was considered to be statistically significant.
Results
The speed of sound, proportional to the square root of the Young’s modulus, of the anterior (200 × 200 μm) region and the posterior (200 × 200 μm) region for treated and control corneas as measured by SAM is given in Tables 1 and 2. There is no statistically significant difference among the cross-linked corneas in the three groups (P = 0.067), as determined with one-way ANOVA.
Table 1. The Speed of Sound (Mean ± SE) of the Anterior and Posterior Parts of The Cross-Linked and Control Corneas in All Groups.
| Cornea | Speed of Sound of the Anterior Cornea, Mean ± SE, ms−1 | Speed of Sound of the Posterior Cornea, Mean ± SE, ms−1 |
|---|---|---|
| CXL, group A | 1677.38 ± 10.70 | 1603.90 ± 9.82 |
| Control, group A | 1595.23 ± 9.66 | 1577.13 ± 8.16 |
| CXL, group B | 1746.33 ± 23.37 | 1631.60 ± 18.92 |
| Control, group B | 1637.57 ± 22.15 | 1612.30 ± 22.23 |
| CXL, group C | 1717.97 ± 18.92 | 1616.62 ± 17.58 |
| Control, group C | 1628.69 ± 9.37 | 1597.68 ± 11.97 |
CXL, cross-linking.
Table 2. The Factorial Increase in the Speed of Sound Between the Anterior and Posterior Parts of the Same Cornea and Between the Cross-Linked and Control Corneas in All Groups With P Values for Comparisons Using Analysis of Variance.
| Cornea | Increase Factor | P Value |
|---|---|---|
| Group A | ||
| CXL ant vs. post | 1.046 | 0.001 |
| Control ant vs. post | 1.011 | 0.190 |
| CXL ant vs. control ant | 1.051 | 0.005 |
| CXL post vs. control post | 1.017 | 0.069 |
| Group B | ||
| CXL ant vs. post | 1.070 | 0.005 |
| Control ant vs. post | 1.015 | 0.564 |
| CXL ant vs. control ant | 1.066 | 0.010 |
| CXL post vs. control post | 1.012 | 0.681 |
| Group C | ||
| CXL ant vs. post | 1.063 | 0.008 |
| Control ant vs. post | 1.019 | 0.568 |
| CXL ant vs. control ant | 1.055 | 0.005 |
| CXL post vs. control post | 1.012 | 0.688 |
ant, anterior; post, posterior.
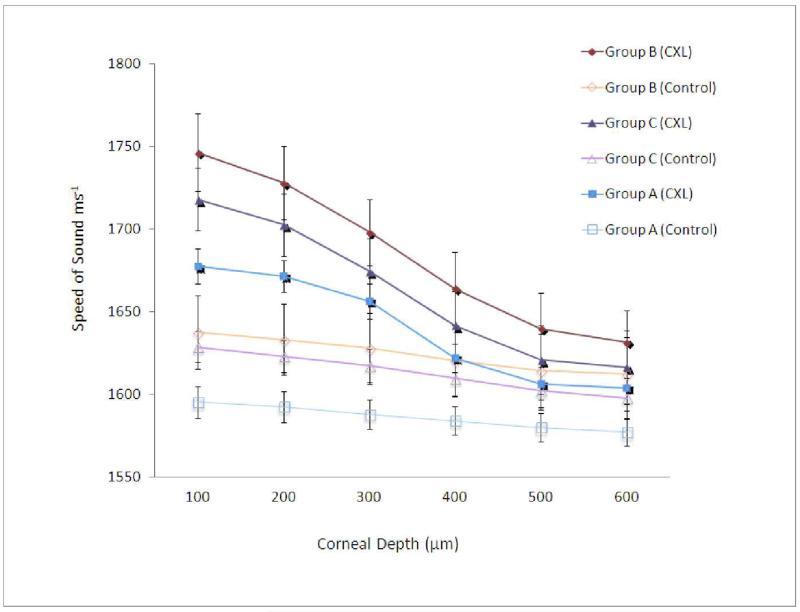
The speed of sound data across the stromal regions is shown in the Figure. The speed of sound in all groups decreased from the anterior region to the posterior region, but with different patterns. In group A, the cross-linked corneas showed a gradual decrease in the speed of sound up to 300 μm, then a sharp drop was noted in the region between 300 and 400 μm. In groups B and C, the cross-linked corneas showed a gradual decrease in the speed of sound through the corneal thickness from the anterior to the posterior part, but sharper than the decrease in the contralateral controls in this group. This decrease in the speed of sound, of the cross-linked corneas, was found to fit a cubic function for the single treatment group (R2 = 0.9938), the two-treatment group (R2 = 0.9999), and the three-treatment group (R2 = 0.9997). The control corneas of all groups showed a steady decrease in the measured speed of sound from the anterior region to the posterior region.
Figure. Speed of sound measurements of cross-linked corneas from groups A, B, and C and their contralateral control from the anterior to the posterior regions of the stroma.
Note the sharp change in speed of sound at 300 to 400 microns in each of the cross-linked corneas.
Discussion
Collagen cross-linking using ultraviolet A and riboflavin is a novel treatment to treat corneal ectasias such as keratoconus. The increase in corneal stiffness after collagen cross-linking is a definite outcome of the treatment proved experimentally, although not yet clinically due to the lack of suitable methods to clinically monitor and measure the changes in corneal biomechanics after cross-linking. Although collagen cross-linking treatment is expected to increase the half-life of the corneal collagen renewal from 5 to 10 years, repeated treatment may be necessary in keratoconic patients who have previously undergone cross-linking in addition to patients with unsatisfactory results after the first treatment. Therefore, studying the effects of repeated treatment is important. In this study, we evaluated the speed of sound changes of collagen cross-linking treatment and repeated treatment carried out on postmortem human corneas in-vitro using SAM.
SAM is a powerful tool to quantify the mechanical properties of tissue in-vitro, pixel by pixel, in different depths. It has been used successfully on human corneas to quantify the speed of sound measurements after collagen cross-linking treatment.6,7 The corneal tissue is not homogeneous or isotropic and the mechanical properties in this setting may be influenced by the interfibrillar ground substance.27 However, in our previous work,28 we have demonstrated that the speed of sound values obtained with SAM closely correlate with collagen content in tissues (in other tissues that were also cryosectioned). Hence, we expect the speed of sound properties in the cornea to be dominated by the collagen content and properties. The SAM technique is sensitive to the properties of extracellular matrix proteins such as collagen.29 Hence, we expect the mechanical properties determined with SAM to be dominated by the fibrous (load-bearing) components of the tissue and reflective of the biomechanical properties of the overall tissue.
The outcomes of this study showed an increase in speed of sound measurements in the anterior region of the cross-linked corneas in all groups, when compared with the posterior part of the same corneal section, and a higher speed of sound was documented in the cross-linked corneas when compared with the contralateral control in all groups. The increase in speed of sound found in the single treatment group was by a factor of 1.05× and this is lower than what was reported previously: 1.5,5 1.6,12 1.8,8 and 1.3×.10 This difference may be due to different species, experimental settings, and procedures used.
The corneas that received repeated treatments (two and three treatments) showed a higher increase in speed of sound (1.066 and 1.055×, respectively) and thus increased stiffness when compared with the single treatment group (1.051×), suggesting further cross-links are induced when collagen cross-linking treatment is repeated. The corneas that received repeated treatments (two and three treatments) also showed an increase in speed of sound (1.066 and 1.055×, respectively). However, the difference among the treated groups was not significant, suggesting no further cross-links in the anterior stroma region are induced when collagen cross-linking treatment is repeated.
The storage time for the three examined groups, where corneas in single, two-, and three-times treatment groups were stored in medium for 48, 72, and 96 hours, respectively. This difference in storage time us unlikely to have influenced the results as corneal tissue is stable in dextran media for 5 days with no change or detriment to the cells, with the deswelling occurring within 24 hours.25,30
The increase of the speed of sound in the cross-linked corneas was depth-dependent, decreasing from the anterior stroma to the posterior part of the stroma. In the single treatment (group A) the cross-linked corneas showed a gradual decrease in the speed of sound up to 300 μm. This has previously been shown to be the region where cross-linking occurs.8,12 A sharp drop was noted in the region between 300 and 400 μm followed by a gradual decrease toward the posterior stroma. The regional depth of the collagen cross-linking treatment effect was investigated previously by Scarcelli et al.31 and similar results were reported after standard collagen cross-linking treatment on porcine corneas. However, in the present study, the cross-linked corneas in the repeated treatment groups (B and C) exhibited a steady gradual decrease from the anterior to the posterior part of the stromal cornea, which may suggest that repeated cross-linking creates new cross-links at a deeper level compared with a single cross-linking treatment. Additionally, as each plotted graph fitted a cubic function (R2 > 0.99), the increase in speed of sound after single and repeated treatment can be calculated at any depth. The safety of repeated treatments with respect to the stromal keratocytes and endothelium is still under investigation by our group. The control corneas of all groups showed a steady decrease in the measured speed of sound from the anterior region to the posterior region, which may be due to their inherently different structural architecture. In a future study, the speed of sound could be measured from the posterior part of the cornea to disassociate the cross-linking from the stromal structure.
The advanced age (mean = 75 years) of the corneas is a limitation in this study as corneas cross-link naturally with age. Nevertheless, differences between the treated corneas and their contralateral controls were found in all treatment groups.
In conclusion, since repeated cross-linking might be necessary for some patients, we assessed in this study the induced stiffness (with acoustic microscopy) after repeated collagen cross-linking treatment and compared it with single treatment results. A greater speed of sound was demonstrated in the treated corneas compared with the controls in all groups. Additionally, a small but insignificant increase in speed of sound measurements were found in the twice-treated group compared with the single- and three-treatment groups, suggesting further cross-links may be induced when collagen cross-linking is carried out a second time. However, consecutive repeated treatment within a few days is ineffective. The safety of repeated cross-linking is yet to be determined. Evidence-based criteria relating to which patients to re-treat and when the optimum time to re-treat is, need to be established.
Acknowledgments
The authors thank Manchester Eye Bank for providing the corneas for this study, and Sebastian Brand (Fraunhofer Institute of Material Mechanics, Freiburg, Germany) and Kay Raum (Julius Wolff Institute & Berlin-Brandenburg School for Regenerative Therapies, Berlin, Germany), who developed the MATSAM software utilized in this study.
Supported by Wellcome Trust (WT085981AIA): development of the SAM; An-Najah National University, Nablus, Palestine (IMB); and Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network (AB).
Footnotes
Disclosure: I.M. Beshtawi, None; R. Akhtar, None; M.C. Hillarby, None; C. O’Donnell, None; X. Zhao, None; A. Brahma, None; F. Carley, None; B. Derby, None; H. Radhakrishnan, None
References
- 1.Beshtawi IM, O’Donnell C, Radhakrishnan H. Biomechanical properties of corneal tissue after ultraviolet-A-riboflavin cross-linking. J Cataract Refract Surg. 2013;39:451–462. doi: 10.1016/j.jcrs.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. Part II. Clinical indications and results. Ocul Surf. 2013;11:93–108. doi: 10.1016/j.jtos.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Meek KM, Hayes S. Corneal cross-linking—a review. Ophthalmic Physiol Opt. 2013;33:78–93. doi: 10.1111/opo.12032. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YT, Conrad AH, Conrad GW. Effects of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking. J Biol Chem. 2011;286:13011–13022. doi: 10.1074/jbc.M110.169813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spoerl E. Biomechanical stiffness of keratoconic cornea, crosslinking and correlation to demarcation line; Paper presented at: 7th International Congress of Corneal Cross-Linking; Zurich, Switzerland. 9–10 December 2011. [Google Scholar]
- 6.Beshtawi IM, Akhtar R, Hillarby MC, et al. Scanning acoustic microscopy for mapping the microelastic properties of human corneal tissue. Curr Eye Res. 2013;38:437–444. doi: 10.3109/02713683.2012.753094. [DOI] [PubMed] [Google Scholar]
- 7.Beshtawi IM, Akhtar R, Hillarby CM, et al. Biomechanical properties of human corneas following low and high intensity collagen cross-linking determined with scanning acoustic microscopy. Invest Ophthalmol Vis Sci. 2013;54:5273–5280. doi: 10.1167/iovs.13-12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 9.Schumacher S, Mrochen M, Wernli J, Bueeler M, Seiler T. Optimization model for UV-riboflavin corneal cross-linking. Invest Ophthalmol Vis Sci. 2012;53:762–769. doi: 10.1167/iovs.11-8059. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011;52:9048–9052. doi: 10.1167/iovs.11-7818. [DOI] [PubMed] [Google Scholar]
- 11.Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol. 2009;87:48–51. doi: 10.1111/j.1755-3768.2008.01190.x. [DOI] [PubMed] [Google Scholar]
- 12.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 13.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan D, Males J. Prospective longitudinal study of corneal collagen crosslinking in progressive keratoconus. Clin Experiment Ophthalmol. 2013;41:531–536. doi: 10.1111/ceo.12035. [DOI] [PubMed] [Google Scholar]
- 16.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Dahl BJ, Spotts E, Truong JQ. Corneal collagen cross-linking: An introduction and literature review. Optometry. 2012;83:33–42. doi: 10.1016/j.optm.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Hafezi F, Iseli HP. Pregnancy-related exacerbation of iatrogenic keratectasia despite corneal collagen crosslinking. J Cataract Refract Surg. 2008;34:1219–1221. doi: 10.1016/j.jcrs.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 19.Spoerl E, Zubaty V, Terai N, Pillunat LE, Raiskup F. Influence of high-dose cortisol on the biomechanics of incubated porcine corneal strips. J Refract Surg. 2009;25:S794–S798. doi: 10.3928/1081597X-20090813-06. [DOI] [PubMed] [Google Scholar]
- 20.Khuriyakub BT. Scanning acoustic microscopy. Ultrasonics. 1993;31:361–372. [Google Scholar]
- 21.Eckardt I, Hein HJ. Quantitative measurements of the mechanical properties of human bone tissues by scanning acoustic microscopy. Ann Biomed Eng. 2001;29:1043–1047. doi: 10.1114/1.1424909. [DOI] [PubMed] [Google Scholar]
- 22.Raum K, Kempf K, Hein HJ, Schubert J, Maurer P. Preservation of microelastic properties of dentin and tooth enamel in vitro—a scanning acoustic microscopy study. Dent Mater. 2007;23:1221–1228. doi: 10.1016/j.dental.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Akhtar R, Sherratt MJ, Watson RE, Kundu T, Derby B. Mapping the micromechanical properties of cryo-sectioned aortic tissue with scanning acoustic microscopy. Mater Res Soc Symp Proc. 2009;1132E doi: 10.1557/PROC-1132-Z03-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, Akhtar R, Nijenhuis N, et al. Multi-layer phase analysis: quantifying the elastic properties of soft tissues and live cells with ultra-high-frequency scanning acoustic microscopy. IEEE Trans Ultrason Ferroelectr Freq Control. 2012;59:610–620. doi: 10.1109/TUFFC.2012.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armitage WJ, Easty DL. Factors influencing the suitability of organ-cultured corneas for transplantation. Invest Ophthalmol Vis Sci. 1997;38:16–24. [PubMed] [Google Scholar]
- 26.Briggs A, Kolosov O. Acoustic Microscopy. 2nd ed. Oxford University Press; Oxford: 2010. [Google Scholar]
- 27.Sondergaard AP, Ivarsen A, Hjortdal J. Reduction of stromal swelling pressure after UVA-riboflavin cross-linking. Invest Ophthalmol Vis Sci. 2013;54:1625–1634. doi: 10.1167/iovs.12-10346. [DOI] [PubMed] [Google Scholar]
- 28.Graham HK, Akhtar R, Kridiotis C, et al. Localised micromechanical stiffening in the ageing aorta. Mech Ageing Dev. 2011;132:459–467. doi: 10.1016/j.mad.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saijo Y, Jørgensen CS, Erling F. Characterization of collagen fibers in atherosclerotic plaques in mice. In: Halliwell M, Wells PNT, editors. Acoustical Imaging. Vol. 25. Springer; New York: 2001. pp. 363–368. [Google Scholar]
- 30.Armitage WJ. Preservation of human cornea. Transfus Med Hemother. 2011;38:143–147. doi: 10.1159/000326632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013;54:1418–1425. doi: 10.1167/iovs.12-11387. [DOI] [PMC free article] [PubMed] [Google Scholar]