Abstract
The ability to localize sound rapidly and accurately depends on the precise organization of inhibitory neuronal circuits in the auditory brainstem. However, the rules and mechanisms by which this precision is established during development are still poorly understood. Although activity-dependent reorganization has been known for over a decade to have a central role in this process, more recent studies have revealed an unanticipated degree of reorganization that occurs on levels ranging from cellular phenotype to network connectivity. These results suggest novel mechanisms by which immature inhibitory sound-localization circuits become optimized. Lessons from auditory brainstem circuits thus could provide insight into inhibitory development in other brain areas, where inhibitory networks are less experimentally accessible.
Introduction
Establishing correctly organized and appropriately adjusted synaptic circuits is a crucial event during brain development. The past few decades have seen tremendous progress in deciphering the mechanisms used to remodel developing excitatory circuits [1,2]. However, despite the central role of inhibition in calibrating and fine-tuning neuronal activity, the processes by which inhibitory circuits are established remain poorly understood. Several recent studies have taken advantage of the precise organization of inhibitory pathways in the auditory system, providing new information about the sequence of events by which inhibitory networks are assembled and giving rise to an emerging conceptual framework for understanding inhibitory circuit development. This short review will focus on studies of developing inhibitory connections in auditory sound-localization circuits. After a brief introduction to the organization of primary sound-localization circuits in the mammalian brain, we will illustrate key processes by which these circuits are optimized during development. We will focus on very recent work and emphasize studies of direct relevance to the issues discussed. For a more comprehensive treatment of auditory development, the reader is referred to previously published reviews [3–5].
Inhibition in primary sound-localization circuits
Animals determine the azimuthal location of incoming sound primarily through interaural level differences (ILDs) and interaural time differences (ITDs). Neurons sensitive to ILDs and/or ITDs are found at almost all levels in the mammalian central auditory system, but are encountered for the first time by incoming sound information in the superior olivary complex (SOC), a collection of about nine auditory nuclei in the ventral brainstem. In the SOC, neurons specialized for encoding ILDs are located in the lateral superior olive (LSO), a nucleus easily recognized in most mammals by its characteristic S-shape [6,7] (Figure 1a). LSO neurons process ILDs by integrating excitatory inputs from the ipsilateral ear with inhibitory inputs from the contralateral ear. The ipsilateral excitatory input is carried by glutamatergic fibers from the cochlear nucleus, whereas the inhibitory contralateral input is carried by glycinergic fibers from the sign-inverting medial nucleus of the trapezoid body (MNTB). The precise tonotopic convergence of these inputs onto individual cells of the LSO enables LSO neurons to extract ILDs in a frequency-specific manner [6].
Figure 1.
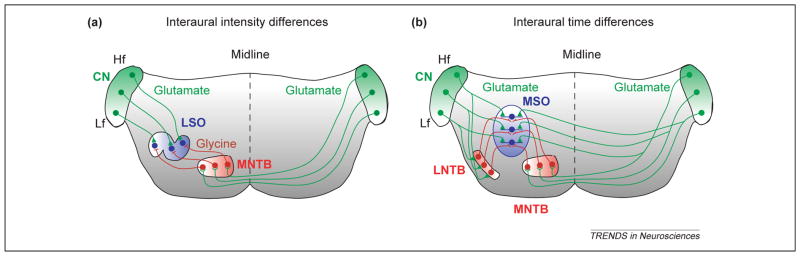
Synaptic organization of primary sound localization circuits. (a) Neurons in the lateral superior olive (LSO) encode interaural intensity differences by integrating excitatory glutamatergic inputs from the ipsilateral cochlea nucleus (CN) with inhibitory glycinergic inputs from the medial nucleus of the trapezoid body (MNTB), which in turn is activated by the contralateral cochlea nucleus. (b) Neurons in the medial superior olive (MSO) encode interaural time differences by integrating bilateral excitatory inputs from both cochlear nuclei and bilateral inhibitory inputs from the lateral nucleus of the trapezoid body (LNTB) and the MNTB. All of these auditory nuclei are tonotopically organized, as indicated by color gradients. Additional abbreviations: Hf, high frequency; Lf, low frequency.
Neurons specialized for processing ITDs are located in the medial superior olive (MSO) (Figure 1b). Mammalian MSO neurons, or their analogs in the avian nucleus laminaris, receive tonotopically matched excitatory inputs from both ears and are sensitive to the relative arrival time of these inputs on a submillisecond scale [8]. This extraordinary temporal resolution is achieved through such specializations as axonal delay lines [9], termination of inputs on opposing dendritic trees [10] and properties of voltage-gated conductances [11,12]. Recently, Grothe and colleagues demonstrated that in mammals, encoding ITDs in the physiological range also depends on fast and precisely timed bilateral inhibition [8,13]. This inhibition is provided by glycinergic neurons in the lateral nucleus of the trapezoid body and the MNTB. In low-frequency-hearing mammals, which localize sound primarily using ITDs, the glycinergic synapses are almost exclusively targeted to somata of MSO neurons – an organization that optimizes fast and temporally precise inhibition.
From excitation to inhibition
Developing GABAergic and glycinergic synapses in the MNTB and LSO, as elsewhere in the brain, undergo a striking switch from a depolarizing to a hyperpolarizing phenotype [14–16]. Depolarization mediated by GABA and glycine results from a high intracellular Cl− concentration ([Cl−]i) that sets the Cl− equilibrium potential (ECl) as positive relative to the resting membrane potential (Vrest) [17,18]. Consequently, activation of GABAA or glycine receptors causes Cl− efflux and resulting membrane depolarization. In the neonatal LSO, GABA and glycine are not only depolarizing but also excitatory, because glycine application or MNTB stimulation increase the intracellular Ca2+ concentration through activation of voltage-gated Ca2+ channels, and can trigger Na+-mediated action potentials [18–20]. This excitatory action of GABA and glycine is restricted to the first postnatal week, during which the progressive shift of ECl towards more negative values gradually converts GABA and glycine into hyperpolarizing neurotransmitters [14,15,17,18,21].
The cellular mechanisms by which immature LSO neurons accumulate Cl− to establish and maintain a high [Cl−]i are incompletely understood. In contrast to its role in many other neuron types [16], the Na+–2Cl−–K+ cotransporter NKCC1 cannot be responsible for the high [Cl−]i of neonatal LSO neurons, because it is not expressed during the depolarizing period [22,23]. A recent study [22] points to the HCO3 −–Cl− exchanger AE3 as a potential candidate, although further functional studies are necessary to establish whether AE3 indeed elevates [Cl−]i in neonatal LSO neurons.
The low [Cl−]i in mature LSO neurons is created and maintained by the K+–Cl− cotransporter KCC2 [23]. Strong evidence for this comes from KCC2-knockdown mice, in which glycine remains a depolarizing neurotransmitter after the normal depolarizing period [23,24]. A major current question is how KCC2 activity is regulated during LSO maturation. Single-cell RT–PCR has shown upregulation of KCC2 mRNA in LSO neurons during the first two postnatal weeks [25]. However, post-translational modification of KCC2 might be the principal determinant of KCC2 activity, because a separate study demonstrated high levels of KCC2 mRNA and protein already in newborn animals. Because this KCC2 protein was located primarily in the cytosol and not in the plasma membrane, KCC2 protein is unlikely to be functional at this age. Concomitant with the switch to hyperpolarization, KCC2 protein translocates to the cell membrane and presumably becomes functional.
The regulation of KCC2 activity through neuronal activity, membrane trafficking and phosphorylation [26–28] raises the possibility that developmental changes in spontaneous activity before hearing onset control KCC2 function [29]. In accordance with this view, ablation of the cochlea, which is thought to abolish spontaneous bursting activity present before hearing onset, decreases KCC2 activity and phosphorylation in auditory midbrain neurons [30]. In LSO neurons, cochlea ablation or systemic application of the glycine receptor antagonist strychnine impedes upregulation of KCC2 mRNA and impairs the switch to hyperpolarizing glycine responses [25,31].
What is the role of excitatory GABA and glycine in SOC neurons? Through the activation of voltage-gated Ca2+ channels, depolarizing GABAergic and glycinergic synapses gain access to various intracellular Ca2+ signaling pathways that have central roles in nearly all major developmental events of the SOC [32–34]. Excitation mediated by GABA and glycine might also be important for synaptic reorganization of the MNTB–LSO pathway [35] and for the processes by which inhibitory and excitatory inputs become tonotopically aligned. Our ability to address the latter possibility will greatly benefit from a better understanding of the spatial and temporal characteristics of glutamate- and glycine-mediated Ca2+ responses and their mutual interactions.
Elimination of GABAergic and glycinergic connections sharpens tonotopic maps
Neurons in the LSO, MSO and MNTB receive synaptic inputs before or around birth, one-to-two weeks before the onset of hearing [11,36–38]. From the outset, these early inputs are topographically organized, suggesting that the basic tonotopic organization of auditory nuclei is achieved using guidance molecules [36,39–42]. The initial topographic patterns, however, lack the precise tonotopy of the mature system, which is achieved through processes that include additional synapse formation and elimination.
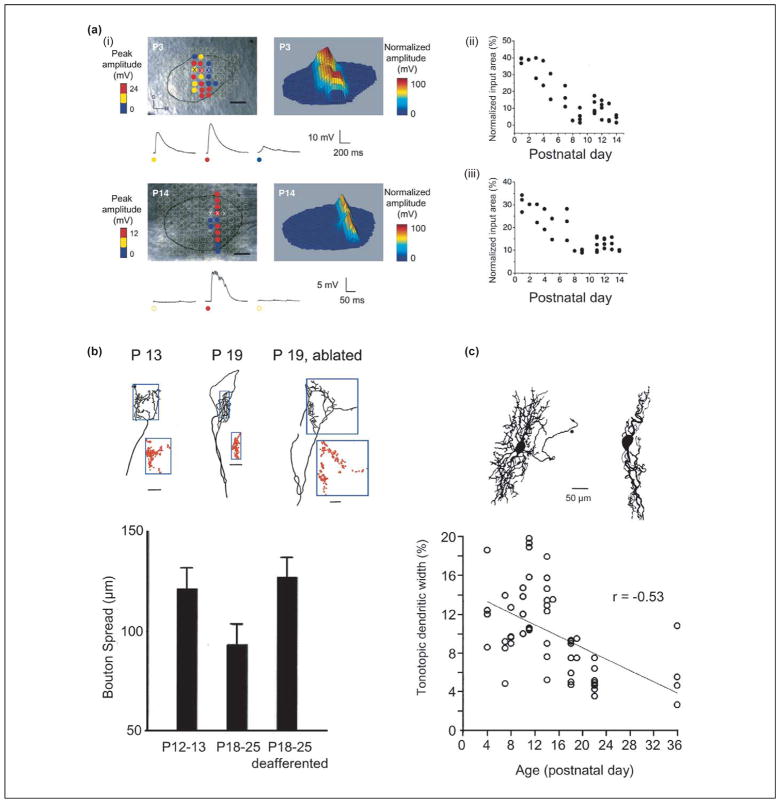
Pioneering studies by Sanes and colleagues in the MNTB–LSO pathway of gerbils demonstrated that the axonal arbors of MNTB neurons and the dendritic trees of LSO neurons both become restricted to tonotopically narrower bands during development [43,44] (Figure 2). The timing of these structural changes during the third postnatal week – the week after hearing onset – suggests that auditory experience has a role in these processes. In support of this view, deafening by cochlea ablation interferes with this pruning and gives rise to less precise MNTB–LSO topography [39,45]. Using focal photolysis of caged glutamate to map MNTB–LSO connectivity in rat brainstem slices, this pruning was recently found to be preceded by functional synaptic refinement [35] (Figure 2). Individual LSO neurons in newborn rats receive synaptic inputs from ~40% of the MNTB cross-sectional area, but one week later receive inputs from only ~8%. Along with the functional loss of most inputs, the synaptic conductance of the remaining inputs increases approximately twelvefold. The contraction of input maps along the tonotopic axis translates to a twofold increase in topographic accuracy – a degree of refinement comparable to that achieved after hearing onset through pruning.
Figure 2.
Functional and anatomical refinement of the developing MNTB–LSO pathway. (a) Developmental decrease in the size of MNTB input area to individual rat LSO neurons. (i) Example input maps as revealed by focal glutamate uncaging in the MNTB at postnatal day (P)3 and P14. (ii, iii) Developmental profile of normalized input map area (ii) and width along the tonotopic axis (iii). Modified, with permission, from Ref. [35]. (b) Developmental refinement of MNTB axonal arbors in the LSO. Red dots indicate the distribution of boutons relative to the tonotopic axis indicated by the 100 μm scale bar. Modified, with permission, from Ref. [5]. (c) Developmental refinement of dendritic arbors of LSO neurons. The ‘tonotopic width’ is defined as the percentage of the mediolateral tonotopic axis over which dendritic trees of single LSO neurons spread. Note the absence of wide dendritic trees after hearing onset (~P14). Modified from Ref. [70], with permission of Wiley-Liss Inc., a subsidiary of John Wiley & Sons, Inc.
Functional weakening of synapses followed quickly by structural elimination is a common sequence during excitatory-circuit refinement [46]. The MNTB–LSO system is unusual in that these two processes appear to be separated by at least a week – a long period in the rapidly developing auditory system of rats. Consequently, functional and structural refinement in the LSO occur in two qualitatively distinct contexts. Functional refinement takes place when MNTB–LSO synapses are excitatory and probably driven by spontaneous activity [29,47,48]. Structural refinement occurs when MNTB–LSO synapses are hyperpolarizing and are driven by auditory experience. Perhaps the functionally defined map provides a default ‘best guess’ topography to guide subsequent pruning. The delay of pruning predicts a transient, functionally silent, inhibitory network [49–51]. Retention of a broadly tuned silent network might allow the system to correct for slight differences in the frequency-place code between the two cochleae [52] by providing room for fine-tuning tonotopic convergence of inputs from the MNTB and cochlear nucleus.
Experience-dependent subcellular repositioning of glycinergic synapses
That sensory experience can direct subcellular location of inhibitory synapses was first demonstrated in the MSO [53]. Before hearing onset, glycinergic synapses are distributed with equal density on somata and dendrites. In gerbils and other low-frequency-hearing animals, glycinergic synapses on dendrites are eliminated after hearing onset, whereas synapses on the soma are maintained, resulting in an age-dependent decrease in the ratio of the number of synapses on dendrites to that of synapses on somata. This synaptic redistribution requires normal auditory experience. If normal binaural cues are destroyed by raising gerbils in omnidirectional white noise, or if one cochlea is ablated, specific elimination of dendritic glycinergic synapses fails to occur. As in the LSO, activity-dependent pruning of glycinergic synapses occurs after glycine becomes hyperpolarizing. Because individual MNTB axons project to both the MSO and LSO, it will be interesting to discover whether depolarizing MNTB–MSO synapses undergo functional changes similar to MNTB–LSO synapses, or whether terminals on different axon collaterals are refined by different mechanisms and on timescales depending on the postsynaptic target.
Adjusting inhibitory synaptic strength
Another necessary step in fine-tuning inhibitory circuits is adjusting synaptic strength. The postsynaptic current is influenced by number of release sites, by probability of release, and by quantal size at GABAergic and glycinergic synapses; it is also influenced by Cl− concentration. In the rat MNTB, inhibitory postsynaptic currents (IPSCs) increase fivefold during the second postnatal week, and continue to increase into the fourth postnatal week by as much as two orders of magnitude [14]. The large increase after hearing onset is due primarily to an increase of glycinergic current caused by more synchronized release and by an increase in quantal amplitude, which probably reflects an increase in size of glycine receptor clusters [54]. This increase is regulated by auditory experience, because glycinergic synapses in the MNTB of congenitally deaf mice fail to achieve normal synaptic strength and exhibit more synaptic sites with fewer receptors at each [55]. In addition, IPSCs in these mice show relatively slower decay times, suggesting that the normal developmental substitution of glycine receptor subunit α1 for α2 is compromised. It will be interesting to determine the synaptic mechanisms that strengthen MNTB–LSO connections during the period of map refinement [35].
Activity-dependent changes in synaptic efficacy, such as long-term potentiation (LTP) and long-term depression (LTD), are understood to be important processes in the developmental reorganization of synaptic circuits. In the LSO of one-to-three-week-old gerbils, low-frequency stimulation of MNTB inputs induces a form of LTD (LFS-LTD) that is expressed postsynaptically, as indicated by the fact that both GABAA-receptor-mediated and glycine-receptor-mediated currents are reduced [34,56]. The mechanisms underlying LTD induced by high-frequency stimulation (HFS-LTD) are complex and likely to involve activation of several signaling pathways. Induction of HFS-LTD requires an increase in intracellular Ca2+ concentration but not depolarization of the membrane or activation of voltage-gated Ca2+ channels. Induction of HFS-LTD also depends on the activation of GABAB receptors and tyrosine kinase receptors, which are activated by brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT-3). These receptors activate the cAMP-dependent protein kinase (PKA), protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase II (CAMKII), each of which participates in the expression of HFS-LTD, perhaps by phosphorylation of GABAA and glycine receptors [57]. Consistent with the hypothesis that HFS-LTD participates in MNTB–LSO refinement, HFS-LTD is greatest around hearing onset, during the period when tonotopic precision is increased through experience-dependent processes involving axonal and dendritic pruning. It is currently unclear whether MNTB–LSO synapses can express additional forms of LTD, or perhaps any forms of LTP [27,58], that might mediate early refinement of depolarizing MNTB–LSO synapses.
Changing neurotransmitter phenotype at MNTB–LSO synapses
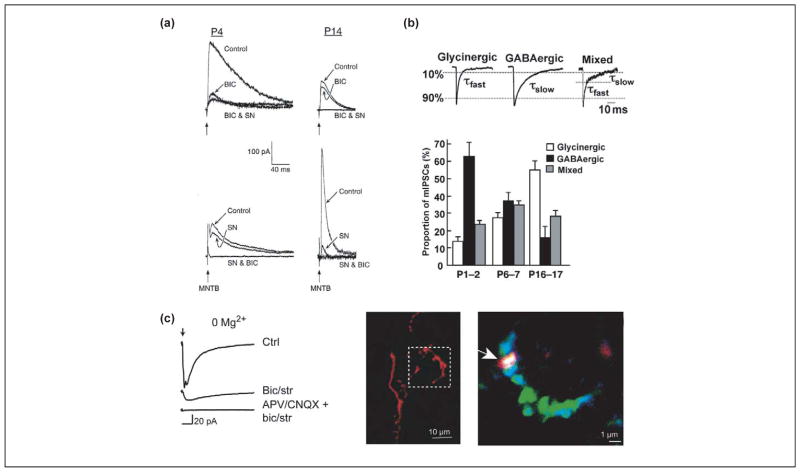
Although mature MNTB–LSO synapses are glycinergic [36,59,60], immature MNTB–LSO synapses are primarily GABAergic before hearing onset [20,21,61]. In neonatal gerbils and rats, MNTB-elicited postsynaptic currents and Ca2+ responses are blocked to a large extent by the GABAA receptor antagonist bicuculline [20,21,61] (Figure 3). Both postsynaptic and presynaptic changes appear to mediate the transition from a primarily GABAergic phenotype to a glycinergic phenotype. Postsynaptically, GABAA receptor subunits β2 and β3 are down-regulated, whereas the glycine-receptor-anchoring protein gephyrin is upregulated [62]. Presynaptically, there is a shift in vesicle content. In neonatal MNTB terminals, a majority of the synaptic vesicles contain GABA, whereas by P16 most vesicles contain glycine only [61]. At all ages there are also mixed GABA–glycine vesicles. Although the function of this mixed GABA–glycine phenotype is unknown in the LSO or elsewhere [63,64], GABA–glycine cotransmission does provide the postsynaptic neuron with various options for regulating inhibitory input through relative receptor number and distribution. The longer decay times of GABAA-receptor-mediated currents might also create a broader window for coincidence detection and allow the interaction of more loosely correlated inputs at the beginning of hearing. Finally, and perhaps most importantly, activation of GABAB receptors(which, as will be discussed in the following paragraph, is necessary for inducing activity-dependent synaptic depression at MNTB–LSO synapses [56]), could enable GABA release to constitute a central mechanism for synapse-specific refinement.
Figure 3.
Multiple neurotransmitters in immature MNTB–LSO synapses. (a) In neonatal gerbils, MNTB-evoked (vertical arrow) synaptic currents are predominantly GABAergic and are blocked by bicuculline (BIC). At P14, these synaptic currents are predominantly glycinergic and are blocked by strychnine (SN). Modified, with permission, from Ref. [21] © (1998) the Society for Neuroscience. (b). Synaptic vesicles in MNTB terminals contain GABA, glycine or both, as distinguished by the decay kinetics of miniature inhibitory postsynaptic currents (mIPSCs). In neonates, most vesicles are purely GABAergic whereas, after hearing onset (P16–P17), most vesicles are purely glycinergic. Modified, with permission, from Ref. [61]. (c) In Mg2+-free solution, blocking GABA and glycine receptors using bicuculline (bic) and strychnine (str) uncovers an MNTB-evoked (arrow) glutamatergic component that is blocked by the glutamate receptor antagonists D-2-amino-5-phosphonopentanoic acid (D-APV) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). In identified MNTB axons in the LSO, labeled extracellularly using Alexa (red), the vesicular glutamate transporter VGLUT3 (blue) colocalizes with the presynaptic marker synaptic vesicle protein SV2 (green; overlay of all three colors results in white color). Modified, with permission, from Ref. [65].
Recently, MNTB–LSO synapses were found to release not only GABA and glycine, but also glutamate [65]. The glutamate component was revealed by recording in Mg2+-free solution, and in fact much of the response was mediated by NMDA receptors. Interestingly, the glutamatergic response was most prominent during the first postnatal week, when the vesicular glutamate transporter VGLUT3 is highly expressed in the SOC [65,66] (Figure 3). These findings suggest that VGLUT3 expression enables glutamate release at nascent inhibitory synapses during the period of functional refinement. Because GABA and glycine are depolarizing during this period, we hypothesize that depolarizing GABA and glycine could relieve Mg2+-block at NMDA receptors, whereas glutamate release at the same synapse activates those receptors. During the early depolarizing period, this would enable inhibitory synapses to borrow mechanisms of excitatory synaptic plasticity, modulating the activity and stability not of AMPA receptors but instead of GABA and glycine receptors [67]. If so, glutamate release could then mediate tonotopic sharpening in the inhibitory MNTB–LSO pathway. Intriguingly, VGLUT3 expression in the SOC falls during the week after hearing onset [66], suggesting that auditory-evoked activity might downregulate VGLUT3 expression and glutamate release. Indeed, changing neurotransmitter phenotype and modulation of transmitter phenotype by neural activity has been shown in other systems [66,68,69], and it will be interesting to know whether neuronal activity or auditory experience regulates the multiple transmitter release seen in the MNTB–LSO pathway.
Concluding remarks
Results from studies in primary sound-localization circuits are giving rise to a framework for conceptualizing the events and mechanisms by which these highly precise inhibitory circuits develop. For example, activity-dependent sharpening of MNTB–LSO topography appears to entail a series of events that fall into two qualitatively distinct phases. The first phase is characterized by functional elimination (silencing) of MNTB–LSO connections during the period of depolarization. This process might use mechanisms that rely on depolarizing GABA and glycine to enable NMDA receptor activation by glutamate that is also released during this time, allowing spontaneous activity to guide synapse refinement before hearing onset. The second phase could use the functionally defined topography as a scaffold for tonotopic sharpening via the axonal and dendritic pruning that occurs after GABA and glycine become hyperpolarizing. This process might use mechanisms that include GABA-dependent and neurotrophin-dependent synaptic depression, enabling auditory experience to drive fine-adjustment of tonotopy.
Many crucial questions remain unanswered. In addition to testing working models, we need to understand better the properties and abilities of immature inhibitory synapses, and their mutual interaction with excitatory synapses. Another burning question concerns what specific physiological and morphological changes during circuit development and tonotopic refinement are influenced by spontaneous versus auditory-evoked activity. Because the processes that translate synaptic activity into circuits must operate first on depolarizing and then on hyperpolarizing synapses, it is possible that some of these processes will involve unexpected mechanisms, such as perhaps glutamate release from GABAergic and glycinergic synapses. Whatever the answers to these questions, the auditory brainstem should continue to yield exciting new insights into the development and plasticity of inhibitory circuits – stay tuned.
Acknowledgments
Work in our laboratory mentioned in this review was supported by the Alfred P. Sloan foundation, a Presidential Early Career Award for Scientists and Engineers, and grants from the National Institute on Deafness and other Communication Disorders.
References
- 1.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 2.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 3.Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- 4.Rubel EW, et al. Development of the Auditory System. Springer-Verlag; 1998. [Google Scholar]
- 5.Sanes DH, Friauf E. Development and influence of inhibition in the lateral superior olivary nucleus. Hear Res. 2000;147:46–58. doi: 10.1016/s0378-5955(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 6.Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- 7.Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grothe B. New roles for synaptic inhibition in sound localization. Nat Rev Neurosci. 2003;4:540–550. doi: 10.1038/nrn1136. [DOI] [PubMed] [Google Scholar]
- 9.Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agmon-Snir H, et al. The role of dendrites in auditory coincidence detection. Nature. 1998;393:268–272. doi: 10.1038/30505. [DOI] [PubMed] [Google Scholar]
- 11.Smith AJ, et al. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. J Physiol. 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svirskis G, et al. Sodium along with low-threshold potassium currents enhance coincidence detection of subthreshold noisy signals in MSO neurons. J Neurophysiol. 2004;91:2465–2473. doi: 10.1152/jn.00717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand A, et al. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- 14.Awatramani GB, et al. Staggered development of GABAergic and glycinergic transmission in the MNTB. J Neurophysiol. 2005;93:819–828. doi: 10.1152/jn.00798.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Ari Y. Excitatory actions of GABA during development, the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 17.Kakazu Y, et al. Regulation of intracellular chloride by cotransporters in developing lateral superior olive neurons. J Neurosci. 1999;19:2843–2851. doi: 10.1523/JNEUROSCI.19-08-02843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrlich I, et al. Shift from depolarizing to hyperpolarizing glycine action in rat auditory neurones is due to age-dependent Cl− regulation. J Physiol. 1999;520:121–137. doi: 10.1111/j.1469-7793.1999.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kullmann PH, Kandler K. Glycinergic/GABAergic synapses in the lateral superior olive are excitatory in neonatal C57Bl/6J mice. Dev Brain Res. 2001;131:143–147. doi: 10.1016/s0165-3806(01)00271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kullmann PH, et al. Glycinergic and GABAergic calcium responses in the developing lateral superior olive. Eur J Neurosci. 2002;15:1093–1104. doi: 10.1046/j.1460-9568.2002.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotak VC, et al. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker M, et al. Differential expression pattern of chloride transporters NCC, NKCC2, KCC1, KCC3, KCC4, and AE3 in the developing rat auditory brainstem. Cell Tissue Res. 2003;312:155–165. doi: 10.1007/s00441-003-0713-5. [DOI] [PubMed] [Google Scholar]
- 23.Balakrishnan V, et al. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, et al. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J Neurophysiol. 2005;93:1557–1568. doi: 10.1152/jn.00616.2004. [DOI] [PubMed] [Google Scholar]
- 25.Shibata S, et al. Experience-dependent changes in intra-cellular Cl− regulation in developing auditory neurons. Neurosci Res. 2004;48:211–220. doi: 10.1016/j.neures.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Rivera C, et al. Mechanism of activity-dependent down-regulation of the neuron-specific K–Cl cotransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodin MA, et al. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- 28.Aguado F, et al. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 29.Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14:1486–1495. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- 31.Kotak VC, Sanes DH. Developmental influence of glycinergic transmission, Regulation of NMDA receptor-mediated EPSPs. J Neurosci. 1996;16:1836–1843. doi: 10.1523/JNEUROSCI.16-05-01836.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohmann C, et al. Development of a topographically organized auditory network in slice culture is calcium dependent. J Neurobiol. 1998;34:97–112. doi: 10.1002/(sici)1097-4695(19980205)34:2<97::aid-neu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Liu SQ, Kaczmarek LK. Depolarization selectively increases the expression of the Kv3.1 potassium channel in developing inferior colliculus neurons. J Neurosci. 1998;18:8758–8769. doi: 10.1523/JNEUROSCI.18-21-08758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotak VC, Sanes DH. Long-lasting inhibitory synaptic depression is age- and calcium-dependent. J Neurosci. 2000;20:5820–5826. doi: 10.1523/JNEUROSCI.20-15-05820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- 36.Kandler K, Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J Comp Neurol. 1993;328:161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- 37.Sanes DH. The development of synaptic function and integration in the central auditory system. J Neurosci. 1993;13:2627–2637. doi: 10.1523/JNEUROSCI.13-06-02627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity, developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanes DH, Takacs C. Activity-dependent refinement of inhibitory connections. Eur J Neurosci. 1993;5:570–574. doi: 10.1111/j.1460-9568.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 40.Kil J, et al. Development of ventral cochlear nucleus projections to the superior olivary complex in gerbil. J Comp Neurol. 1995;353:317–340. doi: 10.1002/cne.903530302. [DOI] [PubMed] [Google Scholar]
- 41.Person AL, et al. Tonotopic gradients of Eph family proteins in the chick nucleus laminaris during synaptogenesis. J Neurobiol. 2004;60:28–39. doi: 10.1002/neu.10330. [DOI] [PubMed] [Google Scholar]
- 42.Leake PA, et al. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanes DH, Siverls V. Development and specificity of inhibitory terminal arborizations in the central nervous system. J Neurobiol. 1991;22:837–854. doi: 10.1002/neu.480220805. [DOI] [PubMed] [Google Scholar]
- 44.Sanes DH, et al. Refinement of dendritic arbors along the tonotopic axis of the gerbil lateral superior olive. Dev Brain Res. 1992;67:47–55. doi: 10.1016/0165-3806(92)90024-q. [DOI] [PubMed] [Google Scholar]
- 45.Sanes DH, et al. The influence of inhibitory afferents on the development of postsynaptic dendritic arbors. J Comp Neurol. 1992;321:637–644. doi: 10.1002/cne.903210410. [DOI] [PubMed] [Google Scholar]
- 46.Colman H, et al. Alterations in synaptic strength preceding axon withdrawal. Science. 1997;275:356–361. doi: 10.1126/science.275.5298.356. [DOI] [PubMed] [Google Scholar]
- 47.Kros CJ, et al. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- 48.Beutner D, Moser T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. J Neurosci. 2001;21:4593–4599. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gubellini P, et al. Activity- and age-dependent GABAergic synaptic plasticity in the developing rat hippocampus. Eur J Neurosci. 2001;14:1937–1946. doi: 10.1046/j.0953-816x.2001.01823.x. [DOI] [PubMed] [Google Scholar]
- 50.Charpier S, et al. Latent inhibitory connections become functional during activity-dependent plasticity. Proc Natl Acad Sci U S A. 1995;92:117–120. doi: 10.1073/pnas.92.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poisbeau P, et al. Silent GABAA synapses during flurazepam withdrawal are region-specific in the hippocampal formation. J Neurosci. 1997;17:3467–3475. doi: 10.1523/JNEUROSCI.17-10-03467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lippe W, Rubel EW. Development of the place principle, tonotopic organization. Science. 1983;219:514–516. doi: 10.1126/science.6823550. [DOI] [PubMed] [Google Scholar]
- 53.Kapfer C, et al. Experience-dependent refinement of inhibitory inputs to auditory coincidence-detector neurons. Nat Neurosci. 2002;5:247–253. doi: 10.1038/nn810. [DOI] [PubMed] [Google Scholar]
- 54.Lim R, et al. Quantal size is correlated with receptor cluster area at glycinergic synapses in the rat brainstem. J Physiol. 1999;516:505–512. doi: 10.1111/j.1469-7793.1999.0505v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leao RN, et al. Differences in glycinergic mIPSCs in the auditory brain stem of normal and congenitally deaf neonatal mice. J Neurophysiol. 2004;91:1006–1012. doi: 10.1152/jn.00771.2003. [DOI] [PubMed] [Google Scholar]
- 56.Chang EH, et al. Long-term depression of synaptic inhibition is expressed postsynaptically in the developing auditory system. J Neurophysiol. 2003;90:1479–1488. doi: 10.1152/jn.00386.2003. [DOI] [PubMed] [Google Scholar]
- 57.Kotak VC, Sanes DH. Postsynaptic kinase signaling underlies inhibitory synaptic plasticity in the lateral superior olive. J Neurobiol. 2002;53:36–43. doi: 10.1002/neu.10107. [DOI] [PubMed] [Google Scholar]
- 58.Gaiarsa JL, et al. Long-term plasticity at GABAergic and glycinergic synapses, mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 59.Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J Neurosci. 1983;3:237–242. doi: 10.1523/JNEUROSCI.03-01-00237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu SH, Kelly JB. Synaptic pharmacology of the superior olivary complex studied in mouse brain slice. J Neurosci. 1992;12:3084–3097. doi: 10.1523/JNEUROSCI.12-08-03084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nabekura J, et al. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- 62.Korada S, Schwartz IR. Development of GABA, glycine, and their receptors in the auditory brainstem of gerbil, a light and electron microscopic study. J Comp Neurol. 1999;409:664–681. doi: 10.1002/(sici)1096-9861(19990712)409:4<664::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 63.Dumoulin A, et al. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci. 2001;21:6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jonas P, et al. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 65.Gillespie DC, et al. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- 66.Boulland JL, et al. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- 67.Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 68.Gutierrez R. The GABAergic phenotype of the ‘glutamatergic’ granule cells of the dentate gyrus. Prog Neurobiol. 2003;71:337–358. doi: 10.1016/j.pneurobio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- 70.Rietzel HJ, Friauf E. Neuron types in the rat lateral superior olive and developmental changes in the complexity of their dendritic arbors. J Comp Neurol. 1998;390:20–40. [PubMed] [Google Scholar]