Abstract
The lateral superior olive (LSO), a binaural nucleus involved in sound localization, receives tonotopically organized inhibitory inputs from the medial nucleus of the trapezoid body (MNTB). During development, the tonotopic organization of this glycinergic/GABAergic MNTB–LSO pathway is established by activity-dependent axonal reorganization. However, the underlying mechanisms by which this reorganization takes place have remained largely unknown. As cytosolic calcium is one of the most important second messengers responsible for inducing synaptic plasticity and reorganization, we examined whether and how activity in the MNTB–LSO pathway changes the intracellular calcium concentration ([Ca2+]i) in developing LSO neurons. By applying calcium imaging techniques to Fura-2-labelled slices from neonatal rats and mice, we found that glycine and GABA (γ-aminobutyric acid) affect [Ca2+]i in LSO neurons in an age-dependent manner; during the first postnatal week, the period at which glycine and GABA are depolarizing in the LSO, glycine and GABA always increased [Ca2+]i. However, in 2-week-old animals, the time around hearing onset when glycine and GABA are hyperpolarizing, glycine and GABA slightly decreased [Ca2+]i. Calcium responses could also be elicited by stimulation of afferent fibres from the MNTB, and these synaptic responses were mediated by glycine and GABAA receptors. Furthermore, GABA, which is a neurotransmitter only in the immature MNTB–LSO pathway, played a major role in generating MNTB-elicited Ca2+ responses. The direct link of glycinergic/GABAergic synaptic activity to intracellular calcium signalling during the period of inhibitory synaptic plasticity could be one of the mechanisms by which tonotopic MNTB–LSO connections become established.
Keywords: caged glutamate, calcium imaging, inhibition, mouse, rat
Introduction
Precise and fast processing of interaural time and intensity differences is required for sound localization. In mammals, interaural intensity differences are first processed in the lateral superior olive (LSO), a binaural auditory nucleus in the auditory brainstem (Boudreau & Tsuchitani, 1968; Caird & Klinke, 1983; for review see Oertel, 1999). The LSO receives information from the ipsilateral ear via excitatory, glutamatergic axons from the cochlear nucleus and receives information from the contralateral ear via inhibitory, glycinergic axons from the medial nucleus of the trapezoid body (MNTB). Both afferent pathways terminate in the LSO in a strictly tonotopic manner and both pathways are spatially aligned such that single LSO neurons are excited and inhibited by axons encoding the same stimulus frequency.
The development of these highly organized connections has been the subject of a number of studies (for recent reviews see Cant, 1998; Sanes & Friauf, 2000). An interesting outcome of these studies was the finding that the precise tonotopic organization of the inhibitory MNTB–LSO pathway emerges gradually through axonal and dendritic reorganization (Sanes & Siverls, 1991; Sanes et al., 1992; Rietzel & Friauf, 1998). Single cell analysis demonstrated that MNTB axons initially innervate relatively broad areas along the tonotopic axis of the LSO and subsequently become refined, terminating in narrow isofrequency bands. This reorganization occurs to a great extent before hearing onset and, thus, in the absence of sound-evoked neuronal activity. Nevertheless, axonal reorganization requires the presence of spontaneous activity, which is endogenously generated in the auditory system before the onset of hearing (Lippe, 1994; Kotak & Sanes, 1995; Kros et al., 1998). Blocking or decreasing neuronal activity in the LSO prevents the refinement of LSO circuitry (Sanes et al., 1992; Sanes & Takacs, 1993). While the importance of glycinergic/GABAergic synaptic activity for normal LSO development is well established, the mechanisms by which MNTB activity exerts its effects on the maturation of neuronal circuits in the LSO are still poorly understood.
Previous studies have demonstrated that during the period in which the LSO circuit becomes reorganized, synaptic transmission in the MNTB–LSO pathway is quantitatively and qualitatively different from that in mature animals. For example, while the MNTB–LSO pathway in mature animals is purely glycinergic (Caspary & Finlayson, 1991; Schwartz, 1992), the MNTB–LSO pathway in neonatal animals is both glycinergic and GABAergic (Kotak et al., 1998; Korada & Schwartz, 1999). Furthermore, GABA (γ-aminobutyric acid) and glycine, which are hyperpolarizing in the LSO of mature animals, are depolarizing in the LSO of neonatal animals (Kandler & Friauf, 1995; Ehrlich et al., 1999) similar to other neuronal systems (Obata et al., 1978; Ben-Ari et al., 1989). It has been suggested that this depolarizing action could increase the intracellular calcium concentration ([Ca2+]i) in LSO neurons — a scenario which has been demonstrated previously in other developing systems (Yuste & Katz, 1991; Reichling et al., 1994; Leinekugel et al., 1995; Obrietan & van den Pol, 1995; Owens et al., 1996). Such glycinergic/GABAergic Ca2+ responses could link MNTB–LSO synapses to calcium-regulated intracellular pathways, many of which play key roles in neuronal differentiation and synaptic plasticity (Bootman et al., 2001).
In the present study we applied calcium imaging techniques to auditory brainstem slices of neonatal rats and mice in order to re-examine the idea that depolarizing glycine and GABA raise [Ca2+]i in immature LSO neurons during the period of synaptic refinement. Contrary to previous studies (Schmanns & Friauf, 1994), we found that pharmacological stimulation of glycine and GABAA receptors, as well as glycinergic and GABAergic synaptic activity, increased [Ca2+]i in LSO neurons during the first postnatal week, when glycine and GABA are depolarizing (Kandler & Friauf, 1995), but not at later ages. Our results also revealed that GABA plays a prominent role in mediating MNTB-evoked Ca2+ responses, indicating that the transient GABAergic nature of the immature MNTB–LSO pathway is important for linking MNTB activity to Ca2+ responses in the developing LSO.
Some of the results have been published in abstract form (Kullmann & Kandler, 1999).
Materials and methods
Animals and slice preparation
Experiments were performed in slices prepared from neonatal rats (Sprague–Dawley, Charles River Laboratories, Wilmington, MA, USA) and mice (C57Bl/6 J, Jackson Laboratory, Bar Harbor, ME, USA). Data presented in this report were obtained from 21 mice aged between postnatal day (P) 1 and P5, six mice aged between P13 and P15, 19 rats P1–P5, and two rats P14. Experimental procedures were in accordance with USA National Institute of Health, guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Brainstem slices were prepared as described previously (Kandler & Friauf, 1995). In short, animals were anaesthetized by hypothermia (P1–P5) or by pentobarbital (P13–P15, 100 mg/kg, i.p.) and decapitated. The brain was removed quickly and transferred to cold (4–8 °C) artificial cerebrospinal fluid (ACSF, composition in mM: NaCl 124, NaHCO3 26, glucose 10, KCl 5, KH2PO4 1.25, MgSO4 1.3, CaCl2 2, pH = 7.4 when aerated with 95% O2/5% CO2). ACSF used for preparation, storage and staining of slices also contained 1 mM kynurenic acid. Coronal slices of the brainstem (200–300 μm thick) were cut on a vibrating microtome (DTK-1500E, Ted Pella, Redding, CA, USA). Slices that contained the superior olivary complex were kept either submerged in ACSF (for Fura-2 bulk labelling, discussed later) or were kept in an interface chamber (for Fura-2 AM pressure injections). Slices were allowed to recover for at least 1 h before being used for recordings.
Fura-2 labelling
Calcium imaging of LSO neurons was performed using the calcium indicator Fura-2 (Grynkiewicz et al., 1985). Slices from animals up to P5 were bulk labelled by submersion in ACSF, which contained 10 μM Fura-2 AM (Molecular Probes, Eugene, OR, USA), prepared from a stock solution of 1 mM Fura-2 AM in a 20% (w/v) solution of pluronic acid in dimethylsulphoxide (DMSO). Slices were stained for 1–3 h at 30–32 °C. In slices from P13–P15 animals, LSO neurons were labelled by injecting Fura-2 AM (100 μM Fura-2 AM, 1% DMSO in ACSF) into the slice using pressure application (Leinekugel et al., 1995). This approach labelled many neurons simultaneously without changing the native intracellular chloride concentration, the main determinant for the polarity of glycinergic and GABAergic membrane potential responses in LSO neurons (Ehrlich et al., 1999; Kakazu et al., 1999).
Retrograde labelling
In some experiments, rhodamine-labelled beads (40 nL, Red Retrobeads, Lumafluor, Naples, FL, USA) were injected bilaterally into the inferior colliculus of P1 rats in order to unequivocally identify Fura-2-labelled cells as neurons. Injected rats were allowed to survive for 2 days and brainstem slices (300 μm) were prepared at P3, as described earlier. Beads were visualized with 548 nm excitation (monochromator, Polychrome II, TILL Photonics, Martinsried, Germany) and a 590-long pass emission filter (Chroma Technology Corp., Brattleboro, VT, USA).
Electrical stimulation
The MNTB–LSO pathway was stimulated with bipolar electrodes (two tungsten microelectrodes at 100–200 μm distance; FHC, Bowdoingham, ME, USA) placed in the ipsilateral MNTB. Most electrical stimulation experiments were performed in the presence of the potassium channel antagonist tetraethylammonium (TEA; 10–20 mM) in order to increase response amplitudes and reliability, while using minimal stimulation currents. The effects of TEA on LSO neurons were characterized using gramicidin-perforated patch-clamp recordings (Wang et al., 1994) in current clamp mode (Fig. 1). In these preliminary studies, amplitudes of MNTB-evoked postsynaptic potentials were increased to 244.5 ± 29.1% (mean ± SEM, n = 3), which is within the range that we also achieved by simply increasing the stimulation strength. The effects of TEA on the decay times of depolarizing postsynaptic potentials were minor and did not result in multipeaked postsynaptic potentials indicative of repetitive presynaptic action potentials, which would result in prolonged and repetitive activation of glycine or GABAA receptors.
Fig. 1.
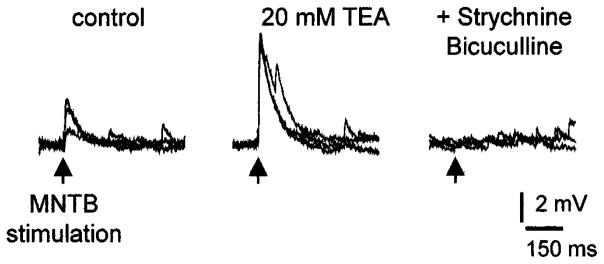
Effect of the potassium channel blocker TEA on medial nucleus of the trapezoid body- (MNTB-) elicited, depolarizing postsynaptic potentials (PSPs) in immature lateral superior olive neurons. TEA (20 mM) more than doubled the amplitude of subthreshold PSPs without significantly changing their decay time (control, τ = 74 ± 23 ms; TEA, τ = 82 ± 3 ms). Depolarizing PSPs were abolished by blocking glycine receptors and GABAA receptors with strychnine and bicuculline (10 μM). Recordings were performed in perforated patch clamp mode and slice was prepared from a P3 rat.
Focal photolysis of caged glutamate
For photolytic cleavage of caged glutamate, 150–200 μM p-hydroxyphenacyl-glutamate (Givens et al., 1997) was added to the ACSF. Focal photolytic cleavage was achieved by guiding the light from a 100-watt mercury arc lamp to the MNTB, using fused silica optical fibres (core diameter, 50 μm; Kandler et al., 1998). The duration of the light pulses (50–100 ms) was regulated by a shutter (Vincent Associates, Rochester, NY, USA), located between the arc lamp and the optical fibre.
Calcium imaging
Individual slices were placed in a submerged-type recording chamber mounted on an upright epifluorescence microscope (BX50WI, Olympus America, Melville, NY, USA). Slices were superfused continuously with oxygenated ACSF (30–32 °C, perfusion rate 2–4 mL/min, chamber volume 2 mL). Fura-2-loaded neurons were alternately excited with light at 340 nm and 380 nm (bandwidth 15 nm, duration 10–100 ms) using a computer-controlled monochromator (Polychrome II, TILL Photonics, Martinsried, Germany). Fluorescence emission was filtered (510/40 nm bandpass filter, Chroma Technology Corp.) and was detected with a CCD camera (IMAGO, TILL Photonics). Image pairs at 340 and 380 nm were acquired every 0.5–10 s using 10 × (NA 0.3) or 20 × (NA 0.5) water-immersion objectives (Olympus America). In photolysis experiments, only single wavelength excitation (380 nm) was used due to absorption of 340 nm light by p-hydroxyphenacyl-glutamate.
Data analysis
Digital images were low pass filtered with a Gaussian 3 × 3 kernel and background fluorescence was subtracted using the program Tillvision (TILL Photonics). Ca2+ responses were analysed by calculating the ratio of images obtained with 340 nm excitation over images obtained with 380 nm excitation (340 : 380 ratio). Due to large variations of Rmin and Rmax (Grynkiewicz et al., 1985) in our preparation and setup, we opted to express the changes in [Ca2+]i in the form of the 340 : 380 ratio which, over physiological concentrations, is linearly related to changes in [Ca2+]i. For photolysis experiments, in which only 380 nm excitation was used, changes in intracellular calcium are expressed as ΔF/F × 100 using the average of five images before photolysis as a baseline.
Fluorescence measurements were performed from the cell soma of LSO neurons (see e.g. Fig. 2). Cells with high resting 340 : 380 ratios (10 × objective > 0.5, 20 × objective > 1.2), indicating high levels of baseline calcium concentrations, and cells that did not return to baseline after stimulation were excluded from analysis.
Fig. 2.
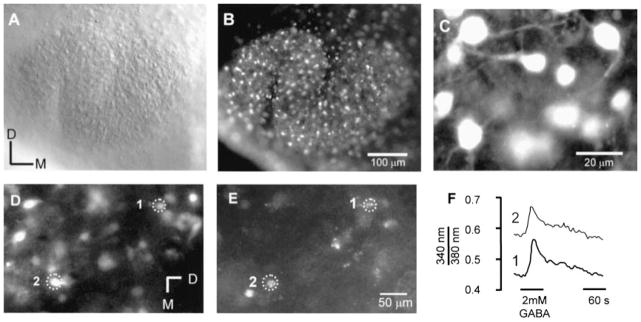
Fura-2 staining of lateral superior olive (LSO) neurons in brainstem slices. (A) Brightfield image of the LSO from a P4 rat. (B) Fluorescence image with 380 nm excitation of the same LSO shown in A. Numerous cells along the extent of the LSO are labelled. (C) Higher magnification reveals that most labelled cells have a typical neuron-like appearance. (D–F) GABA elicited calcium responses in neurons retrogradely labelled from the inferior colliculus. (D) Fura-2-labelled cells in a P3 rat. (E) In the same slice some cells are retrogradely labelled with rhodamine beads, identifying them as inferior colliculus-projecting neurons. (F). Response of two inferior colliculus-projecting neurons to application of 2 mM GABA. D, dorsal, M, medial.
Statistical significance was analysed using Student’s t-test and ANOVA followed by Dunnett’s post hoc test as indicated in the text. Throughout the text, values are expressed as arithmetic mean ± SEM.
Chemicals
Tetrodotoxin (TTX) was purchased from Alomone Laboratories (Jerusalem, Israel), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), DL-amino-5-phosphonovalerate (APV), bicuculline methiodide and SKF 97541 from Tocris (Ballwin, MO, USA) and (+)-α-methyl-4-carboxyphenylglycine (MCPG) from RBI (Natick, MA, USA). If not stated otherwise, all other chemicals used in this study were from Sigma (St Louis, MO, USA).
Results
Incubation of slices from P1–P5 animals in Fura-2 resulted in numerous labelled cells throughout the LSO (Fig. 2A and B). At higher magnification (Fig. 2C), most of these labelled cells depicted morphological features, such as a bipolar shape, that are reminiscent of immature LSO neurons (Henkel & Brunso-Bechtold, 1991; Rietzel & Friauf, 1988). To verify the neuronal nature of Fura-2 labelled cells, rhodamine beads were injected into the inferior colliculus of neonatal rats (P1, n = 2) and slices from these animals labelled with fura-2 at P3 (Fig. 2D–E). Cells that were double-labelled with beads and fura-2 responded to application of GABA (2 mM) indicating that our recordings were indeed from neurons.
We first examined the effects of direct stimulation of glycine and GABA receptors on the [Ca2+]i of developing LSO neurons. In these experiments, glycine and GABA were bath applied in the presence of the sodium channel blocker TTX (0.5–1 μM) to prevent synaptically evoked responses. No differences were observed between mice and rats, and results from both species will be presented together for the rest of this paper.
Calcium responses in neonatal animals (P1–P5)
In newborn animals, bath application of glycine (2 mM) or GABA (2 mM) consistently elicited a transient increase in the [Ca2+]i of LSO neurons (Fig. 3). In preliminary experiments, 2 mM glycine and GABA were found to be saturating concentrations and these concentrations were used in all subsequent experiments. These Ca2+ responses were sensitive to antagonists for glycine receptors and GABAA receptors (Fig. 4). The glycine receptor antagonist strychnine (25 μM) completely blocked glycine-induced Ca2+ responses in 76% of cells (25 out of 33, two slices) and reduced the response in the remaining 24% (eight cells) to 51.4 ± 7.4% (Fig. 4A and B). Consistent with previous studies (Kandler & Friauf, 1995; Ehrlich et al., 1998; Lo et al., 1998), a high strychnine concentration was necessary to block glycinergic responses in neonatal LSO neurons, reflecting the fact that glycine receptors in immature LSO neurons contain the ‘neonatal’ α2 subunit that results in reduced strychnine sensitivity (Kuhse et al., 1995; Friauf et al., 1997). The GABAA receptor antagonist bicuculline (100 μM) completely blocked GABA-induced Ca2+ responses in 95% of cells (56 out of 59, two slices) and reduced the response in the remaining three cells to 46.7 ± 15.8% (Fig. 4C and D). This indicates that GABAergic Ca2+ responses in the LSO are mediated by GABAA receptors without any significant involvement of GABAB receptors. Consistent with this, application of the selective GABAB receptor agonist SKF 97541 (10 μM) did not elicit Ca2+ responses in 68 GABA-responding neurons.
Fig. 3.
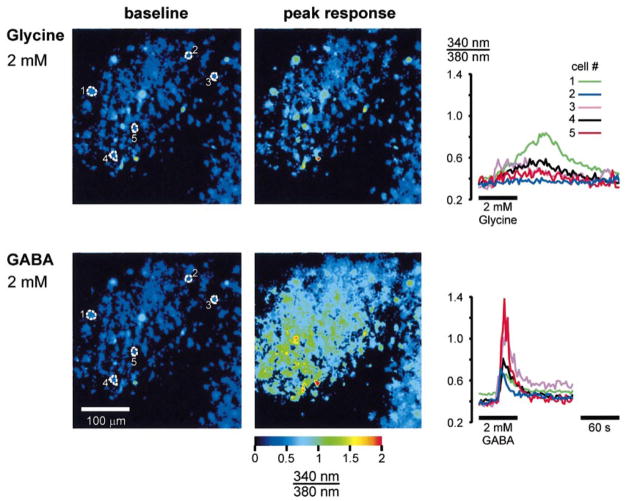
Glycine and GABA-elicited calcium responses in immature lateral superior olive (LSO) neurons. Top row: 340 : 380 ratio images before (baseline) and at the peak of the response following bath application of 2 mM glycine. The graph on the right illustrates the time-course of the response of the five cells outlined in the baseline image. Bottom row: response of the same LSO to bath application of 2 mM GABA. Notice that many more cells respond to GABA than to glycine. GABAergic responses also have a more rapid onset and higher amplitudes. The slice was prepared from a P3 rat. Dorsal is up and medial is to the right in all panels.
Fig. 4.
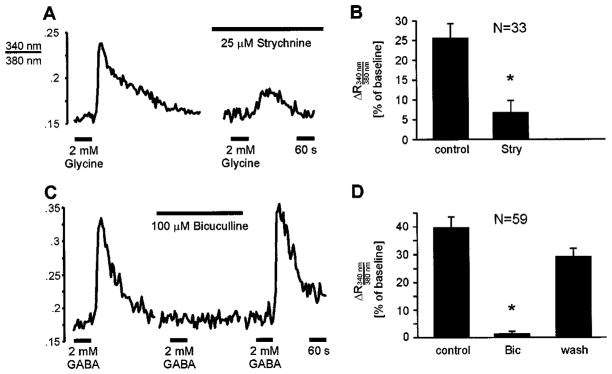
Glycine- and GABA-elicited Ca2+ responses are mediated by glycine and GABAA receptors. (A) Examples of glycine-elicited Ca2+ responses of a lateral superior olive neuron from a P3 rat. Responses are strongly reduced by the glycine receptor antagonist strychnine. (B) Summary of the effects of strychnine. Asterisk indicates value significantly different from controls (P < 0.001, paired t-test). (C) The GABAA receptor antagonist bicuculline blocks GABA-elicited Ca2+ responses (mouse P2). (D) Summary of the effects of bicuculline. Asterisk indicates value significantly different from controls (P < 0.01; ANOVA followed by Dunnett’s test).
Although both glycine and GABA were able to increase [Ca2+]i, GABA appeared to be more effective than glycine. First, GABA induced responses in about twice as many cells as glycine did: 71% of the LSO neurons (163 out of 230, seven slices) responded to GABA, whereas only 34% of the neurons (94 out of 280, seven slices) responded to glycine (Fig. 5A). Second, the amplitudes of GABAergic calcium changes were about twice as large as the amplitudes of glycinergic responses (peak 340 : 380 ratio change relative to baseline: GABA, 41.8 ± 1.4%, n = 163 vs. glycine, 22.6 ± 1.7%, n = 94; P ≤ 0.001, t-test) (Figs 3 and 5B). These differences are not attributable to the presence of two distinct cell populations, one GABA-responsive and one glycine-responsive, because these differences were usually also observed in the same cells. When GABA and glycine were applied to the same slice, 8.2% of cells (six out of 73, three slices) responded to glycine only, 37% (27 cells) responded to both glycine and GABA, and 54.8% (40 cells) responded to GABA only. Thus, the majority of glycine-responding cells (82%, 27 out of 33) also responded to GABA and more than half of neonatal LSO neurons were purely GABA-responsive. Third, GABA increased [Ca2+]i much more rapidly than glycine (see traces in Figs 3, 6 and 7). A quantitative comparison of the responses in dual-responding cells revealed that the rise times (time from onset to peak) of GABAergic Ca2+ responses were about half as long as those for glycinergic responses (GABA 31 ± 3 s vs. glycine 64 ± 5 s, P ≤ 0.001, paired t-test; Fig. 5C).
Fig. 5.
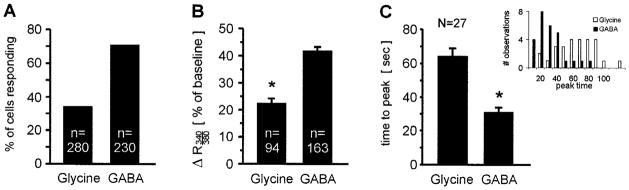
Comparison of glycine and GABA-elicited calcium responses. (A) Glycine (2 mM) elicits responses in 34% of monitored lateral superior olive neurons, whereas GABA (2 mM) elicits responses in 71% of neurons. (B) GABA-evoked peak responses are almost twice as large as glycine-evoked responses (P ≤ 0.001, unpaired t-test). (C) The mean time from response onset to peak is about twice as long for glycine- (64 s) than for GABA-elicited responses (31 s; P < 0.001, paired t-test). Inset shows distribution histograms of peak times for glycine (open bars) and GABA (closed bars).
Fig. 6.
GABA-elicited calcium oscillations. Left column: examples of responses of six lateral superior olive neurons from a P2 mouse. Neurons respond to a single GABA pulse (1 min, indicated by the bar) with longlasting, multipeaked responses that persist for several minutes after the washout of GABA. Right column: glycine-elicited calcium responses with similar amplitudes illustrating that prolonged increase in [Ca2+]i is insufficient to elicit oscillations.
Fig. 7.
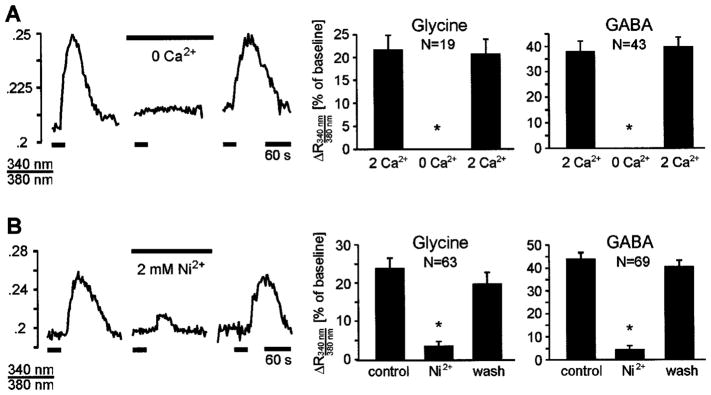
Glycine- and GABA-evoked calcium responses are mediated by influx of extracellular calcium through voltage-gated calcium channels. (A) Glycinergic and GABAergic Ca2+ responses are abolished in calcium-free solution. Traces show glycine-evoked Ca2+ responses in a P2 mouse. (B) Glycinergic and GABAergic Ca2+ responses are blocked by the voltage-gated Ca2+-channel antagonist nickel (2 mM). Traces show glycine-evoked Ca2+ responses in a P2 rat. Asterisks in bar graphs indicate significantly different from controls (P < 0.01; ANOVA followed by Dunnett’s test).
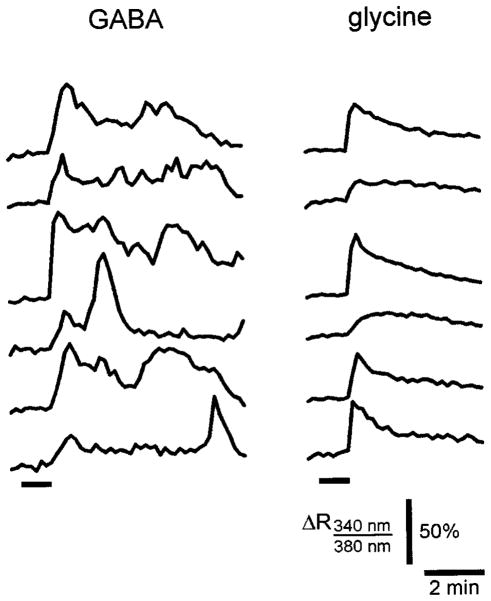
Another effect of GABA was that it could elicit long lasting, irregular oscillating Ca2+-responses. Figure 6 illustrates some of these recurrent oscillations in six LSO neurons. Application of a 1 min GABA pulse caused an initial calcium response that was followed by repetitive calcium changes which outlasted the presence of GABA for several minutes (up to 7 min observed). These oscillations showed no temporal correlation between cells. Such GABA-induced calcium oscillations were observed in 22 cells from 5 out of 12 slices (4/9 from mouse; 1/3 from rat). In contrast, Ca2+-oscillations never occurred following application of glycine. Although we did not further explore the underlying mechanism responsible for these slow calcium oscillations, the fact that they occurred in the presence of TTX argues against the possibility that they were generated by neuronal network activity. In this respect, GABAergic Ca2+-oscillations in immature LSO neurons are similar to glutamatergic or acetylcholinergic Ca2+-oscillations that have been described previously in the immature neocortex (Yuste and Katz, 1991; Flint et al., 1999).
Calcium responses are mediated by voltage-gated calcium channels
The above experiments were performed during the period in which glycine and GABA are depolarizing in the LSO. The observed calcium responses could, thus, have been generated by activation of voltage-gated Ca2+ channels (VGCCs) and the resulting influx of extracellular calcium. To test this possibility, glycine and GABA were applied in the absence of extracellular Ca2+ (nominally free Ca2+-ACSF with 0.15 mM EGTA). Under this condition Ca2+ responses were completely abolished (glycine, n = 19 cells; GABA, n = 43 cells; three slices; Fig. 7A). In addition, in the presence of the calcium channel antagonist Ni2+ (2 mM, five slices), glycinergic Ca2+ responses were blocked in 71% of cells (45 out of 63) and reduced in 29% of cells (18 cells) by 52.0 ± 5.8%. Similarly, Ni2+ abolished GABAergic Ca2+ responses in 83% of cells (57 out of 69) and reduced the responses in the remaining 17% of cells (12 cells) by 43.0 ± 4.2% (Fig. 7B). Nifedipine (20 μM), a specific L-type calcium channels inhibitor (Bean & Mintz, 1994), reduced glycine-evoked calcium responses by 66.4 ± 3.7% (n = 43 cells in five slices, rats P1–P2) and reduced GABA-evoked responses by 71.6 ± 1.6% (n = 87 cells in three slices, rat P1). These results demonstrate that glycine and GABA increase [Ca2+]i in developing LSO neurons primarily by activation of L-type calcium channels and an influx of extracellular calcium.
Calcium responses in animals after hearing onset (P13–P15)
Although glycine and GABA are only depolarizing during the first postnatal week in the LSO, prolonged activation of glycine and GABA receptors (Backus et al., 1998) or repeated activation of dendritic GABA receptors can cause the breakdown of the transmembrane chloride concentration gradient and produce depolarizations (Staley et al., 1995). In the next experiments we therefore tested whether glycinergic and GABAergic increases in [Ca2+]i are restricted to the developmental period during which glycine and GABA are depolarizing, or whether glycine and GABA also increase [Ca2+]i after these neurotransmitters have become primarily hyperpolarizing.
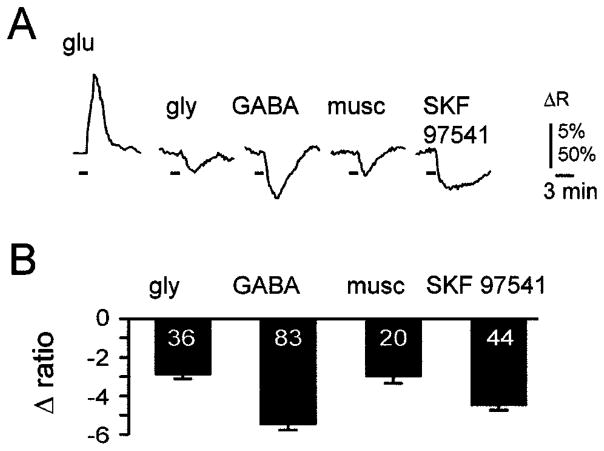
In these experiments, LSO neurons were labelled in slices from 2-week-old animals by injecting Fura-2 AM directly into the extracellular space (Leinekugel et al., 1995), labelling about 10–30 cells per injection site. Because these cells were exposed to higher DMSO concentrations than cells labelled by bulk staining, responses to glutamate or membrane depolarizations with 60 mM KCl were examined at the end of each experiment in order to assess the viability of labelled neurons. In contrast to neonatal slices, in P13–P15 slices (n = 8) neither glycine (2 mM) nor GABA (2 mM) increased [Ca2+]i, but instead consistently elicited a small decrease in [Ca2+]i (Fig. 8). Selective stimulation of GABAA receptors with muscimol (100 μM) or stimulation of GABAB receptors with SKF 97541 (10 μM) also decreased [Ca2+]i. This indicates that in 2-week-old animals, GABA-induced decreases of [Ca2+]i can be attributed to the activation of both GABAA and GABAB receptors. The results obtained from eight animals are summarized in Fig. 8B. On average, glycine reduced the 340 : 380 ratio by 2.9 ± 0.3% (n = 36), GABA by 5.5 ± 0.3% (n = 83), muscimol (100 μM) by 3.0 ± 0.4% (n = 20) and SKF 97541 (10 μM) by 4.5 ± 0.2% (n = 44). Together, these results demonstrate that even strong and prolonged activation of glycine or GABA receptors (90 s of 2 mM glycine or GABA) does not increase [Ca2+]i in LSO neurons after hearing onset when glycine and GABA have become primarily hyperpolarizing.
Fig. 8.
Glycine and GABA slightly decrease [Ca2+]i in 2-week-old animals. (A) Averaged responses of 20 cells from a P14 rat slice following applications of glutamate, glycine or GABA receptor agonists. Horizontal bars indicate agonist applications. Vertical scale bar represents changes in 340 : 380 ratio and is 50% for glutamate response and 5% for all others. (B) Average peak responses from lateral superior olive neurons in slices from eight P13 to P15 animals. Numbers within the bars indicate the number of cells.
Synaptically elicited calcium responses in neonatal LSO neurons
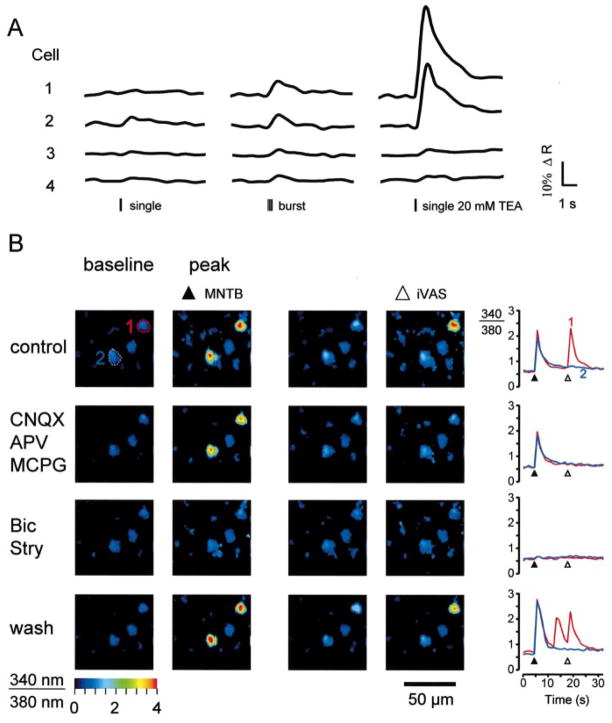
Next, we examined whether Ca2+-responses can also be elicited by selectively stimulating glycine and GABA receptors that are activated by MNTB–LSO synapses in neonatal animals. Single electrical shocks or stimulus trains (five stimuli at 20 Hz) delivered to the ipsilateral MNTB elicited Ca2+-responses in LSO neurons (Fig. 9A). However, with these conditions, responses had small amplitudes and were unreliable; i.e., for a given neuron, they only occurred in about one third of the trials. Detection of these weak responses also required relatively long illumination times (100–500 ms) in order to obtain reasonable signal-to-noise ratios, which in turn increased the possibility of inducing phototoxicity. The small amplitudes and low probability of synaptic Ca2+-responses made it difficult to perform pharmacological experiments, which require reliable responses and repetitive illumination. To circumvent these problems, we performed pharmacological experiments in the presence of the K+-channel blocker tetraethylammonium (TEA, 10–20 mM), which strongly increased response amplitudes but hardly increased the frequency of spontaneously occurring calcium transients (Fig. 9A).
Fig. 9.
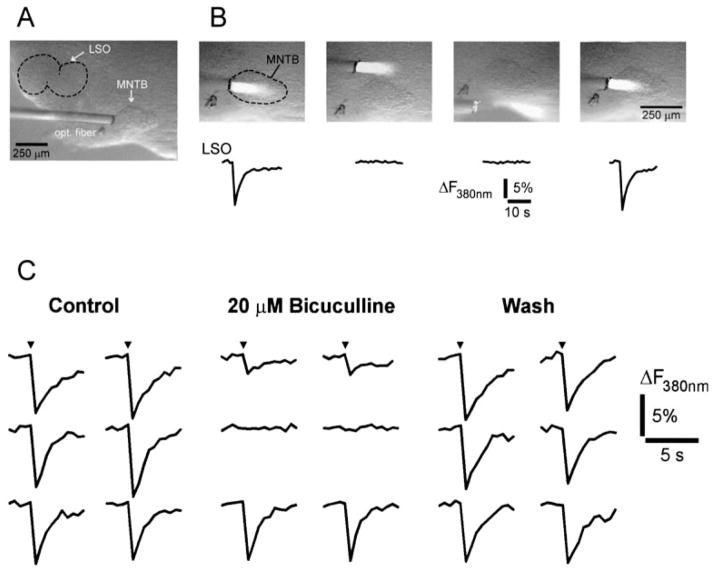
Medial nucleus of the trapezoid body- (MNTB-) evoked synaptic Ca2+ responses in neonatal lateral superior olive (LSO) neurons. (A) Left column: responses in four LSO neurons to single electrical shocks applied to the ipsilateral MNTB. Middle column: responses to five shocks delivered at 20 Hz. Right column: responses to a single shock in the presence of 20 mM TEA. (B) Pharmacology of synaptic Ca2+ responses of two neurons in the medial limb of the LSO (P4 mouse). Both cells responded to stimulation of the MNTB and one cell responded to stimulation of the ipsilateral ventral acoustic stria (iVAS). Blocking ionotropic and metabotropic glutamate receptors with CNQX (10 μM), APV (100 μM), and MCPG (250 μM) abolished iVAS-evoked responses. Blocking glycine and GABA receptors with strychnine and bicuculline (10 μM each) reversibly abolished MNTB-evoked responses. Pseudocolour images were calculated from images just before stimulation (baseline) and at peak of responses.
MNTB-evoked synaptic Ca2+ responses were analysed in 68 cells (P1–P4). Application of ionotropic and metabotropic glutamate receptor antagonists (10 μM CNQX, 100 μM APV, 250 μM MCPG) abolished responses in 26 cells (38%), consistent with previous electrophysiological findings indicating that electrical stimulation of the MNTB can elicit glutamatergic responses in LSO neurons (Wu & Kelly, 1991; Kotak et al., 1998). In the continued presence of glutamate receptor antagonists, bicuculline and strychnine (10 μM each) abolished responses in the remaining 88% of cells (37 of 42) (Fig. 9B). Responses in the remaining five cells were not affected by glutamate and glycine/GABA antagonists, suggesting that these responses were the result of direct electrical activation.
Recently it has been shown that in addition to glycine, which is the only neurotransmitter in the adult MNTB–LSO pathway (Finlayson & Caspary, 1989; Bledsoe et al., 1990; Glendenning et al., 1991), GABA is used transiently as an additional neurotransmitter in the developing MNTB–LSO pathway (Kotak et al., 1998; Korada & Schwartz, 1999). We therefore examined whether, and to what degree, synaptically released GABA contributes to MNTB-evoked Ca2+ responses (Fig. 10; Table 1). Blocking glycine receptors with strychnine (10 μM) completely abolished synaptic Ca2+ responses in 67% of neurons (10 out of 15; three slices, P2–P3) and reduced responses in 13% of neurons (average reduction 50%, two out of 15 cells). Responses in 20% of the neurons (n = 3) were insensitive to strychnine. All strychnine-insensitive Ca2+ responses (n = 5 cells) were blocked by additional application of bicuculline (10 μM). When bicuculline was applied prior to strychnine, it abolished synaptic responses in 31% of neurons (10 out of 32 neurons), reduced responses in 53% of the neurons (average reduction, 52.6 ± 1.8%; n = 17) and had no effect on 16% of the neurons (n = 5). Subsequent addition of strychnine abolished all bicuculline-insensitive responses. These results indicate that for MNTB-elicited responses, glycine partially mediates responses in 80% of LSO neurons, is necessary in 67% of neurons and is solely responsible for eliciting Ca2+ responses in 20% of neurons. Our results also highlight a strong contribution of GABA in eliciting Ca2+ responses, as GABA is not only involved in mediating synaptic Ca2+ responses in 84% of neurons, but is also necessary for Ca2+ responses in 31% of LSO neurons.
Fig. 10.
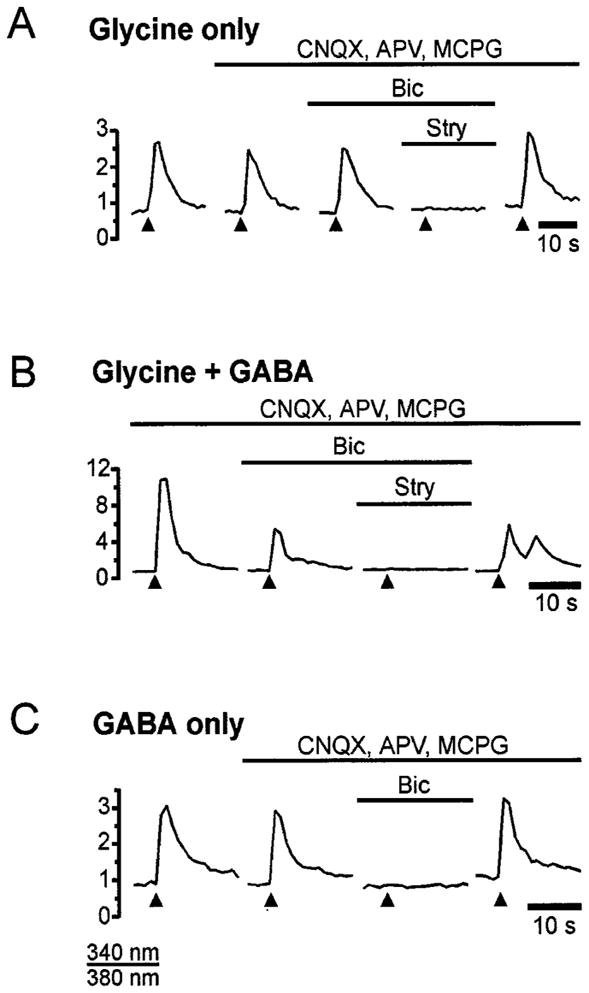
Medial nucleus of the trapezoid body- (MNTB-) elicited Ca2+ responses are mediated by glycine, GABA or both. (A) Example of responses mediated only by glycine. The response is unaffected by glutamate antagonists or bicuculline, but is abolished by strychnine. (B) Example of responses mediated by glycine and GABA. The response is reduced by bicuculline and blocked by the addition of strychnine. (C) Example of responses mediated only by GABA. The response is completely abolished by bicuculline. Bicuculline and strychnine were applied at 10 μM, CNQX at 10 μM, APV at 100 μM and MCPG at 250 μM. Slices in A and B are from a P2 rat and slice in C from a P1 rat.
Table 1.
Effect of strychnine and bicuculline on medial nucleus of the trapezoid body- (MNTB-) evoked calcium responses
| Percentages (and numbers, n) of cells with:
|
||||
|---|---|---|---|---|
| Complete block % (n) | Reduced block % (n) | No effect % (n) | Totals % (n) | |
| Strychnine | 67 (10) | 13 (2) | 20 (3) | 100 (15) |
| Bicuculline | 31 (10) | 53 (17) | 16 (5) | 100 (32) |
n = number of cells.
The strong contribution of GABA in mediating MNTB-evoked Ca2+ responses contradicts previous electrophysiological studies in rats and mice that reported no or only a weak contribution of GABA to contralateral synaptic transmission to the LSO (Wu & Kelly, 1992; Kandler & Friauf, 1995; Ehrlich et al., 1998). This discrepancy could be explained if our electrical stimulation activated other GABAergic afferent pathways that run in close vicinity to the MNTB (Moore & Moore, 1987; Adams & Mugnaini, 1990; Warr & Beck, 1996). In order to address this possibility, we stimulated MNTB neurons using focal photolysis of caged glutamate (p-hydroxyphenacyl-glutamate, 150–200 μM (Givens et al., 1997). This approach allowed us to excite MNTB neurons selectively by completely restricting the excited area to the boundaries of the MNTB and thus avoiding the accidental stimulation of periolivary neurons (Fig. 11A and B). Pure MNTB-evoked Ca2+ responses were examined in 42 LSO neurons. Application of bicuculline (20 μM) completely abolished MNTB-evoked Ca2+ responses in 19% of cells (n = 8), reduced the responses in 29% of cells (n = 12; reduction to 39 ± 5% of control) and did not affect responses in 52% of cells (n = 22; reduction to 99 ± 1%; Fig. 11C). The effect of strychnine (10 μM) was tested in 21 cells, which had bicuculline-insensitive responses. In 42% of these cells (n = 9) bicuculline-resistant responses were completely abolished by strychnine, indicating that these responses were purely glycinergic. In another 12 cells, in which bicuculline partly decreased response amplitudes, additional application of strychnine eliminated all responses (data not shown), indicating that MNTB-evoked responses were mediated by both glycine and GABA. Upon washout of bicuculline and strychnine, responses fully recovered (amplitude after washout 102.6 ± 3.1% compared with control). In summary, these experiments indicate that glycine participates in 81% of MNTB-evoked Ca2+ responses in LSO neurons and GABA participates in at least 48% of responses confirming our results, which were obtained with electrical stimulation.
Fig. 11.
Selective stimulation of medial nucleus of the trapezoid body (MNTB) neurons elicit GABAergic Ca2+ responses in the lateral superior olive (LSO). (A) Overview of the superior olivary complex in a slice (P3 rat) in brightfield illumination to illustrate experimental approach. A thin optical fibre is used to deliver UV light focally to the MNTB. (B) Responses in the LSO are elicited only from neurons within the boundaries of the MNTB. Top row: area of UV-illumination (presumed site of glutamate release). Bottom row: corresponding responses in an LSO neuron (ΔF with 380 nm excitation). (C) Pure MNTB-evoked Ca2+ responses in three neurons. Responses of the upper neurons are partly or completely blocked by bicuculline. Responses in the bottom neuron are unaffected by bicuculline. Ca2+ responses are expressed as fluorescence changes with 380 nm excitation. Glutamate was uncaged in the centre of the MNTB (150 μM p-hydroxyphenacyl-glutamate; 50 ms flash duration indicated by the arrowhead). Slice was prepared from a P3 rat.
Discussion
In this study we characterized the effects of the inhibitory neurotransmitters glycine and GABA on [Ca2+]i in developing LSO neurons. Our results demonstrate that during the first postnatal week, the period during which these neurotransmitters are depolarizing (Kandler & Friauf, 1995; Ehrlich et al., 1999), glycine and GABA increase [Ca2+]i. Around hearing onset (P12–P14), when GABA and glycine have become hyperpolarizing, they slightly decrease [Ca2+]i. In addition, brief stimulation of the MNTB–LSO pathway also increased [Ca2+]i, indicating that glycinergic/GABAergic synaptic activity is directly coupled to postsynaptic [Ca2+]i in neonatal LSO neurons.
GABAergic and glycinergic Ca2+ responses have been described previously in a variety of developing neuronal systems (e.g. Yuste & Katz, 1991; Reichling et al., 1994; Leinekugel et al., 1995; Obrietan & van den Pol, 1995; Owens et al., 1996). In the developing auditory system, an increase in [Ca2+]i by GABA or glycine has been demonstrated in the nucleus magnocellularis of the chick (Lachica et al., 1998) and the inferior colliculus of rodents (Lo et al., 1998; Frech et al., 1999). However, such glycinergic and GABAergic calcium responses appeared to be absent in the immature LSO (Schmanns & Friauf, 1994). Results of the present study are in obvious contrast to this previous finding. The reason for this discrepancy is not clear, but differences in the imaging techniques used in both studies might account for the different findings. For example, the present study used a CCD camera to measure responses from individual neurons, while the previous study used a photomultiplier tube to measure the average response of a large number of cells. Due to this spatial averaging, minor calcium changes in a small number of individual cells might have remained undetected.
Ca2+ responses in the immature LSO differ in several aspects from those reported in the avian nucleus magnocellularis (Lachica et al., 1998) and the inferior colliculus (Lo et al., 1998; Frech et al., 1999). For example, in the avian nucleus magnocellularis, GABAergic Ca2+ responses are mediated by a metabotropic, G-protein-linked GABA receptor and by release of calcium from intracellular stores. In the inferior colliculus, glycinergic and GABAergic Ca2+ responses occur only with prolonged pharmacological stimulation, a condition that can result in a breakdown of the transmembrane chloride gradient and produce depolarizations caused by the efflux of bicarbonate (Staley et al., 1995; Frech et al., 1999). In agreement with this, brief activation of glycinergic or GABAergic synapses in the inferior colliculus produces hyperpolarizing responses, which actually decrease the amplitude of glutamate-evoked Ca2+ responses (Lo et al., 1998). This scenario is distinct from the situation in the neonatal LSO, in which glycinergic or GABAergic synapses consistently increase [Ca2+]i and in which even prolonged activation of glycine and GABAA receptors does not generate calcium responses once inhibitory synapses have become hyperpolarizing.
GABA and glycine can increase [Ca2+]i by several mechanisms, such as activation of VGCCs (Connor et al., 1987; Yuste & Katz, 1991; Reichling et al., 1994; Owens et al., 1996; Lo et al., 1998; Frech et al., 1999), removal of the magnesium block of NMDA receptors (Staley et al., 1995; Leinekugel et al., 1997) or activation of G-protein-coupled metabotropic GABA receptors (Lachica et al., 1998). In the LSO, the primary mechanism by which GABA and glycine increase [Ca2+]i seems to be the activation of VGCCs, and mainly the L-type channels. First, blockage of glycine and GABAA receptors abolished Ca2+ responses, whereas activation of metabotropic GABA receptors failed to have an effect on [Ca2+]i. Second, an increase in [Ca2+]i was observed only during the period when GABA and glycine are depolarizing and third, responses were abolished if extracellular calcium was removed or greatly reduced, or if VGCC were blocked by nickel or nifedipine. Although VGCCs seem to be the main source of calcium influx, our results do not exclude the possibility that calcium-induced calcium release from internal stores, a mechanism which is present in the avian cochlear nucleus (Kato & Rubel, 1999), amplifies the initial calcium influx through VGCC. In the LSO of 2-week-old animals, glycine and GABA slightly, yet consistently, decreased [Ca2+]i. This could be due to glycinergic and GABAergic hyperpolarizations that are present in the LSO at this age and which could inactivate L-type channels that are spontaneously open at the resting membrane potential (Magee et al., 1996).
GABAergic Ca2+ responses in the neonatal LSO
Our results revealed that GABA partly or completely mediated MNTB-evoked Ca2+ responses in over 80% of LSO neurons in newborn rats and mice. This is consistent with recent anatomical and electrophysiological studies that demonstrated the transient use of GABA in the immature MNTB–LSO pathway of gerbils (Kotak et al., 1998; Korada & Schwartz, 1999). However, the extent by which GABA contributes to Ca2+ responses in rats and mice is significantly higher than what was expected from electrophysiological recordings in these species (Kandler & Friauf, 1995; Ehrlich et al., 1998). It is unlikely that the GABAergic Ca2+ responses in the present study were the result of activation of periolivary GABAergic neurons which project to the LSO (Moore & Moore, 1987; Adams & Mugnaini, 1990; Warr & Beck, 1996) because specific stimulation of MNTB neurons with focal photolysis of glutamate still elicited prominent GABAergic responses. Thus, while GABA plays only a minor role in eliciting MNTB-evoked membrane potential responses in the rat LSO, it has a substantial role in mediating MNTB-evoked Ca2+ responses. One possible explanation for this apparent discrepancy could lie in the nonlinear relationship between membrane potential and activation of VGCCs. This possibility is supported by the observation that bicuculline completely abolished Ca2+ responses in over 30% of all LSO neurons, despite the fact that only about 16% of responses were purely GABAergic.
In addition to enhancing MNTB-evoked Ca2+ responses, GABA, when applied externally, could also generate longer-lasting calcium oscillations which exceeded the presence of GABA by several minutes. These calcium oscillations are somewhat reminiscent of glutamate-induced calcium oscillations that have been described in other neuronal systems and which are caused by oscillatory activity of intracellular second messenger pathways (Yuste & Katz, 1991; Kawabata et al., 1996; Gu & Spitzer, 1997; Flint et al., 1999). The long duration and TTX-insensitivity of the oscillations in the LSO suggest that GABA can activate similar pathways in immature LSO neurons. While the exact nature of these pathways is currently unclear, our data indicate a specific requirement of GABAA receptor activation because strong increases in [Ca2+]i per se, as seen after glycine application, were not sufficient to trigger oscillations. As calcium oscillations can activate distinct sets of signalling pathways and regulate the expression of specific genes (Gu & Spitzer, 1995; Bootman et al., 2001), it will be interesting to determine the age or conditions under which synaptic activity can induce GABAergic calcium oscillations in the immature LSO.
The functional role of the transient expression of GABA in the immature MNTB–LSO pathway is currently unknown. It has been suggested that the additional release of GABA provides a negative feedback by activating presynaptic GABAB receptors on glycinergic MNTB terminals (Kotak et al., 1998). The present study indicates that GABA also plays an important role in amplifying or mediating postsynaptic Ca2+ responses. The question remains as to why this effect could not be accomplished by simply increasing the amount of glycine released. A possible explanation could lie in the different time-course of glycinergic and GABAergic postsynaptic currents. Previous studies at spinal interneuron–motoneuron synapses, which corelease glycine and GABA, have demonstrated slower decay times for GABAergic components than for glycinergic components (Jonas et al., 1998; Gao et al., 2001). If such differences also exist in the LSO, GABA would be much more effective in depolarizing LSO neurons and activating VGCCs than glycine.
Functional implications of glycinergic and GABAergic calcium responses in the developing LSO
Numerous studies have demonstrated that spontaneous synaptic activity before hearing onset is necessary for the normal development of the LSO (for review see Sanes & Friauf, 2000). Decreasing or abolishing spontaneous activity in the MNTB or blocking glycinergic transmission in the LSO interferes with the establishment of the precise tonotopic organization of the MNTB–LSO pathway and results in dendritic hypertrophy (Sanes et al., 1992; Sanes & Takacs, 1993), abnormal glycinergic and glutamatergic synaptic transmission, and altered membrane properties (Kotak & Sanes, 1996). The chain of intracellular events by which glycinergic and GABAergic synaptic activities exert their effect on the developing LSO is still largely unknown, but most likely involves calcium-dependent processes (Kirsch & Betz, 1998; Liu & Kaczmarek, 1998; Lohmann et al., 1998; Parks, 1999; Kotak & Sanes 2000). Results of the present study provide a first step in deciphering these mechanisms by demonstrating a direct link between glycinergic/GABAergic synaptic activity and postsynaptic calcium signalling. One possible reason for the paradoxical depolarizing action of immature inhibitory MNTB–LSO synapses could thus be to provide this link during the period of activity-dependent remodelling of sound localization circuits. Finally, in addition to directly increase [Ca2+]i, glycinergic/GABAergic depolarizations could also enhance glutamatergic neurotransmission by removing the magnesium block from NMDA receptors, as has been shown in immature hippocampal neurons (for review see Ben-Ari et al., 1997). Such synergistic action of glycinergic/GABAergic and glutamatergic activity could provide a mechanism for encoding temporally correlated activity in converging inhibitory and excitatory inputs to LSO neurons.
Acknowledgments
We are grateful to Dr Richard S. Givens for the generous gift of phydroxyphenacyl-glutamate and to E. Aizenman, G.-Q. Bi, G. Kim, and D. Goldring for valuable discussions and comments on the manuscript. We also thank the two anonymous reviewers for their constructive critique and insightful suggestions. This work was supported by the NIDCD (DC 04199) and the Alfred P. Sloan foundation (KK). F.A.E. was supported in part by the Center for the Neural Basis of Cognition, University of Pittsburgh.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- APV
amino-5-phosphonovalerate
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DMSO
dimethylsulphoxide
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- GABA
γ-aminobutyric acid
- LSO
lateral superior olive
- MCPG
α-methyl-4-carboxyphenylglycine
- MNTB
medial nucleus of the trapezoid body
- P
postnatal day
- TEA
tetraethylammonium
- TTX
tetrodotoxin
- VGCC
voltagegated calcium channel
References
- Adams JC, Mugnaini E. Immunocytochemical evidence for inhibitory and disinhibitory circuits in the superior olive. Hearing Res. 1990;49:281–298. doi: 10.1016/0378-5955(90)90109-3. [DOI] [PubMed] [Google Scholar]
- Backus KH, Deitmer JW, Friauf E. Glycine-activated currents are changed by coincident membrane depolarization in developing rat auditory brainstem neurones. J Physiol (Lond) 1998;507:783–794. doi: 10.1111/j.1469-7793.1998.783bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, Mintz IM. Pharmacology of different types of calcium channels in rats neurons. In: Peracchia C, editor. Handbook of Membrane Channels: Molecular and Cellular Physiology. Academic Press; San Diego, CA: 1994. pp. 199–210. [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol (Lond) 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Snead CR, Helfert RH, Prasad V, Wenthold RJ, Altschuler RA. Immunocytochemical and lesion studies support the hypothesis that the projection from the medial nucleus of the trapezoid body to the lateral superior olive is glycinergic. Brain Res. 1990;517:189–194. doi: 10.1016/0006-8993(90)91025-c. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling — an overview. Semin Cell Dev Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- Caird D, Klinke R. Processing of binaural stimuli by cat superior olivary complex neurons. Exp Brain Res. 1983;52:385–399. doi: 10.1007/BF00238032. [DOI] [PubMed] [Google Scholar]
- Cant NB. Structural development of the mammalian central auditory pathways. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. Springer Verlag; New York, NY: 1998. pp. 315–413. [Google Scholar]
- Caspary DM, Finlayson PG. Superior olivary complex: Functional neuropharmacology of the principal cell types. In: Altschuler RA, Bobbin RP, Clopton BM, Hoffman DW, editors. Neurobiology of Hearing: the Central Auditory System. Raven Press; New York, NY: 1991. pp. 141–161. [Google Scholar]
- Connor JA, Tseng HY, Hockberger PE. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J Neurosci. 1987;7:1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Ilic V, Lohmann C, Friauf E. Development of glycinergic transmission in organotypic cultures from auditory brain stem. Neuroreport. 1998;9:2785–2790. doi: 10.1097/00001756-199808240-00019. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Lohrke S, Friauf E. Shift from depolarizing to hyperpolarizing glycine action in rat auditory neurones is due to age-dependent Cl-regulation. J Physiol (Lond) 1999;520:121–137. doi: 10.1111/j.1469-7793.1999.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson PG, Caspary DM. Synaptic potentials of chinchilla lateral superior olivary neurons. Hearing Res. 1989;38:221–228. doi: 10.1016/0378-5955(89)90067-1. [DOI] [PubMed] [Google Scholar]
- Flint AC, Dammerman RS, Kriegstein AR. Endogenous activation of metabotropic glutamate receptors in neocortical development causes neuronal calcium oscillations. Proc Natl Acad Sci USA. 1999;96:12144–12149. doi: 10.1073/pnas.96.21.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech MJ, Deitmer JW, Backus KH. Intracellular chloride and calcium transients evoked by gamma-aminobutyric acid and glycine in neurons of the rat inferior colliculus. J Neurobiol. 1999;40:386–396. doi: 10.1002/(sici)1097-4695(19990905)40:3<386::aid-neu10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Friauf E, Hammerschmidt B, Kirsch J. Development of adult-type inhibitory glycine receptors in the central auditory system of rats. J Comp Neurol. 1997;385:117–134. doi: 10.1002/(sici)1096-9861(19970818)385:1<117::aid-cne7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Gao BX, Stricker C, Ziskind-Conhaim L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. J Neurophysiol. 2001;86:492–502. doi: 10.1152/jn.2001.86.1.492. [DOI] [PubMed] [Google Scholar]
- Givens RS, Jung A, Park CH, Weber J, Bartlett W. New photoactivated protecting groups. 7 p-hydroxyphenacyl – a phototrigger for excitatory amino acids and peptides. J Am Chem Soc. 1997;119:8369–8370. [Google Scholar]
- Glendenning KK, Masterton RB, Baker BN, Wenthold RJ. Acoustic chiasm III: Nature, distribution, and sources of afferents to the lateral superior olive in the cat. J Comp Neurol. 1991;310:377–400. doi: 10.1002/cne.903100308. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Breaking the code: regulation of neuronal differentiation by spontaneous calcium transients. Dev Neurosci. 1997;19:33–41. doi: 10.1159/000111183. [DOI] [PubMed] [Google Scholar]
- Henkel CK, Brunso-Bechtold JK. Dendritic morphology and development in the ferret lateral superior olivary nucleus. J Comp Neurol. 1991;313:259–272. doi: 10.1002/cne.903130206. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kakazu Y, Akaike N, Komiyama S, Nabekura J. Regulation of intracellular chloride by cotransporters in developing lateral superior olive neurons. J Neurosci. 1999;19:2843–2851. doi: 10.1523/JNEUROSCI.19-08-02843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Katz LC, Kauer JA. Focal photolysis of caged glutamate produces long-term depression of hippocampal glutamate receptors. Nature Neurosci. 1998;1:119–123. doi: 10.1038/368. [DOI] [PubMed] [Google Scholar]
- Kato BM, Rubel EW. Glutamate regulates IP3-type and CICR stores in the avian cochlear nucleus. J Neurophysiol. 1999;81:1587–1596. doi: 10.1152/jn.1999.81.4.1587. [DOI] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature. 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- Korada S, Schwartz IR. Development of GABA, glycine, and their receptors in the auditory brainstem of gerbil: a light and electron microscopic study. J Comp Neurol. 1999;409:664–681. doi: 10.1002/(sici)1096-9861(19990712)409:4<664::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Synaptically evoked prolonged depolarizations in the developing auditory system. J Neurophysiol. 1995;74:1611–1620. doi: 10.1152/jn.1995.74.4.1611. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Developmental influence of glycinergic transmission: regulation of NMDA receptor-mediated EPSPs. J Neurosci. 1996;16:1836–1843. doi: 10.1523/JNEUROSCI.16-05-01836.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Long-lasting inhibitory synaptic depression is age- and calcium-dependent. J Neurosci. 2000;20:5820–5826. doi: 10.1523/JNEUROSCI.20-15-05820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Kuhse J, Betz H, Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr Opin Neurobiol. 1995;5:318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Kullmann PHM, Kandler K. Glycine and GABA raise [Ca2+]i in developing neurons of the lateral superior olive. Soc Neurosci Abstr. 1999;25:394. [Google Scholar]
- Lachica EA, Kato BM, Lippe WR, Rubel EW. Glutamatergic and GABAergic agonists increase [Ca2+]i in avian cochlear nucleus neurons. J Neurobiol. 1998;37:321–337. doi: 10.1002/(sici)1097-4695(19981105)37:2<321::aid-neu10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA (A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Tseeb V, Ben Ari Y, Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol (Lond) 1995;487:319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14:1486–1495. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Kaczmarek LK. Depolarization selectively increases the expression of the Kv3.1 potassium channel in developing inferior colliculus neurons. J Neurosci. 1998;18:8758–8769. doi: 10.1523/JNEUROSCI.18-21-08758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YJ, Rao SC, Sanes DH. Modulation of calcium by inhibitory systems in the developing auditory midbrain. Neuroscience. 1998;83:1075–1084. doi: 10.1016/s0306-4522(97)00410-7. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Ilic V, Friauf E. Development of a topographically organized auditory network in slice culture is calcium dependent. J Neurobiol. 1998;34:97–112. doi: 10.1002/(sici)1097-4695(19980205)34:2<97::aid-neu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Magee JC, Avery RB, Christie BR, Johnston D. Dihydropyridine-sensitive, voltage-gated Ca2+ channels contribute to the resting intracellular Ca2+ concentration of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1996;76:3460–3470. doi: 10.1152/jn.1996.76.5.3460. [DOI] [PubMed] [Google Scholar]
- Moore JK, Moore RY. Glutamic acid decarboxylase-like immunoreactivity in brainstem auditory nuclei of the rat. J Comp Neurol. 1987;260:157–174. doi: 10.1002/cne.902600202. [DOI] [PubMed] [Google Scholar]
- Obata K, Oide M, Tanaka H. Excitatory and inhibitory actions of GABA and glycine on embryonic chick spinal neurons in culture. Brain Res. 1978;144:179–184. doi: 10.1016/0006-8993(78)90447-x. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annu Rev Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks TN. Cochlear influences on development of the brainstem auditory system. In: Hyson RL, Johnson F, editors. The Biology of Early Influences. Academic Press; New York, NY: 1999. pp. 15–34. [Google Scholar]
- Reichling DB, Kyrozis A, Wang J, Macdermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol (Lond) 1994;476:411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietzel HJ, Friauf E. Neuron types in the rat lateral superior olive and developmental changes in the complexity of their dendritic arbors. J Comp Neurol. 1998;390:20–40. [PubMed] [Google Scholar]
- Sanes DH, Friauf E. Development and influence of inhibition in the lateral superior olivary nucleus. Hear Res. 2000;147:46–58. doi: 10.1016/s0378-5955(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Markowitz S, Bernstein J, Wardlow J. The influence of inhibitory afferents on the development of postsynaptic dendritic arbors. J Comp Neurol. 1992;321:637–644. doi: 10.1002/cne.903210410. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Siverls V. Development and specificity of inhibitory terminal arborizations in the central nervous system. J Neurobiol. 1991;8:837–854. doi: 10.1002/neu.480220805. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Takacs C. Activity-dependent refinement of inhibitory connections. Eur J Neurosci. 1993;5:570–574. doi: 10.1111/j.1460-9568.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Schmanns H, Friauf E. K (+)- and transmitter-induced rises in [Ca2+]i in auditory neurones of developing rats. Neuroreport. 1994;5:2321–2324. doi: 10.1097/00001756-199411000-00028. [DOI] [PubMed] [Google Scholar]
- Schwartz IR. The superior olivary complex and lateral lemniscal nuclei. In: Webster B, Popper AN, Fay RR, editors. The Mammalian Auditory Pathways: Neuroanatomy. Springer-Verlag; New York, NY: 1992. pp. 117–167. [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Wang J, Reichling DB, Kyrozis A, Macdermott AB. Developmental loss of GABA- and glycine-induced depolarization and Ca2+ transients in embryonic rat dorsal horn neurons in culture. Eur J Neurosci. 1994;6:1275–1280. doi: 10.1111/j.1460-9568.1994.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Warr WB, Beck JE. Multiple projections from the ventral nucleus of the trapezoid body in the rat. Hear Res. 1996;93:83–101. doi: 10.1016/0378-5955(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Physiological properties of neurons in the mouse superior olive: Membrane characteristics and postsynaptic responses studied in vitro. J Neurophysiol. 1991;65:230–246. doi: 10.1152/jn.1991.65.2.230. [DOI] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Synaptic pharmacology of the superior olivary complex studied in mouse brain slice. J Neurosci. 1992;12:3084–3097. doi: 10.1523/JNEUROSCI.12-08-03084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]