Abstract
Objective
Periodontal pathogens initiate chronic dysregulation of inflammation and tissue homeostasis that characterize periodontal disease. To better understand oral microbe - host tissue interactions, we investigated expression and activation of MMP-2 in periodontal ligament cells following Treponema denticola challenge.
Design
Cultured PDL cells were challenged with T. denticola, and bacterial adherence, internalization and survival were assayed by immunofluorescence microscopy and antibiotic protection assays, respectively. MMP-2 activation was detected by zymography. MMP-2, MT1/MMP and TIMP-2 expression following T. denticola challenge was determined by qRT-PCR. Promoter methylation of MMP-2 and MT1/MMP was screened by methylation-sensitive restriction analysis and by bisulfite DNA sequencing.
Results
T. denticola adhered to and was internalized by PDL cells but did not survive intracellularly beyond 24 hours. Importantly, while dentilisin activity in PDL culture supernatants gradually decreased following T. denticola challenge, MMP-2 activation persisted for up to 5 days, suggesting involvement of other regulatory mechanisms. Transcription and expression of MT1/MMP and TIMP-2 increased in response to T. denticola challenge. However, consistent with previously reported constitutive pro-MMP-2 expression in PDL cells, the MMP-2 promoter was hypomethylated, independent of T. denticola challenge.
Conclusions
MMP-2 promoter hypomethylation is consistent with constitutive pro-MMP-2 expression in PDL cells. This, coupled with T. denticola-mediated upregulation of MMP-2-related genes and chronic activation of pro-MMP-2, mimics key in vivo mechanisms of periodontal disease chronicity, in particular MMP-2-dependent matrix degradation and bone resorption. Adherence and/or internalization of T. denticola may contribute to these processes by one or more regulatory mechanisms, including contact-dependent signal transduction or other epigenetic mechanisms.
Keywords: Treponema, proteases, MMP-2, TIMP-2, MT1/MMP, epigenetics, regulation
Introduction
Periodontal pathogens, including Treponema denticola, initiate the dysregulation of inflammation and tissue homeostasis that characterize periodontal disease. Early studies proposed a direct role of bacterial proteases in periodontal tissue destruction (1-3). It has more recently became apparent that host enzymes involved in inflammatory responses and tissue remodeling were the direct causes of periodontal destruction (4) while the specific roles of bacterial enzymes in the destructive processes remain unresolved (5, 6).
The dynamics of attachment loss in periodontal disease are centered in tissues comprising the junctional epithelium and periodontal ligament (7, 8). Breakdown of this tissue results in apical migration of the junctional epithelium and eventual alveolar bone loss. During periodontal disease, host-derived proteases cleave extracellular matrix (ECM) components and release ECM fragments, including fibronectin fragments, into the inflammatory milieu. Specific fibronectin fragments (40-, 68- and 120-kDa) in gingival crevicular fluid are markers of periodontal disease status (9). Evidence from cell culture studies suggests that these fibronectin fragments induce apoptosis or suppress osteoblast differentiation of periodontal ligament (PDL) cells (10, 11). The mechanisms by which this proteolytic signature of fibronectin fragments is generated, including the relative contributions of bacterial and host-derived proteases, are not clearly understood.
Inactive pro-MMP-2 is constitutively expressed and secreted by PDL cells (12). This is of interest because, while many cell types express MMP-2, high-level expression of MMP-2 correlates with disease, such as in both the metastatic state of tumor cells (13) and early stages of aneurysm formation (14). The T. denticola dentilisin protease activates pro-MMP-2 secreted by PDL cells, inducing MMP-2-dependent fibronectin fragmentation (15). While the mechanism by which dentilisin activates pro-MMP-2 has not been conclusively determined, this activity suggests that the constitutive pro-MMP-2 expression combined with its activation by bacterial proteases could play a pivotal role in periodontal disease.
The mechanisms controlling MMP-2 expression in PDL cells have not been previously investigated. Emerging evidence suggests that epigenetic modifications play a major role in inflammatory diseases, perhaps including periodontal disease (16). Disease-associated DNA methylation changes can result in either hypermethylation, which tends to suppress gene expression, or hypomethylation and resultant elevation of gene expression (17). Several etiologic and contributing factors mediating periodontal disease pathogenesis (including levels of periodontopathic bacteria, smoking and diabetes status) are associated with marked epigenetic changes in certain periodontal tissue components (18, 19). Herein we report persistent activation of pro-MMP-2 and upregulation of associated regulatory proteins subsequent to infection of cultured PDL cells with T. denticola. Furthermore, we present evidence that constitutive expression of pro-MMP-2 in PDL cells is due to hypomethylation of the MMP-2 promoter, while T. denticola dentilisin activity induces persistent activation of pro-MMP-2.
Materials and Methods
Primary periodontal ligament cells (PDL) culture
PDL cells were obtained from extracted third molars of healthy subjects. Cells were cultured as described previously (12) in minimal essential medium (αMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin and used from passages 2 to 6. For use in experiments, culture medium was replaced with serum- and antibiotic-free αMEM. Use of human PDL cells for these studies was approved by the University of Michigan Health Sciences Institutional Review Board.
T. denticola culture conditions
T. denticola ATCC 35405 and an isogenic dentilisin mutant (20) were grown at 37°C under anaerobic conditions in NOS broth or semisolid agar medium as previously described (21, 22). Culture purity was monitored by darkfield microscopy.
Treatment of PDL cells with T. denticola
PDL cells were challenged with T. denticola at a multiplicity of infection (MOI) =100 in serum- and antibiotic-free medium for indicated times, as described previously (15). Bacteria were removed by washing with PBS. Fresh medium was added and changed at indicated intervals.
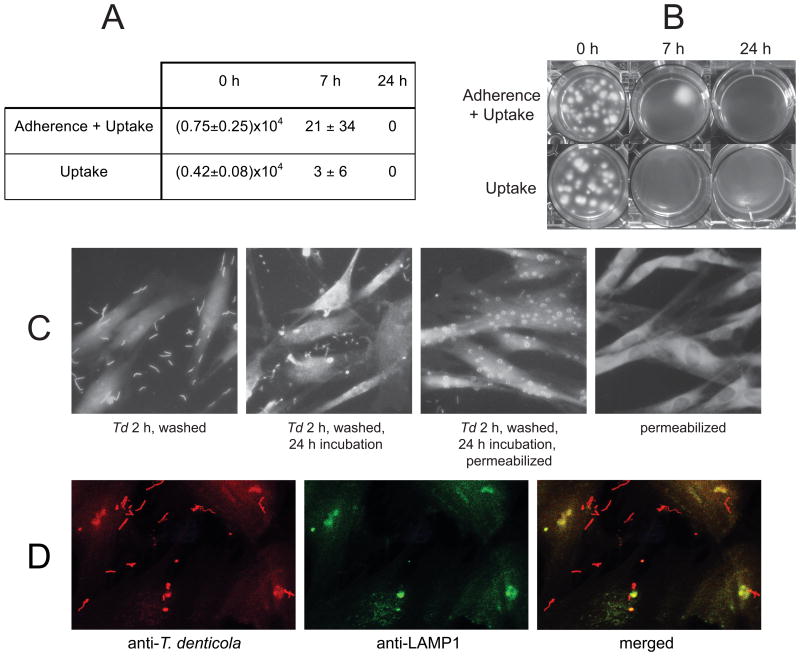
To detect uptake and survival of T. denticola within PDL cells, we used the well-established antibiotic protection assay (23, 24). Briefly, T. denticola at MOI=100 was added to PDL cultures and incubated at 37°C for 2h, after which PDL cells were treated (“uptake”) or not treated (“adherence + uptake”) with 200 μg ml-1 gentamicin for 1h. Gentamicin (10 μg ml-1, 45 min) completely inhibits subsequent growth of planktonic T. denticola (data not shown). After washing and incubation in fresh αMEM for the indicated times, PDL cells were lysed with sterile water, and serial dilutions of the lysates were mixed with NOS semisolid medium and incubated anaerobically at 37°C for 2 weeks.
Immunofluorescence microscopy
PDL cells, 75% confluent on glass coverslips, were challenged with T. denticola (2h, MOI=100), washed with PBS and incubated in serum-free medium for the indicated times. PDL cells were then fixed (3.5% glutaraldehyde, 15 min), washed with PBS, blocked (PBS, 1% BSA, 0.05% Tween-20) for 1h and probed with rabbit anti-T. denticola Msp IgG followed by Alexa 555-conjugated goat anti-rabbit IgG to detect T. denticola and phalloidin-647 (Invitrogen, Carlsbad, CA) to detect cytoskeletal actin of PDL cells, respectively. To detect intracellular T. denticola, PDL cells were permeabilized (0.2% Triton X-100) prior to probing and examination under fluorescence illumination using a Nikon TI (Eclipse) instrument. For immunofluorescence microscopy to detect colocalization of T. denticola and LAMP1, PDL cells were treated, challenged and fixed as above, then permeabilized with (0.2% Triton X-100) prior to probing with rabbit anti-T. denticola whole cell IgG and mouse anti-LAMP1 IgG (Enzo Life Sciences, Farmingdale, NY) followed by fluor-conjugated secondary antibodies. The slides were examined under fluorescence illumination using a Nikon Eclipse TE300 instrument, and red-filter, green filter and merged images were prepared.
Gelatin zymography
Gelatin zymography of T. denticola-challenged PDL cells and culture supernatants was conducted as described previously (15).
Western immunoassays
Detection of proteins of interest in PDL cell lysates or culture supernatants by Western immunoblotting was done as described previously (15) using antibodies specific for MT1/MMP (Abcam, Eugene, OR), actin (Abcam) and TIMP-2 (Triple Point Biologics, Forest Grove, OR).
Purification of T. denticola dentilisin complex
The dentilisin complex was purified from T. denticola outer membrane extracts as described previously (15).
Quantitative RT-PCR
PDL cell RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA), reverse transcribed to cDNA and amplified by qRT-PCR using gene-specific primers for MMP-2, MT1/MMP, TIMP-2(25) and 18S rRNA (26). Cycle threshold values of the genes of interest and the quantitative gene expression levels normalized to 18S rRNA were determined and compared with unchallenged control.
Methylation assays
For DNA methylation screening, equal portions genomic DNA purified from T. denticola-challenged and unchallenged PDL cell cultures using the DNeasy Blood and Tissue Kit (Qiagen) were digested with methylation-sensitive or -resistant enzymes using the EpiTect MethylDNA Restriction Kit (Qiagen). DNA regions of interest were amplified in qPCR using primers for CpG islands in MMP-2 and MT1/MMP promoter regions provided with the EpiTect Primer Assay (Qiagen). Data were analyzed using the EpiTect Methyl qPCR assay template (Qiagen).
Bisulfite DNA sequence analysis was performed on genomic DNA from T. denticola-challenged and unchallenged PDL cell cultures. DNA was purified from PDL cultures as described above. Unmethylated cytosines were converted to thymidines using the EpiTect Bisulfite Kit (Qiagen) following the manufacturer's instructions. Regions of interest, amplified by nested PCR using the primer sets described by Chernov et al. (27), were cloned in E. coli plasmid vector pSTBlue-1 (EMD Biosciences). Nine clones from each experimental condition were selected for DNA sequencing at the University of Michigan DNA Sequencing Core Facility. The entire experimental procedure was replicated using PDL cells independently isolated from another subject.
Statistical analysis
Data on expression and methylation status of MMP-2, MT1/MMP and TIMP-2 were analyzed using Student's t-test.
Results
Persistence of MMP-2 activation and dentilisin activity following T. denticola challenge
T. denticola dentilisin mediates MMP-2-dependent fibronectin fragmentation and these effects are detectable up to 48 h following replacement of culture medium (15). We extended the experiment to five days with daily medium changes to assay persistence of MMP-2 activation. Zymography of culture supernatants shows MMP-2 activation from 72-kDa pro-MMP-2 to 64-kDa active MMP-2 throughout the experiment (Fig. 1A). In contrast, while pro-MMP-2 is present in PDL cell lysates, activated MMP-2 is absent in cell lysates at all time points (Fig. 1B). This is consistent with activation of pro-MMP2 following its secretion. Dentilisin activity is absent in unchallenged controls (Fig. 1A, B). In T. denticola-challenged PDL cultures, dentilisin activity decreased over the course of the experiment, yet remained detectable for at least five days in both culture supernatants (Fig. 1A) and in lysates of washed PDL cells (Fig. 1B).
Figure 1. Zymograms showing gelatinase activity of T. denticola dentilisin protease, pro-MMP-2 and activated MMP-2.
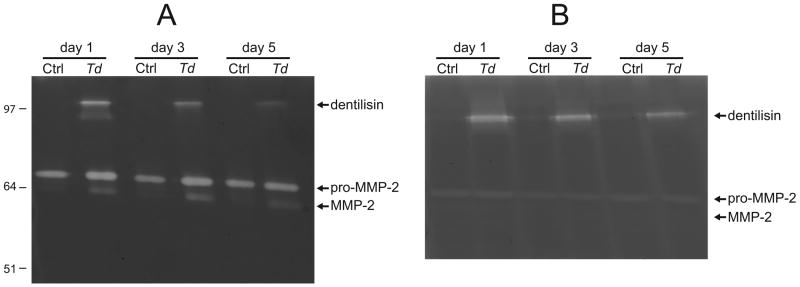
PDL cells were challenged with T. denticola at MOI=100 for 2h, washed twice in PBS and incubated in serum- and antibiotic-free medium with daily changes. Panel A: Gelatinase activity in equal volumes of conditioned medium collected on the indicated days following T. denticola challenge. Panel B: Gelatinase activity in lysates of PDL cells collected on the indicated days following T. denticola challenge and medium replacement as in Panel A. Equal amounts of protein were loaded per lane. The locations of the active dentilisin complex (95-100 kDa), 72-kDa pro-MMP-2 and 64-kDa activated MMP-2 are indicated, as are the positions of relative molecular mass markers in kDa.
Uptake and intracellular survival of T. denticola
Persistence of PDL cell-associated dentilisin activity suggested strong association between PDL cells and either intact T. denticola or secreted dentilisin. Using a standard antibiotic protection “invasion assay,” we tested the ability of PDL cells to internalize T. denticola. As shown in Fig. 2A and 2B, large numbers of viable T. denticola were recovered immediately following gentamicin treatment and washing of challenged PDL cultures. Intracellular T. denticola viability decreased rapidly, but was detectable in gentamicin-treated cultures up to 7h following treatment. No viable T. denticola were recovered after 24 h under aerobic cell culture conditions, with or without gentamicin treatment. Uptake of an isogenic T. denticola dentilisin mutant was not observably different from that of the parent strain (data not shown).
Figure 2. T. denticola adherence to and uptake by PDL cells.
Panels A and B: T. denticola at MOI=100 was added to PDL cultures for 2h, after which PDL cells were treated (“uptake”) or not treated (“adherence + uptake”) with 200 μg ml-1 gentamicin for 1h to kill extracellular bacteria. After washing and incubation in fresh αMEM for the indicated times, PDL cells were lysed with sterile water, and lysates were mixed with NOS semisolid medium and incubated anaerobically at 37°C. Panel A: T. denticola colony forming units recovered per well of PDL cells (approximately 105 cells) after 0, 7 and 24 h post-challenge incubation. The data represent two independent experiments conducted in triplicate. Panel B: T. denticola colonies recovered from PDL cell lysates in a representative experiment. Panel C: Immunofluorescence microscopy of PDL cells with or without 2h T. denticola challenge (2h, MOI=100) followed by washes, with or without further incubation in culture medium and membrane permeabilization. Slides were probed with rabbit anti-T. denticola Msp IgG followed by Alexa 555-conjugated goat anti-rabbit IgG to detect T. denticola and phalloidin-647 to detect cytoskeletal actin in PDL cells. Panel D: Imunofluorescence microscopy of PDL cells challenged with T. denticola (2h, MOI=100) followed by washes and membrane permeabilization, probed with rabbit anti-T. denticola whole cell IgG and mouse anti-LAMP1 IgG followed by fluor-conjugated secondary antibodies.
We then performed standard immunofluorescence microscopy under conditions permitting differentiation of intracellular and extracellular T. denticola. As shown in Fig. 2C, T. denticola is adherent to the surface of washed PDL cells after 2h challenge and is present within PDL cells after 2h challenge followed by 24h incubation in fresh medium. Extracellular T. denticola exhibit typical spirochete morphology whereas intracellular T. denticola appear as coiled structures apparently within vacuoles, suggesting that T. denticola is internalized by a phagocytic mechanism and does not survive within vacuoles. To further examine the fate of intracellular T. denticola, we performed confocal immunofluorescence microscopy (Fig. 2D) using antibodies specific for T. denticola (red fluorescence) and lysosomal-associated membrane protein 1 (LAMP1, green fluorescence). The merged image indicates close association between T. denticola and LAMP1 in what appear to be endocytic vacuoles.
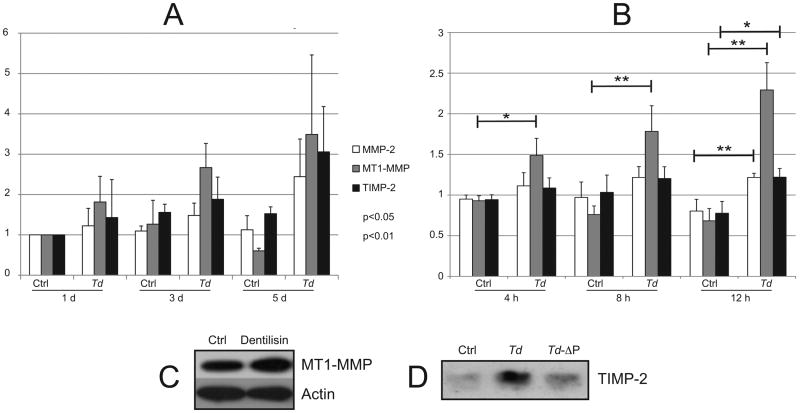
T. denticola challenge induces MMP-2, MT1/MMP and TIMP-2 expression
Transcription of MMP-2 and its modulators MT1/MMP and TIMP-2 was significantly increased at one or more timepoints during the five-day period following T. denticola challenge (Fig. 3A). With increased (12h) T. denticola challenge time, transcription of all three genes increased after further 24h incubation (Fig. 3B). Immunoblots of PDL cell lysates probed with antibodies against MT1/MMP (Fig. 3C) and PDL cell culture supernatants probed with antibodies against TIMP-2 (Fig. 3D) confirmed increased protein expression following challenge with purified dentilisin or T. denticola, respectively for 2h, followed by washing and incubation in fresh medium for 5 days. Interestingly, the post-challenge increase in TIMP-2 was quite marked compared with that of MT1/MMP.
Figure 3. Expression of MMP-2, MT1/MMP and TIMP-2 following T. denticola challenge.
Panels A and B: Transcript levels in PDL cells after T. denticola challenge and incubation in fresh medium for indicated times, assayed by qRT-PCR. The Y-axis in each panel represents fold-expression level of each gene relative to unchallenged control at day 1 shown in Panel A. Panel A: gene expression after 2h T. denticola challenge and incubation in fresh medium for 1, 3 or 5 days. Panel B: gene expression after 4h, 8h or 24h T. denticola challenge and incubation in fresh medium for 24h. Data were analyzed using Student's t-test. Panel C: Western blots showing levels of MT1-MMP expression in PDL cells treated with purified dentilisin (50 ng/ml, 2h) or media control (Ctrl), then washed and maintained in fresh media for 5 days (MT1-MMP1 antibody; Abcam; actin antibody). Panel D: Western blot of TIMP-2 expression in PDL cells (conditioned media) treated for 2h with wildtype T. denticola, T. denticola dentilisin mutant (Td-ΔP) or media control (Ctrl), then washed and maintained in antibiotic supplemented media for 5 days (TIMP-2 antibody, Triple Point Biologics).
Promoter methylation status of genes controlling MMP-2 expression following T. denticola challenge
Persistence of MMP-2 activation after washing and medium changes suggested that T. denticola challenge might have long-term effects on PDL cells. We screened for changes in DNA methylation status of CpG islands in MMP-2 and MT1/MMP promoters 24h after 2h T. denticola challenge. As shown in Fig. 4A, T. denticola challenge resulted in detectable decreases in methylation of the already hypomethylated MMP-2 promoter (p<0.05). To confirm hypomethylation of the MMP-2 promoter, we conducted bisulfite DNA sequencing of the same CpG island in the MMP-2 promoter region under the same challenge conditions. As shown in Fig. 4B, CpG island DNA from both control and challenged PDL cultures showed extremely low levels of methylated cytosine residues. This experiment was repeated with PDL cells independently isolated from a different subject, yielding similar results (data not shown). We also performed methylation screens of the CpG-rich promoter region of the MT1/MMP gene, with inconclusive results. While the methylation-sensitive restriction assay suggested significant decreases in CpG methylation 24h after 2h T. denticola challenge, these results could not be confirmed by the more reliable bisulfite DNA sequencing method (data not shown).
Figure 4. Analysis of MMP-2 promoter methylation in PDL cells following T. denticola challenge.
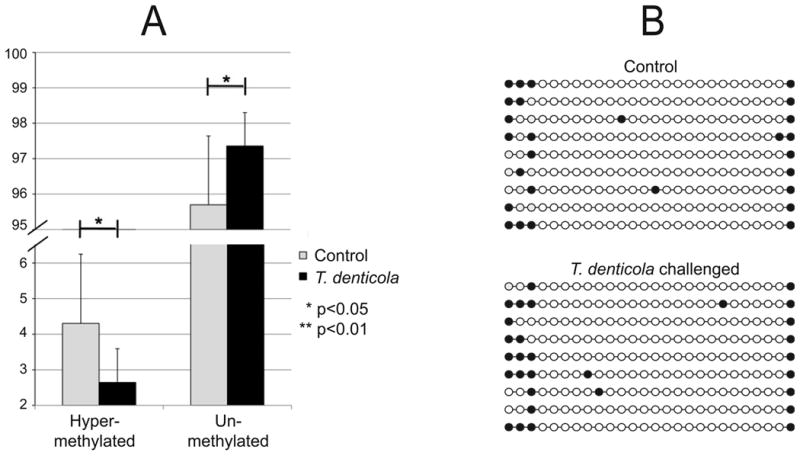
PDL cells were challenged with T. denticola for 2h, washed, and incubated in fresh medium for 24 h prior to analysis. The vertical scale indicates percent of total DNA. Panel A: methylation analysis of the MMP-2 promoter region as determined by MethylDNA Restriction Screen (Qiagen). Results are expressed as the percentage of hyper-methylated and unmethylated DNA, which sum to 100%. Data were analyzed using Student's t-test. Panel B: DNA methylation analysis of the MMP-2 promoter region as determined by sequencing of independent PCR clones of bisulfite-converted DNA from T. denticola challenged and unchallenged PDL cell cultures. Each circle represents an individual CpG within residues 236-456 of MMP2-exon 1-transcript variant 1. Open and closed circles indicate unmethylated and methylated CpGs, respectively within residues 236-456 of MMP2-exon 1.
Discussion
Emerging evidence suggests epigenetic modifications to gene regulatory sequences play a significant role in inflammatory diseases, including periodontal disease. Important questions as to the identity and importance of factor(s) instigating epigenetic regulation of gene expression remain largely unanswered. Several etiologic and contributing factors mediating periodontal disease pathogenesis, including bacteria and their byproducts, smoking and diabetes are associated with apparent epigenetic changes in certain periodontal tissue components (16, 18, 19). Epigenetic processes are consistent with and may underlie the chronic nature of periodontal disease and thus may contribute to the relative ineffectiveness of standard treatment modalities in arresting and reversing periodontal pathogenesis.
Previous studies by our group characterized the role of T. denticola dentilisin in activation of pro-MMP2 secreted into PDL cell culture supernatants and subsequent MMP-2-dependent degradation of PDL cell-associated fibronectin into a consistent pattern of fragments (15). A similar pattern of specific fibronectin fragments in gingival crevicular fluid is a marker of periodontal disease status (9) and fibronectin fragments induce apoptosis in and suppress osteoblast differentiation of PDL cells (10, 11). Clearly, determining the mechanisms by which disease-associated fibronectin fragments are generated is of high interest, and our results support the hypothesis that bacterial protease-induced MMP-2 activation is key factor in this process. We hypothesize that T. denticola and its dentilisin protease contribute to both MMP-2 activation and to activation of expression of genes regulating MMP-2 activation. Results of the present study implicate T. denticola and the dentilisin protease as contributors to increased expression of genes controlling MMP-2 expression and activity. Further studies will focus on distinguishing between direct activation of pro-MMP-2 by T. denticola proteolytic activity and indirect activation by T. denticola-dependent influences on expression of MMP-2 and related genes.
Our previous work demonstrated that dentilisin is necessary but not sufficient for MMP-2-dependent fibronectin fragmentation in cultured PDL cell supernatants (15). The present study focused on potential mechanisms responsible for persistence of MMP-2 activation in PDL cell cultures. While T. denticola dentilisin activity was detectable for at least 5 days in cultures that were challenged with T. denticola and washed to remove unattached bacteria, the level of dentilisin activity decreased markedly while the level of MMP-2 expression increased significantly, and the activation level was consistent over the 5-day period. This suggests that the mechanisms driving MMP-2 expression and activation extend beyond the putative direct activation of pro-MMP-2 by dentilisin cleavage of the MMP-2 pro-peptide.
In addition to MMP-2 activation, T. denticola induced increased transcription of MMP-2 and modulators of its activation: TIMP-2, one of the tissue inhibitors of metalloproteinases, and MT1/MMP, a member of the metalloproteinase family that, when complexed with TIMP-2, induces MMP-2 activation (28). Our previous study showed that T. denticola dentilisin induces increased MMP-2 protein levels in PDL cell culture supernatants. Here we show that T. denticola challenge also induces increased TIMP-2 and MT1/MMP protein levels. In the present study, increased MMP-2 transcription was seen only at endpoints of T. denticola challenge experiments, a result consistent with prior studies reporting that PDL cells constitutively express pro-MMP-2 (12). Similarly, TIMP-2 expression was significantly increased only at the last timepoint. In contrast, MT1/MMP transcription was increased at all timepoints. These results suggest that PDL cell responses to T. denticola are both persistent and, surprisingly, increase over time despite the removal of most of the T. denticola challenge by repeated washing.
To explore a mechanism that could be responsible for the observed long-term effects of T. denticola, we assayed epigenetic modifications to the promoter regions of MMP-2 and MT1/MMP. Both of these genes contain 1.0-1.4 kb CpG-rich islands in their promoter regions, which may be subject to epigenetic control or modification. Both MMP-2 and MT1/MMP showed significantly decreased DNA methylation in CpG-rich promoter regions of these genes in PDL cells challenged with T. denticola, consistent with higher levels of expression at the endpoint of the 5-day experiment. Bisulfite DNA sequence analysis confirmed the hypomethylation of the MMP-2 promoter, independent of T. denticola challenge and identified a likely molecular basis for the unusually high level of constitutive pro-MMP-2 expression in this particular cell type. Pretreatment of PDL cells with chemical epigenetic inhibitors prior to T. denticola challenge resulted in slightly decreased levels of MMP-2 activation (data not shown), though determination of the biological significance of this observation will require more extensive characterization of the effects of these inhibitors on PDL cell growth and behavior. The extremely low DNA methylation level of the MMP-2 promoter in untreated cells suggests that any T. denticola-dependent change in methylation status may not be biologically significant by itself and that other modes of gene regulation may be involved. The potential roles of other epigenetic mechanisms such as histone modification remain to be tested.
The mechanism by which T. denticola challenge results in persistent changes in expression of genes controlling MMP-2 activity is not yet known. Using bisulfite DNA sequencing, we were unable to confirm methylation changes in the MT1/MMP promoter that were suggested by methylation-sensitive restriction screening. Another likely possibility is that increases in MMP-2 expression and activity are a consequence of T. denticola adherence to and uptake into PDL cells. Numerous bacterial pathogens, including several periodontal pathogens, survive uptake by actin-mediated phagocytic mechanisms in epithelial and other cell types and have intracellular effects that contribute to pathogenesis (29-31), To determine the persistence and localization of T. denticola following PDL cell challenge, we measured adherence and uptake of T. denticola using an antibiotic protection assay and performed immunofluorescence microscopy under conditions that distinguish intra- and extracellular compartments. While T. denticola did not remain viable in the intracellular environment, detection of T. denticola antigens within PDL cells suggests that they may contribute to intracellular signaling pathways important to maintenance of intracellular dynamics. Such a process has been described by Visser et al. as a potential mechanism for the role of T. denticola Msp protein in modulating intracellular actin dynamics in neutrophils and fibroblasts (32, 33).
In contrast to well-characterized cellular invasion behavior of Porphyromonas gingivalis (23) and Aggregatibacter actinomycetemcomitans (34), uptake and intracellular survival of T. denticola has not been extensively examined, nor has any specific role in disease been proposed. A few studies have reported detection of apparently intracellular T. denticola by immunofluorescence or transmission electron microscopy (31, 35). A recent manuscript reported uptake and survival of T. denticola within immortalized epithelial cells (36). To our knowledge, the present work is the first to both distinguish intra- and extracellular T. denticola and to simultaneously assay its intracellular viability within primary human cell cultures. Additionally, ours is the first report of uptake of viable bacteria by cultured PDL cells. A recent report by Konerman et al. reported that PDL cells, which have documented collagen phagocytosis activity (37), take up heat-killed bacterial cells into phagosomes (38). Studies are in progress to determine the fate of intracellular T. denticola cells and proteins including dentilisin, as well as potential intracellular signaling pathways induced in response to T. denticola adherence and uptake.
Taken together, these data suggest that T. denticola infection may chronically “imprint” periodontal tissues toward a destructive phenotype through persistent activation of MMP-2 that is constitutively expressed at high levels in a key cell population in the periodontium. It is important to note that we are not claiming that the consequences of dentilisin activity are unique in the complex subgingival environment. Several reports have suggested that P. gingivalis may also have the ability to activate MMP-2, though the mechanism has not been determined (39-41). Rather, this systematic approach to understanding the pathways by which T. denticola modulates tissue homeostasis serves as a useful model to study biological roles of bacterial proteases in periodontal disease. This model can be used to generate new knowledge on the ongoing communication between oral microbiota and host tissue required both for maintenance of health and induction of disease.
From a clinical standpoint, this suggests retooling our thinking about therapeutic approaches used to treat severe or refractory periodontal disease. PDL cells, by constitutively expressing pro-MMP-2, are essentially “primed” for responding to challenge by bacterial proteases capable of activating pro-MMP-2 and initiating a cascade of tissue-destructive processes. Targeting of periodontal pathogen proteolytic activity is problematic due to the fact that these proteases are members of ubiquitous conserved protease families. A more refined therapeutic approach might target mechanisms required for stability of specific protease complex types (dentilisin, gingipain) rather than conserved catalytic domains. For example, studies in our laboratory have identified specific domains in non-protease components of the dentilisin complex that are required for expression of dentilisin activity (42, 43). These surface-expressed proteins are unique to oral spirochetes and are being examined as potential targets for therapeutic agents capable of blocking formation of the active dentilisin protease complex.
Our in vitro results mimic key in vivo mechanisms of periodontal disease chronicity, in particular MMP-2-dependent matrix degradation and bone resorption (44). Adherence and/or internalization of T. denticola may contribute to these processes by one or more regulatory mechanisms, including contact-dependent signal transduction or other epigenetic mechanisms. This new paradigm of chronic disease pathogenesis rooted in epigenetic “imprinting” may help explain part of our failure to control periodontal disease despite an array of non-surgical and surgical treatments combined with local delivery and systemic therapeutics.
T. denticola dentilisin activates constitutively expressed pro-MMP-2 of PDL cells.
MMP-2 activation persists for up to 5 days after a brief T. denticola challenge.
T. denticola is taken up into vacuoles by PDL cells and rapidly loses viability.
PDL cells' MMP-2 promoter is hypomethylated, independent of T. denticola challenge.
T. denticola dentilisin upregulates expression of TIMP-2 and MT1/MMP.
Acknowledgments
This work was supported by Public Health Service grants DE013725 (Y.L.K.) and DE018221 (J.C.F.), and by a China Scholarship Council fellowship (D.M.). We thank Alexander H. Rickard for consultation on microscopy. The authors report no conflicts of interest related to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uitto VJ, Larjava H, Heino J, Sorsa T. A protease of Bacteroides gingivalis degrades cell surface and matrix glycoproteins of cultured gingival fibroblasts and induces secretion of collagenase and plasminogen activator. Infect Immun. 1989 Jan;57(1):213–8. doi: 10.1128/iai.57.1.213-218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slots J, Genco RJ. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–21. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 3.Uitto VJ, Larjava H, Peltonen J, Brunette DM. Expression of fibronectin and integrins in cultured periodontal ligament epithelial cells. J Dent Res. 1992;71(5):1203–11. doi: 10.1177/00220345920710051301. [DOI] [PubMed] [Google Scholar]
- 4.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010 Jun 1;8(7):481–90. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 5.Mäkinen KK, Mäkinen PL. The peptidolytic capacity of the spirochete system. Med Microbiol Immunol. 1996;185(1):1–10. doi: 10.1007/s004300050008. [DOI] [PubMed] [Google Scholar]
- 6.Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000. 2000 Oct;24:153–92. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- 7.Brunette DM, Melcher AH, Moe HK. Culture and origin of epithelium-like and fibroblast-like cells from porcine periodontal ligament explants and cell suspensions. Arch Oral Biol. 1976;21(7):393–400. doi: 10.1016/0003-9969(76)90001-7. [DOI] [PubMed] [Google Scholar]
- 8.Somerman MJ, Archer SY, Imm GR, Foster RA. A comparative study of human periodontal ligament cells and gingival fibroblasts in vitro. J Dent Res. 1988 Jan;67(1):66–70. doi: 10.1177/00220345880670011301. [DOI] [PubMed] [Google Scholar]
- 9.Huynh QN, Wang S, Tafolla E, Gansky SA, Kapila S, Armitage GC, et al. Specific fibronectin fragments as markers of periodontal disease status. J Periodontol. 2002 Oct;73(10):1101–10. doi: 10.1902/jop.2002.73.10.1101. [DOI] [PubMed] [Google Scholar]
- 10.Dai R, Iwama A, Wang S, Kapila YL. Disease-associated fibronectin matrix fragments trigger anoikis of human primary ligament cells: p53 and c-myc are suppressed. Apoptosis. 2005 May;10(3):503–12. doi: 10.1007/s10495-005-1880-5. [DOI] [PubMed] [Google Scholar]
- 11.Joseph J, Kapila YL, Hayami T, Kapila S. Disease-associated extracellular matrix suppresses osteoblastic differentiation of human periodontal ligament cells via MMP-1. Calcif Tissue Int. 2010 Feb;86(2):154–62. doi: 10.1007/s00223-009-9321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapila YL, Kapila S, Johnson PW. Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol. 1996 Sep;15(4):251–61. doi: 10.1016/s0945-053x(96)90116-x. [DOI] [PubMed] [Google Scholar]
- 13.Tryggvason K, Hoyhtya M, Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993;24(3):209–18. doi: 10.1007/BF01833261. [DOI] [PubMed] [Google Scholar]
- 14.Goodall S, Crowther M, Hemingway DM, Bell PR, Thompson MM. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001 Jul 17;104(3):304–9. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- 15.Miao D, Fenno JC, Timm JC, Joo NE, Kapila YL. Treponema denticola chymotrypsin-like protease (dentilisin) induces MMP-2-dependent fibronectin fragmentation in periodontal ligament cells. Infect Immun. 2011 Nov 29;79:806–11. doi: 10.1128/IAI.01001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Offenbacher S, Barros SP, Beck JD. Rethinking periodontal inflammation. J Periodontol. 2008 Aug;79(8 Suppl):1577–84. doi: 10.1902/jop.2008.080220. [DOI] [PubMed] [Google Scholar]
- 17.Grummt I, Pikaard CS. Epigenetic silencing of RNA polymerase I transcription. Nat Rev Mol Cell Biol. 2003 Aug;4(8):641–9. doi: 10.1038/nrm1171. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Barros SP, Niculescu MD, Moretti AJ, Preisser JS, Offenbacher S. Alteration of PTGS2 promoter methylation in chronic periodontitis. J Dent Res. 2010 Feb;89(2):133–7. doi: 10.1177/0022034509356512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andia DC, de Oliveira NF, Casarin RC, Casati MZ, Line SR, de Souza AP. DNA methylation status of the IL8 gene promoter in aggressive periodontitis. J Periodontol. 2010 Sep;81(9):1336–41. doi: 10.1902/jop.2010.100082. [DOI] [PubMed] [Google Scholar]
- 20.Bian XL, Wang HT, Ning Y, Lee SY, Fenno JC. Mutagenesis of a novel gene in the prcA-prtP protease locus affects expression of Treponema denticola membrane complexes. Infect Immun. 2005;73:1252–5. doi: 10.1128/IAI.73.2.1252-1255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haapasalo M, Singh U, McBride BC, Uitto VJ. Sulfhydryl-dependent attachment of Treponema denticola to laminin and other proteins. Infect Immun. 1991;59(11):4230–7. doi: 10.1128/iai.59.11.4230-4237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan ECS, DeCiccio A, McLaughlin R, Klitorinos A, Siboo R. An inexpensive solid medium for obtaining colony-forming units of oral spirochetes. Oral Microbiol Immunol. 1997;12(6):372–6. doi: 10.1111/j.1399-302x.1997.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 23.Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infection & Immunity. 1995;63(10):3878–85. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang P, Foubister V, Pucciarelli MG, Finlay BB. Methods to Study Bacterial Invasion. J Microbiol Meth. 1993 Oct;18(3):227–40. [Google Scholar]
- 25.Shelton L, Rada JS. Effects of cyclic mechanical stretch on extracellular matrix synthesis by human scleral fibroblasts. Exp Eye Res. 2007 Feb;84(2):314–22. doi: 10.1016/j.exer.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, et al. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng Part A. 2008 May;14(5):667–80. doi: 10.1089/tea.2007.0272. [DOI] [PubMed] [Google Scholar]
- 27.Chernov AV, Sounni NE, Remacle AG, Strongin AY. Epigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells. J Biol Chem. 2009 May 8;284(19):12727–34. doi: 10.1074/jbc.M900273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007 Mar;8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JD, Chen R, Lenton PA, Zhang G, Hinrichs JE, Rudney JD. Persistence of extracrevicular bacterial reservoirs after treatment of aggressive periodontitis. J Periodontol. 2008 Dec;79(12):2305–12. doi: 10.1902/jop.2008.080254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer DH, Mintz KP, Fives-Taylor PM. Models of invasion of enteric and periodontal pathogens into epithelial cells: a comparative analysis. Crit Rev Oral BiolMed. 1997;8(4):389–409. doi: 10.1177/10454411970080040301. [DOI] [PubMed] [Google Scholar]
- 31.Kirschbaum M, Schultze-Mosgau S, Pfister W, Eick S. Mixture of periodontopathogenic bacteria influences interaction with KB cells. Anaerobe. 2010 Apr 7; doi: 10.1016/j.anaerobe.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Visser MB, Koh A, Glogauer M, Ellen RP. Treponema denticola major outer sheath protein induces actin assembly at free barbed ends by a PIP2-dependent uncapping mechanism in fibroblasts. PloS One. 2011;6(8):e23736. doi: 10.1371/journal.pone.0023736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visser MB, Sun CX, Koh A, Ellen RP, Glogauer M. Treponema denticola major outer sheath protein impairs the cellular phosphoinositide balance that regulates neutrophil chemotaxis. PloS One. 2013;8(6):e66209. doi: 10.1371/journal.pone.0066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer DH, Lippmann JE, Fives-Taylor PM. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64(8):2988–97. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uitto VJ, Pan YM, Leung WK, Larjava H, Ellen RP, Finlay BB, et al. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect Immun. 1995;63(9):3401–10. doi: 10.1128/iai.63.9.3401-3410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin J, Choi Y. The fate of Treponema denticola within human gingival epithelial cells. Mol oral microbiol. 2012 Dec;27(6):471–82. doi: 10.1111/j.2041-1014.2012.00660.x. [DOI] [PubMed] [Google Scholar]
- 37.van der Pauw MT, Van den Bos T, Everts V, Beertsen W. Phagocytosis of fibronectin and collagens type I, III, and V by human gingival and periodontal ligament fibroblasts in vitro. J Periodontol. 2001 Oct;72(10):1340–7. doi: 10.1902/jop.2001.72.10.1340. [DOI] [PubMed] [Google Scholar]
- 38.Konermann A, Deschner J, Allam JP, Novak N, Winter J, Baader SL, et al. Antigen-presenting cell marker expression and phagocytotic activity in periodontal ligament cells. J Oral Pathol Med. 2011 Sep 22; doi: 10.1111/j.1600-0714.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- 39.Pattamapun K, Tiranathanagul S, Yongchaitrakul T, Kuwatanasuchat J, Pavasant P. Activation of MMP-2 by Porphyromonas gingivalis in human periodontal ligament cells. J Periodontal Res. 2003 Apr;38(2):115–21. doi: 10.1034/j.1600-0765.2003.01650.x. [DOI] [PubMed] [Google Scholar]
- 40.Grayson R, Douglas CW, Heath J, Rawlinson A, Evans GS. Activation of human matrix metalloproteinase 2 by gingival crevicular fluid and Porphyromonas gingivalis. J Clin Periodontol. 2003 Jun;30(6):542–50. doi: 10.1034/j.1600-051x.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 41.Euzebio Alves VT, Bueno da Silva HA, de Franca BN, Eichler RS, Saraiva L, de Carvalho MH, et al. Periodontal treatment downregulates protease-activated receptor 2 in human gingival crevicular fluid cells. Infect Immun. 2013 Dec;81(12):4399–407. doi: 10.1128/IAI.01107-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godovikova V, Goetting-Minesky MP, Fenno JC. Composition and localization of Treponema denticola outer membrane complexes. Infect Immun. 2011 Dec;79(12):4868–75. doi: 10.1128/IAI.05701-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godovikova V, Wang HT, Goetting-Minesky MP, Ning Y, Capone RF, Slater CK, et al. Treponema denticola PrcB is required for expression and activity of the PrcA-PrtP (dentilisin) complex. J Bacteriol. 2010 Jul;192(13):3337–44. doi: 10.1128/JB.00274-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohshiba T, Miyaura C, Inada M, Ito A. Role of RANKL-induced osteoclast formation and MMP-dependent matrix degradation in bone destruction by breast cancer metastasis. Brit J Cancer. 2003 Apr 22;88(8):1318–26. doi: 10.1038/sj.bjc.6600858. [DOI] [PMC free article] [PubMed] [Google Scholar]