Abstract
In order to create an effective immunization approach for a potential vaccine to heroin, liposomes containing monophosphoryl lipid A [L(MPLA)] were tested as an adjuvant system to induce antibodies to heroin hapten analogs. Four synthetic haptens and two immunization strategies were employed. In the first strategy, a hydrophobic 23 amino acid immunogenic peptide derived from the membrane proximal external region of gp41 from HIV-1 envelope protein was embedded as a carrier in the outer surface of L(MPLA), to which was conjugated a 15 amino acid universal T cell epitope and a terminal heroin hapten analog. In the second strategy, tetanus toxoid (TT) carrier protein was decorated with haptens by conjugation, and the hapten-conjugated protein was mixed with L(MPLA). After immunization of mice, each of the immunization strategies was effective for induction of IgG anti-hapten antibodies. The first immunization strategy induced a mean end-point IgG titer against one of two haptens tested of approximately 12,800; however, no detectable antibodies were induced against the liposome-associated HIV-1 carrier peptide. In the second immunization strategy, depending on the hapten used for decorating the TT, end-point IgG titers ranged from 100,000 to 6,500,000. In this strategy, in which hapten was conjugated to the TT, end-point IgG titers of 400,000 to the TT carrier were observed with each conjugate. However, upon mixing unconjugated TT with L(MPLA), anti-TT titers of 6,500,000 were observed. We conclude that L(MPLA) serves as a potent adjuvant for inducing antibodies to candidate heroin haptens. However, antibodies to the carrier peptide or protein were partly or completed inhibited by the presence of conjugated hapten.
Keywords: Liposomes containing monophosphoryl lipid A, adjuvant system, opiate vaccine, antibodies to heroin haptens
1. Introduction
In the field of vaccines, adjuvants and adjuvant formulations are becoming increasingly important as factors for increasing the potency and efficiency of the immune response [1,2]. Liposomes (L), which were originally introduced as biodegradable model membranes and as therapeutic drug carriers, have emerged as highly adaptable and useful carriers for vaccine formulations [1-3]. Liposomes containing monophosphoryl lipid A [L(MPLA)] as a potent adjuvant system have been employed in human trials for proposed vaccines to malaria, HIV-1, meningococcal type B disease; breast cancer, prostate cancer, and colon cancer [4].
The fundamental basis for using L(MPLA) as an adjuvant system has been that it engages a variety of adjuvant mechanisms that have additive effects, including a depot effect, binding and activation of membrane-associated toll-like receptor type 4 (TLR4) during uptake of the particles by antigen presenting cells, and intracellular processing by both major histocompatibility antigens type 1 and type 2, to enhance and direct antigen-specific induction of antibodies and cell-mediated immunity to a variety of antigens [2,4]. Because of its function as a synthetic membrane that emulates cell membranes, L(MPLA) has also served as a platform in which chemical interactions of peptides with membrane phospholipids induced conformation-specific antibodies to membrane-associated antigens [5,6], and a commercial candidate peptide vaccine to induce conformation-specific antibodies for treatment of Alzheimer's disease is currently being tested in humans [4,7].
The peptide antigens, and certain protein antigens, cited in the above and other studies were very poorly immunogenic by themselves in the absence of strong adjuvants, and this is often a problem that limits vaccine development. However, another category of antigens consists of molecules known as “haptens” that are completely non-immunogenic as separate molecules [8], but when haptens are conjugated to carrier macromolecules, generally protein or polypeptide carriers, hapten-specific antibodies can be induced in addition to carrier-specific antibodies. In recognition of this phenomenon, haptenic drugs have long been conjugated to carriers as a general method to induce hapten-specific antibodies for diagnostic use in clinical immunoassays of drugs [9,10]. The success of the clinical development of anti-hapten antibodies for immunoassay of drugs has led to a growing interest in the possibility of developing human vaccines to so-called drugs of abuse in which haptenic compounds conjugated to carriers are used as antigens in combination with adjuvants to induce antibodies that react with psychoactive drugs such as nicotine, cocaine, methamphetamine, or heroin [11-14]. The underlying rationale for such vaccines is that the offending neurotropic drugs are captured by vaccine-induced antibodies to the drug in the blood, thus blocking penetration through the blood-brain barrier and preventing the drug from binding to drug-specific receptors in the brain.
The theoretical possibility of using liposomes as carriers for inducing anti-hapten antibodies was shown in earlier studies in rodents with liposomal phospholipid-conjugated haptens when emulsified with complete Freund's adjuvant, a toxic emulsion that is unsuitable for use in humans [15]. In previous work in the development of antibodies to opiate (morphine or heroin) haptens, a variety of adjuvants have been employed, including: complete Freund's adjuvant [16-20]; Imject® Alum [21,22], which is an aluminum and magnesium salt adjuvant that is not considered equivalent to aluminum salt adjuvants which are present in licensed human vaccines [23]; and aluminum hydroxide [24], an adjuvant that is widely used in human vaccines. Although aluminum hydroxide is an acceptable adjuvant for human use, it is relatively weak and induces lower titers of antibodies when compared with liposomes containing MPLA [25,26]. In the present study we investigated the feasibility of creating synthetic liposomal formulations containing MPLA that could induce high levels of specific antibodies to synthetic opiate haptens that might be useful as a candidate vaccine to heroin and similar opiates.
2. Materials and Methods
2.1 Materials and reagents
1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG); 1,2-dimyristoyl-sn-glycero-3-phosphate (DMPC), galactosyl ceramide (GalCer), monophosphoryl lipid A (PHAD™) (MPLA), and cholesterol (CHOL) were purchased from Avanti Polar Lipids (Alabaster, AL). Sulfo-3-GalCer (sulfatide), gelatin and bovine serum albumin (BSA) used as an ELISA blocker were purchased from Sigma-Aldrich (Saint Louis, MO). Tetanus toxoid (TT) was purchased from Statens Serum Institut (Copenhagen, Denmark). BSA used for coupling to haptens, SM(PEG)2 linker and BCA total protein assay kits were purchased from Pierce Protein Research/Thermo Fisher Scientific (Rockford, IL). Fmoc-dPEG4-OH was obtained from Quanta BioDesign (Powell, OH), while other Fmoc-protected amino acids and peptide synthesis reagents were purchased from Applied Biosystems (Foster City, CA). N,N′-maleoyl-γ-aminobutyric acid and S-trityl-mercaptopropionic acid were obtained from Chem-Impex International (Wood Dale, IL). Immunolon 2HB flat and “U” bottom ELISA plates were purchased from Thermolab Systems. Peroxidase-linked sheep anti-mouse IgG (γ-chain specific) was purchased from The Binding Site. 2,2′-azino-di(3-ethylbenzthiazoline-6-sulfonate) peroxidase substrate system was purchased from KPL, Inc. 4E10 was purchased from Polymun Scientific Immunbiologische Forschung GmbH, Klosterneuburg, Austria. WR339, a murine monoclonal antibody to gp41, was developed in our laboratory following immunization with gp145. Pichia pastorisi-expressed recombinant ectodomain of gp41 (amino acids 541–682) from HIV-1 was purchased from The Biotech Source (Franklin, MA).
2.2 Haptens and peptide synthesis
The structures of the synthetic haptens and peptides used are shown in Fig. 1A and 1B, respectively. HerHap was synthesized as previously described [22]. OMAHap was synthesized from β-oxymorphamine, MorHap was synthesized from morphine, and 6-AcMorHap was synthesized from hydromorphone (manuscript in preparation). The MPER carrier contains a 23 amino acid peptide derived from the membrane proximal external region (MPER) from the gp41 transmembrane HIV-1 envelope protein and a universal T cell epitope peptide. Peptides were synthesized using an Applied Biosystems 433A peptide synthesizer and a conventional Fmoc chemistry [27]. The peptide sequences intended for conjugation with heroin/morphine haptens were capped with N,N′-maleoyl-γ-aminobutyric acid using standard carbodiimide/HOBt coupling chemistry [28], followed by cleavage with a mixture of 95% TFA, 2.5 % water, and 2.5% triisopropylsilane [29]. All peptides were purified by reverse-phase HPLC using a Shimadzu Prominence LC-6AD semi-preparative system, and characterized by mass-spectrometry using a Shimadzu LCMS-IT-TOF system (Shimadzu Scientific Instruments; Columbia, MD, USA). The purity of the peptides MPER carrier and PEG-MPER carrier were >95% by HPLC. The molecular weights of MPER carrier and PEG-MPER carrier were determined to be 5,034.7 and 5447.9, respectively, by ESI-MS.
Fig. 1. Heroin haptens (A) and hapten coupling strategy to peptide (B) and protein (C) carriers used in the study. The MPER carrier contains both a 23 amino acid.
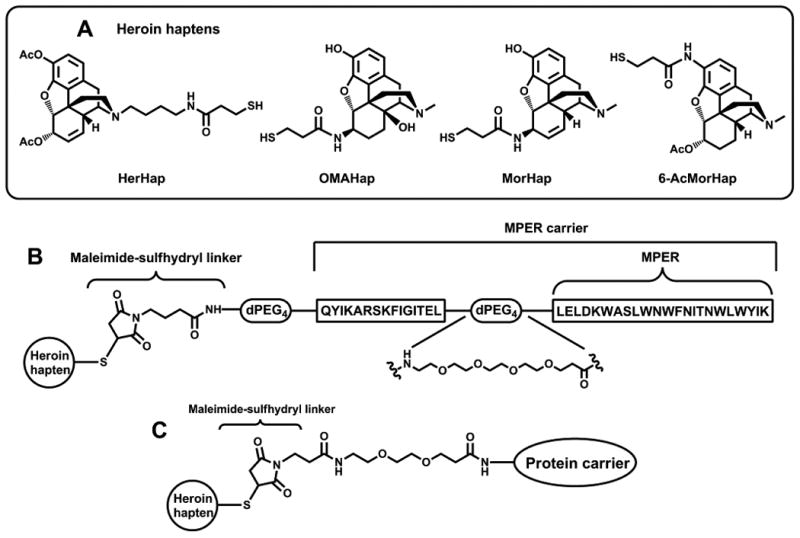
2.3 Coupling of haptens to peptides and protein carriers
Conjugation of haptens with MPER carrier was performed by 1) removing the trityl protecting group from the haptens with 3% TFA, 3% triisopropylsilane in chloroform (30 min), followed by evaporation of volatile components under vacuum; 2) suspension of the residue in pH 7.4 PBS buffer at the concentration of about 10 mg/ml; 3) mixing the resulting suspension with a solution of MPER-carrier in 1:1 methanol-water at the concentration of about 2-5 mg/ml; 4) agitating the resulting mixture by sonication in a cleaning bath for 1-2 h. The progress of the conjugation was monitored by LC-MS using a Shimadzu LCMS-IT-TOF system equipped with a RP-UFLC column (Shim-Pack XR-ODS II, 2.0 mm × 150 mm) (Shimadzu Scientific Instruments; Columbia, MD). Purification of the conjugates was accomplished by HPLC on a Vydac 218TP152022 C18 column (Grace Davison Discovery Sciences; Deerfield, IL) using a Shimadzu Prominence LC-6AD semi-preparative HPLC system. The HPLC fractions containing the conjugates with the target masses, as determined by LC-MS, were pooled and lyophilized to yield hapten-MPER carrier conjugates in a powder form. The purity of HerHap-PEG-MPER carrier and OMAHap-PEG-MPER carrier were >95% by HPLC. The molecular weights were of HerHap-PEG-MPER carrier and OMAHap-PEG-MPER were determined to be 5962.1 and 5838.1, respectively, by ESI-MS.
Conjugation of heroin/morphine haptens with TT and BSA carriers was performed by 1) activation of the protein carrier with SM(PEG)2 linker by mixing the protein and the linker solutions (1-2 mg of linker per 1 mg of protein) in pH 7.4 PBS at concentration of about 10 mg/ml and agitation by gentle shaking (1 h); 2) purification of the activated protein by dialysis in pH 7.4 PBS buffer; 3) removing the trityl protecting group from the haptens with 3% TFA, 3% triisopropylsilane in chloroform (30 min), followed by evaporation of volatile components under vacuum; 4) suspension of the residue in pH 7.4 PBS buffer at the concentration of about 10 mg/ml, followed by filtering off the insoluble particulate using Pall 0.2 μm Acrodisc syringe filters; 3) mixing the resulting filtrate with the activated protein solution, at about 1 mg of hapten per 1 mg of protein ratio; 4) agitating the resulting mixture by gentle shaking for 1 h; and 5) purification of the hapten-protein conjugate by dialysis in pH 6.5 PBS buffer. The resulting conjugate concentrations were measured by a microplate BCA total protein assay.
2.4 Preparation of liposomes and immunization
Liposomes were prepared as previously described [30]. Four different liposome formulations were used for immunization with L(MPLA + HerHap-PEG-MPER carrier) and L(MPLA + OMAHap-PEG-MPER carrier). L(43% Chol) contained DMPC:CHOL:DMPG (molar ratio, 9:7.5:1) with 51 mg/ml total lipid. L(sulfo-GalCer) contained DMPC:CHOL:DMPG:sulfo-GalCer (9:7.5:1:1.11) (5 mg/ml sulfo-GalCer) with 56 mg/ml total lipid. L(71% Chol) contained DMPC:CHOL:DMPG (9:25:1) (71% CHOL) with 45.4 mg/ml total lipid. L(GalCer) contained DMPC:CHOL:DMPG:GalCer (9:7.5:1:1.28) (2 mg/ml GalCer) with 56 mg/ml total lipid. The liposome formulations contained 400 μg/ml of MPLA (20 μg/mouse) and either 200 μg/ml of HerHap-PEG-MPER carrier, OMAHap-PEG-MPER carrier, MPER carrier, or MPER (10 μg/mouse), which were dissolved in methanol and added to the chloroform:methanol lipid mixture prior to rotary evaporation. The final phospholipid concentration of the liposomes was 50 mM in PBS, pH 6.4. Liposomes containing MPLA that were mixed with for hapten-TT conjugates for immunization were L(43% Chol) containing the MPLA, but lacking peptide. The lyophilized liposomes were hydrated in PBS, pH 6.4, and mixed with hapten-TT conjugates to give a final dose of 10 μg TT/0.05 ml/mouse. Female BALB/c mice (5 mice/group; 6-8 weeks of age) (Charles River Laboratories, Indianapolis, IN) were immunized intramuscularly with 0.05 ml of liposomal vaccines.
2.5 ELISA
Hapten-BSA (0.1 μg BSA/0.1 ml/well in PBS, pH 6.4) was added to flat bottom plates and incubated at 4 °C, overnight. The plates were blocked with 20 mM Tris-HCl-154 mM sodium chloride–1% BSA, pH 7.4 (blocker) (0.3 ml/well) at room temperature for 2 h. Serum (0.1 ml/well) diluted in blocker starting at 1:50 in serial 2-fold dilutions in triplicate was added to the plate. Following incubation at room temperature 1 h, the plates were washed 4 times with 0.5 ml/well of TBS–0.05% Tween 20®. Peroxidase-linked sheep anti-mouse IgG (0.1 μg in 0.1 ml blocker) was added and the plates incubated for 1 hr at room temperature. The plates were washed and 0.1 ml/well substrate was added. After incubation for 1 h at room temperature, the absorbance was read at 405 nm. The above procedure was used for measurement of antibodies to TT using TT (0.1 μg/well) as the coating antigen in PBS, pH 7.4. ELISA with peptides and gp41 were conducted as described [31]. End point titer is defined as the dilution at which the absorbance is twice background as defined from wells lacking primary sera.
3. Results
3.1. Immune response to heroin hapten-MPER peptide carrier attached to liposomes containing MPLA
Four liposome compositions were used as vehicles for immunization of 5 mice each with L(MPLA + HerHap-PEG-MPER carrier): liposomes containing 43% CHOL; liposomes containing 43% CHOL and sulfo-GalCer; liposomes containing 43% CHOL and GalCer; and liposomes containing 71% CHOL. Liposomes containing 43% CHOL have been routinely used in many clinical trials [4], and 71% CHOL changes the biophysical structure to allow the induction of anti-CHOL antibodies [32,33].
As shown in Fig. 2A, each of the five mice in each group (20 mice total) mounted an immune response to the attached HerHap hapten, but no distinctive differences were observed between the liposome compositions. The average end point titer of antibodies to the hapten of all of the 20 mice taken together was approximately 12,800 (Fig. 2C). It is clear that the L(MPLA + HerHap-PEG-MPER carrier) was an excellent immunogen for induction of antibodies to a heroin hapten, and neither the induction nor the binding of antibodies was affected by sulfo-GalCer, GalCer, or 71% CHOL. In contrast to HerHap, no detectable antibodies to OMAHap were observed in 20 mice after immunization with L(MPLA + OMAHap-PEG-MPER) containing the same liposomal lipid combinations (Fig. 2B).
Fig. 2.
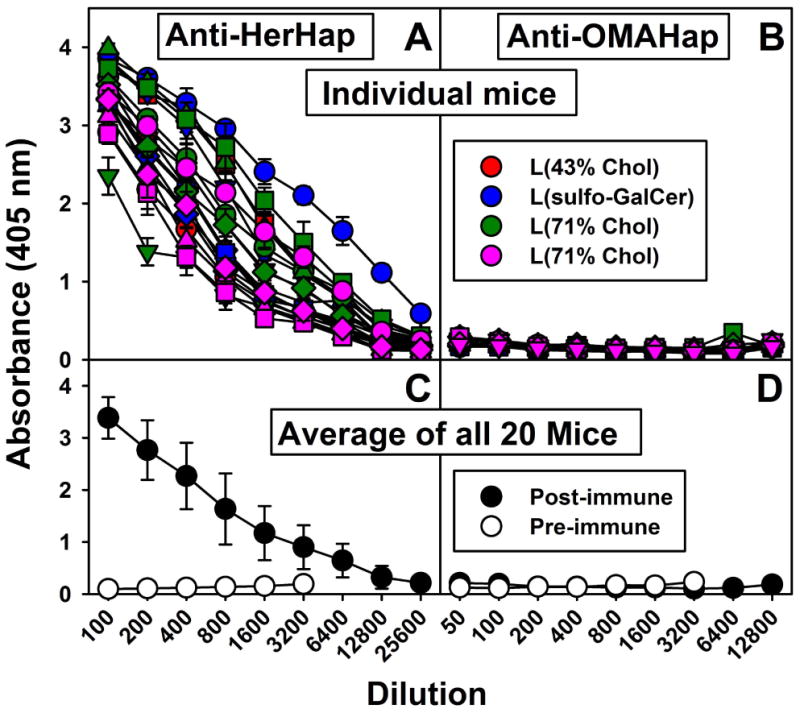
Serum IgG responses to heroin haptens coupled to MPER carrier 9 weeks after primary immunization. Mice were immunized with L(MPLA + HerHap-PEG-MPER carrier) (A, C) or L(MPLA + OMAHap-PEG-MPER) (B, D) at week 0, 3 and 6. ELISA was conducted with the appropriate hapten coupled to BSA. Each curve represents an individual mouse (5 mice/liposome formulation). Each symbol (circle, triangle, square, diamond, inverted triangle) represents a different mouse in the group. Values represent the mean ± standard deviation of triplicate determinations.
Despite the immune response to the hapten observed after immunization with L(MPLA + HerHap-PEG-MPER carrier), none of the 20 mice had any detectable antibodies either to MPER or MPER carrier (Fig. 3A and 3C). Absence of antibodies to MPER was also observed after immunization with L(MPLA + OMAHap-PEG-MPER) (data not shown). Immune responses to MPER after immunization with L(MPLA + MPER) and liposomal MPLA with MPER-conjugated lipopetides have been previously documented [31,34], and antibodies to MPER and to MPER carrier were observed in many of the mice immunized with L(MPLA + MPER-carrier) (Fig. 3B and 3D). In addition to the absence of anti-MPER antibodies induced when using L(HerHap-MPER carrier) (Fig. 3a and 3C), antibodies to the native gp41 protein were not detected (Fig. 3E and 3F). Thus, the presence of either HerHap or OMAHap attached to the MPER carrier had a profound inhibitory effect on the ability of the mice to induce antibodies either to MPER itself or to the complete MPER carrier or to the native gp41 protein.
Fig. 3.
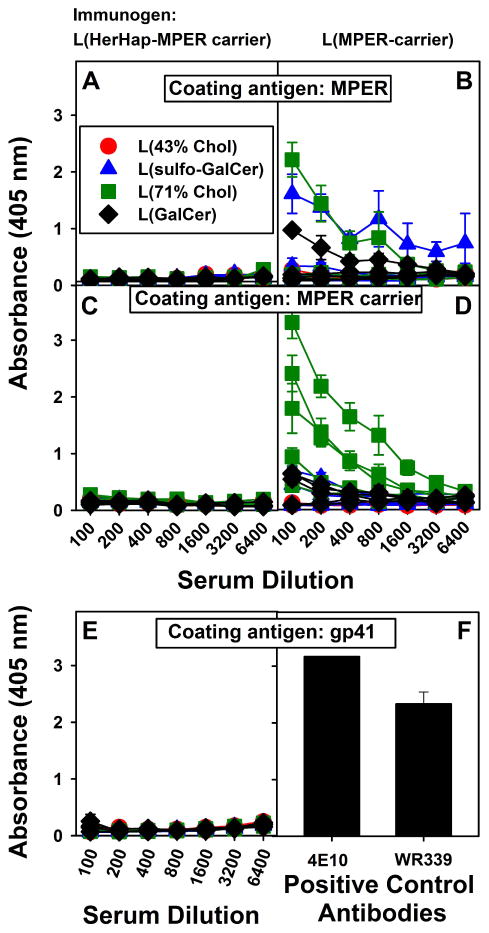
Serum IgG responses to MPER, MPER carrier and gp41 9 weeks after primary immunization. Mice were immunized with L(MPLA + HerHap-PEG-MPER carrier) (A, C, E) or L(MPLA + MPER carrier) (B, D), at weeks 0, 3 and 6. Each curve represents an individual mouse (5 mice/liposome formulation). Monoclonal antibodies 4E10 and WR339 (5 ng/well) were used as positive controls for the assay for gp41 antibodies (F). Values represent the mean ± standard deviation of triplicate determinations.
3.2 Immune response to heroin hapten-TT carrier mixed with liposomes containing MPLA
Each of the haptens, HerHap, OMAHap, MorHap, and 6-AcMorHap, was conjugated to TT and mixed with liposomes containing MPLA. Although each of the conjugate formulations was highly immunogenic in mice, the relative respective anti-hapten titers observed differed greatly, with MorHap (6,500,000) (Fig 4C) > HerHap (3,000,000) (Fig 4A) > 6-AcMorHap (500,000) (Fig 4D) > OMAHap (100,000) (Fig 4B). The relatively lower anti-hapten response of OMAHap-TT was consistent with the previously mentioned absence of anti-hapten antibodies observed after immunization with L(MPLA + OMAHap-PEG-MPER).
Fig. 4.
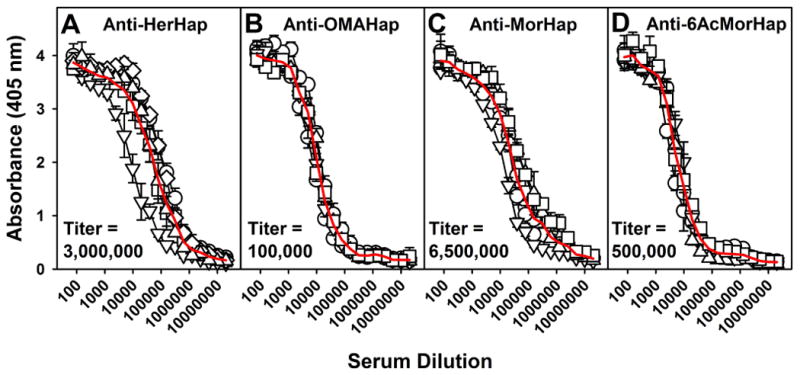
Serum IgG responses to heroin haptens coupled to TT 9 weeks after primary immunization. Mice were immunized with L(MPLA) + HerHap-TT (A) or L(MPLA) + OMAHap (B), L(MPLA) + MorHap (C), or L(MPLA) + 6-AcMorHap (D) at 0 and 6 weeks. ELISA was conducted with the appropriate hapten coupled to BSA. Each curve represents an individual mouse (5 mice/liposome formulation). Values represent the mean ± standard deviation of triplicate determinations. The red line is an average of the 5 mice. Pre-immune sera were not elevated above assay background levels.
Remarkably, despite the above differing antibody titers to the different haptens conjugated to TT, with every conjugated hapten the immune response to the TT carrier was the same (400,000) (Figs 5A-D). However, as with the HerHap-MPER carrier formulations attached to liposomes containing MPLA (Fig 3), the immune response to the hapten-free carrier itself [TT mixed with L(MPLA)] was much higher than to any of the TT conjugates (Fig 5E). Thus, the conjugated hapten inhibited, but did not eliminate, the immune response to TT. The end result is that the titer to TT was greater than the titer to hapten after immunization with either 6-AcMorHap-TT or OMAHap-TT, but much lower than after immunization with MorHap-TT or HerHap-TT.
Fig. 5.
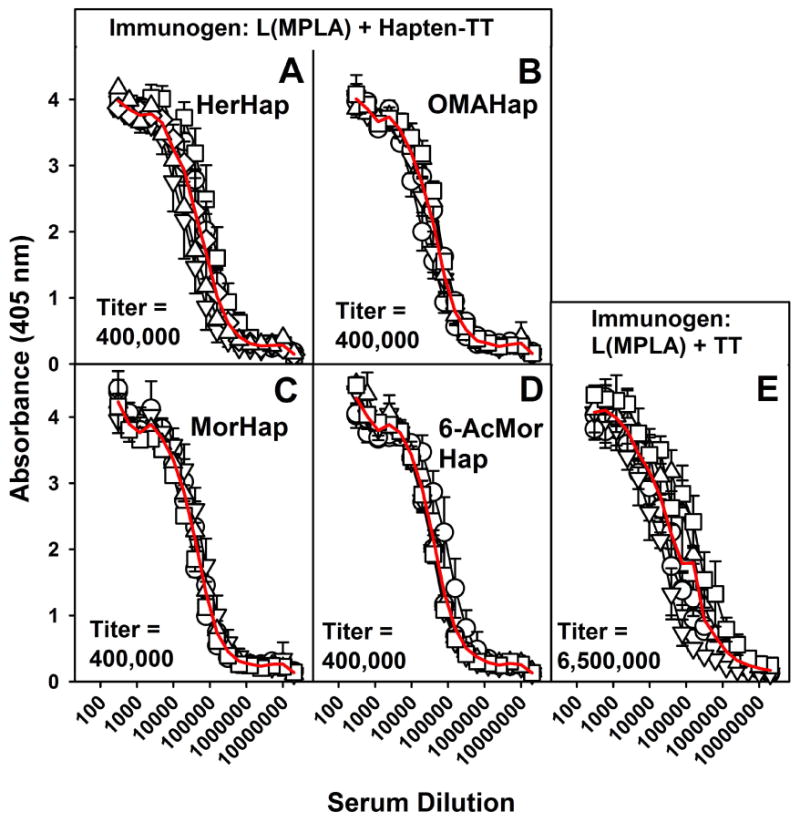
Serum IgG responses to TT 9 weeks after primary immunization. Mice were immunized with L(MPLA + HerHap-TT) (A) or L(MPLA + OMAHap) (B), L(MPLA + MorHap) (C), L(MPLA +6-AcMorHap) (D), or L(MPLA) + TT (E) at 0 and 6 weeks. Each curve represents an individual mouse (5 mice/liposome formulation). Values represent the mean ± standard deviation of triplicate determinations. The red line is an average of the 5 mice. Pre-immune sera were not elevated above assay background levels.
4. Discussion
The theoretical basis that underlies a potential vaccine against a haptenic drug such as nicotine, cocaine, methamphetamine, or an opiate (such as heroin or morphine), each of which drug has a receptor in the brain, is that specific antibodies to the drug will retard or block transmission of the drug across the blood-brain barrier [13,14]. The major challenge for such a vaccine, therefore, is to induce high titers of specific IgG antibodies that will block the drug, and this challenge is best addressed by using both a highly potent and safe adjuvant formulation and an appropriate hapten that is conjugated to a suitable carrier. In the present study we used liposomes containing MPLA as an adjuvant system, and four synthetic haptens were tested, HerHap, OMAHap, MorHap, and 6-AcMorHap, each of which might be used in a candidate vaccine to heroin.
In this study two different carrier/adjuvant strategies for haptens were employed. In the first strategy, HerHap or OMAHap was conjugated to a hydrophobic carrier that contained a 23 amino acid MPER peptide that spontaneously associates with the outer surface of bilayers of liposomes containing MPLA during liposome formation (Fig. 6A). Under the conditions used this resulted in a mean titer of 12,800 to HerHap, but no detectable antibodies were induced to OMA-Hap. However, after immunization of mice by using the second carrier-adjuvant strategy, in which each of the four haptens was directly conjugated to TT for immunization and each hapten conjugate was simply mixed with liposomes containing MPLA (Fig. 6B), even higher IgG endpoint titers of 6,500,000 for MorHap, 3,000,000 for HerHap, 500,000 for 6-AcMorHap, and 100,000 for OMAHap were observed. The epitope density of HerHap conjugated to TT might have differed from that on the surface of L(MPLA + HerHap-PEG-MPER), possibly explaining the different titers observed with the two adjuvant systems. In addition, although there were differences in titers obtained with the two different carriers and the four different haptens, experience has shown that direct translation of titers in mice with different adjuvant systems cannot be reliably used as a predictive measure of anticipated titers that would occur in humans [2].
Fig. 6.
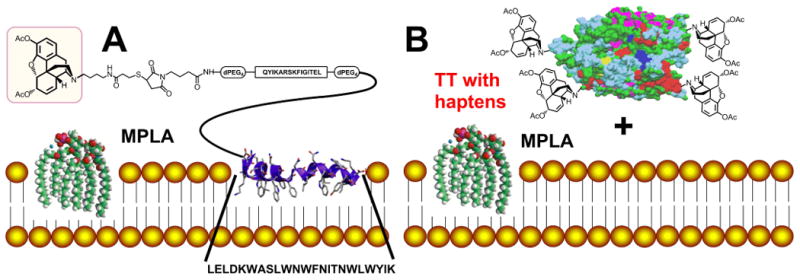
Schematic illustrations of two hapten/adjuvant formulations used for immunization of mice to induce antibodies to heroin hapten. (A) L(MPLA) having surface-attached 23 amino acid MPER peptide to which the T helper peptide and heroin hapten were coupled. (B) Heroin hapten was coupled to TT and the coupled TT-hapten was mixed with L(MPLA) that served as an adjuvant. The entire TT was used for coupling with heroin hapten analogs, but for simplicity the graphic illustrates is the 2.3 Å X-ray crystal structure of tetanus neurotoxin light chain, obtained from the RCSB Protein Data Bank (PDB ID: 1Z7H) [35].
Comparisons of titers obtained with various haptens, carriers, and adjuvants in other studies in the literature are complicated by the widespread use of “mid-point” titers (i.e., serum required for 50% of maximum absorbance in ELISA) [22, 36-38]. With mid-point titers it is difficult to differentiate whether the plateau of absorbance in the ELISA is due to a plateau of substrate in the assay as opposed to an immunological plateau. Although titers obtained in mice and rats might differ because of species differences, in one study examination of the IgG titration curve of serum from rats 10 days after being immunized 4 times with a morphine/heroin hapten coupled to keyhole limpet hemocyanin with complete and incomplete Freund's adjuvant appeared to suggest an end point titer of approximately 100,000 [39]. Clearly, the down-selection of a hapten that would be most suitable for further testing for a candidate human vaccine to heroin would be dependent both on the titer and specificity of the induced antibodies based on affinity to heroin and its degradation products, and detailed studies of affinities are underway. We conclude from these results that liposomal MPLA can induce potent immune responses to heroin haptens, and liposomal MPLA might serve as a useful adjuvant system for inducing antibodies for a candidate opiate vaccine.
Interestingly, both HerHap and OMAHap blocked the induction of antibodies to the MPER peptide carrier, a highly immunogenic antigen when attached to liposomes containing MPLA. This observation was unexpected because according to immunological theory both the hapten itself and the carrier of the hapten should be immunogenic [40]. It is possible that the conformation of the MPER peptide, sandwiched between the conjugated hapten and the outer surface of the liposomal membrane to which the MPER was attached precluded the appropriate display of the MPER as an antigen. Alternatively, some sort of immunological competition between the hapten and the MPER peptide might have occurred. Immunization of mice with each of the four hapten-TT conjugates using the second immunization strategy (Fig. 6B) also resulted in high titers (400,000) against the TT itself that were markedly less than the observed titer (6,500,000) after immunization of unconjugated TT was mixed with liposomes containing MPLA. Thus, it appears that competition or inhibition by the haptens and TT, or steric hindrance by numerous conjugated hapten molecules on the outer surface of TT (as schematically indicated in Fig. 6B), may have reduced the immune response to the TT carrier. Thus, in contrast to the results with MPER-carrier, hapten-TT conjugates induce antibodies, albeit with reduced titers, against the TT carrier.
Highlights.
Liposomes with monophosphoryl lipid A were formulated as adjuvants for haptens
Heroin analog haptens were conjugated either to the liposomes or to tetanus toxoid
Enhanced antibody titers were induced to liposome-conjugated haptens
Enhanced antibody titers were also induced to haptens conjugated to the toxoid
Liposomal monophosphoryl lipid A could strongly adjuvant an anti-heroin vaccine
Acknowledgments
This work was supported through a Cooperative Agreement Award (no.W81XWH-07-2-067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine and the U.S. Army Medical Research and Materiel Command (MRMC). The work was partially supported by an interagency agreement (Y1-DA0121-01 to CRA) between MRMC and the National Institute on Drug Abuse. The work of KC, FL, MRI, AEJ, and KCR was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism, NIH, DHHS. KJ was supported in part by NIH grant no. DA 026625. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition. The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of the Army, Department of Defense, or the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dey AK, Srivastava IK. Novel adjuvants and delivery systems for enhancing immune responses induced by immunogens. Expert Rev Vaccines. 2011;10:227–51. doi: 10.1586/erv.10.142. [DOI] [PubMed] [Google Scholar]
- 2.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24(3):310–5. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;16(13):2256–72. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alving CR, Rao M, Steers NJ, Matyas GR, Mayorov AV. Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev Vaccines. 2012;11(6):733–744. doi: 10.1586/erv.12.35. [DOI] [PubMed] [Google Scholar]
- 5.Alving CR, Koulchin V, Glenn GM, Rao M. Liposomes as carriers of peptide antigens: induction of antibodies and cytotoxic T lymphocytes to conjugated and unconjugated peptides. Immunol Rev. 1995;145:5–31. doi: 10.1111/j.1600-065x.1995.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 6.Nicolau C, Greferath R, Balaban TS, Lazarte JE, Hopkins RJ. A liposome-based therapeutic vaccine against beta -amyloid plaques on the pancreas of transgenic NORBA mice. Proc Natl Acad Sci USA. 2002;99(4):2332–7. doi: 10.1073/pnas.022627199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickman DT, López-Deber MP, Ndao DM, Silva AB, Nand D, Pihlgren M, et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J Biol Chem. 2011;286(16):13966–76. doi: 10.1074/jbc.M110.186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landsteiner K. Revised ed. New York: Dover; 1945. The specificity of serological reactions. [Google Scholar]
- 9.Butler VP., Jr The immunological assay of drugs. Pharmacol Rev. 1977;29(2):103–84. [PubMed] [Google Scholar]
- 10.Zhang H, Wang S, Fang G. Applications and recent developments of multi-analyte simultaneous analysis by enzyme-linked immunosorbent assays. J Immunol Methods. 2011;368(1-2):1–23. doi: 10.1016/j.jim.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Kosten T, Owens SM. Immunotherapy for the treatment of drug abuse. Pharmacol Ther. 2005;108(1):76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Kinsey BM, Jackson DC, Orson FM. Anti-drug vaccines to treat substance abuse. Immunol Cell Biol. 2009;87(4):309–14. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- 13.Janda KD, Treweek JB. Vaccines targeting drugs of abuse: is the glass half-empty or half-full? Nat Rev Immunol. 2011;12(1):67–72. doi: 10.1038/nri3130. [DOI] [PubMed] [Google Scholar]
- 14.Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther. 2012;91(1):60–70. doi: 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uemura K, Nicolotti RA, Six HR, Kinsky SC. Antibody formation in response to liposomal model membranes sensitized with N-substituted phosphatidylethanolamine derivatives. Biochemistry. 1974;13(8):1572–8. doi: 10.1021/bi00705a003. [DOI] [PubMed] [Google Scholar]
- 16.Spector S, Berkowitz B, Flynn EJ, Peskar B. Antibodies to morphine, barbiturates, and serotonin. Pharmacol Rev. 1973;25(2):281–91. [PubMed] [Google Scholar]
- 17.Van Vunakis H, Wasserman E, Levine L. Specificities of antibodies to morphine. J Pharmacol Exp Ther. 1972;180(2):514–21. [PubMed] [Google Scholar]
- 18.Wainer BH, Fitch FW, Fried J, Rothberg RM. Immunochemical studies of opioids: specificities of antibodies against codeine and hydromorphone. Clin Immunol Immunopathol. 1974;3(2):155–70. doi: 10.1016/0090-1229(74)90001-4. [DOI] [PubMed] [Google Scholar]
- 19.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature. 1974;252(5485):708–10. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 20.Usagawa T, Itoh Y, Hifumi E, Takeyasu A, Nakahara Y, Uda T. Characterization of morphine-specific monoclonal antibodies showing minimal cross-reactivity with codeine. J Immunol Methods. 1993;157(1-2):143–148. doi: 10.1016/0022-1759(93)90080-q. [DOI] [PubMed] [Google Scholar]
- 21.Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24(16):3232–40. doi: 10.1016/j.vaccine.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, et al. A vaccine strategy that induces protective immunity against heroin. J Med Chem. 2011;54(14):5195–204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hem SL, Johnston CT, HogenEsch H. Imject Alum is not aluminum hydroxide adjuvant or aluminum phosphate adjuvant. Vaccine. 2007;25(27):4985–6. doi: 10.1016/j.vaccine.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 24.Farhangi A, Akbarzadeh A, Mehrabi MR, Chiani M, Saffari Z, Ghassemi S, et al. Safety of human therapeutic morphine vaccine employing Lohmann specific pathogen free eggs. Pak J Biol Sci. 2010;13(21):1047–51. doi: 10.3923/pjbs.2010.1047.1051. [DOI] [PubMed] [Google Scholar]
- 25.Richards RL, Alving CR, Wassef NM. Liposomal subunit vaccines: effects of lipid A and aluminum hydroxide on immunogenicity. J Pharm Sci. 1996;85(12):1286–9. doi: 10.1021/js9601593. [DOI] [PubMed] [Google Scholar]
- 26.Rao M, Peachman KK, Li Q, Matyas GR, Shivachandra SB, Borschel R, et al. Highly effective generic adjuvant systems for orphan or poverty-related vaccines. Vaccine. 2011;29(5):873–7. doi: 10.1016/j.vaccine.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan WC, White PD, editors. Fmoc Solid Phase Peptide Synthesis: A Practical Approach. New York: Oxford University Press; 2000. [Google Scholar]
- 28.König W, Geiger R. Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexylcarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen. Chemische Berichte. 1970;103:788–98. doi: 10.1002/cber.19701030319. [DOI] [PubMed] [Google Scholar]
- 29.Sole NA, Barany G. Optimization of solid-phase synthesis of [Ala8]-dynorphin A. J Org Chem. 1992;57:5399–403. [Google Scholar]
- 30.Matyas GR, Muderhwa JM, Alving CR. Oil-in water liposomal emulsions for vaccine delivery. Methods Enzymol. 2003;373:34–50. doi: 10.1016/S0076-6879(03)73003-1. [DOI] [PubMed] [Google Scholar]
- 31.Matyas GR, Wieczoreck L, Beck Z, Ochsenbauer-Jambor C, Kappes JC, Michael N, et al. Neutralizing antibodies induced by liposomal HIV-1 gp41 peptide simultaneously bind to both the 2F5 or 4E10 epitope and lipid epitopes. AIDS. 2009;23:2069–77. doi: 10.1097/QAD.0b013e32832faea5. [DOI] [PubMed] [Google Scholar]
- 32.Swartz GM, Jr, Gentry Mk, Amende LM, Blanchette-Mackie EJ, Alving CR. Antibodies to cholesterol. Proc Natl Acad Sci USA. 1988;85(6):1902–1906. doi: 10.1073/pnas.85.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alving CR, Swartz GM., Jr Antibodies to cholesterol, cholesterol conjugates and liposomes: implications for atherosclerosis and autoimmunity. Crit Rev Immunol. 1991;10(5):441–53. [PubMed] [Google Scholar]
- 34.Watson DS, Szoka FC., Jr Role of lipid structure in the humoral immune response in mice to covalent lipid-peptides from the membrane proximal region of HIV-1 gp41. Vaccine. 2009;27(34):4672–83. doi: 10.1016/j.vaccine.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breidenbach MA, Brunger AT. 2.3 Å Crystal Structure of Tetanus Neurotoxin Light Chain. Biochemistry. 2005;44:7450–7. doi: 10.1021/bi050262j. [DOI] [PubMed] [Google Scholar]
- 36.Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, et al. Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin. 2009;5(4):214–29. doi: 10.4161/hv.5.4.7556. [DOI] [PubMed] [Google Scholar]
- 37.Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci USA. 2001;98(4):1988–92. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 2006;184(3-4):409–16. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- 39.Li QQ, Luo YX, Sun CY, Xue YX, Zhu WL, Shi HS, et al. A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J Neurochem. 2011;119(6):1271–81. doi: 10.1111/j.1471-4159.2011.07502.x. [DOI] [PubMed] [Google Scholar]
- 40.Berzofsky JA, Berkower IJ. Immunogenicity and antigen structure. In: Paul WE, editor. Fundamental Immunology. 6th. Philadelphia: Lippincott-Raven; 2008. pp. 631–83. [Google Scholar]


