Abstract
The lateral superior olive (LSO), a nucleus involved in sound localization, receives tonotopically organized, inhibitory input from the medial nucleus of the trapezoid body (MNTB). To better understand the development of this glycinergic/GABAergic pathway, we used Gramicidin-perforated patch clamp recordings to characterize MNTB-evoked postsynaptic potentials in LSO neurons of neonatal C57Bl/6J mice. We found that during the first postnatal week, MNTB-evoked responses change from being depolarizing to being hyperpolarizing. Most interestingly, depolarizing glycinergic/GABAergic synaptic potentials were able to trigger action potentials, demonstrating that the MNTB-LSO pathway can act as a true excitatory pathway. This transient excitatory action of immature MNTB-LSO synapses might play an important role in activity-dependent sharpening of the tonotopic organization of inhibitory connections in the LSO.
Keywords: Brainstem slice, Auditory system, Development, Sound localization, Chloride
The lateral superior olive (LSO) is the first binaural nucleus in the ascending auditory pathway of mammals. Neurons in the LSO encode interaural intensity differences and thus play an important role in localizing sound in space (for a review see [1]). Information from the contralateral ear reaches the LSO via a highly tonotopically organized, inhibitory pathway that arises in the medial nucleus of the trapezoid body (MNTB). This MNTB-LSO pathway uses the neurotransmitter glycine and, in neonatal animals, also uses GABA [12]. During early postnatal development, the spatial connectivity pattern of this inhibitory MNTB-LSO pathway is sharpened by activity-dependent mechanisms resulting in the precise tonotopic organization seen in adult animals (for review see [15,16]). During the first postnatal week, glycinergic/GABAergic MNTB-LSO synapses are not hyperpolarizing as in adult animals, but are depolarizing due to a high internal chloride concentration of LSO neurons [7,11]. It has been suggested that this depolarization renders the MNTB pathway excitatory and that the excitatory nature is part of the mechanism by which activity in the MNTB-LSO pathways exerts its effect on neuronal growth, survival, and synaptic organization in the LSO [16]. However, evidence that immature MNTB-LSO synapses are indeed excitatory, i.e. that they elicit action potentials (APs) in LSO neurons, is still missing.
In the present study, we examined whether and at what age the MNTB-LSO pathway is depolarizing and whether it can elicit action potentials in LSO neurons of mice. While the development of the MNTB-LSO pathway has been intensively studied in rats and gerbils [16], very little is known about its development in mice. However, as the mouse has become a favored species for developmental studies due to its genetic possibilities, information about the immature MNTB-LSO pathway in mice has become important.
Experiments were performed in C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME). Experimental procedures were in accordance with NIH guidelines and were approved by the IACUC at the University of Pittsburgh. Coronal slices (250 µm thick) of the auditory brainstem were prepared from animals aged postnatal day (P) 1–P7 as previously described [10]. ACSF used in this study consisted of (in mM): NaCl 124, NaHCO3 26, Glucose 10, KCl 5, KH2PO4 1.25, MgSO4 1.3, CaCl2 2, Kynurenic acid 1, pH = 7.4 when aerated with 95% O2/5% CO2.
Recordings were performed in a submerged-type recording chamber in which slices were continuously superfused (1–4 ml/min) with oxygenated artificial cerebrospinal fluid at 23–26°C. Patch clamp electrodes had resistances of 5–8 MΩ. Two pipette solutions with different [Cl−] were used: Solution 1 had a total [Cl−] of 14 mM and consisted of (in mM) 100 K-Gluconate, 10 KCl, 10 HEPES, 11 EGTA, 1 MgCl2, 1 CaCl2, 1.5 MgATP, 0.3 Na2 GTP, pH 7.2 with KOH. Solution 2 had a total [Cl−] of 132 mM and consisted of 130 KCl, 10 HEPES, 5 EGTA, 1 MgCl2, 2 MgATP, 0.3 Na2 GTP, pH 7.2 with KOH. All chemicals and drugs were purchased from Sigma (St. Louis, MO). Junction potentials of these solutions were 7 and 1 mV, respectively, and potentials were corrected for these values. Most recordings were performed in the Gramicidin-perforated patch clamp configuration (40–50 µg/ml), a technique with leaves the native intracellular chloride concentration intact [13]. Synaptic reversal potentials in whole cell mode were determined 5–10 min after establishing whole cell access. Access resistance (≤40 MΩ) was compensated using the bridge circuitry of the recording amplifier (BVC-700, Dagan Corp., Minneapolis, MN). The ipsilateral MNTB was stimulated every 5–15 s with single electrical shocks (0.2 ms duration, 15–200 µA) delivered via a bipolar electrode (tip distance 100–200 µm). Data were digitized at a rate of 5 kHz and analyzed using custom-written software (P.H.M.K.) running in the LabVIEW environment (National Instruments, Austin, TX). The reversal potential of synaptic responses was determined by fitting a linear regression line to the membrane potential–response amplitude plot; the zero response amplitude point of the fitted line was taken as the synaptic reversal potential. Spike threshold was determined by injecting positive current pulses and was measured as the absolute membrane potential at the onset of an action potential. Throughout the text average values are expressed as mean±S.E.M.
Electrical stimulation of the MNTB in the presence of the glutamate antagonist Kynurenic acid (1 mM) consistently elicited short-latency postsynaptic potentials (PSPs) in LSO neurons. These PSPs had onset latencies of 5.5±0.8 ms (n = 6) in P1–P2 mice and 5.0±0.5 ms (n = 13) in P4–P7 mice. These values are comparable to those reported for newborn rats [11] and gerbils [15] and about 3 ms longer than the latencies reported for 3-week-old mice [17]. In slices prepared from P1 and P2 mice MNTB-evoked PSPs (recorded in perforated-patch mode) were always depolarizing from the resting membrane potential (n = 8, Vrest −51.8 ± 1.4 mV) (Fig. 1). In contrast, in P4–P7 animals, MNTB-evoked PSPs were always hyperpolarizing (n = 15, Vrest −55.4 ± 0.9 mV). Thus, similar to rats [9], the glycinergic/GABAergic MNTB input to the LSO in mice is depolarizing during early postnatal development.
Fig. 1.
MNTB-evoked PSPs in neonatal LSO neurons of C57Bl/6J mice. A: Example of MNTB-evoked PSPs recorded at the cells’ resting potentials. In the 1-day-old animal (P1), the PSP is depolarizing while at P6 it is hyperpolarizing. Arrows point to stimulus artifact. B: MNTB-evoked PSPs in a P2 neuron at different membrane potentials. With perforated-patch recordings (upper row) the synaptic reversal potential is −34 mV (closed arrowhead in PSP amplitude-membrane potential plot), well above the resting potential of −57 mV (open arrowhead). After gaining whole-cell access with 14 mM Cl− pipette solution (lower row), the synaptic reversal potential shifts to −50 mV. C: MNTB-evoked PSPs in a P7 neuron. In perforated-patch mode the synaptic reversal potential is −74 mV, below the resting potential of −54 mV. Changing to whole-cell mode with 132 mM Cl− pipette solution changes the reversal potential to −6 mV. Dashed vertical lines in the voltage traces indicate time of MNTB-stimulation.
Glycinergic depolarizations in LSO neurons of rats are due to an increased intracellular chloride concentration ([Cl−]i) [6,9]. To test whether this mechanism also underlies the depolarizing nature of synaptic glycinergic/GABAergic responses in mice, we examined the effect of altered [Cl−]i on PSP polarity and amplitudes by switching from perforated-patch mode to whole-cell mode. Results from such experiments are illustrated in Fig. 1 for a P2 neuron and a P7 neuron. In the P2 neuron, MNTB-evoked PSPs in perforated-patch mode are depolarizing at the neuron’s resting membrane potential (−57 mV). At more depolarized membrane potentials, the amplitude of these PSPs decreases until the neuron is depolarized above the reversal potential (Erev) of −34 mV and PSPs become hyperpolarizing. Switching to whole-cell mode and dialyzing the neuron with 14 mM Cl− shifts Erev to −50 mV, a value close to the theoretical chloride equilibrium potential of −58 mV as determined by the Nernst equation for this chloride concentration. This result indicates that Erev of MNTB-evoked PSPs is primarily determined by the internal chloride concentration and, thus, that depolarizing PSPs have been generated by an efflux of chloride through glycine and/or GABA gated channels. In the P7 neuron, MNTB-evoked PSPs are hyperpolarizing at the resting membrane potential and they have an Erev of −74 mV. Switching the recording configuration to whole cell mode and dialyzing with 132 mM Cl− shifts Erev to −6 mV, a value close to the theoretical value of 0 mV for this chloride concentration. Similar results as those in Fig. 1 were obtained in four more LSO neurons (one P2 and three >P4). Together, these results indicate that both depolarizing MNTB-evoked PSPs at P1–P2 and hyperpolarizing PSPs at P4–P7 are mediated primarily by chloride. A small positive deviation from the calculated Erev was observed in whole-cell recordings in P1–P2 animals (Erev measured: −49.5 ± 0.9 mV (n = 8); Erev calculated: of −58 mV) which could be due to a contribution of bicarbonate [2] or a higher internal Cl− concentration in proximal dendrites caused by incomplete cell dialysis or transmembrane Cl− accumulation [4].
In order to examine the developmental changes of MNTB-evoked PSPs, Erev’s were determined in 31 neurons from P1 to P7 mice. As illustrated in Fig. 2, Erev gradually shifted to more negative values during the first postnatal week (P<0.001, One way ANOVA; −28.3 ± 4.7 mV at P1, n = 3, −71.0 ± 1.2 mV at P7, n = 4). No significant change was observed for the resting membrane potential over the same period (P = 0.268, One way ANOVA; P1: 50.3 ± 2 mV, P7: 55.5 ± 2 mV). These data indicate that MNTB-evoked PSPs are primarily depolarizing up to P3 and hyperpolarizing thereafter. Thus, compared to rats [11] or gerbils [12] MNTB-evoked PSPs in mice become hyperpolarizing 5–7 days earlier, consistent with the somewhat faster maturation of the mouse auditory system [5].
Fig. 2.
Developmental profile of synaptic reversal potentials and membrane resting potentials in mouse LSO neurons as determined by Gramicidin-perforated patch recordings. Synaptic reversal potentials (closed diamonds) become more negative during the first postnatal week while membrane resting potentials (open triangles) do not change. Lines connect measurements from the same neuron.
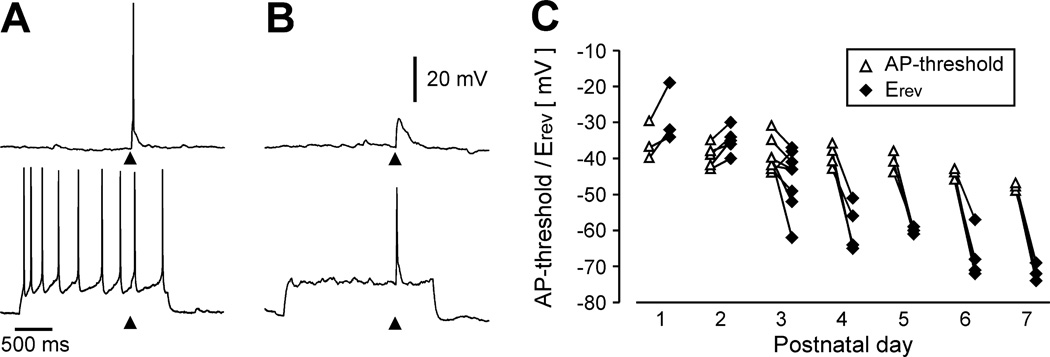
Recently, it has been demonstrated that glycine can elicit action potentials (APs) in immature rat LSO neurons [7] suggesting that glycine can act as an excitatory neurotransmitter in the LSO. However, in these studies glycine was applied iontophoretically to the cell body, a somewhat artificial situation because it activates both synaptic, as well as extrasynaptic, glycine receptors. We therefore tested whether APs also occur with more physiological stimuli, i.e. with synaptic stimulation. In these experiments MNTB-axons were stimulated while recording LSO neurons in perforated patch clamp mode at their resting membrane potential. Under these conditions, MNTB-evoked APs were observed in three out of nine P1–P3 neurons, all of which had depolarizing PSPs (Fig. 3A). In addition, in two neurons APs could be elicited by pairing MNTB-stimulation with membrane depolarization (Fig. 3B). Although MNTB-evoked APs were not encountered in all P1–P3 neurons, AP-thresholds were lower than the Erev of MNTB-evoked PSPs in eight out of nine P1–P3 neurons (Fig. 3C) suggesting that larger PSPs (elicited by higher stimulation intensities or by temporal integration of synaptic potentials) could generate APs in these neurons. Taken together, these results provide strong evidence that glycinergic/GABAergic MNTB-LSO synapses in neonatal mice are not only depolarizing but also can act as true excitatory synapses in the sense that they are able to elicit postsynaptic APs either per se or in cooperation with other depolarizing inputs.
Fig. 3.
During the first postnatal days, MNTB-evoked PSPs can elicit action potentials in LSO neurons. A: Examples of an MNTB-elicited action potential in a P2 neuron at resting membrane potential (−57 mV, upper trace) and when depolarized by current injection. At the depolarized membrane potential synaptic stimulation elicits an additional spike interspersed amount depolarization-induced spikes. B: Example of a P1 neuron in which pairing of synaptic stimulation with depolarization is necessary to generate APs. Upper trace shows subthreshold response at resting membrane potential (−47 mV) lower trace shows suprathreshold response when the neuron is depolarized to about −32 mV. Triangles in A and B indicate electrical stimulation. C: Developmental profile of AP-threshold and synaptic reversal potential. Lines connect measurements from the same neurons. Both reversal potentials and AP-thresholds gradually become more negative over the first postnatal week (reversal potential: P<0.001, One way ANOVA; AP-threshold: P = 0.002, One way ANOVA). Up to P2, the synaptic reversal potential is always more positive than the AP-threshold in the same neuron.
The present study provides the first demonstration that glycinergic/GABAergic synapses can elicit APs in auditory neurons. The excitatory effect of depolarizing glycinergic/GABAergic synapses in the immature LSO is in contrast to the inhibitory effect of other depolarizing GABAergic synapses previously described in the auditory system. In the avian cochlear nucleus GABAergic synapses are depolarizing but they act in an inhibitory fashion due to shunting of glutamatergic potentials [8]. In the inferior colliculus of neonatal gerbils, GABA and glycine are depolarizing and even increase intracellular calcium concentration, yet glycinergic and GABAergic synapses decrease spike activity and glutamate-evoked calcium responses [14]. The excitatory effect of the MNTB-LSO pathway, however, is similar to what has been described for GABAergic synapses in the developing hippocampus and neocortex [3]. In these systems, it has been suggested that GABA-elicited spike activity plays an important role in synapse formation and in guiding the organization of neuronal circuits. The excitatory nature of the immature MNTB-LSO pathway could serve a similar role in the establishment of LSO circuits. The fact that MNTB-LSO synapses are excitatory when neuronal circuits in the LSO undergo activity-dependent reorganization supports this idea.
Acknowledgements
This work was supported by the NIDCD (DC 04199) and the Alfred P. Sloan foundation.
References
- 1.Altschuler RA, Bobbin RP, Clopton BM, Hoffman DW. Neurobiology of Hearing: The Central Auditory System. New York: Raven Press; 1991. p. 491. [Google Scholar]
- 2.Backus KH, Deitmer JW, Friauf E. Glycine-activated currents are changed by coincident membrane depolarization in developing rat auditory brainstem neurones. J. Physiol. 1998;507(Pt 3):783–794. doi: 10.1111/j.1469-7793.1998.783bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 4.DeFazio RA, Keros S, Quick MW, Hablitz JJ. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J. Neurosci. 2000;20:8069–8076. doi: 10.1523/JNEUROSCI.20-21-08069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J. Am. Audiol. Soc. 1976;1:179–184. [PubMed] [Google Scholar]
- 6.Ehrlich I, Ilic V, Lohmann C, Friauf E. Development of glycinergic transmission in organotypic cultures from auditory brain stem. Neuroreport. 1998;9:2785–2790. doi: 10.1097/00001756-199808240-00019. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich I, Lohrke S, Friauf E. Shift from depolarizing to hyperpolarizing glycine action in rat auditory neurones is due to age-dependent Cl− regulation. J. Physiol. 1999;520(Pt 1):121–137. doi: 10.1111/j.1469-7793.1999.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyson RL, Reyes AD, Rubel EW. A depolarizing inhibitory response to GABA in brainstem auditory neurons of the chick. Brain Res. 1995;677:117–126. doi: 10.1016/0006-8993(95)00130-i. [DOI] [PubMed] [Google Scholar]
- 9.Kakazu Y, Akaike N, Komiyama S, Nabekura J. Regulation of intracellular chloride by cotransporters in developing lateral superior olive neurons. J. Neurosci. 1999;19:2843–2851. doi: 10.1523/JNEUROSCI.19-08-02843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandler K, Friauf E. Development of electrical membrane properties and discharge characteristics of superior olivary complex neurons in fetal and postnatal rats. Eur. J. Neurosci. 1995;7:1773–1790. doi: 10.1111/j.1460-9568.1995.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 11.Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J. Neurosci. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J. Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J. Neurosci. Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- 14.Lo YJ, Rao SC, Sanes DH. Modulation of calcium by inhibitory systems in the developing auditory midbrain. Neuroscience. 1998;83:1075–1084. doi: 10.1016/s0306-4522(97)00410-7. [DOI] [PubMed] [Google Scholar]
- 15.Sanes DH. The development of synaptic function and integration in the central auditory system. J. Neurosci. 1993;13:2627–2637. doi: 10.1523/JNEUROSCI.13-06-02627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanes DH, Friauf E. Development and influence of inhibition in the lateral superior olivary nucleus. Hear. Res. 2000;147:46–58. doi: 10.1016/s0378-5955(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 17.Wu SH, Kelly JB. Synaptic pharmacology of the superior olivary complex studied in mouse brain slice. J. Neurosci. 1992;12:3084–3097. doi: 10.1523/JNEUROSCI.12-08-03084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]