Abstract
Mycosin protease-1 (MycP1) cleaves ESX secretion-associated protein B (EspB) that is a virulence factor of Mycobacterium tuberculosis, and accommodates an octapeptide, AVKAASLG, as a short peptide substrate. Because peptidoboronic acids are known inhibitors of serine proteases, the synthesis and binding of a boronic acid analog of the pentapeptide cleavage product, AVKAA, was studied using MycP1 variants from M. thermoresistible (MycP1mth), M. smegmatis (MycP1msm) and M. tuberculosis (MycP1mtu). We synthesized the boropentapeptide, HAlaValLysAlaAlaB(OH)2 (1) and the analogous pinanediol PD-protected HAlaValLysAlaAlaBO2(PD) (2) using an Fmoc/Boc peptide strategy. The pinanediol boropentapeptide 2 displayed IC50 values 121.6±25.3 μM for MycP1mth, 93.2±37.3 μM for MycP1msm and 37.9±5.2 μM for MycP1mtu. Such relatively strong binding creates a chance for crystalizing the complex with 2 and finding the structure of the unknown MycP1 catalytic site that would potentially facilitate the development of new anti-tuberculosis drugs.
Mycobacterium tuberculosis exerts a staggering human and economic toll: in 2012, an estimated 8.6 million people developed tuberculosis and 1.3 million died from the disease1. M. tuberculosis secretes several highly immunogenic proteins across the cell wall using the ESX-1 transport system2, and these virulence factors cause lung tissue inflammation and necrosis3. In vivo inhibition of MycP1 protease, in a mouse model of infection4, led to a lower mortality rate than in untreated animals. In addition, a M. tuberculosis strain with a mutation affecting the catalytic activity of MycP1 was less virulent than a wild type strain4. Inhibition of MycP1 protease, which is one of the components of the ESX-1 transport system, is an attractive target for drug development5-11
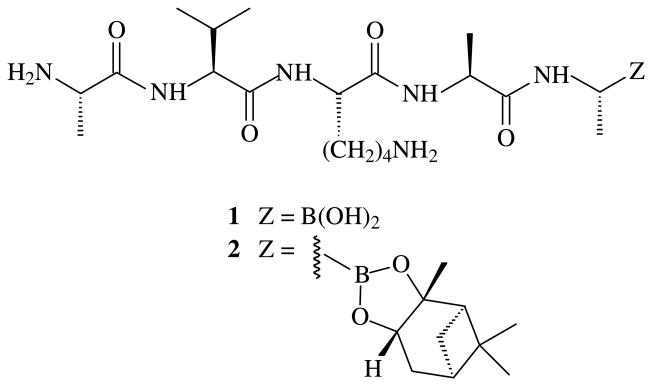
It was recently shown that M. smegmatis MycP111 and M. thermoresistibile MycP112 process M. tuberculosis EspB at positions Ala358 and Ala386. We confirmed that the octapeptide, (H)AVKAASLG(OH), mimicked the natural substrate in a fluorescent resonance energy transfer (FRET) experiment using an internally quenched peptide, (Abz)AVKAASLG(DNP) with an N-terminal, ortho-aminobenzoic acid (Abz) fluorescent group and a C-terminal, 2,4-dinitrophenyl (DNP) quencher12. Cleavage of this octapeptide substrate by MycP1 liberated the readily measured, fluorescent pentapeptide (Abz)AVKAA(OH), and this FRET system also provided a convenient means for screening potential MycP1 inhibitors13. Peptidyl boronic acids and their cyclic boronic esters with 1,2-diols are known inhibitors of various serine proteases14-17 in the nanomolar range, including peptidoboronic acids that show selectivity towards M. tuberculosis.18 The mechanism of action of peptidoboronate inhibitors involves the formation of tetrahedral complexes with active-site serines.19-21 Variability in the activity of structurally related peptidoboronic acids and peptidoboronates in the literature22 prompted us to determine if either the boronic acid analog 1 or the boronate 2 (Fig. 1) of the pentapeptide (H)AVKAA(OH) would inhibit the MycP1 protease and provide lead structures for the development of still other, clinically useful inhibitors.
Figure 1. Boronic acid analogs, HAlaValLysAlaAlaB(OH)2 (1) and the pinanediol PD-protected HAlaValLysAlaAlaBO2(PD) (2).
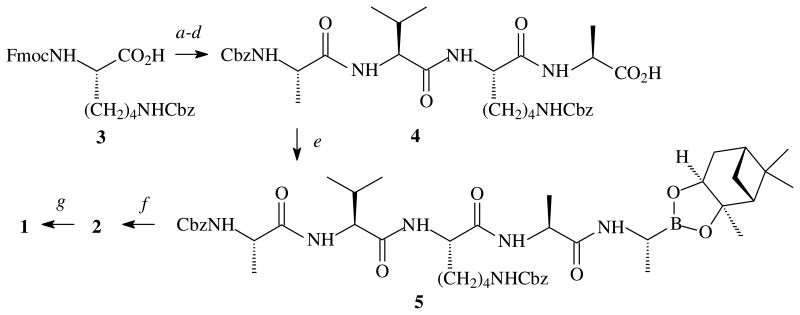
Solution-phase synthesis of HAlaValLysAlaAlaB(OH)2 (1) and the pinanediol PD-protected HAlaValLysAlaAlaBO2(PD) (2) involved the initial coupling of FmocLys(Cbz)OH (3) to HAlaOtBu to afford the dipeptide, FmocLys(Cbz)AlaOtBu, Fmoc-deprotection, and an additional coupling to (Cbz)AlaVal(OH) to provide the tetrapeptide intermediate, (Cbz)AlaValLys(Cbz)AlaOtBu (4) (Fig. 2). Acid-catalyzed removal of the tert-butoxy group furnished the Cbz-protected tetrapeptide, and HATU-promoted condensation23 with ((R)-boroalanine-(1S,2S,3R,5S)-(+)-2,3-pinanediol ester provided the protected pentapeptide 5, purified by a combination of flash silica gel and preparative layer silica gel chromatography. Hydrogenolysis24 afforded 2 and acid-catalyzed hydrolysis of 2 provided (H)AlaValLysAlaAlaB(OH)2 (1), albeit in low yield.
Figure 2. Synthesis of HAlaValLysAlaAlaB(OH)2 (1) and HAlaValLysAlaAlaBO2(PD) (2).
Legend : a, HAlaOtBu, hydroxybenzotriazole (HOBt), iPr2NEt, EDC-HCl, N,N-dimethylformamide (DMF), 0°C to 25°C, 24 h; b, piperidine, DMF, 25°C, 1 h; c, CbzAlaValOH, HOBt, iPr2NEt, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, DMF, 0° to 25°C, 24 h; d, CF3CO2H, CH2Cl2, 25°C, 24 h; e, ((R)-boroalanine-(1S,2S,3R,5S)-(+)-2,3-pinanediol ester hydrochloride, i-Pr2NEt, 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU), DMF, 0°C, 0.5 h; f, H2, 10% Pd/C, 25°C, 17 h; g, 2-methylpropylboronic acid, 3M HCl, 25°C, 17 h.
We have previously characterized the activity of MycP1 variants from M. thermoresistible (MycP1mth) and M. smegmatis (MycP1msm) using a quenched fluorescent peptide assay13. In addition to these variants, we also expressed and purified MycP1 from M. tuberculosis (MycP1mtu). We characterized the activity MycP1mtu and found significant differences in enzyme activity relative to other MycP1 homologs. In particular, the specific activity of MycP1mtu was 28.2±2.0 nmol/min/mg, which was four times higher than that of MycP1mth homolog (Table 1). This difference in enzyme activity was not surprising because the peptide substrate, (Abz)AVKAASLGK(DNP)OH was based on the cognate M. tuberculosis substrate EspBmtu residues 354-362 (AVKAASLG). This recognition region displayed sequence variations in M. thermoresistible EspBmth (i.e., SVKPAAGG) and in M. smegmatis EspBmsm (i.e., SLKPASAG), and consequently, the affinity determinants in the MycP1mth and MycP1msm binding sites differed from those of MycP1mtu. Nevertheless, all three species variants had measureable activity using the quenched fluorescent octapeptide as a substrate.
Table 1. Michaelis-Menten parameters for the three MycP1 variants.
| MycP1 variant | Km (μM) | Vmax (nmol/min/mg) |
|---|---|---|
| M. tuberculosis | 79±11 | 28.2±2.0 |
| M. thermoresistible | 60±12 | 6±0.6 |
| M. smegmatis | 86±42 | 1.8±0.4 |
The potency of the synthetic boronic acid analogs 1 and 2 was tested using the three MycP1 variants. Because compound 1 exhibited poorer inhibition than compound 2 in preliminary testing, we focused on the characterization of compound 2. As expected, all were inhibited to some extent, but the inhibitor showed relatively tighter binding to the cognate MycP1mtu enzyme (Fig. 3) than their binding to the two others. The concentrations necessary to achieve 50% inhibition of MycP1 (IC50 values) were as follows: MycP1mtu = 37.9±5.2 μM, MycP1mth = 121.6±25.3 μM, and MycP1msm = 93.2±33.7 μM. It has been reported that the inhibition of boronic acid peptides could vary over several orders of magnitude depending on the chemical structure of amino acid at critical positions of peptide18. While the inhibition of MycP1 variants by the boronic acid analog 2 is relatively moderate, it could be improved by using a combinatorial chemistry approach to analyze a library of peptides with variable sequence. For example, MycP1 displays a higher activity against a substrate peptide with Met in P1 position.12 therefore, boronic acid analogs with aliphatic side groups in this position could be explored in the future.
Figure 3. Determination of IC50 of HAlaValLysAlaAlaBO2(PD) (2) for MycP1 variants.
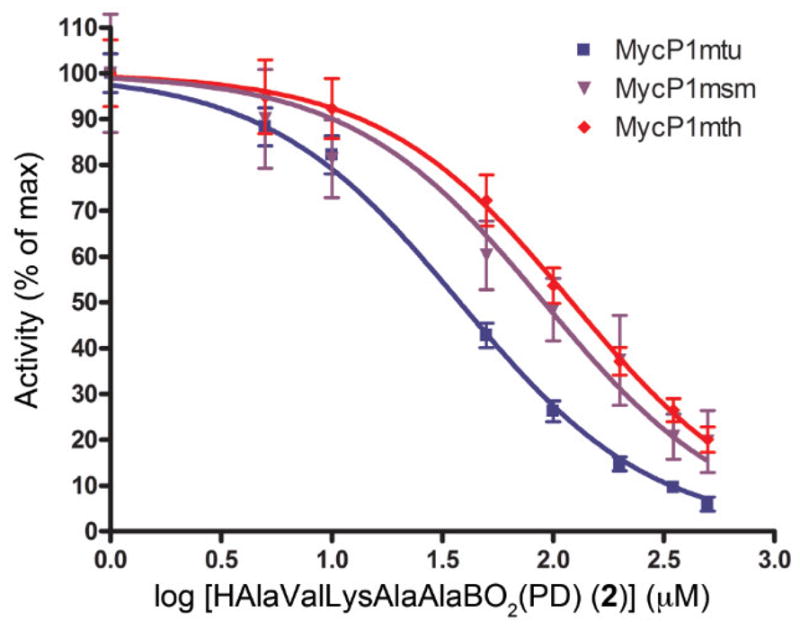
The IC50 of 2 was measured for MycP1 variants from M. tuberculosis (MycP1mtu), M. thermoresistible (MycP1mth), and M. smegmatis (MycP1msm), using a quenched fluorescent peptide (Abz)AVKAASLGK(DNP)OH). Activity of MycP1 is plotted as a function of the logarithm of the concentration of 2. Calculated IC50 values were: MycP1mtu = 37.9±5.2 μM, MycP1mth = 121.6±25.3 μM, and MycP1msm = 93.2±33.7 μM.
Supplementary Material
Acknowledgments
DSW was supported by the Office of the Dean of the College of Medicine and by NIH Grant Number P20 RR020171 from the National Institute of General Medical Sciences to L. Hersh, PI. KVK was supported by the NIH/NIGMS grant P20GM103486. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.World Health Organization. 2013. Global Tuberculosis Report. http://www.who.int/iris/handle/10665/91355.
- 2.Stenley SA, Raghavan S, Hwang WW, Cox JS. Proc Natl Acad Sci U S A. 2003;100:13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simeone R, Bottai D, Brosh R. Curr Opin Microbiol. 2009;12:4–10. doi: 10.1016/j.mib.2008.11.003. 2009. [DOI] [PubMed] [Google Scholar]
- 4.Ohol YM, Goetz DH, Chan K, Shiloh MU, Craik CS, Cox JS. Cell & Host Microbe. 2010;7:210–220. doi: 10.1016/j.chom.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feltcher ME, Sullivan JT, Braunstein M. Future Microbiol. 2010;5:1581–1597. doi: 10.2217/fmb.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villemagne B, Crauste C, Flipo M, Baulard AR, Deprez B, Willand N. Eur J Med Chem. 2012;51:1–16. doi: 10.1016/j.ejmech.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Lechartier B, Rybniker J, Zumla A, Cole ST. EMBO Mol Med. 2014;6:158–168. doi: 10.1002/emmm.201201772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JM, Pojer F, Blasco B, Cole ST. Drug Discov Today Dis Mech. 2010;7:e25–e31. [Google Scholar]
- 9.Roberts DM, Personne Y, Ollinger J, Parish T. Future Microbiol. 2013;8:621–631. doi: 10.2217/fmb.13.25. [DOI] [PubMed] [Google Scholar]
- 10.Bottai D, Serafini A, Cascioferro A, Brosch R, Manganelli R. Curr Pharm Des. 2013 doi: 10.2174/1381612819666131118170717. [DOI] [PubMed] [Google Scholar]
- 11.Solomonson M, Huesgen PF, Wasney GA, Watanabe N, Gruninger RJ, Prehna G, Overall CM, Strynadka NC. J Biol Chem. 2013;228:17782–17790. doi: 10.1074/jbc.M113.462036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner JM, Evans TJ, Chen J, Zhu H, Houben ENG, Bitter W, Korotkov KV. J Struct Biol. 2013;184:115–128. doi: 10.1016/j.jsb.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamza A, Wagner JM, Evans TJ, Frasinyuk MS, Kwiatkowski S, Zhan CG, Watt DS, Korotkov KV. J Chem Inf Model. 2014 doi: 10.1021/ci500025r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smoum R, Rubinstein A, Dembitsky VM, Srebnik M. Chem Rev. 2012;112:4156–4220. doi: 10.1021/cr608202m. [DOI] [PubMed] [Google Scholar]
- 15.LeBeau AM, Singh P, Isaacs JT, Denmeade SR. Chem & Biol. 2008;15:665–674. doi: 10.1016/j.chembiol.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams J, Kauffman M. Cancer Inves. 2004;22:304–311. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 17.Touchet S, Carreaux F, Carboni B, Buillon A, Boucher JL. Chem Soc Rev. 2011;40:3895–3914. doi: 10.1039/c0cs00154f. [DOI] [PubMed] [Google Scholar]
- 18.Lin G, Tsu Ch, Dick L, Zhou XiK, Nathan C. J Biol Chem. 2008;283:34423–34431. doi: 10.1074/jbc.M805324200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bone R, Shenvi AB, Kettner CA, Agard DA. Biochem. 1987;26:7609–7614. doi: 10.1021/bi00398a012. [DOI] [PubMed] [Google Scholar]
- 20.Bachovchin WW, Wong WY, Farr-Jones S, Shenvi AB, Kettner CA. Biochem. 1988;27:7689–7697. doi: 10.1021/bi00420a018. [DOI] [PubMed] [Google Scholar]
- 21.Knorr R, Trzeciak A, Banwarth W, Gillessen D. Tetrahedron Lett. 1989;30:1927–1930. [Google Scholar]
- 22.Gozhina OV, Svendsen JS, Lejon T. J Pept Sci. 2014;20:20–24. doi: 10.1002/psc.2583. [DOI] [PubMed] [Google Scholar]
- 23.Kinder DH, Ames MM. U S Patent. 1990;4:963–655. [Google Scholar]
- 24.Zhu Y, Zhu X, Wu G, Mo Y, Li Y, Zhao X, Yuan Y, Yang J, Yu S, Shao F, Li R, Ke Y, Lu A, Lin Z, Zhang L. J Med Chem. 2010;53:1990–1999. doi: 10.1021/jm901407s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.