Abstract
Background
In non-small cell lung cancer (NSCLC), the well-developed epidermal growth factor receptor (EGFR) is an important therapeutic target. EGFR activating gene mutations have been proved strongly predictive of response to EGFR-tyrosine kinase inhibitors (TKI) in NSCLC. However, both in daily clinical practice and clinical trials, patients with unknown EGFR gene status (UN-EGFR-GS) are very common. In this study, we assessed efficacy and tolerability of sequential treatment of first-line pemetrexed followed by icotinib in Chinese advanced lung adenocarcinoma with UN-EGFR-GS.
Patients and methods
We analyzed 38 patients with advanced lung adenocarcinoma with UN-EGFR-GS treated with first-line pemetrexed-based chemotherapy followed by icotinib as maintenance or second-line therapy.
Results
The response rates to pemetrexed and icotinib were 21.1% and 42.1%, respectively. The median overall survival was 27.0 months (95% CI, 19.7-34.2 months). The 12-month overall survival probability was 68.4%. The most common toxicities observed in icotinib phase were rashes, diarrheas, and elevated aminotransferase. Subgroup analysis indicated that the overall survival is correlated with response to icotinib.
Conclusions
The sequence of first-line pemetrexed-based chemotherapy followed by icotinib treatment is a promising option for advanced lung adenocarcinoma with UN-EGFR-GS in China.
Keywords: Non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR), tyrosine kinase inhibitor (TKI), chemotherapy
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers, which are the leading cause of cancer-related death worldwide (1). The most common histological subtype of NSCLC is adenocarcinoma. Most lung cancers are diagnosed at late stage, conferring a bad prognosis. Less than 5% of stage IV patients live longer than five years. Platinum plus a third-generation agents is the standard regimen for patients with advanced NSCLC (2). One of the third-generation agents, pemetrexed, has been demonstrated efficacy in advanced non-squamous NSCLC as first-line, second-line or maintenance therapy (3-6). A meta-analysis, including four randomized trials, compared the efficacy and toxicities of the doublets of pemetrexed and platinum versus other platinum regimen in advanced NSCLC in the first-line setting (7). The analysis concluded that pemetrexed/platinum combination appear to offer significant survival advantage and acceptable toxicities, especially for NSCLC of non-squamous histology (7).
However, in advanced NSCLC the therapeutic plateau has been reached with conventional chemotherapy (8). The treatment paradigm for NSCLC patients is changing with the improved understanding of the molecular signaling pathways. Some biomarkers of associated target therapies are established. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) was an important progress made towards treating NSCLC in last decade. Two individual studies have reported that EGFR gene activating mutation is strongly predictive of response to EGFR-TKIs in NSCLC (9,10). EGFR-TKIs are recommended for all lines of treating advanced NSCLC with EGFR activating mutations, but the role of EGFR-TKIs in EGFR wild-type NSCLC is still on debating (11).
In western countries, two reversible EGFR-TKIs are commercially available: gefitinib and erlotinib. A China company (Betta Pharmaceuticals Co., Ltd.) developed the third orally EGFR-TKI named icotinib hydrochloride (Conmana®) (12). The large, randomized, head-to-head, phase III clinical trial (ICOGEN) demonstrated that icotinib has comparable efficacy to gefitinib in Chinese pre-treated NSCLC (13). The most commonly observed side effects of icotinib were rash (41.0%) and diarrhea (22%), which was significantly less than gefitinib (13). The recommended dose for clinical treatment is 125 mg three times per day orally. Icotinib is becoming more widely used in clinical practice in China.
Either in daily clinical practice, or in clinical trials, patients with unknown EGFR gene status (UN-EGFR-GS) are very common to see (13-15). The optimal treatment for advanced NSCLC with UN-EGFR-GS is not established yet. In East Asian patients with lung adenocarcinoma, the incidence of EGFR activating mutations, about 40%, is much higher than in western population (14,16,17). Sequential first-line pemetrexed followed by icotinib seems to be a reasonable option for Chinese patients with UN-EGFR-GS advanced lung adenocarcinoma. Thus we conducted this retrospective study, aiming to assess the efficacy and tolerability of the treatment modality in selected patients.
Methods
Study design and treatment
The institutional ethics committee of the First Affiliated Hospital of Zhejiang University approved this study. Informed consent was obtained from each patient. We retrospectively analyzed the data of 38 patients with advanced lung adenocarcinoma and with UN-EGFR-GS between 2010 and 2012, who were treated with first-line pemetrexed-based chemotherapy, and subsequently treated with icotinib as second-line or maintenance therapy. All cases were histologically confirmed. The inclusion criteria were as follows: patients diagnosed with advanced lung adenocarcinoma; the EGFR mutation status was unknown; receive at least one cycle of pemetrexed-based chemotherapy; no more than six cycles of chemotherapy; switch to icotinib for the purpose of second-line or maintenance therapy; at least one measurable lesion according to RECIST criteria (18). Patients with known EGFR gene status (mutant or wild type) were excluded. We defined UN-EGFR-GS as: (I) no tumor sample was sent for detection of EGFR gene mutations; (II) tumor samples were sent for EGFR gene mutation test, but the results were not clear whether mutant or wild type.
The primary objective of this study is to assess overall survival (OS) and tolerability of treating advanced lung adenocarcinoma with the sequence. The OS was defined as the time of starting pemetrexed treatment to death or lost follow-up. The clinical characteristics, toxicity and survival status were collected through reviewing medical records, electronic preserved data, interviewing with patients or their family members. Pemetrexed was administered intravenously at the standard dose of 500 mg/m2 on day 1 of 21-day cycle. The treatment was scheduled up to six cycles unless intolerable toxicity or progressive disease (PD). Switching to icotinib treatment when progression documented, or investigators consider icotinib maintenance therapy for patients not progressing after at least four cycles of pemetrexed-based chemotherapy. Icotinib was administered orally at the standard dose of 125 mg thrice per day until PD or intolerable treatment-related toxicity. Dose reduction or interruption/delay was permitted in the two-phase treatment.
Clinical assessments
The clinical course of included patients and treatment were prospectively monitored. Side effects were graded according to Common Terminology Criteria for Adverse Events, Version 3.0. Tumor response was assessed with RECIST criteria (version 1.1) (18). Clinical follow-up including physical examination, complete blood count, chemistry were performed every 2-3 weeks. Computed tomography (CT) or magnetic resonance imaging (MRI) was performed every 2-3 cycles of pemetrexed, and four weeks after initiating icotinib therapy, then every two months.
Statistical analysis
Statistical analyses were conducted through IBM SPSS 20 for Mac OSX. The median OS were calculated by Kaplan-Meier method, accompanying by 95% CI. Differences among subgroups were tested using the log-rank test. P<0.05 was considered statistically significant.
Results
Patient characteristics
During 2010 and 2012, about 350 cases with advanced lung adenocarcinoma in our electronic medical record were registered. Seventy patients were clear with EGFR gene status. Thirty-eight patients were included in this study according to the inclusion criteria. The patient characteristics are listed in Table 1. The whole cohort patients included 23 females and 15 males. The median age was 58.6 years old (range, 40-75 y). Among the 38 patients, 27 were non-smokers and 11 were smokers. All the patients at least received one cycle of pemetrexed chemotherapy, and then were treated with icotinib. Ten cases were received pemetrexed combined with carboplatin, 22 combined with cisplatin, and 6 as single use. The icotinib use of 11 patients was for the purpose of maintenance therapy, 27 for second line. The average time of patients taking icotinib was 38.7 weeks (range, 3.7-130 weeks).
Table 1. Patients’ characteristics (n=38).
| Variables | Median (range)/frequency (%) |
|---|---|
| Age [years] | 58.6 [40-75] |
| Sex | |
| Male | 15 (39.5) |
| Female | 23 (60.5) |
| Smoking status | |
| Smoker | 11 (28.9) |
| Never-smoker | 27 (71.1) |
| Performance status | |
| 0-1 | 32 (84.2) |
| 2 | 6 (15.8) |
| First-line chemotherapy | |
| Pemetrexed plus cisplatin | 22 (57.9) |
| Pemetrexed plus carboplatin | 10 (26.3) |
| Pemetrexed single use | 6 (15.8) |
| Icotinib treatment | |
| Second line | 27 (71.1) |
| Maintenance | 11 (28.9) |
| Post-study treatment | |
| Paclitaxel | 12 (31.5) |
| Platinum | 10 (26.3) |
| Docetaxel | 9 (23.6) |
| Gemcitabine | 8 (21.0) |
| None | 6 (15.7) |
| Other EGFR-TKIs | 6 (15.7) |
EGFR-TKIs, epidermal growth factor receptor tyrosine kinase inhibitors.
Of the 38 patients, EGFR mutations failed to detect in 9 patients without enough specimens. Tumor sample were not available in 29 patients, who refused to repeat biopsy or send tumor specimen to screen EGFR mutations.
Objective response and toxicities
The mean cycles of pemetrexed given to patients were 3.8 (1-6 cycles). The response rate (RR) to pemetrexed was 21.1% (8/38), stable disease (SD) 34.2% (13/38), PD 44.7% (17/38). One patient stopped pemetrexed plus cisplatin chemotherapy because of grade 3 vomiting. The RR to icotinib was 42.1% (16/38), SD 28.9% (11/38), PD 28.9% (11/38). Table 2 shows the objective response to pemetrexed and icotinib. All grades of side effects observed in the pemetrexed phase included neutropenia (57.8%; 22/38), vomiting (50%; 19/38), nausea (50%; 19/38), anemia (44.7%; 17/38), thrombocytopenia (31.6%; 12/38), and rashes (7.8%; 3/38). Totally, we observed grade 3-4 toxicities in 32 of 146 cycles during the pemetrexed treatment phase. There was no grade 3-4 toxicities observed during the icotinib treatment phase. The most common grade 1-2 toxicities were rashes (36.8%; 14/38), diarrheas (31.5%; 12/38), elevated amino-transferase (13.1%; 5/38) and elevated BUN (7.8%; 3/38). There was no dose reduction or interruption caused by icotinib therapy. No interstitial lung diseases were observed in this study.
Table 2. Objective responses (n=38).
| Pemetrexed n (%) | Icotinib n (%) | |
|---|---|---|
| CR | 0 (0) | 0 (0) |
| PR | 8 (21.1) | 16 (42.2) |
| SD | 13 (34.2) | 11 (28.9) |
| PD | 17 (44.7) | 11 (28.9) |
| ORR (CR + PR) | 8 (21.1) | 16 (42.2) |
| DCR (CR + PR + SD) | 21 (55.3) | 27 (71.1) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate.
Overall survival
The median follow-up time was 28.0 months (7.3-42.4 months). At the end of follow-up, 31 patients died, and 7 patients were still alive. Six patients were still on icotinib treatment. The median OS was 27.0 months (95% CI, 19.7-34.2 months) (Figure 1). The 12-month OS probability was 68.4%. The OS was correlated with response to icotinib. The median OS in patients obtained PR was 32.0 months (95% CI, 15.9-48.0 months), however the median OS in patients with SD and PD, was 22.1 months (95% CI, 5.4-38.8 months) and 12.1 months (95% CI, 0-27.0 months), respectively, (P=0.02) (Figure 2). The median OS was numerically longer in the maintenance group than the second-line group, which was 38.0 months (95% CI, 15.5-60.4 months) and 27.0 months (95% CI, 20.1-33.8 months), respectively. However, the difference was not statistically significant (P=0.35). According to the combined drugs, the patients were divided into cisplatin group or non-cisplatin group. The median OS in cisplatin group was 14.5 months (95% CI, 2.5-26.5 months) and the non-cisplatin group was 29.1 months (95% CI, 25.6-32.5 months). The difference was not statistically significant (P=0.98). There was also no significant OS difference between subgroups stratified by sex, performance score, smoking status, and response to pemetrexed.
Figure 1.
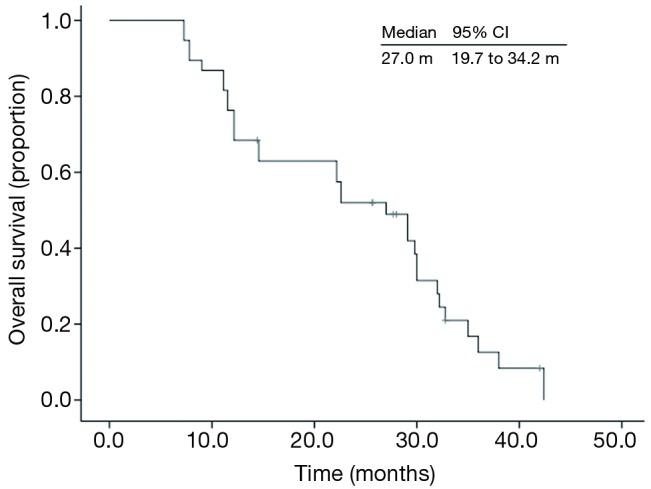
Kaplan-Meier overall survival curve of 38 advanced lung adenocarcinoma patients with unknown EGFR gene status. EGFR, epidermal growth factor receptor.
Figure 2.
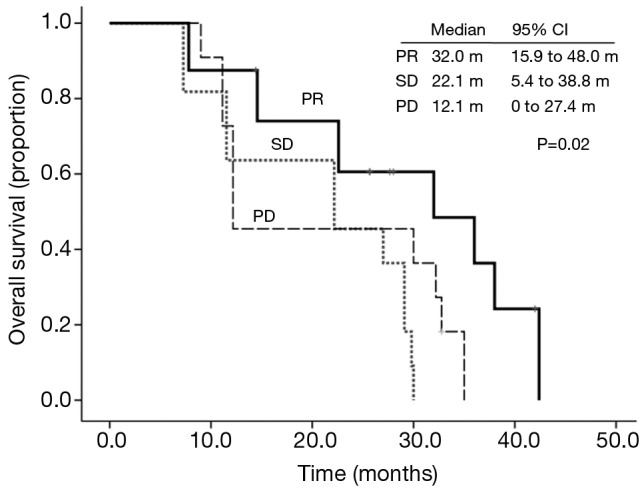
Kaplan-Meier overall survival curve stratified by response to icotinib.
Post-study treatment
Thirty-two patients documented PD from icotinib treatment. On disease progression, 6 patients received other EGFR-TKIs. Twelve patients received paclitaxel chemotherapy; 10 received platinum; 9 docetaxel; and 8 gemcitabine (see Table 1). Six patients received no further anti-cancer treatment.
Discussion
In the present study, we assessed the efficacy and tolerability of first-line pemetrexed followed by icotinib treatment in Chinese advanced lung adenocarcinoma with UN-EGFR-GS. To our best knowledge, this is the first study to discuss sequential pemetrexed and icotinib for selected NSCLC patients. The results showed that the sequential model is a promising treatment choice for advanced lung adenocarcinoma of Chinese patients.
According to EGFR gene status, NSCLC could be divided into three sub-categories: EGFR wild type, EGFR mutant type, and UN-EGFR-GS. Many randomized trials have compared EGFR-TKIs with chemotherapy for NSCLC with EGFR-sensitizing mutations in the first-line setting (14,19-22). EGFR-TKIs yield durable responses, prolonged progression free survival (PFS) and improved quality of life when compared to first-line chemotherapy. Additionally, the toxicities are much less than conventional chemotherapy. All the trials did not produce significant OS improvement, probably due to crossover. In patients with EGFR wild type, the first-line EGFR-TKI treatment seems to be unsuitable (11,14,23). Beyond first-line setting, the role of EGFR-TKIs in treating NSCLC with wild-type EGFR is still a controversy (11,15,24-26).
Therefore performing EGFR gene testing may help managing NSCLC patients. However, either in daily clinical practice, or in clinical trials, NSCLC patients with UN-EGFR-GS are not rare (13-15). For example, in a recent published study tumor samples were available for EGFR testing from only 67% of patients and could be analyzed from 63%; UN-EGFR-GS for 47% of patients (27,28). In TAILOR and IPASS study, 23% and 64.1% patients were ineligible for EGFR gene testing, respectively (14,15). In ICOGEN study, tissue samples were available for only 38% (152/395) patients; 134 samples were eligible for EGFR gene testing; 66% (261/395) patients were with UN-EGFR-GS (13). In clinical trials, UN-EGFR-GS was mainly due to lack of sufficient tissue. In addition, more other reasons might cause UN-EGFR-GS in real world practice including: high cost of testing; re-biopsy not acceptable for some patients; limited testing technology. Especially in developing countries, the prevalence of NSCLC with UN-EGFR-GS would be probably much higher. In our institute, during 2010 to 2012 the EGFR gene status was tested in only about 20% of advanced NSCLC patients.
Treatment for advanced NSCLC is no longer a one-size-fits-all model. However, the demand is still there for one-size-fits-all approach currently because of high prevalence of UN-EGFR-GS. Based on our study, it is feasible to develop one size to fit Chinese advanced lung adenocarcinoma with UN-EGFR-GS. Pemetrexed-based chemotherapy is an appropriate first-line treatment option for advanced lung adenocarcinoma. In this study, the RR to the first-line pemetrexed-based chemotherapy is comparable to that of other studies (4,7,29). Gefitinb has been proved efficacy for East Asian patients with advanced NSCLC either in the second-line setting or maintenance therapy (30,31). So it is reasonable to choose icotinib as second line treatment or maintenance therapy for Chinese advanced lung adenocarcinoma in our study. In present study, the disease control rate was up to 71.0%, and the toxicity was mild, which both are similar to ICOGEN trial (13). In East Asian, EGFR activating mutations present in about 40% of lung adenocarcinoma, which strong drive the benefit of EGFR-TKIs (16,17). In selected patients (east Asian, never-smoker or light smoker, adenocarcinoma), the EGFR mutation rate is up to 60% (14,32). Such sub-group would possibly benefit from EGFR-TKIs without detecting the EGFR gene status.
We observed no significant survival difference between subgroups of sex, age, performance status, smoking status, platinum, maintenance or second-line therapy of icotinib and response to pemetrexed. We consider two reasons might be included to interpret our result. First, our study included relative small number of cases. Second, the overall survival was confounded by subsequent treatment of icotinib. Interestingly, we did find a relationship between overall survival and response to icotinib. The PR group lived longer than SD and PD group. Tsujino and colleagues (33) found that response rate is associated with median survival in clinical trials with EGFR-TKIs, which is consistent with our findings.
Sequential treatment strategies have been attracted more interests in recent lung cancer research. Fiala and colleagues (25) assessed the efficacy of second-line pemetrexed followed by third-line erlotinib to treatment with the reverse sequence in advanced lung adenocarcinoma with wild-type EGFR gene. The result demonstrated about 2-fold longer PFS (3.6 vs. 7.8 months; P=0.029) and 3-fold longer OS (7.9 vs. 26.3 months; P=0.006) for patients treated with erlotinib followed by pemetrexed than the reverse sequence. Another similar designed study showed significantly longer OS for patients managed with second-line erlotinib followed by third-line pemetrexed (23.6 vs. 16.3 months; P=0.042) in Chinese advanced lung adenocarcinoma (34). These studies support the use of EGFR-TKIs in the second-line setting in advanced NSCLC with adenocarcinoma histology. In our study, sequential therapy with first-line pemetrexed followed by icotinib yielded 27.0 months of median OS, which is comparable to second-line erlotinib followed by third-line pemetrexed.
In the first-line treatment, we included pemetrexed or combined with cisplatin or carboplatin. The cisplatin group lived no longer than non-cisplatin group. However meta-analysis demonstrated a significant survival improvement when cisplatin was used for patients with non-squamous histology. We speculate that this result was mainly interfered by the subsequent icotinib treatment.
In our study, we included patients treated with icotinib as either second-line or maintenance therapy. We suggest a sequential treatment is to treat patients with one therapy after another, whatever it is with or without interruption. Clinical trials have confirmed that erlotinib or gefitnib may prolong PFS or OS regardless of the response to prior chemotherapy (30,35,36). The present study shows that the median OS is numerically longer in the maintenance group than the second-line group. Sequential icotinib maintenance therapy after pemetrexed in advanced lung adenocarcinoma probably is better than the second-line model, which need more investigations.
PFS is increasingly used as an important endpoint in clinical trials. However, we didn’t discuss PFS in this study because the frequency of evaluation was different between patients. We think the major limitations of this study are its retrospective nature and relative small number of patients included. Selection bias might present in this study.
Conclusions
In conclusion, sequential therapy of first-line pemetrexed followed by icotinib for patients with advanced lung adenocarcinoma with UN-EGFR-GS seems to be an appealing treatment option. The sequence yielded promising results with acceptable toxicity. The sequential model for selected patients deserves further investigation in the future.
Acknowledgements
Zhejiang Provincial Natural Science Fund (LY13H160007), and Zhejiang Medicines & Health Science and Technology Project (201348801) supported this research. The authors thank the patients and family members for their participation in this study.
Disclosure: The authors declare no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90 [DOI] [PubMed] [Google Scholar]
- 2.Fisher MD, D’Orazio A. Phase II and III trials: comparison of four chemotherapy regimens in advanced non small-cell lung cancer (ECOG 1594). Clin Lung Cancer 2000;2:21-2 [PubMed] [Google Scholar]
- 3.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97 [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51 [DOI] [PubMed] [Google Scholar]
- 5.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40 [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55 [DOI] [PubMed] [Google Scholar]
- 7.Li M, Zhang Q, Fu P, et al. Pemetrexed plus platinum as the first-line treatment option for advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. PLoS One 2012;7:e37229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gridelli C, Rossi A, Maione P.Treatment of non-small-cell lung cancer: state of the art and development of new biologic agents. Oncogene 2003;22:6629-38 [DOI] [PubMed] [Google Scholar]
- 9.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500 [DOI] [PubMed] [Google Scholar]
- 10.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39 [DOI] [PubMed] [Google Scholar]
- 11.Laurie SA, Goss GD. Role of epidermal growth factor receptor inhibitors in epidermal growth factor receptor wild-type non-small-cell lung cancer. J Clin Oncol 2013;31:1061-9 [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Fang W, Liu X, et al. New EGFR-TKI: a case report of recurrent lung adenocarcinoma successfully treated with icotinib. Tumori 2012;98:e102-4 [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953-61 [DOI] [PubMed] [Google Scholar]
- 14.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57 [DOI] [PubMed] [Google Scholar]
- 15.Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8 [DOI] [PubMed] [Google Scholar]
- 16.Liam CK, Wahid MI, Rajadurai P, et al. Epidermal growth factor receptor mutations in lung adenocarcinoma in Malaysian patients. J Thorac Oncol 2013;8:766-72 [DOI] [PubMed] [Google Scholar]
- 17.Choi YL, Sun JM, Cho J, et al. EGFR mutation testing in patients with advanced non-small cell lung cancer: a comprehensive evaluation of real-world practice in an East Asian tertiary hospital. PLoS One 2013;8:e56011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47 [DOI] [PubMed] [Google Scholar]
- 19.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8 [DOI] [PubMed] [Google Scholar]
- 20.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8 [DOI] [PubMed] [Google Scholar]
- 21.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42 [DOI] [PubMed] [Google Scholar]
- 22.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46 [DOI] [PubMed] [Google Scholar]
- 23.Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 2012;30:3002-11 [DOI] [PubMed] [Google Scholar]
- 24.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32 [DOI] [PubMed] [Google Scholar]
- 25.Fiala O, Pesek M, Finek J, et al. Sequential Treatment of Advanced-stage Lung Adenocarcinoma Harboring Wild-type EGFR Gene: Second-line Pemetrexed Followed by Third-line Erlotinib versus the Reverse Sequence. Anticancer Res 2013;33:3397-402 [PubMed] [Google Scholar]
- 26.Okano Y, Ando M, Asami K, et al. Randomized phase III trial of erlotinib (E) versus docetaxel (D) as second- or third-line therapy in patients with advanced non-small cell lung cancer (NSCLC) who have wild-type or mutant epidermal growth factor receptor (EGFR): Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2013;31:abstr 8006.
- 27.Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777-86 [DOI] [PubMed] [Google Scholar]
- 28.Hirsch FR, Gandara DR. FASTACT-2: but don’t act too fast. Lancet Oncol 2013;14:684-5 [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues-Pereira J, Kim JH, Magallanes M, et al. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:1907-14 [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75 [DOI] [PubMed] [Google Scholar]
- 31.Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer 2012;118:6234-42 [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsujino K, Kawaguchi T, Kubo A, et al. Response rate is associated with prolonged survival in patients with advanced non-small cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol 2009;4:994-1001 [DOI] [PubMed] [Google Scholar]
- 34.Hong T, Zhang R, Cai D, et al. Second-line epidermal growth factor receptor inhibitors followed by third-line pemetrexed or the reverse sequence: a retrospective analysis of 83 Chinese patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol 2012;138:285-91 [DOI] [PubMed] [Google Scholar]
- 35.Wu YL, Kim JH, Park K, et al. Efficacy and safety of maintenance erlotinib in Asian patients with advanced non-small-cell lung cancer: a subanalysis of the phase III, randomized SATURN study. Lung Cancer 2012;77:339-45 [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Liu Y, Røe OD, et al. Gefitinib or erlotinib as maintenance therapy in patients with advanced stage non-small cell lung cancer: a systematic review. PLoS One 2013;8:e59314. [DOI] [PMC free article] [PubMed] [Google Scholar]


