Abstract
Background
Although hypertension is associated with atrial fibrillation (AF), the impact of hypertension on the electromechanical properties and outcome of catheter ablation in AF patients is unclear.
Methods
AF patients [n=213, 136 paroxysmal AF (PAF) patients and 77 persistent AF patients] undergoing circumferential pulmonary vein (PV) isolation guided by CARTO mapping were enrolled, and then were divided into normotension group and hypertension group. Several left atrial (LA) electroanatomical parameters determined by the CARTO system were compared between groups.
Results
The LA bipolar voltage was lower in PAF patients with than without hypertension (1.44±1.09 vs. 1.92±0.76 mV, P=0.048); a significant difference was also observed in persistent AF patients. Hypertension significantly increased the size of the LA scar and low-voltage zones (LVZs) in both PAF and persistent AF patients. However, hypertension did not significantly affect recurrence in either PAF or persistent AF patients. The LA bipolar voltage was higher in PAF patients without recurrence than in those with recurrence (1.77±1.01 vs. 1.29±0.93 mV, P=0.048); a significant difference was also observed in persistent AF patients. PAF and persistent AF patients with AF recurrence had significantly larger LA scar and LVZs than patients without recurrence.
Conclusions
Hypertension has a significant impact on the LA electromechanical properties in AF patients, and the LA substrate has an important influence on the outcome of catheter ablation.
Keywords: Hypertension, voltage, atrial fibrillation (AF), pulmonary vein (PV), catheter ablation, radiofrequency (RF) current
Introduction
Atrial fibrillation (AF) is the most common tachyarrhythmia in clinical practice (1,2), and there is a significant increase in the prevalence of AF in patients with hypertension. Circumferential ablation of all four pulmonary veins (PVs) with conduction block between the PVs and left atrium has become the standard procedure to eliminate paroxysmal AF (PAF) (3). However, the patients in those studies always included younger subjects and often excluded very elderly subjects who have more underlying diseases such as hypertension. Hypertension plays an important role in AF genesis (1). Hypertension could lead to left atrial (LA) dilatation (4). An enlarged atrial size modulates the substrate for AF by increasing non-uniform anisotropy and conduction disturbances (5,6). A heterogeneous structure with different thicknesses of the cardiac chambers could play a crucial role in rotor dynamics, leading to wave splitting and stabilization of reentrant circuit (7). Hypertension could lead to fibrosis in the left atrium. Fibrosis plays an important role in the dynamics of AF, since local fibrotic areas could serve as anchors for reentrant circuits and alter wave-front propagation, causing fractionated electrograms, wave breaks, and conduction delays (8). These mechanisms may be relevant in explaining the nature of the arrhythmogenic substrates present in patients with hypertension.
The aim of this study was to investigate the impact of hypertension on the properties of atrial substrate and the outcome of catheter ablation in patients with AF.
Methods
Study patients
A total of 213 consecutive patients (58.3±21.1 years old, 112 males and 101 females, 136 PAF patients and 77 persistent AF group patients) with drug-refractory AF were enrolled. All patients were undergoing circumferential PV isolation (CPVI) guided by the CARTO mapping system (CARTOTM XP, Biosense-Webster Inc, Diamond Bar, CA, USA). AF patients were divided into normotension group and hypertension group. According to the guidelines of JCN-7 (9), Hypertension was defined as blood pressure ≥140/90 mmHg and/or a history of treated hypertension. A total of 40 of these hypertension patients were prescribed with different ACEI or ARB or NDHP-CCBs to treat hypertension in our hospital. The other patients used no drugs to treat hypertension. All antiarrhythmic drugs were discontinued for at least five half-lives before the procedure. Patients who underwent a repeat ablation procedure were excluded from the study. Patients with diabetes or serious structural heart diseases (rheumatic heart disease, hypertrophic cardiomyopathy, dilated cardiomypathy, coronary heart disease or 3rd degree atrioventricular block) were also excluded. This retrospective protocol was approved by the Institutional Review Board of the Dalian Medical University.
Echocardiography was performed in all patients before the ablation procedure. The anteroposterior diameter of the left atrium at end-systole was measured by M mode echocardiography in the parasternal short axis view, and all measurements were performed by two independent observers.
Electrophysiological study and catheter ablation
The details of the electrophysiological study and 3-dimensional (3D) mapping were the same as described previously (3,10). After femoral venous access was obtained, a 7-French deflectable decapolar catheter was inserted into the coronary sinus. Dual transseptal puncture was performed under fluoroscopic guidance, with delivery of two 8 F long sheaths (SL1 and SR0, St. Jude Medical, St. Paul, MN, USA) into the LA. In PAF, the PV ostia were identified by enography and the drop-off site of the 3.5-mm tip ablation catheter (Navi-Star, Biosense-Webster), while dragging it out of the vein. Continuous circumferential lesions were created encircling the right and left PV ostia using the ablation catheter guided by the CARTO system. Radiofrequency (RF) current energy output was limited to a maximum of 70 W and 55 °C. After completion of the circumferential lesion set, the ipsilateral superior and inferior PVs were mapped and ablated carefully. The end point of ablation was complete electrical disconnection of the PV antrum from the LA. After successful isolation of all four PVs, high current pulse duration stimulation from the proximal and distal CS was performed. If induced AF was sustained for >1 minute, an additional linear ablation was performed at the anterior roof and the mitral isthmus. In persistent AF, PV isolation plus linear ablation was performed as the first and second steps. If AF did not stop, sinus rhythm was restored by electric cardioversion.
Atrial substrate analysis using atrial activation maps and 3-dimensional (3D) electroanatomical maps
Sequential activation maps of LA were constructed in all 213 patients. Bipolar electrograms were recorded before catheter ablation from more than 80 sites in LA, and these sites were approximately equally distributed. The bipolar electrograms were filtered between 32 and 300 Hz and recorded digitally. The absolute peak of the waveform was selected as the point of local atrial activation. The ablation catheter was selected as the roving catheter. The roving catheter was moved to various points on the LA walls to determine local activation times (relative to a reference signal). The signals from the roving catheter were used to build a sequential activation map. The LA total activation time was defined as the time interval from the earliest to the latest activation point in the LA; the total activation time of both atria was defined as the time interval from the start of P wave of the most clear lead in the surface electrocardiogram (ECG) to the latest point of the LA.
After completion of the sequential activation maps, the LA bipolar electrograms were used to construct detailed electroanatomical maps using CARTO software. This analysis was performed offline, and the software determined the voltage contribution to the surface area of each point using the distance to the nearest neighboring point, and presented the results as the weighted voltage (nearest distance × voltage)/mean overall nearest distance. There were 126±15 bipolar LA mapping sites used by the CARTO software to construct the electroanatomical maps. Scar was defined as the absence of any voltage or a bipolar voltage amplitude ≤0.05 mV that was indistinguishable from noise. The scar-zone index was defined as the scar surface area/total LA surface area. The low-voltage zone (LVZ) in the AF patients was defined as an amplitude ≤0.2 mV. The LVZ index was defined as the LVZ surface area/total LA surface area.
Clinical variables
The clinical variables that may influence the voltage were analyzed including gender, AF duration, LA enlargement (LA diameter ≥4 cm), hypertension and age.
AF recurrence during follow-up
After discharge, the patients underwent follow-up (1 day after catheter ablation, and then at 1, 3 and 6 months) at our cardiology clinic or with their referring physicians. Antiarrhythmic drugs were prescribed for 8 weeks to prevent the early recurrence of AF in patients with persistent AF. When patients experienced symptoms suggesting a tachycardia after ablation, 24-hour Holter monitoring was performed to define the cause of the clinical symptoms. If more than one episode of recurrent symptomatic AF was documented, the patients were encouraged to receive a second ablation procedure, or antiarrhythmic drugs were prescribed to control the recurrent AF. AF recurrence was defined as an episode confirmed by an ECG that lasted more than 1 minute and occurred 3 months or more after the ablation procedure. The successful AF ablation was defined as no AF recurrence was found during follow up after the AF catheter ablation.
Statistical analysis
All data for continuous variables are reported as the mean ± SD or the median. A chi-square test was used to compare categorical variables between groups. A student’s test or a Wilcoxon rank-sum test was used to compare continuous variables between groups. The mean LA bipolar voltage was compared between the groups. A value of P<0.05 was considered to be statistically significant. All analyses were performed using SPSS software version 11.0 (SPSS, Chicago, Illinois, USA).
Results
Clinical characteristics of the patients with and without hypertension
The baseline characteristics of the patients with and without hypertension are presented in Table 1. The cohort in this study included 213 patients, 136 PAF patients and 77 persistent AF patients. In the 136 PAF patients, there were 94 in the normotensive group (53 males), and 42 patients in the hypertensive group (24 males); in the 77 persistent AF patients, there were 42 in normotensive group (22 males), and 35 in the hypertensive group (18 males). The gender, mean duration of AF and LA diameter were similar between the hypertensive and normotensive groups.
Table 1. Clinical characteristics of AF patients with and without hypertension.
| Group | Case number (male/female) | Age | LA diameter (mm) | AF duration (years) | Systolic pressure (mmHg) |
|---|---|---|---|---|---|
| PAF | |||||
| Normotensive group | 94 (53/41) | 56.2±19.6 | 36.6±8.7 | 5 | 124.4±21.5 |
| Hypertensive group | 42 (24/18) | 60.6±21.6 | 37.4±9.5 | 6 | 140.9±18.4* |
| Persistent AF | |||||
| Normotensive group | 42 (22/20) | 51.6±21.2 | 44.7±13.4 | 4 | 132.3±16.9 |
| Hypertensive group | 35 (18/17) | 57.9±17.4 | 45.9±12.8 | 5 | 139.1±16.3* |
*, P<0.05 normotensive group vs. hypertensive group. AF, atrial fibrillation; LA, left atrial; PAF paroxysmal AF.
Effect of hypertension on the electroanatomical properties of the atrial substrate
The AF substrate variables determined by electranatomical mapping are shown in Table 2. The mean LA bipolar voltage was higher in the normotensive group than the hypertensive group in both PAF (1.92±0.76 vs. 1.44±1.09, respectively, P=0.048) and persistent AF patients (0.74±0.59 vs. B: 0.51±0.39, respectively, P=0.004) (Table 2, Figure 1). The total atrial activation time and LA activation time recorded during sinus rhythm were not significantly different between the normotensive and hypertensive PAF groups (activation time could not be assessed in persistent AF patients). The LA scar-zone index and LVZ index were significantly smaller in the normotensive group than in the hypertensive group in both PAF and persistent AF patients.
Table 2. Atrial substrate characteristics in the normotensive and hypertensive groups.
| Group | Case number | LA bipolar voltage (mV) | Scar-zone index | LVZ index | LA single voltage (mV) | Atrial TAT [ms] | LA TAT [ms] |
|---|---|---|---|---|---|---|---|
| PAF | |||||||
| Normotensive group | 94 | 1.92±0.76 | 0 (0-0) | 0.064 (0.03-0.106) | 3.9±1.9 | 229 [171-264] | 207 [136-244] |
| Hypertensive group | 42 | 1.44±1.09* | 0.0002* (0-0.018) | 0.136* (0.063-0.176) | 3.2±1.8 | 221 [164-266] | 201 [130-240] |
| Persistent AF | |||||||
| Normotensive group | 42 | 0.74±0.59 | 0.007 (0-0.022) | 0.195 (0.123-0.241) | |||
| Hypertensive group | 35 | 0.51±0.39* | 0.013* (0-0.037) | 0.265* (0.164-0.372) |
TAT, total activation time; LVZ, low voltage zone; AF, atrial fibrillation; LA, left atrial; PAF paroxysmal AF. *, P<0.05 normotensive group vs. hypertensive group.
Figure 1.
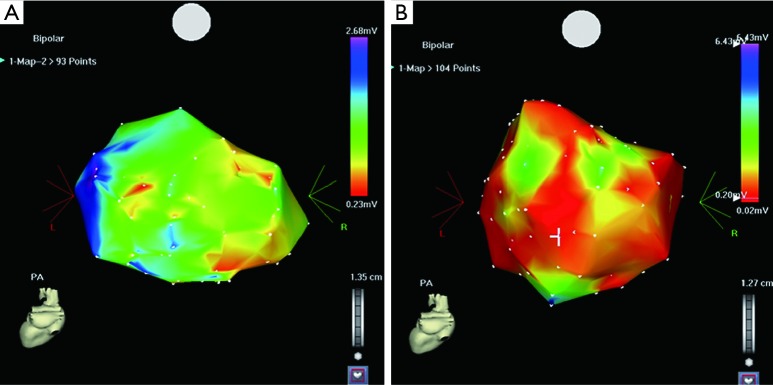
(A) is a CARTO map in a normotensive patient. The red color indicates the low-voltage zone (LVZ); (B) is a CARTO map in a hypertensive patient. Comparison of the two maps shows that there is a much larger LVZ in the patient with hypertension.
Several clinical factors that could affect the mean LA bipolar voltage were also analyzed (Table 3). Gender, mean duration of AF before ablation and LA diameter did not affect the mean LV bipolar voltage in either PAF or persistent AF patients. Hypertension significantly influenced the mean LA bipolar voltage in both PAF and persistent AF patients. Age significantly influenced the mean LA bipolar voltage only in patients with persistent AF.
Table 3. Clinical variables affecting the atrial substrate properties in PAF and persistent AF.
| Group | PAF |
Persistent AF |
|||
|---|---|---|---|---|---|
| Mean LA bipolar voltage | P value | Mean LA bipolar voltage | P value | ||
| Gender | 0.323 | 0.323 | |||
| Male | 1.78±1.16 | 0.78±0.46 | |||
| Female | 1.58±1.13 | 0.68±0.67 | |||
| Age | 0.096 | 0.011 | |||
| ≤65 | 1.71±1.05 | 0.73±0.6 | |||
| >65 | 1.64±1.07 | 0.51±0.36 | |||
| Hypertension | 0.048 | 0.004 | |||
| Yes | 1.92±0.76 | 0.74±0.59 | |||
| No | 1.44±1.09 | 0.51±0.39 | |||
| LA diameter | 0.132 | 0.105 | |||
| ≤40 mm | 1.71±1.1 | 0.75±0.6 | |||
| >40 mm | 1.6±0.86 | 0.55±0.44 | |||
| AF duration | 0.147 | 0.147 | |||
| ≤2 years | 1.78±1.12 | 0.68±0.59 | |||
| >2 years | 1.57±0.92 | 0.59±0.52 | |||
AF, atrial fibrillation; LA, left atrial; PAF paroxysmal AF.
The effect of hypertension on the outcome of AF ablation
The overall incidence of AF recurrence in our patients was 20.6% (44 patients). Although the success rate of ablation in hypertensive patients seemed to be smaller than that in normotensive patients (71.4% vs. 83.8%), the difference did not reach statistical significance (Table 4). A total of 14 of 44 AF recurrence patients receive a second ablation procedure, the others were prescribed with antiarrhythmic drugs (amiodarone or β-adrenoceptor blockers) to control the recurrent AF.
Table 4. The effect of hypertension on the outcome of AF ablation.
| Patients | Ablation success | Ablation recurrence | Success rate (%) | P value |
|---|---|---|---|---|
| PAF | 0.067 | |||
| Normotensive | 78 | 16 | 82.9 | |
| Hypertensive | 29 | 13 | 69 | |
| Persistent AF | 0.207 | |||
| Normotensive | 36 | 6 | 85.7 | |
| Hypertensive | 26 | 9 | 74.2 | |
| All patients | 0.095 | |||
| Normotensive | 114 | 22 | 83.8 | |
| Hypertensive | 55 | 22 | 71.4 |
AF, atrial fibrillation; PAF paroxysmal AF.
The clinical characteristics of patients and the outcome of AF ablation
The baseline characteristics of the patients with and without successful AF ablation are presented in Table 5. In the cohort of PAF patients, the ablation procedure was successful in 107 patients (66 males), whereas 29 patients (16 males) had AF recurrence. In the persistent AF patients, the ablation procedure was successful in 62 patients (32 males), whereas 15 patients (8 males) had AF recurrence. The gender, age, mean duration of AF, LA diameter and proportion of patients with hypertension were similar between the patients with and without AF recurrence (Table 5).
Table 5. Clinical characteristics of patients and the outcome of AF ablation.
| Group | Number of cases (male/female) | Age | LA diameter (mm) | AF duration (years) | Hypertension |
|---|---|---|---|---|---|
| PAF | |||||
| Success group | 107 (66/41) | 58.1±20.8 | 36.9±9.1 | 5 | 31 |
| Recurrence group | 29 (16/13) | 59.3±23.0 | 37±9.3 | 6 | 11 |
| Persistent AF | |||||
| Success group | 62 (32/30) | 54.5±19.6 | 45±13.4 | 4 | 28 |
| Recurrence group | 15 (8/7) | 54.3±24.8 | 45.8±12 | 5 | 7 |
AF, atrial fibrillation; LA, left atrial; PAF paroxysmal AF.
The atrial substrate characteristics and the outcome of AF ablation
The AF substrate properties determined by electroanatomical mapping in patients with and without successful AF ablation are shown in Table 6. The mean LA bipolar voltage was higher in the PAF patients with successful ablation than in those with AF recurrence (1.77±1.01 vs. 1.29±0.93, respectively, P=0.048). Likewise, the mean LA bipolar voltage was also higher in the persistent AF patients with successful ablation than those with AF recurrence (1.31±0.96 vs. 0.78±0.35, respectively, P=0.046) (Table 6). The total atrial activation time and total LA activation time recorded during sinus rhythm were not significantly different in the PAF patients with successful ablation from those had AF recurrence. The LA scar-zone index and LVZ index were smaller in both the PAF and persistent AF patients with a successful ablation compared with those who had AF recurrence (Table 6, Figure 2).
Table 6. The atrial substrate characteristics and the outcome of AF ablation.
| Group | Case number | LA bipolar voltage (mV) | Scar-zone index | LVZ index | LA single voltage (mV) | Atrial TAT [ms] | LA TAT [ms] |
|---|---|---|---|---|---|---|---|
| PAF | |||||||
| Success group | 107 | 1.77±1.01 | 0 (0-0) | 0.067 (0.07-0.116) | 3.72±1.9 | 228 [174-265] | 217 [137-245] |
| Recurrence group | 29 | 1.29±0.93* | 0.0004* (0-0.018) | 0.139* (0.067-0.178) | 2.79±1.4 | 222 [165-260] | 201 [135-241] |
| Persistent AF | |||||||
| Success group | 62 | 1.31±0.96 | 0.005 (0-0.024) | 0.147 (0.068-0.207) | |||
| Recurrence group | 15 | 0.78±0.35* | 0.012* (0-0.036) | 0.285* (0.165-0.378) |
TAT, total activation time; LVZ, low voltage zone; AF, atrial fibrillation; LA, left atrial; PAF paroxysmal AF. *, P<0.05 success group vs. recurrence group.
Figure 2.
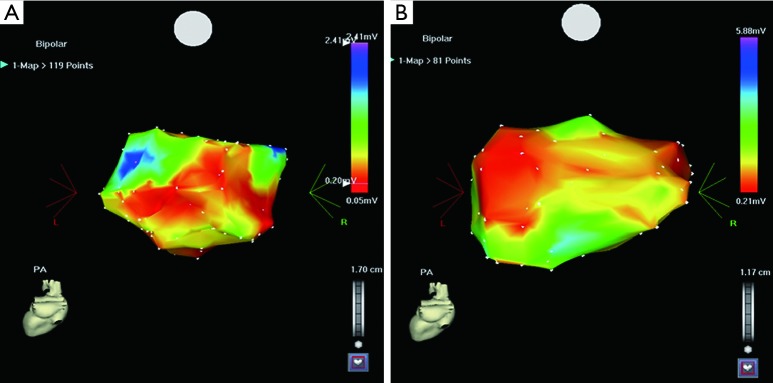
(A) is a CARTO map in a patient who did not have AF recurrence. The red color indicates the low-voltage zone (LVZ); (B) is a CARTP map in a patient who had AF recurrence. Comparison of the two maps shows that the LVZ is smaller in the patient without AF recurrence.
Discussion
The mean LA bipolar voltage was significantly reduced, and the scar-zone index and LVZ index were significantly increased in AF patients with hypertension. However, hypertension did not appear to affect the incidence of AF recurrence after the first catheter ablation procedure. When patients with and without hypertension were combined, the mean LA bipolar voltage was significantly reduced, and the scar-zone index and LVZ index were significantly increased in the AF patients that had recurrence.
In contrast to the effect of hypertension on the mean LA bipolar voltage and size of the scar-index and LVZ index, we found that hypertension did not affect the LA activation time. Although the explanation for the lack of an effect of hypertension on LA activation time is not clear, it may be that both the magnitude and duration of hypertension may influence this activation time. Therefore, it is possible that the blood pressure was not high enough for a long enough period to alter LA activation time in the hypertensive patients in this study.
Effect of hypertension on the outcome of catheter ablation of AF
Our study has shown that the benefits of the PV isolation extend equally to all AF patients, and that the presence of hypertension does not significantly increase the risk of AF recurrence after catheter ablation. However, there was a non-significant trend towards a higher recurrence rate in the AF patients with hypertension (28.6% vs. 16.2%, Table 4). The possible explanation is that the process of electrical and structural remodeling in the atrium may differ between patients with and without hypertension. Increased automaticity and increased triggered activity might occur in human diseased atrial fibers. Furthermore, fibrillatory wavelets could be more easily induced and maintained in larger atria, which might increase the risk of AF recurrence event in the absence of PV discharge (11). It is possible that the blood pressure was not high enough for a long enough period to alter the ablation results significant in the hypertensive patients in this study and we had a relative small patient number.
Relationship between the LA substrate properties and recurrence
A decrease in the mean LA bipolar voltage, and increase in the LA scare-zone index and LVZ index were associated with recurrence after catheter ablation of AF in the present study. These findings can be explained by one of the following mechanisms: (I) the LVZ may aggravate intra-atrial conduction delay, resulting in the formation of reentrant circuits and thus promote AF perpetuation; and (II) the LVZ may serve as sites of ectopic beats that could trigger AF in the absence of PV discharge (12). In addition, LA scarring can be used as a strong predictor of procedural failure in patients undergoing catheter ablation of AF.
Study limitations
In this study, lack of detailed electoanatomical mapping of the right atrium was a limitation. Second, this was an observational study, and the associations that we observed do not prove the presence of cause and effect relationships. Third, atrial activation maps could not be obtained in the patients with persistent AF, and the detailed LA electroanatomical maps were constructed in these patients during AF rather than during sinus rhythm. Forth, the methods for detecting AF recurrence are suboptimal.
Conclusions
Hypertension has a significant impact on the properties of the LA substrate in AF, and AF patients with hypertension have a significantly lower mean LA bipolar voltage. The LA substrate has an important influence on the outcome of catheter ablation in AF patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995;155:469-73 [PubMed] [Google Scholar]
- 2.Anyukhovsky EP, Sosunov EA, Chandra P, et al. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res 2005;66:353-63 [DOI] [PubMed] [Google Scholar]
- 3.Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104:2539-44 [DOI] [PubMed] [Google Scholar]
- 4.Gottdiener JS, Reda DJ, Williams DW, et al. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol 1997;29:651-8 [DOI] [PubMed] [Google Scholar]
- 5.Schotten U, Neuberger HR, Allessie MA. The role of atrial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol 2003;82:151-62 [DOI] [PubMed] [Google Scholar]
- 6.Sanders P, Morton JB, Davidson NC, et al. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation 2003;108:1461-8 [DOI] [PubMed] [Google Scholar]
- 7.Chang SL, Tai CT, Lin YJ, et al. The role of left atrial muscular bundles in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2007;50:964-73 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Zlochiver S, Vikstrom KL, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res 2007;101:839-47 [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72 [DOI] [PubMed] [Google Scholar]
- 10.Ouyang F, Bänsch D, Ernst S, et al. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation 2004;110:2090-6 [DOI] [PubMed] [Google Scholar]
- 11.Chang SL, Tai CT, Lin YJ, et al. Biatrial substrate properties in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:1134-9 [DOI] [PubMed] [Google Scholar]
- 12.Lo LW, Tai CT, Lin YJ, et al. Progressive remodeling of the atrial substrate--a novel finding from consecutive voltage mapping in patients with recurrence of atrial fibrillation after catheter ablation. J Cardiovasc Electrophysiol 2007;18:258-65 [DOI] [PubMed] [Google Scholar]


