Abstract
Background
Recurrence following complete resection of esophageal squamous cell carcinoma (SCC) still remains common. The aim of this study was to investigate the prognostic factors in patients with recurrence after complete resection of esophageal SCC.
Methods
The medical records of 190 patients with recurrent disease after complete resection of esophageal SCC were retrospectively reviewed. Recurrence pattern was classified as loco-regional recurrence and distant metastases. The Kaplan-Meier method was used for the survival analysis. Cox proportional hazards model was used for multivariate analysis.
Results
Mediastinal nodal clearance area was the most common sites of loco-regional recurrence, whereas lung, liver and bone were the most common sites for distant metastases. The median survival after recurrence was 8 months. The 1, 3, 5-year post-recurrence survival rates were 45.9%, 10.6% and 6.4%, respectively. The overall 1, 3, 5-year survival rates were 76.6%, 27.3% and 12.3%, respectively. The independent prognostic factors included time of recurrence (≥12 months vs. <12 months, HR: 3.228, 95% CI: 2.233-4.668), pattern of recurrence (local-regional recurrence vs. distant metastases, HR: 1.690, 95% CI: 1.170-2.439), and treatment of recurrence [no treatment vs. treatment (radiotherapy or surgery or chemotherapy), HR: 0.642, 95% CI: 0.458-0.899].
Conclusions
Our retrospective study showed that time of recurrence, pattern of recurrence and treatment of recurrence were independent prognostic factors in patients with recurrence after complete resection of esophageal SCC.
Keywords: Esophageal carcinoma, esophagectomy, recurrence, prognostic factor
Introduction
Esophageal cancer is one of the most common cancers worldwide, causing more than 400,000 deaths annually (1). Surgical resection is generally recommended for the treatment of esophageal carcinoma in early stage. Advances in anesthesia and surgical techniques and improvements in perioperative management have reduced the postoperative mortality to acceptable levels. However, the overall 5 years survival rate after esophagectomy remains about 25% (2). This dismal result is attributed to recurrences after resection of the primary tumor. Local-regional recurrences or/and distant organ metastases are found in approximately half of patients within 2-3 years of surgical treatment (3-5). The pattern of recurrence after esophagectomy has been well documented (3,5,6). Histologic tumor depth invasion (3,5), local-regional lymph node metastases (5) and intramural metastasis (6) have been reported to predict recurrence.
Recent advances in chemotherapy and radiation techniques may particularly benefit patients with recurrent disease after esophagectomy. In fact, recurrent disease sometimes responds better to anticancer treatment, and those patients can achieve relatively long-term survival. Thus, the factors affecting this survival after recurrence in patients with esophageal SCC need to be further investigated. In this study, we sought to better understand factors affecting overall survival in patients with disease of recurrence following complete resection of esophageal SCC.
Methods
Patients
A total of 773 patients with esophageal cancer received surgical resection at the Department of Thoracic Surgery, Sun Yat-sen University Cancer Center, from January 2001 to December 2005. All tumors were pathologically confirmed as squamous cell carcinoma (SCC) by biopsy under endoscopy. Other preoperative examinations included plain chest radiography, barium swallow, chest and abdominal computed tomography (CT) scan and cervical ultrasonography. Bronchoscopy was also done in patients with tumor located in the upper third thoracic esophagus.
Clinical data regarding cancer recurrence after surgery were collected. Patients were excluded if: (I) there was no complete follow-up data or no pathologic or radiographic examination results to confirm recurrence; (II) surgery was non-curative resection (R1 or R2); (III) the patient received neoadjuvant radiotherapy or chemotherapy; or (IV) there was a second primary cancer. This study was officially approved by the Ethics Committee of our hospital.
A total of 190 patients were included in the study. There were 146 males and 44 females. The median patient age was 55 years (range, 32-76 years). Primary sites of 13 (6.8%) patients were in the upper thoracic esophagus, 129 (67.9%) in the middle thoracic esophagus and 48 (25.3%) in the lower thoracic esophagus.
Esophageal cancer patients with a primary tumor in the lower thoracic esophagus were treated using the left transthoracic procedure or Ivor-Lewis procedure (if there was evidence of mediastinal lymphadenopathy on chest CT) with intrathoracic anastomosis. Patients with a primary tumor in the upper thoracic esophagus underwent cervical-thoracoabdominal procedure with a left cervical anastomosis. Patients with tumor in the middle thoracic esophagus were treated using the left transthoracic procedure with intrathoracic anastomosis (if no evidence of mediastinal lymphadenopathy on chest CT) or the cervical-thoracoabdominal procedure with a left cervical anastomosis (if mediastinal lymphadenopathy was showed on chest CT). All patients underwent esophageal reconstruction using stomach to replace the esophagus. Lymph node dissection was performed including the left and right tracheobronchial, subcarinal, paraesophageal, diaphragmatic lymph nodes as well as the paracardial, lesser gastric curvature, left gastric artery and celiac nodes. The lymph nodes next to the left and right recurrent laryngeal nerves were removed when a right thoracotomy was used. Nine patients developed a post-operative anastomotic leakage, ten atelectasis and pneumonia, and one chylothorax. No postoperative adjuvant radiotherapy or chemotherapy was administered.
The pathologic staging was based on the UICC sixth edition [2002] for staging criteria of esophageal cancer (7). There was one stage I patient (one case of pT1N0M0), 68 stage IIA (30 pT2N0M0 and 38 pT3N0M0) patients, 28 stage IIB (28 pT2N1M0) patients, and 93 stage III (93 pT3N1M0) patients.
Recurrence identification
All patients were examined every 3 to 4 months during the first 2 years after surgery, followed by every 6 months for 3 to 5 years after surgery, and once a year thereafter. The patients were asked to visit the hospital for examination if any symptoms such as hoarseness or dysphagia occurred. Follow-up examinations included physical examination, chest X-Ray, barium swallow and abdominal and cervical ultrasonography. If any suspected recurrence or metastasis was found on X-ray or ultrasonography, or enlarge supraclavicular lymph nodes palpated, chest and abdominal CT scans and esophageal endoscopy were performed. Patients with bone pain had a bone scintigraphy scan.
Tumor diagnosed at least 6 months or more after surgery were considered a recurrence. Loco-regional recurrences was defined as anastomotic recurrence or/and occurring either in the mediastinum or upper abdomen at the site of previous esophageal resection and nodal clearance or in the cervical area where no lymphadenectomy had been performed. Distant metastasis was defined as occurring in distant organs, pleura and peritoneum. If both loco-regional recurrence and distant metastases occurred, the case was considered as distant metastases (3). Diagnosis of recurrence was made using cytological and histopathological results which were obtained by biopsy of suspected recurrence at anastomotic site or supraclavical lymph nodes and tumor imaging studies. Criteria used to determine CT lymph node metastases were a lymph node diameter greater than 1 cm.
Treatment of recurrent disease
The modalities of treatment of recurrent disease are showed in Table 1. In the surgical group, a solitary metastasis to the lung or brain was confirmed by the imaging tests including chest and abdominal CT scan, brain magnetic resonance imaging (MRI). One patient with a solitary metastasis to the lung survived 71 months after pulmonary wedge resection. Among the non-treatment group, 83 patients received only symptomatic and supportive treatment due to age, poor general health status, financial considerations, or patient choice.
Table 1. Treatment of recurrent diseases.
| Modality | Cases | Dose or regimen |
|---|---|---|
| Radiotherapy | 81 pts (74 pts with radiotherapy alone, 7 pts with chemoradiotherapy) | Median radiation dose: 60 Gy (range, 24-75 Gy) |
| Chemotherapy | 19 pts | Cisplatin and 5-fluorouracil: 2-6 cycles |
| Surgery | 7 pts {metastectomy of lung [3], brain [1] and skin [1], liver metastases treated with RFA [2]} | |
| No treatment | 83 pts |
Abbreviations: pts, patients; RFA, radiofrequency ablation.
Follow-up
The follow-up department of our hospital was responsible for postoperative follow-up of all patients. Patients, follow-up data were obtained by reviewing records of clinical re-examination or by directly contacting the patient or their family by telephone. Follow up was stopped upon patient’s death or on January 2012. Eight cases were lost to follow-up and defined as censored cases. The follow-up rate was 95.8%. The mean follow-up was 30.15±28.90 months (range, 2-131 months). Progression-free survival was defined as time from date of surgery to the first recurrence or last follow-up. Post-recurrence survival was calculated as time between the first recurrence and death or last follow-up. Overall survival time was calculated as the time between surgery and death or last follow-up.
Statistical analysis
SPSS16.0 (SPSS, Chicago, IL, USA) statistical software was used for statistical analysis. Quantitative data were expressed as mean ± standard deviation (x̄ ± s), and a t-test was performed for comparison. Survival analysis was performed using the Kaplan-Meier method, and compared by means of the log-rank test. Hazard ratios were calculated using a Cox regression model. Multivariable analysis for prognostic factors was performed using a Cox proportional hazard model. A P value of less than 0.05 was considered statistically significant.
Results
Clinical and pathological features of the patients are shown in Table 2. A total of 106 (55.8%) patients recurred within 1 year, 154 (81.1%) within 2 years, and 169 (88.9%) within 3 years after surgery. The pattern of recurrence after esophagectomy is showed in Table 3. The overall median time to recurrence was 10 months (range, 6-94 months). The median time to recurrence in patients with loco-regional recurrence and distant metastases was 13 months (range, 6-94 months) and 8 months (range, 6-44 months), respectively. The mean time to recurrence in patients with loco-regional recurrence and distant metastases was 18.66±16.30 months and 11.05±7.77 months (P=0.001), respectively.
Table 2. Clinical and histopathologic characteristic of 190 patients with disease of recurrence after esophagectomy.
| Characteristics | Local-regional recurrence (n=124) | Distant metastases (n=66) |
|---|---|---|
| Gender | ||
| Male | 96 | 50 |
| Female | 28 | 16 |
| Tumor location | ||
| Upper third | 10 | 3 |
| Middle third | 83 | 46 |
| Lower third | 31 | 17 |
| Anastomotic site | ||
| Cervical | 27 | 15 |
| Intrathoracic | 97 | 51 |
| Differentiation | ||
| Well | 32 | 13 |
| Moderately | 61 | 34 |
| Poorly | 31 | 19 |
| Depth of invasion | ||
| pT1 | 0 | 1 |
| PT2 | 41 | 17 |
| PT3 | 83 | 48 |
| Lymph node metastasis | ||
| pN0 | 48 | 21 |
| PN1 | 76 | 45 |
| pTNM stage | ||
| I | 0 | 1 |
| IIa | 48 | 20 |
| IIb | 19 | 9 |
| III | 57 | 36 |
Table 3. Patterns of recurrence after esophagectomya.
| Patterns of recurrence | Cases |
|---|---|
| Local-regional recurrenceb (n=138) | |
| Cervical | 43 |
| Mediastinal | 95 |
| Abdominal | 11 |
| Anastomotic | 13 |
| Distant metastasesc (n=66) | |
| Lung | 21 |
| Liver | 18 |
| Bone | 15 |
| Brain | 4 |
| Subcutaneous | 4 |
| Adrenal | 3 |
| Stomach | 2 |
| Pleura | 3 |
| Peritoneum | 1 |
a, Local-regional recurrence and distant metastasis were recognized simultaneously in 14 patients. These patients were classified into distant metastases group; b, recurrence in multiple areas were recognized in 23 patients; c, metastases in multiple distant organs were recognized in 5 patients.
The 1, 3, 5-year progression-free survival rates were 52.2%, 14.9% and 4.2%, respectively (Figure 1). The overall median survival time after recurrence was 8 months. The 1, 3, 5-year post-recurrence survival rates were 45.9%, 10.6% and 6.4%, respectively (Figure 2). The median overall survival time was 21.5 months. The overall 1, 3, 5-year survival rates were 76.6%, 27.3% and 12.3%, respectively (Figure 3).
Figure 1.
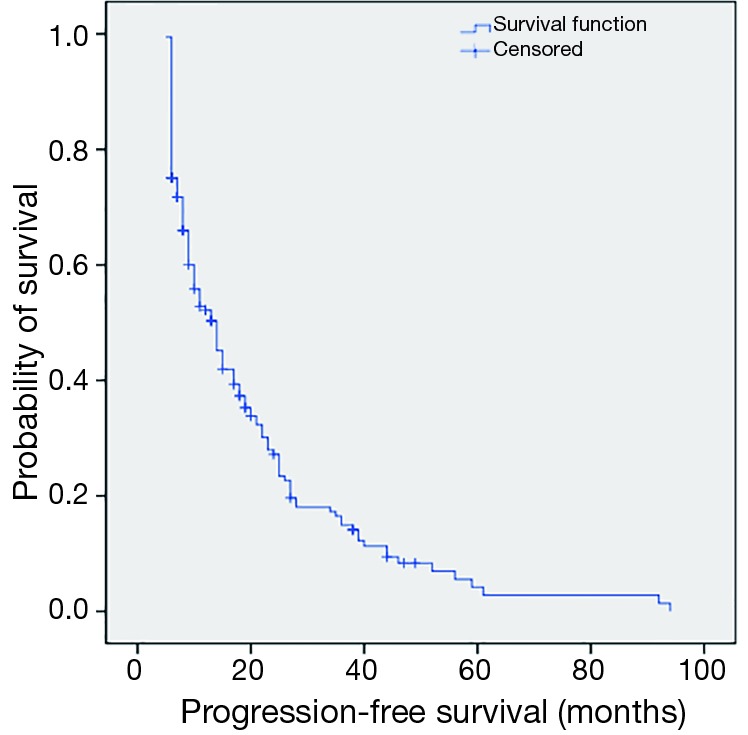
Kaplan-Meier estimating the probability of progression-free survival among 190 patients with disease of recurrence after esophagectomy.
Figure 2.
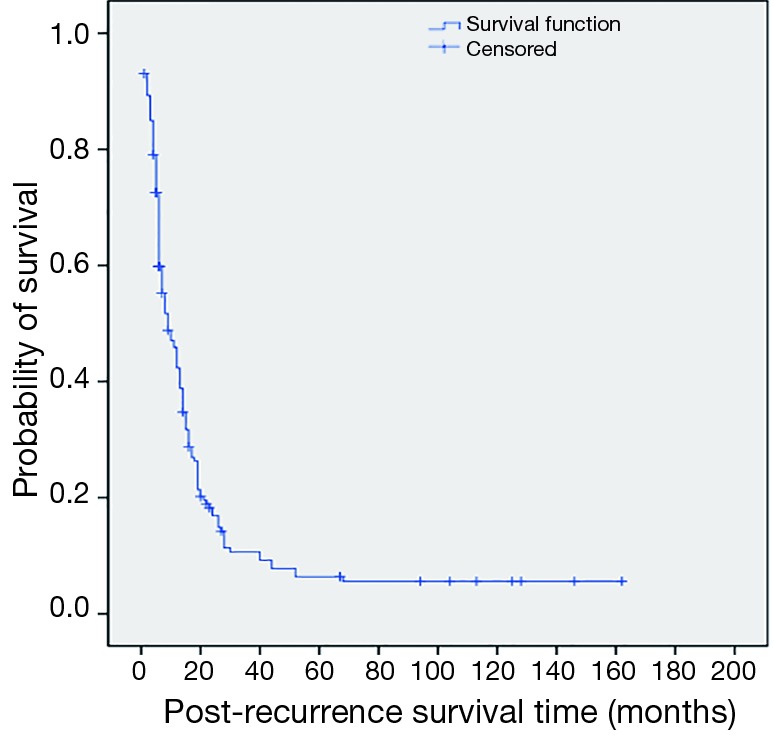
Kaplan-Meier estimating the probability of post-recurrence survival among 190 patients with disease of recurrence after esophagectomy.
Figure 3.
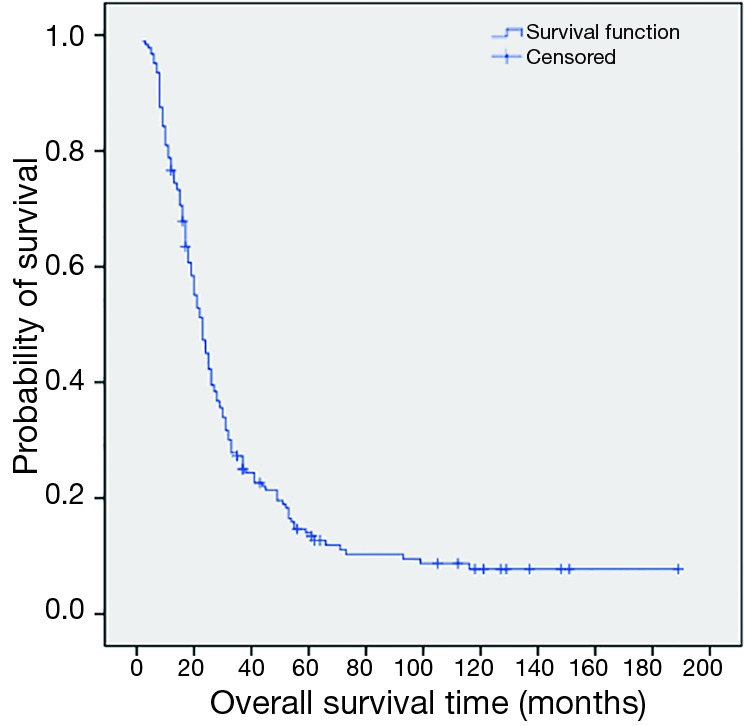
Kaplan-Meier estimating the probability of overall survival among 190 patients with disease of recurrence after esophagectomy.
The median survival time after recurrence in the loco-regional recurrence group and the distant metastasis group was 10 months (range, 2-125 months) and 5 months (range, 1-162 months), respectively. The mean survival time after recurrence in the loco-regional recurrence group and the distant metastasis group was 13.71±14.71 months and 7.18±7.21 months (P=0.001), respectively.
Univariate analysis showed that tumor location, depth of invasion, lymph node metastasis, pathological stage, time of tumor recurrence, type of recurrence and treatment after recurrence were significantly related to survival. Gender, age and tumor tissue differentiation were not related to survival (Table 4). Factors related to survival in the univariate analysis were evaluated with the Cox model. Time of tumor recurrence, type of recurrence and treatment after recurrence were found to be independent factors that affected prognosis (Table 5, Figure 4).
Table 4. Univariate analysis of prognostic factors in 190 patients with disease of recurrence after esophagectomy.
| Variable | n | Median survival time (months) | 5-year survival rate | P value | HR | 95% CI for HR |
|---|---|---|---|---|---|---|
| Gender | 0.737 | 1.057 | 0.696-1.605 | |||
| Male | 146 | 23 | 11.2 | |||
| Female | 44 | 21 | 15.9 | |||
| Age (years) | 0.837 | 0.976 | 0.593-1.606 | |||
| <65 | 155 | 23 | 12.2 | |||
| ≥65 | 35 | 22 | 11.9 | |||
| Tumor location | 0.002 | 1.189 | 1.011-1.445 | |||
| Upper third | 13 | 10 | 0 | |||
| Middle third | 129 | 25 | 15 | |||
| Lower third | 48 | 18 | 6.8 | |||
| Differentiation | 0.097 | 1.088 | 0.856-1.383 | |||
| Well | 43 | 22 | 11.9 | |||
| Moderately | 95 | 23 | 10.1 | |||
| Poorly | 52 | 24 | 8.7 | |||
| Depth of invasion | 0.011 | 1.237 | 1.031-1.533 | |||
| pT1 | 1 | 13 | 0 | |||
| pT2 | 58 | 23 | 19.8 | |||
| pT3 | 131 | 23 | 5.2 | |||
| Lymph node metastasis | 0 | 1.487 | 1.071-2.065 | |||
| N0 | 69 | 28 | 18.8 | |||
| N1 | 121 | 21 | 7.6 | |||
| pTNM stage | 0.029 | 1.242 | 1.046-1.476 | |||
| I | 1 | 13 | 0 | |||
| IIa | 68 | 28 | 19.7 | |||
| IIb | 28 | 20 | 16.9 | |||
| III | 93 | 21 | 8.4 | |||
| Time of recurrence | 0 | 0.32 | 0.231-0.443 | |||
| <12months | 103 | 15 | 4.5 | |||
| ≥12months | 87 | 37 | 24.1 | |||
| Pattern of recurrence | 0 | 1.832 | 1.320-2.543 | |||
| Local-regional | 124 | 26 | 17.3 | |||
| Distant metastases | 66 | 17 | 2.3 | |||
| Treatment of recurrence | 0.004 | 0.734 | 0.587-0.917 | |||
| No treatment | 83 | 19 | 10.5 | |||
| Chemotherapy | 19 | 21 | 14 | |||
| Radiotherapy | 81 | 30 | 14.8 | |||
| Surgery | 7 | 71 | 16.7 |
Abbreviations: HR, hazard Ratio; CI, confidential interval.
Table 5. Multivariate analysis of prognostic factors in 190 patients with disease of recurrence after esophagectomy.
| Variable | B | Wald | P | Exp.(ß) | 95% CI for Exp.(ß) |
|---|---|---|---|---|---|
| Time of recurrencea | 1.172 | 38.831 | 0 | 3.228 | 2.233-4.668 |
| Pattern of recurrenceb | 0.524 | 7.836 | 0 | 1.69 | 1.170-2.439 |
| Treatmentc | –0.443 | 6.633 | 0.01 | 0.642 | 0.458-0.899 |
a, ≥12 months vs. <12 months; b, local-regional recurrence vs. distant metastases; c, no treatment vs. treatment (radiotherapy or surgery or chemotherapy). Abbreviations: Exp., Experiences; CI, Confidence interval.
Figure 4.

Kaplan-Meier estimating the overall survival among 190 patients with disease of recurrence after esophagectomy. A, survival according to time of recurrence; B, survival according to pattern of recurrence; C, survival according to treatment of recurrence disease.
Discussion
Esophagectomy with radical lymph node dissection is the main treatment for esophageal cancer. However, the rich lymphatic capillary network in the esophageal mucosa and submucosa facilitate local recurrences or distant metastases after surgery. It has been reported that more than half of esophageal cancer patients have recurrence or metastases within 2-3 years after resection (3,4). Mariette et al. (5) have reported 90% of recurrence occurred within 38 months after surgery. 88.9% of our patients had recurrence or metastasis within 3 years after surgery. The fact that nearly half of patients [in both Mariette et al. (5) and our study] had stage III disease may have contributed to these high recurrent rates. Loco-regional recurrence of esophageal cancer, especially in lymph nodes located in the mediastinum and cervicothoracic junction, was common. Different methods have been used to try to reduce loco-regional recurrence. There is no standard lymph node dissection used in the treatment of esophageal cancer. In theory, a three-field lymphadenectomy is the most thorough method, and might reduce loco-regional recurrence. However, even with three-field lymphadenectomy, loco-regional recurrence in esophageal cancer has been 14.2-20.4% (5,8). This is not significantly different from two-field lymphadenectomy (9). Neoadjuvant chemoradiotherapy is now widely used because it is believed to improve local-regional control and prolong survival in patients with local advanced esophageal cancer. Local-regional recurrence is still seen in 13-22% of patients who obtained a pathologically complete response after neoadjuvant therapy (10,11). Therefore, recurrences following complete resection of esophageal cancer still remain common.
We found the presence of distant metastases to be a poor prognostic factor. Liver, lung and bone were the most common sites of reported hematogenous metastases (3-5,12). In a study by Smit et al. (13), 40.3% of distant recurrences were present in the skin or soft tissue, which were the most frequent sites for distant recurrence. In our study, the most common sites for distant recurrence were still the lung, liver and bone, accounting for 81.8% of all metastases. The median time to distant metastasis was significantly shorter than to loco-regional recurrence (8 vs. 13 months), similar to that reported by Mariette et al. (5) (11 vs. 13 months). This suggests that tumor micrometastases were present at the time of surgery (14). Loco-regional lymph node recurrences do not always occur before distant metastases. Hematogenous metastases and loco-regional recurrences are thought to occur independently (15,16). 27.5% of our patients had only distant metastases, that supporting this view. O’Sullivan et al. (17) found preoperative rib and iliac bone marrow micrometastases in respectively 88% and 15% of patients with esophageal cancer. Mariette et al. (5) reported that distant metastases occurred within one year after surgery among some stage I and stage IIA patients. These findings suggest that even at an early stage, esophageal cancer is already a systemic disease, and once distant metastases occur, prognosis becomes very poor (3,12). We found the 5-year survival rate of patients with distant metastasis to be only 2.3%.
The time interval between esophageal resection and recurrence was also a prognostic factor. Hsu et al. (12) reported that recurrence within 10 months after surgery was an independent factor for poor prognosis. Shimada et al. (18) reported that patients who recurred within one year after treatment had one year shorter subsequent survival than patients that recurred later. Osugi et al. (19) showed that the time to recurrence correlated with survival after recurrence in esophageal carcinoma patients who underwent esophagectomy and extended lymphadenectomy We found that the 5-year survival rate of patients who had recurrence within 12 months after surgery was significantly lower than those who had recurrence after 12 months (4.5% vs. 24.1%), consistent with previous reports. Distant metastases occurred sooner than loco-regional recurrence, hence recurrences that occurred within 1 year after surgery were mostly distant metastasis (3). This suggests that patients with distant metastases had highly malignant tumors and that effective treatments of distant metastases are still lacking.
While recurrent diseases may not be curative, multidisciplinary treatments can improve survival. Miyata et al. (20) found that radiotherapy, chemotherapy and surgery increased survival in 196 esophageal cancer patients with postoperative recurrence, and 26.2% of patients who received radiotherapy survived longer than 2 years after recurrence. Zhang et al. (21) and Maruyama et al. (22) reported that radiochemotherapy of recurrent mediastinal lymph nodes improved survival. The treatment result was found to be related to radiation dose (21,23). We also found that treatment was an independent prognostic factor for survival. Surgical resection was effective in some patients with solitary metastases. We treated one patient with a solitary lung metastasis that survived 71 months after surgery. In a retrospective study on the clinical outcome of patients who developed pulmonary metastasis after undergoing radical esophagectomy, Takemura et al. (24) showed that survival was significantly worse in patients who did not undergo resection than in those who did, and concluded that metastasectomy was an acceptable choice of treatment for solitary pulmonary metastasis. However, only few patients were included in the study (24) and in our series, they may be considered as cases of personalized therapy. Nakamura et al. (25) reported that surgical resection plus adjuvant chemotherapy for mediastinal lymph node recurrence achieved significantly better survival than chemotherapy alone, but was not significantly different from concurrent chemoradiotherapy. Mediastinal lymph node recurrences are mostly multi-station recurrences and the patients are generally in poor health. As a consequence, most patients cannot tolerate surgery and radio-chemotherapy may become the main modality for patients with local-regional recurrences (20-23).
There are several limitations in this retrospective study. There may be bias regarding patient selection. Because of this, we did not analyze factors that may be associated with tumor recurrence. Secondly, nearly half of patients were in the disease of stage III. Since neoadjuvant chemoradiotherapy have shown survival benefit in the patients with esophageal carcinoma (26), it has become the main modality in the management of stage III disease in our institution. Finally, because different doses and schedules were used in the study, the optimum treatment regimen cannot be established for recurrence.
We found clinical and pathological features before recurrence did not correlate with prognosis after recurrence. Prognostic factors after recurrence included time of recurrence, type of recurrence and treatment after recurrence. Similar findings have been reported in the literature (12,18,19). These prognostic factors are useful in evaluating the prognosis of patients with postoperative recurrence of esophageal cancer in order to provide appropriate treatment.
Conclusions
Our retrospective study showed that time of recurrence, pattern of recurrence and treatment of recurrence were independent prognostic factors in patients with recurrence after complete resection of esophageal carcinoma. Postoperative recurrence of esophageal cancer is relatively common. Close follow-up should be performed for 2-3 years after surgery in order to timely detect tumor recurrence. For patients with local regional recurrence, chemo-radiotherapy should be given to improve survival.
Acknowledgements
Disclosure: The authors declare no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90 [DOI] [PubMed] [Google Scholar]
- 2.Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 2007;8:545-53 [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004;198:205-11 [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Wang Z, Liu XY, et al. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg 2007;31:1107-14 [DOI] [PubMed] [Google Scholar]
- 5.Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616-23 [DOI] [PubMed] [Google Scholar]
- 6.Kosugi S, Kanda T, Yajima K, et al. Risk factors that influence early death due to cancer recurrence after extended radical esophagectomy with three-field lymph node dissection. Ann Surg Oncol 2011;18:2961-7 [DOI] [PubMed] [Google Scholar]
- 7.Sobin LH, Wittekind C. eds. TNM Classification of Malignant Tumours Sixth Edition. UICC International Union Against Cancer. New York: Wiley-Liss, 2002. [Google Scholar]
- 8.Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72; discussion 972-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana M, Kinugasa S, Yoshimura H, et al. Extended esophagectomy with 3-field lymph node dissection for esophageal cancer. Arch Surg 2003;138:1383-9; discussion 1390 [DOI] [PubMed] [Google Scholar]
- 10.van Hagen P, Wijnhoven BP, Nafteux P, et al. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg 2013;100:267-73 [DOI] [PubMed] [Google Scholar]
- 11.Meguid RA, Hooker CM, Taylor JT, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg 2009;138:1309-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu PK, Wang BY, Huang CS, et al. Prognostic factors for post-recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg 2011;15:558-65 [DOI] [PubMed] [Google Scholar]
- 13.Smit JK, Pultrum BB, van Dullemen HM, et al. Prognostic factors and patterns of recurrence in esophageal cancer assert arguments for extended two-field transthoracic esophagectomy. Am J Surg 2010;200:446-53 [DOI] [PubMed] [Google Scholar]
- 14.van Lanschot JJ, Tilanus HW, Voormolen MH, et al. Recurrence pattern of oesophageal carcinoma after limited resection does not support wide local excision with extensive lymph node dissection. Br J Surg 1994;81:1320-3 [DOI] [PubMed] [Google Scholar]
- 15.Morita M, Kuwano H, Ohno S, et al. Characteristics and sequence of the recurrent patterns after curative esophagectomy for squamous cell carcinoma. Surgery 1994;116:1-7 [PubMed] [Google Scholar]
- 16.Matsubara T, Ueda M, Kaisaki S, et al. Localization of initial lymph node metastasis from carcinoma of the thoracic esophagus. Cancer 2000;89:1869-73 [DOI] [PubMed] [Google Scholar]
- 17.O’sullivan GC, Sheehan D, Clarke A, et al. Micrometastases in esophagogastric cancer: high detection rate in resected rib segments. Gastroenterology 1999;116:543-8 [DOI] [PubMed] [Google Scholar]
- 18.Shimada H, Kitabayashi H, Nabeya Y, et al. Treatment response and prognosis of patients after recurrence of esophageal cancer. Surgery 2003;133:24-31 [DOI] [PubMed] [Google Scholar]
- 19.Osugi H, Takemura M, Higashino M, et al. Causes of death and pattern of recurrence after esophagectomy and extended lymphadenectomy for squamous cell carcinoma of the thoracic esophagus. Oncol Rep 2003;10:81-7 [PubMed] [Google Scholar]
- 20.Miyata H, Yamasaki M, Kurokawa Y, et al. Survival factors in patients with recurrence after curative resection of esophageal squamous cell carcinomas. Ann Surg Oncol 2011;18:3353-61 [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Peng F, Li N, et al. Salvage concurrent radio-chemotherapy for post-operative local recurrence of squamous-cell esophageal cancer. Radiat Oncol 2012;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama K, Motoyama S, Anbai A, et al. Therapeutic strategy for the treatment of postoperative recurrence of esophageal squamous cell carcinoma: clinical efficacy of radiotherapy. Dis Esophagus 2011;24:166-71 [DOI] [PubMed] [Google Scholar]
- 23.Zhu YL, Li Q, Gao JM, et al. A retrospective evaluation of radiotherapy for the treatment of local esophageal squamous cell carcinoma recurrence after initial complete surgical resection. J Investig Med 2013;61:34-9 [DOI] [PubMed] [Google Scholar]
- 24.Takemura M, Sakurai K, Takii M, et al. Metachronous pulmonary metastasis after radical esophagectomy for esophageal cancer: prognosis and outcome. J Cardiothorac Surg 2012;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura T, Ota M, Narumiya K, et al. Multimodal treatment for lymph node recurrence of esophageal carcinoma after curative resection. Ann Surg Oncol 2008;15:2451-7 [DOI] [PubMed] [Google Scholar]
- 26.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92 [DOI] [PubMed] [Google Scholar]


