Abstract
Background
Studies have indicated that therapy with inhaled corticosteroids (ICS) can be associated with a higher risk of pneumonia. However, it is not known whether ICS increases the risk of mycobacterium. Most of these published studies were small, and the conclusions were inconsistent.
Methods
A meta-analysis was conducted into whether ICS increases the risk of mycobacterium in patients with chronic respiratory diseases. PubMed, OVID, EMBASE and Cochrane Library databases were searched.
Results
Five studies involving 4,851 cases and 28,477 controls were considered in the meta-analysis. From the pooled analyses, there was significant association between ICS and risk of mycobacterium in all patients with chronic respiratory diseases [risk ratio (RR) =1.81; 95% confidence interval (CI), 1.23-2.68; P=0.003]. Among patients with chronic respiratory diseases, the relationship between ICS and risk of tuberculosis (TB) was also significant (RR =1.34; 95% CI, 1.15-1.55; P=0.0001). And meta-analysis of four studies in patients with chronic obstructive pulmonary disease (COPD) (RR =1.42; 95% CI, 1.18-1.72; P=0.0003) or two studies in patients who have prior pulmonary TB (RR =1.61; 95% CI, 1.35-1.92; P<0.00001) or three studies in patients with high-dose ICS (RR =1.60; 95% CI, 1.28-1.99; P<0.0001) showed a relationship between ICS and risk of mycobacterium.
Conclusions
Significant relationship has been shown between ICS use and risk of mycobacterium in all patients with chronic respiratory diseases. ICS use also increases the risk of TB among the patients with chronic respiratory diseases. Use of ICS increases the risk of mycobacterium in patients with COPD or patients with prior pulmonary TB or patients inhaling high-dose corticosteroids. Further research is required to establish the potential adverse effect of ICS as a therapy for chronic respiratory diseases.
Keywords: Inhaled corticosteroids (ICS), mycobacterium, risk, meta-analysis
Introduction
Inhaled corticosteroids (ICS) play an important role in the treatment of patients with chronic respiratory diseases, especially in the case of asthma, for which they are the most effective anti-inflammatory drugs. ICS control airway inflammation by reducing synthesis of inflammatory mediators, thus controlling the airway hypersensitivity to viral infection, allergens and irritants.
The systemic side effects of long-term treatment with ICS include easy bruising (1), adrenal suppression (2), and decreased bone mineral density (3). ICS have also been associated with cataracts (4) and glaucoma (5) in cross-sectional studies. Several recent studies of patients with chronic obstructive pulmonary disease (COPD) have reported an increased risk of pneumonia among patients treated with ICS, mainly fluticasone propionate (6-9). A recent meta-analysis (10) indicated that ICS have important immunomodulatory effects on airways with COPD that may explain both their beneficial effects and the enhanced risk of pneumonia. Bahçeciler’s study (11) suggested that inhaled corticosteroid therapy is safe in tuberculin-positive asthmatic children. Although systemic administration of corticosteroids is a known risk factor for tuberculosis (TB) (12), there is no obvious evidence that use of ICS is associated with the risk of mycobacterium. Sporadic reports (13,14) and several studies (15-21) have evaluated whether ICS increase the risk of mycobacterium. However, the sample sizes were relatively modest, and the results were inconclusive. In order to investigate the precise relationship between ICS and mycobacterium, we carried out this meta-analysis.
Methods
Search strategy
We conducted an exhaustive search for studies that examined the association of ICS with mycobacterium. We searched PubMed, EMBASE, OVID, and the Cochrane library to identify available articles published before August 2013 concerning the relationship between ICS and the risk of mycobacterium. The Medical Subject Heading (MeSH) terms and/or free words that were entered were ‘ICS’ or ‘inhaled corticosteroid’ in combination with ‘TB’ or ‘mycobacterium’. Reviews and reference lists of relevant articles were also screened for additional articles of interest. There were no restrictions placed on language, race, ethnicity or geographic area.
Inclusion and exclusion criteria
Studies fulfilling the following selection criteria were included in this meta-analysis: (I) evaluation of the ICS and mycobacterium risk or TB; (II) using a case-control or cohort design; (III) exposure to ICS in both cases and controls should be available for estimating risk ratio (RR) and 95% confidence interval (CI); (IV) patients with chronic respiratory diseases. The exclusion criteria included: (I) reviews and abstracts; (II) the number exposure to ICS in both teams was not reported.
Quality score assessment
The quality of studies was independently assessed by two reviewers who used the quality assessment scores of the Newcastle-Ottawa Scale (NOS). The NOS assesses cohort and case-control studies in terms of selection of participants (sources and selection of cases and controls), and comparability of cases and controls and exposure (ascertainment of exposure and non-response rates) (22). Total scores ranged from 0 to 9, with a higher score meaning that the quality of the study was higher. There are only five included studies, so we cannot conduct a funnel plot.
Data term
Two investigators independently extracted data and appraised them. The data were compared and any discrepancies were resolved by discussion or a third author. From each study, we extracted the following information: first author, year of publication, study design, the number of cases and controls, and the study population (Table 1).
Table 1. Characteristic of included studies.
| Author | Study design | No. of subjects: all (cases/controls) | Population | NOS | Follow-up (months) | Cases |
|---|---|---|---|---|---|---|
| Brassard et al. 2011 (16) | A population-based cohort design with a nested case-control | 6,204 (564/5,640) | Respiratory diseases | 6 | 12 | TB |
| Andrejak et al. 2013 (18) | Case-control | 1,232 (112/1,120) | Chronic respiratory diseases | 8 | 6 | NTM |
| Shu et al. 2010 (15) | A case-control | 554 (16/538) | COPD | 5 | 46 | Pulmonary TB |
| Lee et al. 2013 (20) | A nested case-control study | 24,722 (4,139/20,583) | Chronic airway diseases | 7 | 12 | TB |
| Kim et al. 2013 (19) | Retrospective cohort study | 616 (20/596) | COPD | 6 | 37 | Pulmonary TB |
NO, number; NOS, Newcastle-Ottawa Scale; TB, tuberculosis; COPD, chronic obstructive pulmonary disease; NTM, non-tuberculosis mycobacteria.
Statistical analyses
We performed a meta-analysis to assess whether exposure to ICS was associated with a risk of mycobacterium. Subgroup analyses were performed for different populations. All statistical tests were performed using Revman Software (version 5.1, Cochrane Collaboration, United Kingdom London), using RR and 95% CI for dichotomous outcomes. Cochran’s Q test (23), based on the χ2 test, was used to assess the heterogeneity between the studies. If the result of the heterogeneity test had a P value >0.10, RR was pooled according to the fixed effect model. Otherwise, the random effect model was used. A P value <0.05 was considered statistically significant.
Results
Studies included in the meta-analysis
Figure 1 outlines our study selection process. A total of 125 articles were identified after an initial search. After removing duplications, 30 articles were excluded. After reading the titles and abstracts, 76 articles were excluded because they were abstracts or reviews, or irrelevant to ICS, TB or mycobacterium. After review of the full-text articles, 14 articles were excluded from the meta-analysis, and five remained. There were two article (17,20) were excluded, for although they were well designed case-control study and the exposed factor was ICS, we could not conduct an effective fourfold table according to the data provided. Consequently, a total of five eligible studies were included in this meta-analysis, with a total sample size of 4,851 cases and 28,477 controls. One was performed in European populations, one in North American populations, and three in Asian populations. Four involved TB, and one involved non-tuberculous mycobacterium (NTM). Details of the studies are summarized in Table 1.
Figure 1.
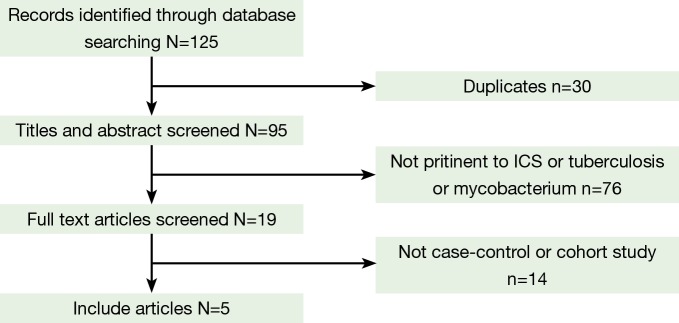
Study flow chart. ICS, inhaled corticosteroids.
ICS and mycobacterium in chronic respiratory disease patients
A summary of the findings of the meta-analysis regarding an association between ICS and the risk of mycobacterium is provided in Figure 2. Meta-analysis of the five studies (4,851 cases and 28,477 control subjects) indicated significant association between ICS and the risk of mycobacterium (RR =1.81; 95% CI, 1.23-2.68; P=0.003). In addition, the outcomes of subgroup analyses are shown in Figures 3,4,5,6,7 and Table 2. Association was similarly found between ICS and risk of TB (Figure 3: RR =1.34; 95% CI, 1.15-1.55; P=0.0001). Compared with the control group, the patients who have prior pulmonary TB (Figure 4: RR =1.61; 95% CI, 1.35-1.92; P<0.00001) or patients with COPD (Figure 5: RR =1.42; 95% CI, 1.18-1.72; P=0.0003) after using ICS show an increased mycobacterium risk. There was also significant relationship between mycobacterium and ICS in patients who inhaling high-dose corticosteroids (fluticasone >500 µg/day) (Figure 6: RR =1.60; 95% CI, 1.28-1.99; P<0.0001). Accordingly, the equivalent doses for ICS were 100 mg beclomethasone, 50 mg beclomethasone HFA, 80 mg budesonide, 200 mg triamcinolone, 32 mg ciclesonide, 50 mg fluticasone and 200 mg flunisolide. All doses were converted to fluticasone equivalents. But we found that exposure to ICS does not increase the risk of mycobacterium in the presence of oral corticosteroids (OCS) (Figure 7: RR =1.12; 95% CI, 0.80-1.56; P=0.53).
Figure 2.

The relationship between ICS and mycobacterium in chronic respiratory diseases. ICS, inhaled corticosteroids.
Figure 3.
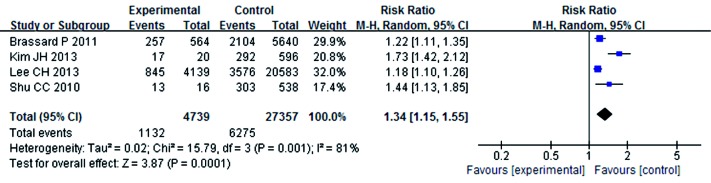
The relationship between ICS and tuberculosis risk. ICS, inhaled corticosteroids.
Figure 4.
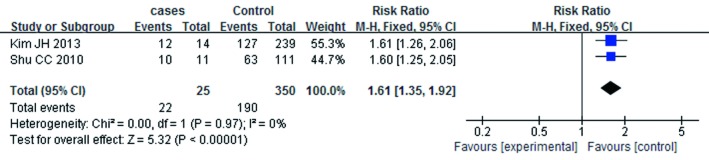
The relationship between ICS and mycobacterium in the patients prior to pulmonary TB. ICS, inhaled corticosteroids; TB, tuberculosis.
Figure 5.
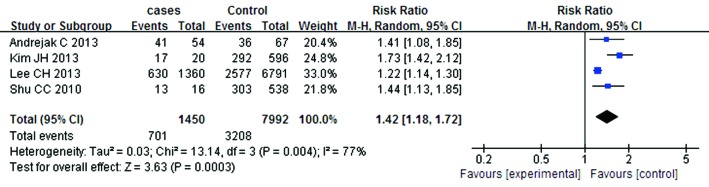
The relationship between ICS and mycobacterium in the patients with COPD. ICS, inhaled corticosteroids; COPD, chronic obstructive pulmonary disease.
Figure 6.
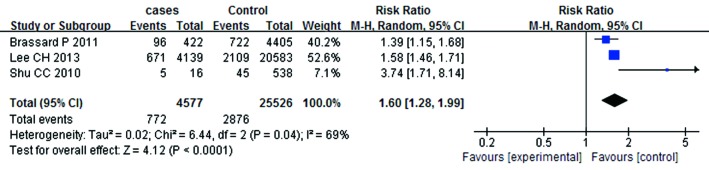
Relationship between mycobacterium and high-dose ICS. ICS, inhaled corticosteroids.
Figure 7.

Relationship between mycobacterium and ICS in patients who using OCS. ICS, inhaled corticosteroids; OCS, oral corticosteroids.
Table 2. Subgroup analyses by populations between ICS and mycobacterium.
| Population | NO. of study | RR (95% CI) | P1 | P2 |
|---|---|---|---|---|
| Overall | 5 | 1.81 (1.23-2.68) | 0.003 | <0.00001 |
| Subgroup by population | ||||
| TB | 4 | 1.34 (1.15-1.55) | 0.0001 | 0.001 |
| Prior TB | 2 | 1.61 (1.35-1.92) | <0.00001 | 0.97 |
| High doses | 3 | 1.60 (1.28-1.99) | <0.0001 | 0.04 |
| COPD | 4 | 1.42 (1.18-1.72) | 0.0003 | 0.004 |
| OCS | 2 | 1.12 (0.80-1.56) | 0.53 | 0.002 |
NO, number; RR, risk ratio; CI, confidential interval; P1, P value of pooled effect; P2, P value of heterogeneity test; TB, tuberculosis; COPD, chronic obstructive pulmonary disease; OCS, oral corticosteroids; ICS, inhaled corticosteroids.
Discussion
The sorts of mycobacterium are more, can be divided into mycobacterium TB, NTM and mycobacterium laprae. Mycobacterium TB is the cause of TB. Among infectious diseases, TB is one of the leading causes of mortality and morbidity worldwide, with over 95% of TB deaths occurring in low- and middle-income countries (24). Mycobacterium TB has developed strategies involving proteins and other compounds called virulence factors to subvert human host defences and damage and invade the human host. Therefore, it is an important task for us to prevent mycobacterium infection. ICS is frequently used to treat patients with chronic airway diseases, including asthma and COPD, as this is the most efficient anti-inflammatory treatment. ICS has become the first choice for long-term treatment of persistent asthma. ICS are metabolized via the cytochrome P450 system, which is involved in biotransformation of the majority of all drugs current available. Of the various ICS available, ciclesonide has less systemic activity, and therefore is likely to be associated with fewer adverse effects of the upper airway (25). ICS do have various systemic side effects, although less than OCS, which are a recognized risk factor for the development of active TB (26). This meta-analysis was therefore performed under the hypothesis that ICS increase the risk of mycobacterium in respiratory diseases.
Overall, we can find that ICS could increase a risk of mycobacterium or TB in all patients with chronic respiratory diseases. The mechanism behind any association between ICS use and increased mycobacterium risk remains unclear. ICS could reduce local defences in peripheral airway and may exert systemic effects through partial but consistent systemic absorption. The structural abnormalities of poorly controlled chronic airway disease with remodeling may be a risk factor for mycobacterium infection. Clinicians should realize this association and do their best to confirm or exclude definitive mycobacterium infection.
Our meta-analysis reveals that patients with prior pulmonary TB have a higher risk of mycobacterium on ICS treatment. The result could provide a warming for the patients with prior pulmonary TB. The main immune protection mechanism of TB is cellular immunity and ICS could decrease local immunity of lung (27). Therefore, ICS could easily make the latent infection of mycobacterium TB to reproduction for the patients with prior pulmonary TB. For these patients, we can use less ICS or prevent it earlier. In our study, we also find that patients with COPD using ICS are at high risk for mycobacterium. While ICS have a smaller therapeutic role in COPD than asthma, patients are more at risk from side effects (28). This could be due to several reasons: firstly, patients with COPD usually use higher doses of ICS, and many side-effects are dose-related; Secondly, patients with COPD are often older and therefore may have several co-morbid conditions, making them more susceptible to the potential side effects of ICS, such as steroid bursts for COPD exacerbation; Lastly, immune suppression may be involved in the link between steroid use and increased risk of mycobacterium among COPD patients (12). Furthermore even though the guidelines points out those COPD patients of group C and D with frequent COPD exacerbations are candidates for ICS, many patients of group A and B were treated with ICS. Evidence-based guidelines have been released that support a limited role for ICS in COPD treatment (29). There are few studies examining this, and so further interventional and/or observational studies are required to investigate the relationship between ICS and mycobacterium risk in patients with COPD.
There were two prior studies (15-16) showing high dose ICS could increase the risk of TB. And our study also shows a significant relationship between high dose ICS treatment (>500 µg/day fluticasone) and the risk of mycobacterium. Glucocorticoids can interfere with the division and proliferation of lymphoid tissue under the action of antigen, and blockade the accumulation of monocytes and macrophages induced by sensitized T lymphocyte, and then suppress the immunization. ICS can attenuate the local and systemic immunity, as adverse effect is dose dependent, therefore high dose can enlarge the effect which makes it easily for the patients with high dose ICS to infect mycobacterium. Adverse effects caused by higher doses can be avoided by using combinations of different therapeutic classes. If some patients cannot avoid to use long-term high dose ICS, they should be considered to using an ICS with less systemic activity such as ciclesonide.
Some limitations of this meta-analysis should be taken into account. Firstly, this meta-analysis is limited to five studies with 33,328 subjects. This sample size is not large enough to provide decisional clinical evidence and is not well balanced. Secondly, the studies were conducted in Asian, North American and European populations only, therefore our conclusions may only be applicable to these ethnic groups. Finally, some relevant published and unpublished studies with insufficient information or with null results were excluded, which may have biased our results.
Conclusions
In conclusion, based on this meta-analysis, it can be concluded that there was significant association between ICS and mycobacterium susceptibility or TB when all patients with chronic respiratory diseases were included. Patients with COPD or prior pulmonary TB treated with ICS or patients inhaling high dose corticosteriods are at a highly increased risk of mycobacterium. More large-scale, well designed, high quality studies are required to validate our findings. More ethnicities and different types of respiratory diseases should also be considered in future studies.
Acknowledgements
Authors’ contributions: Conceived and designed the experiments: NSS FZX. Performed the experiments: NSS LH. Analyzed the data: NSS ZJ. Contributed analysis tools: NSS LH. Wrote the paper: NSS FZX.
Funding: This study was supported by grants from: National Natural Science Foundation of China [No.30971306]; Nantong Social Development Project [No. S2009023]; Subject of Six Peek Talent of Jiangsu province [batch 7. No.033]; and the Project of the Fourth “226” High Level Talent of Nantong.
Disclosure: The authors declare no conflict of interest.
References
- 1.Mak VH, Melchor R, Spiro SG. Easy bruising as a side-effect of inhaled corticosteroids. Eur Respir J 1992;5:1068-74 [PubMed] [Google Scholar]
- 2.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med 1999;159:941-55 [DOI] [PubMed] [Google Scholar]
- 3.Lung Health Study Research Group Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000;343:1902-9 [DOI] [PubMed] [Google Scholar]
- 4.Ernst P, Baltzan M, Deschênes J, et al. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J 2006;27:1168-74 [DOI] [PubMed] [Google Scholar]
- 5.Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med 1997;337:8-14 [DOI] [PubMed] [Google Scholar]
- 6.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89 [DOI] [PubMed] [Google Scholar]
- 7.Kardos P, Wencker M, Glaab T, et al. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:144-9 [DOI] [PubMed] [Google Scholar]
- 8.Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008;177:19-26 [DOI] [PubMed] [Google Scholar]
- 9.Ernst P, Gonzalez AV, Brassard P, et al. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 2007;176:162-6 [DOI] [PubMed] [Google Scholar]
- 10.Jen R, Rennard SI, Sin DD. Effects of inhaled corticosteroids on airway inflammation in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2012;7:587-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahçeciler NN, Nuhoglu Y, Nursoy MA, et al. Inhaled corticosteroid therapy is safe in tuberculin-positive asthmatic children. Pediatr Infect Dis J 2000;19:215-8 [DOI] [PubMed] [Google Scholar]
- 12.Jick SS, Lieberman ES, Rahman MU, et al. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum 2006;55:19-26 [DOI] [PubMed] [Google Scholar]
- 13.Shaikh WA. Pulmonary tuberculosis in patients treated with inhaled beclomethasone. Allergy 1992;47:327-30 [DOI] [PubMed] [Google Scholar]
- 14.Smeenk FW, Klinkhamer PJ, Breed W, et al. Opportunistic lung infections in patients with chronic obstructive lung disease; a side effect of inhalation corticosteroids? Ned Tijdschr Geneeskd 1996;140:94-8 [PubMed] [Google Scholar]
- 15.Shu CC, Wu HD, Yu MC, et al. Use of high-dose inhaled corticosteroids is associated with pulmonary tuberculosis in patients with chronic obstructive pulmonary disease. Medicine (Baltimore) 2010;89:53-61 [DOI] [PubMed] [Google Scholar]
- 16.Brassard P, Suissa S, Kezouh A, et al. Inhaled corticosteroids and risk of tuberculosis in patients with respiratory diseases. Am J Respir Crit Care Med 2011;183:675-8 [DOI] [PubMed] [Google Scholar]
- 17.Hojo M, Iikura M, Hirano S, et al. Increased risk of nontuberculous mycobacterial infection in asthmatic patients using long-term inhaled corticosteroid therapy. Respirology 2012;17:185-90 [DOI] [PubMed] [Google Scholar]
- 18.Andréjak C, Nielsen R, Thomsen VØ, et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013;68:256-62 [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Park JS, Kim KH, et al. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest 2013;143:1018-24 [DOI] [PubMed] [Google Scholar]
- 20.Lee CH, Lee MC, Shu CC, et al. Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect Dis 2013;13:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CH, Kim K, Hyun MK, et al. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax 2013;68:1105-13 [DOI] [PubMed] [Google Scholar]
- 22.Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013;7:e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vangel MG, Rukhin AL. Maximum likelihood analysis for heteroscedastic one-way random effects ANOVA in interlaboratory studies. Biometrics 1999;55:129-36 [DOI] [PubMed] [Google Scholar]
- 24.Assam Assam JP, Penlap Beng V, Cho-Ngwa F, et al. Mycobacterium tuberculosis is the causative agent of tuberculosis in the southern ecological zones of Cameroon, as shown by genetic analysis. BMC Infect Dis 2013;13:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulet LP, Bateman ED, Voves R, et al. A randomized study comparing ciclesonide and fluticasone propionate in patients with moderate persistent asthma. Respir Med 2007;101:1677-86 [DOI] [PubMed] [Google Scholar]
- 26.Brassard P, Lowe AM, Bernatsky S, et al. Rheumatoid arthritis, its treatments, and the risk of tuberculosis in Quebec, Canada. Arthritis Rheum 2009;61:300-4 [DOI] [PubMed] [Google Scholar]
- 27.Suissa S, McGhan R, Niewoehner D, et al. Inhaled corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007;4:535-42 [DOI] [PubMed] [Google Scholar]
- 28.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet 2009;373:1905-17 [DOI] [PubMed] [Google Scholar]
- 29.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179-91 [DOI] [PubMed] [Google Scholar]


