ABSTRACT
The development of gastritis during Helicobacter pylori infection is dependent on an activated adaptive immune response orchestrated by T helper (Th) cells. However, the relative contributions of the Th1 and Th17 subsets to gastritis and control of infection are still under investigation. To investigate the role of interleukin-21 (IL-21) in the gastric mucosa during H. pylori infection, we combined mathematical modeling of CD4+ T cell differentiation with in vivo mechanistic studies. We infected IL-21-deficient and wild-type mice with H. pylori strain SS1 and assessed colonization, gastric inflammation, cellular infiltration, and cytokine profiles. Chronically H. pylori-infected IL-21-deficient mice had higher H. pylori colonization, significantly less gastritis, and reduced expression of proinflammatory cytokines and chemokines compared to these parameters in infected wild-type littermates. These in vivo data were used to calibrate an H. pylori infection-dependent, CD4+ T cell-specific computational model, which then described the mechanism by which IL-21 activates the production of interferon gamma (IFN-γ) and IL-17 during chronic H. pylori infection. The model predicted activated expression of T-bet and RORγt and the phosphorylation of STAT3 and STAT1 and suggested a potential role of IL-21 in the modulation of IL-10. Driven by our modeling-derived predictions, we found reduced levels of CD4+ splenocyte-specific tbx21 and rorc expression, reduced phosphorylation of STAT1 and STAT3, and an increase in CD4+ T cell-specific IL-10 expression in H. pylori-infected IL-21-deficient mice. Our results indicate that IL-21 regulates Th1 and Th17 effector responses during chronic H. pylori infection in a STAT1- and STAT3-dependent manner, therefore playing a major role controlling H. pylori infection and gastritis.
IMPORTANCE
Helicobacter pylori is the dominant member of the gastric microbiota in more than 50% of the world’s population. H. pylori colonization has been implicated in gastritis and gastric cancer, as infection with H. pylori is the single most common risk factor for gastric cancer. Current data suggest that, in addition to bacterial virulence factors, the magnitude and types of immune responses influence the outcome of colonization and chronic infection. This study uses a combined computational and experimental approach to investigate how IL-21, a proinflammatory T cell-derived cytokine, maintains the chronic proinflammatory T cell immune response driving chronic gastritis during H. pylori infection. This research will also provide insight into a myriad of other infectious and immune disorders in which IL-21 is increasingly recognized to play a central role. The use of IL-21-related therapies may provide treatment options for individuals chronically colonized with H. pylori as an alternative to aggressive antibiotics.
INTRODUCTION
Helicobacter pylori is a Gram-negative microaerophilic bacterium and a dominant member of the gastric microbiota harbored by approximately 50% of the world’s population. A hallmark of H. pylori infection is a gastric mucosal inflammatory response, termed superficial gastritis (1). The presence of H. pylori increases the risk for development of duodenal ulcer disease, gastric ulcer disease, noncardia gastric adenocarcinoma, and B-cell malignancies, such as gastric mucosa-associated lymphoid tumors (MALT lymphomas) and high-grade lymphomas (reviewed in references 2 and 3). Conversely, there is also increasing evidence that H. pylori colonization protects against esophageal and cardial pathologies (4–7), childhood asthma (8–10), and childhood allergies (9, 11). The gastritis associated with H. pylori infection reflects the recruitment and activation of immune cells representing both innate and adaptive immunity (12 reviewed in reference 13). Actual treatment for H. pylori involves an aggressive triple-antibiotic treatment that unbalances the gastric microbiota. Furthermore, recent studies suggest that H. pylori is finding strategies to bypass the treatment by developing resistance to clarithromycin (14). Other studies have pointed out that during chronic H. pylori infection, the exacerbated immune response in the gastric lamina propria is driving more epithelial cell damage than the bacterium itself (15). Therefore, new strategies to treat chronic H. pylori infections are needed. H. pylori infection of humans and experimental infection of rodents typically results in a mixed T helper 1 (Th1)/Th17-mediated immune response (12, 16–26). The long-term chronic inflammatory response to H. pylori is believed to drive or initiate the pathways which lead to the adverse outcomes of colonization, including chronic gastritis, intestinal metaplasia, and gastric cancer. Our mouse model of H. pylori infection is set up to investigate this critical pathway during chronic infection, focusing on the outcome of gastritis.
T cells play a decisive role in initiating and shaping pathological and protective responses in tissues. Classical examples of T cell-mediated diseases are inflammatory bowel disease (IBD), type 1 diabetes, psoriasis, rheumatoid arthritis, and multiple sclerosis. Relevant to this study, interleukin-21 (IL-21) is a cytokine produced mostly by activated CD4+ T cells, especially Th17 cells, T follicular helper (Tfh) cells, and NKT cells. IL-21 induces proliferation and increases cell survival and cytokine synthesis in many immune cells (reviewed in reference 27). H. pylori also upregulates IL-21 during infection, correlating the IL-21 expression with levels of gastritis in the mouse model (28). Moreover, IL-21 was associated with H. pylori infection in a study of infected humans (29).
Immunoinformatic approaches cannot replace traditional experimentation; however, they can be used to synthesize, organize, and integrate diverse types of data and theoretical frameworks to help generate new knowledge and target in vivo experimentation. Indeed, computer simulations of immunological processes can predict novel experimental behaviors, correlations, and interactions between components of a complex system, such as the signaling pathways controlling the differentiation and function of Th cells (30, 31). A CD4+ T cell computational model was built, calibrated, and validated to investigate interactions of external cytokines and transcription factors within a CD4+ T cell in the absence of infection (32). Our initial CD4+ T cell modeling studies investigated the importance of the peroxisome proliferator-activated receptor gamma (PPARγ) in regulating the plasticity between Th17 and inducible T regulatory cells (iTreg) (15). To not only observe intracellular events but also have a cellular understanding of the immune response toward H. pylori, we also used a published tissue-level model to study how CD4+ T cell subsets influenced the initiation, progression, and outcome of disease (15).
This study leverages our published CD4+ T cell model (32) and cellular H. pylori model (15) to establish a chronic-H. pylori-specific CD4+ T cell differentiation model that allowed us to investigate the role of IL-21 in the maintenance of the T cell-mediated gastric mucosal responses to chronic H. pylori infection. Thus, we combined computational modeling and mechanistic experimental studies in mice to dissect the effects of H. pylori infection on the intracellular pathways by which IL-21 modulates CD4+ T cell responses during chronic infection. Our in silico and in vivo data suggest that IL-21 is a key cytokine for maintenance of both the Th1 and Th17 response during H. pylori infection. Furthermore, we provide novel evidence that IL-21 is required for the development of gastritis and control of the H. pylori bacterial burden, as well as for the modulation of T cell-derived IL-10 and phosphorylation of both STAT1 and STAT3. These data together represent key knowledge that could help in the development of novel IL-21-centered immunotherapeutics for controlling infectious and immune-mediated diseases.
RESULTS
IL-21 deficiency leads to increased H. pylori colonization in the mouse model.
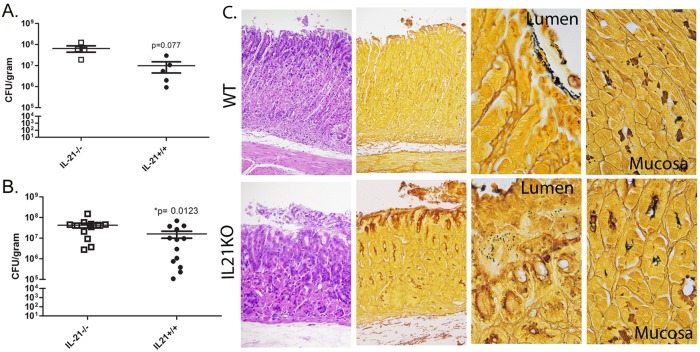
The first step to evaluate the role of IL-21 during H. pylori infection in vivo was to determine any effects on H. pylori burden in the mouse stomach. IL-21-deficient (IL-21−/−) mice and their wild-type littermates were infected with H. pylori strain SS1. At time points up to 3 months postinfection, mice were sacrificed and H. pylori colonization was measured by culture using serial dilution colony counting. At 1 month postinfection, there was no significant difference in the levels of colonization of IL-21−/− mice and wild-type mice (data not shown). However, at later time points, as chronicity developed, IL-21−/− mice had significantly higher levels of H. pylori colonization than their wild-type littermates on the B6;129 background at both 2 months (Fig. 1A) and 3 months postinfection (Fig. 1B). We also observed this increase in bacterial burden in infected IL-21−/− mice from the C57BL/6 background (see Fig. S3A in the supplemental material), suggesting that IL-21 modulates the immune response, resulting in greater H. pylori clearance in the gastric mucosa of wild-type mice. In order to localize the presence of H. pylori bacteria in the gastric tissue, a modified Steiner stain was performed on sections from the gastric tissue at 3 months postinfection. When inflammation was present in the H. pylori-infected wild-type mice (Fig. 1C), bacteria localized to the mucus on the lumen side of the tissue and were rarely observed deeper in the tissue. In contrast, in the H. pylori-infected IL-21−/− mice, where there was minimal inflammation, as well as in areas of the stomach in wild-type mice where inflammation was low, bacteria were present both in the mucus and in the glands and were also deeper in the tissue (Fig. 1C).
FIG 1 .
IL-21 is required to control H. pylori infection in the mouse model. IL-21−/− mice and wild-type littermates were infected with H. pylori strain SS1 for up to 3 months. (A and B) Levels of colonization were measured by plating serial dilutions of stomach homogenates. The CFU counts were then calibrated to the weight of the tissue and are presented in the graphs as log(CFU/gram) at 2 months (A) and 3 months (B) postinfection. Bars and error bars represent means ± standard errors of the means (SEM). Results are representative of 3 independent experiments. (C) Steiner and hematoxylin and eosin stains were performed in gastric mucosa sections of H. pylori-infected wild-type (WT) and IL-21−/− mice (from left to right, sections are at ×200, ×200, ×1,000, and ×1,000). Sections are representative of those from 10 wild-type and 10 IL-21−/− mice at 3 months postinfection.
IL-21 deficiency protects H. pylori-infected mice from chronic gastritis.
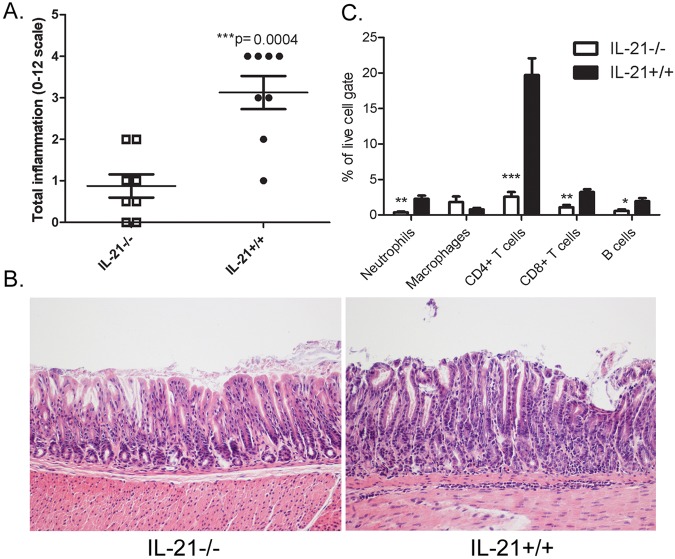
We next sought to evaluate the level of gastritis following H. pylori infection, and tissues were scored for inflammation. Scoring the tissue for acute and chronic inflammation in both the antrum and the corpus provided a quantitative method for assessing the presence of the neutrophils (acute inflammation) and lymphocytes (chronic inflammation). H. pylori-infected IL-21−/− mice had significantly reduced inflammation compared to the levels in H. pylori-infected wild-type mice on both the B6;129 (Fig. 2A) and C57BL/6 background (see Fig. S3B in the supplemental material). Representative photomicrographs demonstrated the lack of inflammation present in the IL-21−/− mice compared to that in their wild-type littermates at 3 months postinfection on the B6;129 (Fig. 2B) and C57BL/6 backgrounds (Fig. S3C). These data suggest that IL-21 plays a role in controlling chronic H. pylori colonization and makes a significant contribution to the generation of chronic gastritis.
FIG 2 .
Inflammation is reduced in H. pylori-infected IL-21−/− mice compared to the levels in their H. pylori-infected wild-type littermates. Levels of acute and chronic inflammation were scored for stomach tissue (in the corpus and antrum) at 3 months postinfection. (A) Total inflammation was scored on a scale of 0 to 12 (bars represent means, and error bars represent upper and lower interquartile ranges). (B) Representative sections of the gastric mucosa from 3 months postinfection are presented (×200). (C) Flow cytometric analysis was performed on dissociated stomach tissue at 3 months postinfection (n = 8 per genotype). Percentages of neutrophils (Gr1+ CD11c+), macrophages (CD11b+ Gr1−), CD4+ CD3+ T cells, CD8+ CD3+ T cells, and B cells (B220+) were calculated in the live-cell gate from the H. pylori-infected mice. Bars and error bars represent means ± SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. Results are representative of 3 independent experiments.
To evaluate specifically the effects of IL-21 deficiency on cell migration to the stomach, we immunophenotyped isolated gastric lamina propria cells by flow cytometry. The most striking finding was that IL-21 deficiency significantly affected the numbers of CD4+ T cells in the H. pylori-infected stomachs. In addition, reduced numbers of CD8+ T cells, B lymphocytes, and neutrophils were found in the gastric mucosa of H. pylori-infected IL-21−/− mice compared to the numbers of these cells in H. pylori-infected wild-type littermates (Fig. 2C).
Chemokine and cytokine expression are abrogated in IL-21-deficient mice.
To investigate how the levels of chemokines and other inflammatory cytokines were affected by the IL-21 deficiency, a multiplex protein assay was performed at 2 and 3 months postinfection. Our data demonstrate that many interferon gamma (IFN-γ)-induced chemokines, including RANTES, IFN-inducible protein-10 (IP-10), and macrophage inflammatory protein-1β (MIP-1β), were present at significantly lower levels in the H. pylori-infected IL-21−/− mice than in their H. pylori-infected wild-type littermates (see Fig. S4A in the supplemental material). Moreover, the levels of the IL-17-induced chemokine KC (a mouse homologue of IL-8) were significantly lower in the H. pylori-infected IL-21−/− mice than in their H. pylori-infected wild-type littermates (Fig. S4B). The levels of the proinflammatory cytokines TNF-α and IL-1β, which can enhance inflammation or induce further effector T cell differentiation, were significantly lower in the H. pylori-infected IL-21−/− mice than in their H. pylori-infected wild-type littermates both at the protein (Fig. S4C) and RNA levels (Fig. S4D).
IL-21 deficiency leads to abrogated Th17 and Th1 effector responses.
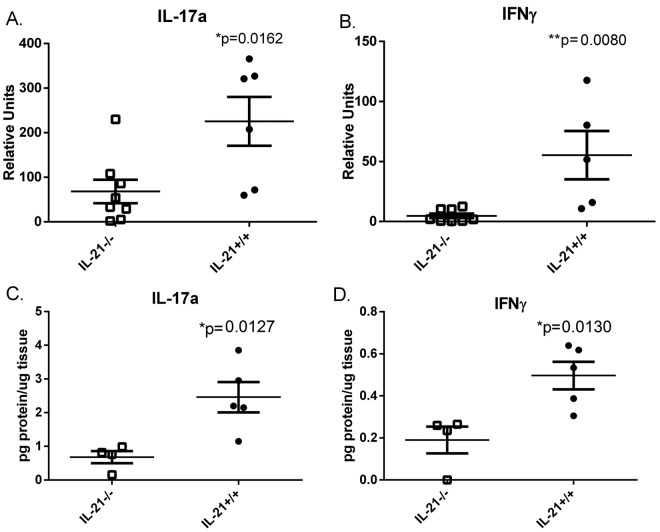
IL-21 plays a role in the maintenance of the Th17 responses (33). To investigate the role of IL-21 in the production of mucosal T cell-derived cytokines in the context of H. pylori infection, real-time reverse transcription (RT)-PCR was performed on RNA isolated from the stomachs of H. pylori-infected IL-21−/− mice and their H. pylori-infected wild-type littermates. The expression of both IL-17 (Fig. 3A) and IFN-γ (Fig 3Β) was significantly reduced in the H. pylori-infected IL-21−/− mice compared to their expression in H. pylori-infected wild-type littermates by 3 months postinfection. We found similar results with our C57BL/6 mice (Fig. S3D). These significant differences were confirmed by using a protein-based assay on stomach lysates (Fig. 3C and D).
FIG 3 .
Th17 and Th1 responses are reduced in H. pylori-infected IL-21−/− mice compared to the levels in H. pylori-infected wild-type mice. (A and B) Real-time RT-PCR was performed on stomach tissue of H. pylori-infected mice. Relative units of IL-17A (A) and IFN-γ (B) were measured at 3 months postinfection. Relative units are normalized using the relative expression calibrated to that in uninfected wild-type mice, with GAPDH as the endogenous control. Results are representative of 3 independent experiments. Bars and error bars represent means ± SEM. *, P ≤ 0.05; **, P ≤ 0.01. (C and D) Gastric protein levels were measured in the stomach tissue using a Milliplex assay at 2 and 3 months postinfection. Protein levels are reported as picogram of protein per microgram of total tissue. IL-21−/− mice express reduced protein levels of IL-17A (C) and IFN-γ (D) during chronic infection. Graphs are representative of 2 independent experiments. Bars and error bars represent means ± SEM. *, P ≤ 0.05.
IL-21 contributes to Th17 differentiation and modulates Th1 responses upon infection with H. Pylori.
CD4+ T cell responses are believed to drive chronic inflammation and contribute to long-term damage associated with adverse outcomes of chronic H. pylori infection. Based on our experimental data, IL-21 plays a role in maintaining the activation of CD4+ T cells, since H. pylori-infected IL-21−/− mice have significantly reduced inflammation in their gastric mucosa compared to that in wild-type littermates, with the most striking reduction in CD4+ T cells. To gain a more comprehensive mechanistic understanding of why the lack of IL-21 has such an impact on the development and maintenance of CD4+ T cells and their function in the gastric mucosa of H. pylori-infected mice, we used our cytokine data to leverage a computational and mathematical model that simulates CD4+ T cell differentiation. Specifically, the original CD4+ T cell differentiation model (32) was calibrated with real-time RT-PCR data from wild-type or IL-21−/− mice infected with H. pylori for 3 months (Fig. 3; see also Text S1 in the supplemental material) to represent the influence of the cytokine environment that a CD4+ T cell encounters during infection. To determine the effect of the loss of IL-21 in CD4+ T cell differentiation and function, we engineered and modeled an IL-21-deficient system (Text S1). Modulation of transcription factors is critical for Th cell differentiation and cytokine production (reviewed in reference 34). To broadly investigate the contribution of specific molecules to IL-21, sensitivity analysis was run on internal CD4+ T cell-specific IL-21. The simulation results showed a positive correlation for Th17-related molecules, such as phosphorylated STAT3 (P-STAT3), IL-17, and RORγt (Fig. 4A). T-bet, IFN-γ, and phosphorylated STAT1 (P-STAT1) were also found to be positively correlated with IL-21 (Fig. 4A). Interestingly, the FOXP3 and IL-10 results from the sensitivity analysis showed a negative correlation to IL-21 (Fig. 4A). In silico experimentation indicated that there is a dramatic downregulation of RORγt, IL-17, and phosphorylation of STAT3 during Th17 differentiation when IL-21 production is deleted (Fig. 4B). Our results also demonstrated a downregulation of IFN-γ and T-bet following Th1 differentiation in the IL-21-deficient model compared to their expression in the wild-type model (Fig. 4C). Next, we sought to determine the effect of an in silico upregulation of IL-21 in Th17-differentiated CD4+ T cells and found that P-STAT3, IL-17, and RORγt were upregulated with increasing doses of IL-21 (Fig. 4D). These effects were abrogated in the IL-21-deficient system (Fig. 4E). Of note, when evaluating Th1 and Th17 populations in our H. pylori tissue-level model, we also found reduced levels of Th1 and Th17 populations in the IL-21-deficient model compared to the levels in the wild-type model during the chronic stage of infection (Fig. S6). These in silico results provided evidence that IL-21 plays a key role in CD4+ T cell modulation during H. pylori-induced Th1/Th17 CD4+ T cell responses at chronic time points.
FIG 4 .
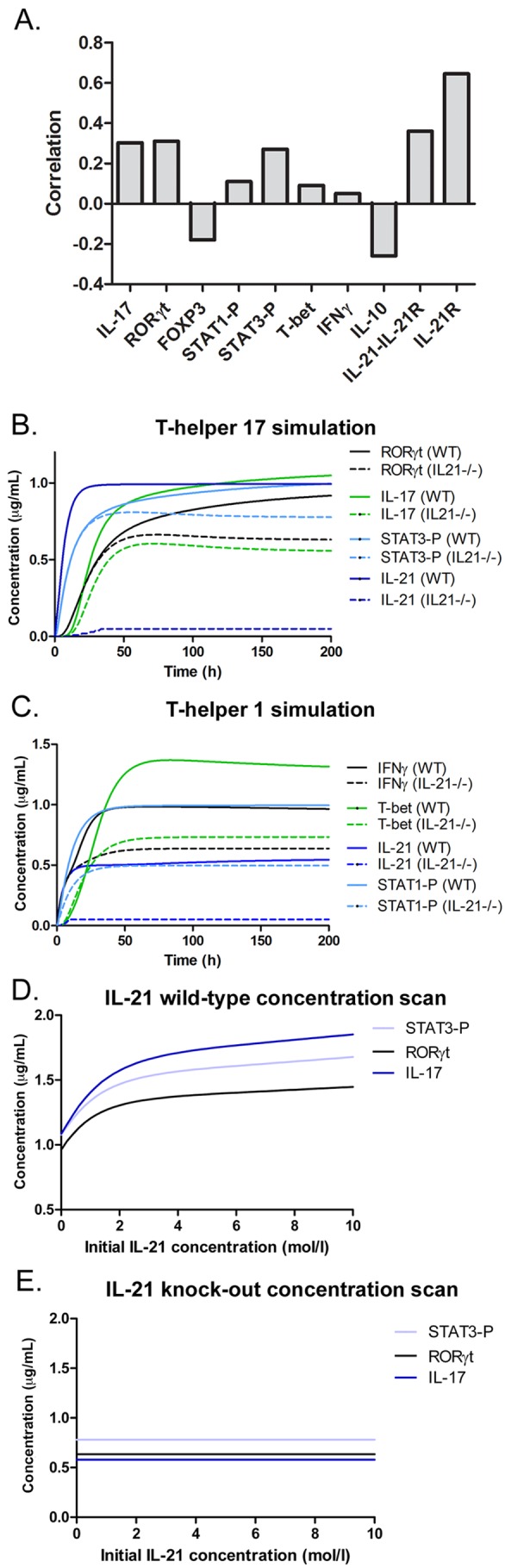
The CD4+ T cell computational model predicts modulation of differentiation and maintenance by IL-21 in silico. (A) Sensitivity analysis of IL-21 over Th1- and Th17-related molecules showing positive or negative correlation to IL-21. (B) Effect of IL-21 deficiency on modulation of RORγt, IL-17, IL-21, and STAT3 in the computed Th17 system. (C) Effect of IL-21 deficiency on modulation of T-bet and IFN-γ in the computed Th1 system. (D and E) Upregulation of RORγt, IL-17, and STAT3-P in a Th17 state with increasing concentrations of IL-21 in the wild-type system (D) but not in the IL-21−/− system (E) during a time course of 200 hours.
Experimental validation of model predictions: IL-21 modulates the expression of Th1 and Th17 transcription factors.
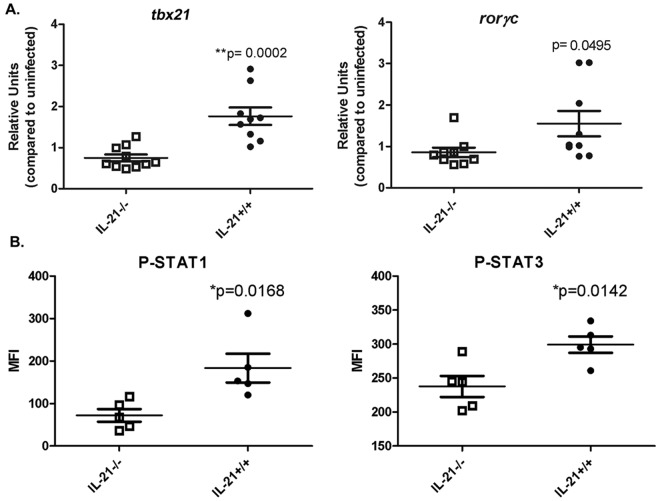
Guided by the model predictions, we sought next to investigate whether IL-21 affects the gene expression of CD4+ T lymphocyte transcription factors tbx21 and rorc (which translate to T-bet and RORγt) in vivo. CD4+ T cells were isolated from spleens of H. pylori-infected IL-21−/− mice and wild-type littermates at 3 months postinfection, and real-time RT-PCR was performed. As predicted, the expression of tbx21 and rorc was significantly reduced in the CD4+ cells from H. pylori-infected IL-21−/− mice compared to their expression in CD4+ cells from H. pylori-infected wild-type littermates (Fig. 5A).
FIG 5 .
Transcription factors associated with Th1 and Th17 are affected by IL-21 deficiency in the mouse model. (A) CD4 lymphocytes isolated from H. pylori-infected IL-21−/− mice express reduced levels of tbx21 and rorc. Gene expression levels of transcription factors tbx21 and rorc were measured in CD4+ T cells isolated from the spleens of H. pylori-infected IL-21−/− and wild-type mice. Relative units are normalized using the relative expression calibrated to expression in CD4+ splenocytes from uninfected mice, with GAPDH as the endogenous control. (B) The levels of phospho-STAT1 and phospho-STAT3 in unstimulated cells from infected mice were measured by flow cytometry, and the mean fluorescence intensities (MFI) of the CD4+ splenocytes are reported. Bars and error bars represent means ± SEM. *, P ≤ 0.05; **, P ≤ 0.01.
Our computational model also predicted that IL-21 correlates positively with phosphorylation of STAT1 and STAT3. Indeed, the in silico IL-21−/− model showed a downregulation of P-STAT3 in Th17 cells (Fig. 4B) and P-STAT1 in Th1 cells (Fig. 4C). To test this prediction in vivo, the levels of P-STAT1 and P-STAT3 were measured in CD4+ splenocytes from H. pylori-infected IL-21−/− and H. pylori-infected wild-type mice at 3 months postinfection. The data indicate that, while total phosphorylation of STAT1 and STAT3 is low when the cells are not restimulated ex vivo, the mean fluorescence intensities of the P-STAT1 and P-STAT3 staining are significantly lower in the CD4+ splenocytes from IL-21−/− mice than in those from wild-type mice (Fig. 5B).
Expression of markers of T cell regulation (FOXP3 and IL-10) in IL-21-deficient mice.
Since we observed a negative correlation of IL-10 and FOXP3 in relation to IL-21 (Fig. 4A), we investigated whether there was a change in IL-10 and FOXP3 expression in iTreg cells in the gastric mucosa, since the major expression of FOXP3 comes from regulatory T cells. To do so, we performed computational simulations in the CD4+ T cell differentiation model and evaluated the level of IL-10 in Th17 and the level of FOXP3 in iTreg cells. We hypothesized that an increase in Treg cells may explain the decrease in gastritis found in IL-21−/− mice. However, in silico results differentiating the mathematical model with transforming growth factor-β (TGF-β) and IL-2 showed no difference between wild-type and IL-21−/− mice in the expression of FOXP3 within the Treg cell subset (Fig. 6A). Furthermore, our mathematical model predicted a dramatic upregulation of IL-10 in Th17 cells in the IL-21-deficient system compared to the response in the wild-type system when induced with IL-6 and TGF-β together (Fig. 6B). To validate these predictions, we measured foxp3 transcript levels in CD4+ T cells isolated from the spleens of H. pylori-infected IL-21−/− mice and wild-type littermates at 3 months postinfection. The experimental results showed no significant difference in the CD4+ T lymphocytes, validating the model’s prediction and suggesting that the reduction in Th1 and Th17 effector responses is not mediated through an increase in iTreg cells (Fig. 6C). We similarly measured IL-10 expression in CD4+ T lymphocytes isolated from the spleens of H. pylori-infected IL-21−/− mice and wild-type mice. As the model predicted, IL-10 expression was significantly higher in CD4+ T lymphocytes from H. pylori-infected IL-21−/− mice than in those from H. pylori-infected wild-type littermates (Fig. 6D).
FIG 6 .
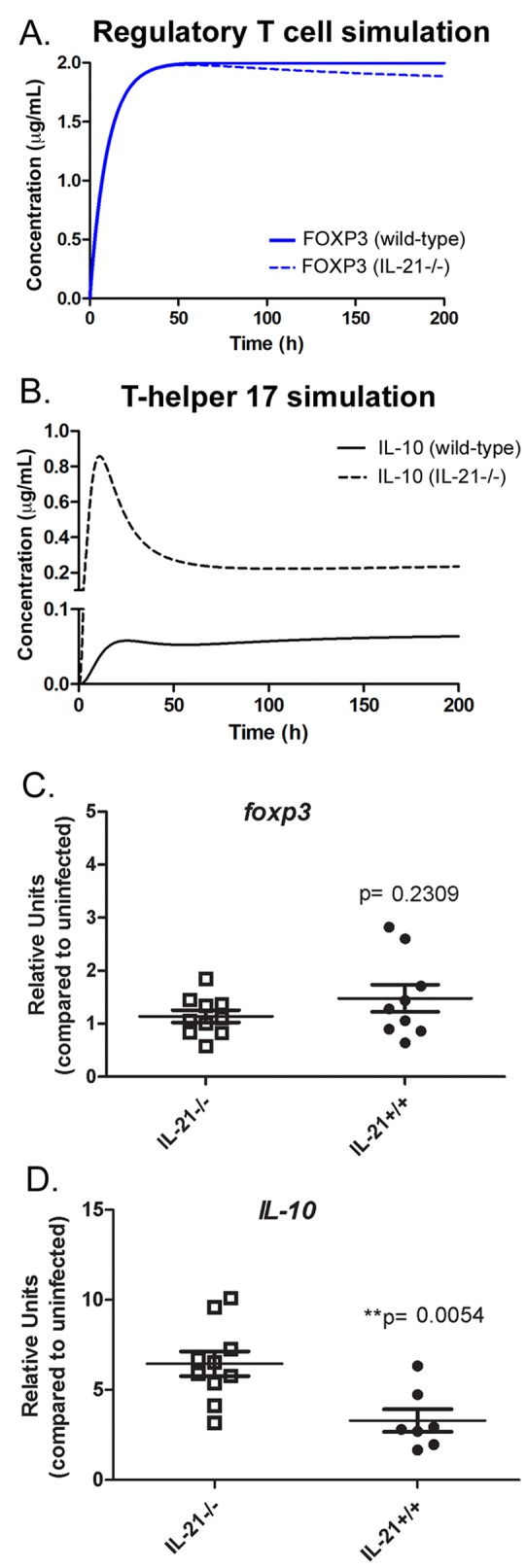
IL-21 modulates the expression of IL-10 during Helicobacter pylori infection. (A and B) The CD4+ T cell model predicted no differences in the expression of FOXP3 in wild-type and IL-21−/− mice (A) and a higher production of IL-10 in IL-21−/− mice (B). (C and D) To validate those predictions, the levels of mRNA in CD4+ T cells of spleens of H. pylori-infected mice were assessed for foxp3 transcripts (C) and il-10 transcripts (D). Bars and error bars represent means ± SEM.
DISCUSSION
Since the identification of Th17 cells almost a decade ago, we have gained a better understanding of how CD4+ T helper cells help control bacterial colonization beyond providing B cell help and activating macrophage function. Th17 cells play a central role in controlling many bacterial infections through the activation of chemokine pathways and antimicrobial responses. They activate these pathways through the production of IL-17A, IL-17F, and IL-22. In addition, IL-21, which is produced by Th17 cells, plays a role in amplifying the Th17 cell effector response (35). In this study, we find not only that IL-21 is required for the maintenance of IL-17 production in the gastric mucosa following H. pylori infection but also that IL-21 plays an important role in the development and maintenance of the IFN-γ response. As a result, a deficiency in IL-21 protected H. pylori-infected mice from chronic gastritis, at the expense of increased bacterial burden. Since IFN-γ and IL-17 can induce chemokine expression, the lack of Th1 and Th17 responses in the gastric mucosa of the H. pylori-infected IL-21−/− mice leads to reduced chemokine expression and fewer neutrophils. Since neutrophils are likely controlling the bacterial burden, IL-21−/− mice have more bacterial colonization and, interestingly, Steiner stains indicated that the bacteria in the IL-21−/− mice are typically localized not only in the mucus layer but also deeper in the tissues (i.e., in glands) (Fig. 1C). Despite the increased bacterial burden and deeper localization of H. pylori in the tissue, in our mouse model, the infected IL-21−/− mice did not show any signs of discomfort or distress. In wild-type mice, where inflammation occurs in a localized manner, the H. pylori bacteria localized to the mucus layer, but the bacteria were not observed in the gland. It is worth noting that there are areas in wild-type mice where inflammation is low, and in those areas, H. pylori can also be found deeper in the tissue.
Immunoinformatic approaches are increasingly useful tools to provide insight into potential trends through integrating current datasets and knowledge and detecting behavioral responses at the systems level. In this specific study, after observing the significantly higher bacterial burden in the stomachs of IL-21−/− mice (Fig. 1), we found that these mice in fact have less leukocytic infiltration, including very few CD4+ T cells in the gastric lamina propria (Fig. 2). One potential explanation is that IL-21 impairs the ability of CD4+ T cells to mount a proper inflammatory response and affects a specific T cell product that would maintain or promote CD4+ T cell recruitment in the gastric lamina propria. This would explain the lack of inflammation in the gastric lamina propria. The ability of the innate immune compartment to clear out the bacteria could then be less effective without the proper effector response driven by CD4+ T cells. As a matter of fact, since CD4+ T cells were our focus, we leveraged the existing CD4+ T cell differentiation model (32) by recalibrating the model with H. pylori-specific data (Fig. 3) to understand what intracellular events were occurring within the CD4+ T cell compartment, in order to target experimental studies with predicted hypotheses. Indeed, by using a modeling approach that simulates the time period of CD4+ T cell differentiation and calibrating with chronic H. pylori data, we could determine which are the pathways most affected by the presence or lack of IL-21. Moreover, we could characterize cross talk between pathways within the CD4+ T cell differentiation model at the chronic stages of H. pylori infection. The results of previous studies suggest that IL-21 is a key cytokine for Th17 cell maintenance. Indeed, our in silico results demonstrate that differences between the wild-type and the IL-21 knockout models are not noticeable at the first stages of differentiation. However, when Th17 is fully differentiated, IL-21 is required for the upregulation of IL-17 and RORγt and phosphorylation of STAT3. In fact, the results of the tissue-level model show a clear difference in Th1 and Th17 populations when comparing the IL-21-deficient system with the wild-type system. Furthermore, our computational simulations show a dramatic downregulation of IL-17 and RORγt and phosphorylation of STAT3 in a fully differentiated Th17 cell. IL-21 also plays an important role in Th1 responses, as illustrated by the smaller amounts of P-STAT1 and IFN-γ and reduced T-bet expression. This computational approach allowed us to target experimental studies and be able to confirm and validate all these findings in our in vivo model of H. pylori infection.
In this regard, IL-10 has long been known to play an important role in the regulation of Th1 cell responses to pathogens (36). It has also been shown that Th1 effector cells are themselves coproducers of IL-10 and IFN-γ, since they use IL-10 as a self-regulation mechanism (37, 38), and that TGF-β and IL-6 can induce a CD4+ T cell subset that coproduces IL-17 and IL-10 (39). These coproducers are considered self-regulatory Th17 cells. Our modeling results predict that IL-21 might be involved in controlling the balance between regulatory and effector responses during H. pylori infection through an IL-10 mechanism. Indeed, the CD4+ T cell computational model predicted an upregulation of Th17-specific IL-10 in IL-21−/− mice, supporting the notion that IL-21 negatively modulates the expression of IL-10 in Th17 cells. Taken together, these findings indicate that IL-21 has emerged as a central molecule in CD4+ T cell differentiation, which promotes effector responses in Th1 and Th17 cells through P-STAT1 and P-STAT3, respectively, and also downregulates the gene expression of il-10 in Th17 cells (Fig. 7A). In this study, our modeling approaches were predictive of the in vivo outcomes (Fig. 7B).
FIG 7 .
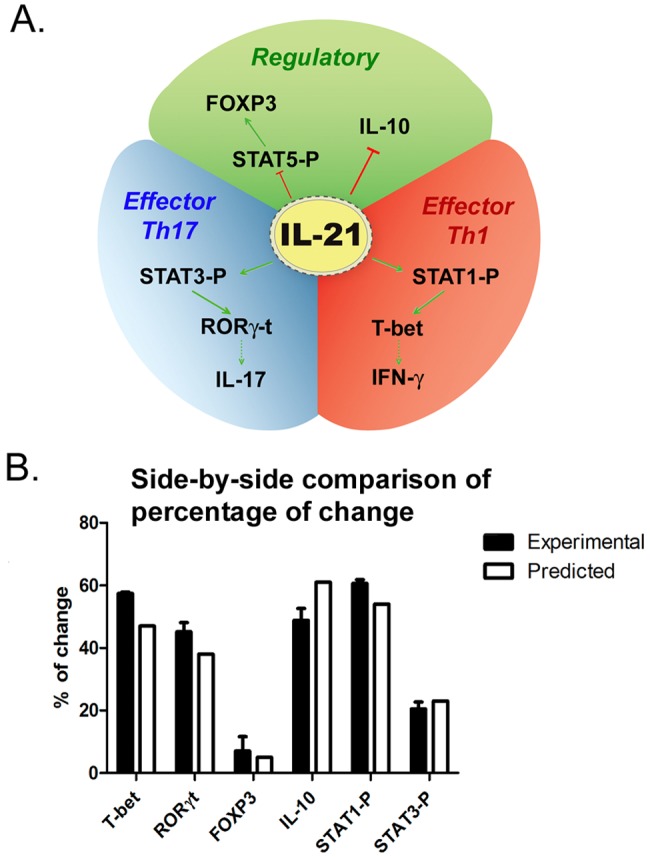
Predictive value of the CD4+ T cell differentiation model. (A) Cartoon representation of IL-21 interaction within the CD4+ T cell subset. (B) Side-by-side comparison assessing the differences between the model’s predicted percentages of change between the wild-type and the IL-21−/− systems and the experimental results for wild-type and IL-21−/− mice. Bars and error bars represent means ± SEM.
While several studies have investigated the role of Th17 versus Th1 cells during control of H. pylori infection and the development of gastritis, there does not appear to be a clear indication that one Th subset plays a more important role than the other. Murine studies have shown that Th1 responses are associated with increased gastritis, since IFN-γ−/− mice have decreased levels of gastric inflammation (40). These studies have also shown that an insufficient Th1 response is associated with increased bacterial colonization (40, 41). However, there is also evidence that adoptive transfer into SCID mice of CD4+ T cells from T-bet−/− mice, which do not exhibit IFN-γ production and Th1 differentiation, still results in gastritis (42), leaving the door open for a role for Th17 cells or other effector immune cell subsets in gastritis. Some studies have suggested that, in the absence of IL-17, there is a decrease in inflammation and an increase in Th1 effector responses, which then drive down bacterial infection. On the other hand, the results of our previously published study suggest that, in the absence of IL-17RA (and IL-17 signaling), the Th1 response is not affected and, while there is a decrease in acute inflammation driven by neutrophils, the loss of IL-17 signaling leads to increased bacterial burden and increased chronic inflammation, especially B lymphocyte density (28). The increased bacterial burden and chronic inflammation were accompanied by an increase in IL-21 expression as well. We hypothesize that IL-21 may drive the chronic inflammation in the IL-17RA−/− mice infected with H. pylori. Our studies in IL-21−/− mice infected with H. pylori presented here demonstrate that IL-21 is required for both Th1 and Th17 responses to be maintained during chronic infection.
A hypothesis for why both Th1 and Th17 responses are suppressed in IL-21−/− mice is that there may be an increase in Treg cells in the absence of IL-21 signaling. Whereas sensitivity analysis performed with our CD4+ T cell computational model indicated that FOXP3 could potentially be negatively correlated to IL-21, the additional time course, loss-of-function, and in silico experiments and our data suggest that the number of Tregs is not affected by the deficiency of IL-21. Indeed, the suppression of Th1 and Th17 responses is a more direct effect on the CD4+ T cells as a consequence of the IL-21 deficiency. Our data indicate that at 3 months postinfection, there is no increase of Treg cells in the stomachs or in the spleens of the H. pylori-infected IL-21−/− mice compared to the Treg cell numbers in the stomachs or spleens of their wild-type littermates, but this is not surprising, since H. pylori IL-21−/− mice have little inflammation in their stomachs and there is an overall decrease in T cells in the stomachs of the IL-21−/− mice. Even in the periphery (spleen), the levels of foxp3 expression are not significantly different in the H. pylori-infected IL-21−/− mice and their H. pylori-infected wild-type littermates. IL-21 may still affect the efficiency of the Treg response during infection, but our data indicate that it does not affect the levels of foxp3 expression. However, we cannot rule out the possibility that Tregs contribute to the higher levels of CD4+-derived IL-10 in the IL-21−/− mice.
IL-21 has been implicated in expanding both Th1 and Th17 cells in our study with H. pylori, but it is also a key modulator in intestinal CD4+ T cell populations (43). These studies suggest IL-21 as a promising therapeutic target for treatment of T cell-mediated diseases, such as inflammatory bowel disease (IBD). IL-21 is upregulated in mouse models of IBD (trinitrobenzene sulfonic acid [TNBS]-induced relapsing colitis and dextran sulfate sodium [DSS]-induced colitis) and during chronic bacterial infections like H. pylori infection (reviewed in reference 27). In models of experimental colitis, IL-21−/− mice were largely protected against both DSS-induced colitis and TNBS-induced relapsing colitis (44). In these models, the IL-21−/− mice were unable to upregulate Th17-associated molecules during gut inflammation (44). Blockade of IL-21 with IL-21R.Fc inhibits disease progression in a lupus-prone mouse model (45) and ameliorates disease in a mouse model of rheumatoid arthritis (46). Recent findings in systemic lupus erythematosus (SLE) patients demonstrate that IL-21 expression correlates with alterations of T cell and B cell subsets and suggest that targeting IL-21 could provide beneficial effects on both T cell and B cell alterations (47). In our IL-21−/− model, B cell responses are also affected. We observed that H. pylori-infected IL-21−/− mice lack an H. pylori-specific IgG1 or IgG2a antibody response (measured in the serum; see Fig. S8 in the supplemental material), but B cells have never been reported to play a pathogenic role during H. pylori gastritis. Of note, an anti-IL-21 receptor monoclonal antibody is being tested in a phase I clinical trial (14), positioning IL-21 as a promising, host-targeted therapeutic that could potentially replace the current aggressive triple-antibiotic treatment in the context of H. pylori infection.
In summary, we find that IL-21-deficient mice are protected from H. pylori-induced gastritis, similar to the protection observed in models of chemically induced colitis. Protection from H. pylori-induced gastritis was associated with marked decreases in IL-17 and IFN-γ in infected IL-21-deficient mice compared to their levels in wild-type mice. The results of our combined approach, utilizing mathematical modeling and in vivo H. pylori infections in the mouse model, indicate that IL-21 has a role in sustaining both Th1 and Th17 effector cell responses through the induction of phosphorylation of STAT1 and STAT3 and induced expression of tbx21 and rorc. These data suggest that chronic maintenance of the T cell-mediated inflammation during H. pylori infection requires IL-21. Hence, IL-21 may be an ideal target for the development of immunotherapeutics, although caution should be employed when Th1 and Th17 responses are necessary for controlling more virulent infections.
MATERIALS AND METHODS
Animals.
Male and female interleukin-21+/− mice (B6;129S5-Il21tm1Lex/Mmucd, stock number MMRRC:011723-UCD, backcrossed to C57BL/6 mice, stock number MMRRC:032800-UCD) were obtained from the NIH Consortium (University of California, Davis) for the establishment of a breeding colony. Helicobacter-free IL-21−/− and IL-21+/+ (wild-type) male littermates, 8 to 10 weeks old, were used in all experiments. The IL-21+/− breeding pairs tested negative for intestinal Helicobacter. Feces from sentinel mice housed in the same room consistently tested negative for pinworms, mouse parvovirus, and several other murine pathogens. Mice were housed and maintained according to the requirements of the Vanderbilt University Institutional Animal Care and Use Committee (protocol M/11/055).
Culture of H. Pylori.
A mouse-passaged derivative of H. pylori strain SS1 was used in these experiments. Bacteria were grown on Trypticase soy agar (TSA) plates containing 5% sheep blood. Alternatively, bacteria were grown in Brucella broth containing 10% heat-inactivated fetal bovine serum (FBS) and 10 µg/ml vancomycin. Plate cultures were grown at 37°C either in room air supplemented with 5% CO2 or under microaerobic conditions generated by a CampyPak plus* hydrogen-plus-CO2 system with integral palladium catalyst (BD). Liquid cultures for infection were grown under microaerobic conditions with shaking at 150 rpm.
Infection of mice with H. pylori.
One day prior to infection of mice, H. pylori strain SS1 was inoculated into liquid medium and was cultured for 18 h under microaerobic conditions as described above. Mice were orogastrically inoculated with a suspension of 5 × 108 CFU H. pylori (in 0.5 ml of brucella broth) twice over 5 days.
Processing of mouse stomachs.
Mouse stomachs were processed as previously described (48). In brief, after rinsing in phosphate-buffered saline (PBS), the stomach tissue was cut into longitudinal strips that were used for bacterial culture, RNA analysis, histology, and/or protein quantification. For histological analyses, sections were stained with hematoxylin and eosin to assess inflammation (scoring described below). Moreover, a Steiner stain was performed to localize the H. pylori bacteria within the gastric tissue, as previously described (49).
Mathematical modeling.
To assert the dynamics of IL-21-related pathways in CD4+ T cells, an ordinary differential equation (ODE)-based CD4+ T cell differentiation model was used in its wild-type and IL-21−/− setup. Briefly, the model was calibrated with H. pylori-specific experimental data (Fig. 3), and time courses were performed with a Th1-, Th17-, or iTreg-specific initialization to assess the role of IL-21 in these phenotypes. More information about the calibration and simulation process can be found in Text S1 in the supplemental material.
Culture of H. pylori from mouse stomach.
Gastric tissue was homogenized using a Tissue-Tearor (Biospec Products, Inc.) in brucella broth with 10% FBS. Serial dilutions of the homogenate were plated as previously described (49). After 5 to 7 days of culture under microaerobic conditions, H. pylori colonies were counted.
Stomach inflammation scoring.
Acute and chronic inflammation in the gastric antrum and corpus were graded on a scale from 0 to 3 (50–52). Acute inflammation was graded by a blinded pathologist (M.K.W. or M.B.P.) based on density of neutrophils, and chronic inflammation was graded based on the density of lamina propria (LP) mononuclear cell infiltration independent of lymphoid follicles. Total gastric inflammation was calculated as the sum of acute and chronic inflammation scores for the corpus and the antrum, allowing quantification of total inflammation on a scale from 0 to 12.
RNA extraction and real-time RT-PCR.
Total RNA was isolated from the stomach using the TRIZOL isolation protocol (Invitrogen) with slight modifications as previously described (49). CD4+ T cell RNA was isolated using Qiagen’s RNeasy kit as directed by the manufacturer. RNA was reverse transcribed using the high-capacity cDNA reverse-transcription kit (Life Technologies). For real-time reverse transcription-PCR (RT-PCR), we used the relative gene expression method (50). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the normalizer, and tissue from uninfected mouse stomachs (of the same genotype) served as the calibrator sample, as previously described (49).
Flow cytometric analysis.
To analyze gastric cellular infiltrates, whole mouse glandular stomachs were harvested and processed (with dispase and collagenase) using the gentleMACS dissociator (Miltenyi Biotec) as previously described (49). The gastric cells were stained with anti-CD4, anti-CD8, anti-CD3, anti-Gr1, anti-CD11b, and anti-B220 antibodies (BD Biosciences) as previously described (49). Samples were collected and analyzed on a BD LSR II flow cytometer (BD Biosciences).
Analysis of cytokine and chemokine protein levels in gastric tissues.
Freshly excised glandular stomach tissues were rinsed in PBS and homogenized in CelLytic MT mammalian tissue lysis/extraction buffer (Sigma). Twenty-five cytokine analytes were measured in tissue lysates using the Milliplex MAP mouse cytokine/chemokine magnetic bead panel kit according to the manufacturer’s instructions (Millipore). Standards were also prepared for all 25 cytokine analytes according to the manufacturer’s instructions. Protein concentrations were measured using the DC protein assay kit (Bio-Rad Laboratories). The concentration of each cytokine is presented as picograms of protein per microgram of tissue.
CD4+ T cell isolations.
CD4+ T cells were isolated from the spleens of H. pylori-infected IL-21−/− mice and H. pylori-infected wild-type littermates between 2 and 3 months postinfection. Spleens were harvested, and after red blood cell lysis, the cells were magnetically labeled with CD4 microbeads (clone L3T4; Miltenyi Biotec). CD4+ cells were positively selected using the positive selection program in sensitive mode on the autoMACS machine (Miltenyi Biotec) according to the manufacturer’s protocol. The resulting population was 92 to 96% CD4+ by flow cytometry analysis.
STAT1 and STAT3 phosphorylation assays.
The levels of STAT phosphorylation were measured by flow cytometry. Briefly, 1 million splenocytes were either unstimulated or stimulated with 10 ng/ml recombinant IL-6 or recombinant IL-21 (Peprotech) for 15 min. After fixation with a final concentration of 1.5% paraformaldehyde, the cells were permeabilized with cold methanol. The cells were stained with either anti-phospho-STAT3 (tyr705, D3A7) antibody (Cell Signaling Technology) or anti-phospho-STAT1 (tyr 701, D4A7) antibody for 45 min. After several washes, anti-rabbit IgG-Alexa Fluor 647 was added for 30 min. Cells were washed three times and analyzed by flow cytometry.
Statistical analysis.
Four to seven mice per group per time point were used for all of the studies. Colonization, inflammation, Luminex assays, and cytokine real-time RT-PCR were all performed as distinct experiments at least three times. To compare results obtained with different groups of mice, statistical analysis was performed using one-way analysis of variance, followed by a Student-Neuman-Keuls post hoc test. For analyses of bacterial numbers and cell numbers, the data were normalized by log transformation prior to statistical analysis. For histology scores, the Mann-Whitney U test was applied to compare results between wild-type and IL-21−/− mice.
SUPPLEMENTAL MATERIAL
Creation of CD4+ T cell differentiation model, calibrations, and in silico experimentation addendum. In-depth explanation of the computational modeling approach taken to calibrate and simulate the CD4+ T cell differentiation model, as well as the strategy to create the in silico IL-21-null system. Download
Calibration process using the CD4+ T cell computational model to adjust dynamics to H. pylori-specific data. A top-down approach was used to calibrate IL-21-specific pathways with H. pylori infection data. Briefly, the dynamics of the CD4+ T cell computational model were adjusted by using a calibration database with data obtained from published repositories or generated in house. After the parameter estimation process was completed and the model fitted to the experimental data, computational simulations were performed. These first sets of simulations were published in reference 32. H. pylori-specific data regarding the expression of IL-17 and IFN-γ were used for further calibration of the model by running parameter estimations iteratively. Download
Computational fitting of computational model parameters from H. pylori-derived RT-PCR and protein data in COPASI. IFN-γ (A) and IL-17 (B) were fitted by COPASI using the Particle Swarm algorithm (2,000 iterations). The fitted value (dark blue dots) could reproduce the behavior of the measured value (red dots). The weighted error (green dots) is low, indicating that the fitting has been performed successfully. After completion, a new set of hypotheses regarding the role of IL-21 in Th1 and Th17 during H. pylori infection were generated and experimental animal studies were run in order to validate such computational predictions. Download
IL-21 is required for control of bacterial burden and enhances gastritis and Th1 and Th17 cytokine production in C57BL/6 mice. (A) The number of CFU was calibrated to the weight of the tissue, and CFU/gram from tissues harvested between 2 and 3 months postinfection are presented. (B) Levels of acute and chronic inflammation in stomach tissue (in the corpus and antrum) harvested between 2 and 3 months postinfection were scored, and total inflammation was scored on a scale of 0 to 12. (C) Representative sections of the gastric mucosa are presented (×200). (D) Real-time RT-PCR was performed on stomach tissue of H. pylori-infected mice. Relative units of IL-17A and IFN-γ were measured. Relative units are normalized using the relative expression calibrated to the level of expression in uninfected wild-type mice, with GAPDH as the endogenous control. Data are representative of 2 independent experiments. *, P < 0.05; **, P < 0.01. Download
H. pylori-infected IL-21−/− mice express reduced levels of proinflammatory cytokines and chemokines during chronic infection. Gastric protein levels were measured in the stomach tissue using a Milliplex assay at 2 and 3 months postinfection. Protein levels are reported as picograms of protein per microgram of total tissue. (A to C) Levels of IFN-γ-induced chemokines (RANTES, IP-10, and MIP-1β) (A), IL-17A-induced chemokine (KC) (B), and proinflammatory cytokines IL-1β and TNF-α (C) are reduced at the protein level in H. pylori-infected IL-21−/− mice. Real-time RT-PCR was performed on stomach tissue of H. pylori-infected mice. (D) Relative units of IL-1β and TNF-α were measured. Relative units are normalized using the relative expression calibrated to the expression level in uninfected wild-type mice, with GAPDH as the endogenous control. Data are representative of 2 independent experiments. *, P < 0.05; **P < 0.01; ***, P ≤ 0.001. Abundances of IL-12p40, G-CSF, GM-CSF, and MCP-1 are not affected by IL-21 deficiency during H. pylori infection (data not shown). Download
Parameter estimation results and differential reactions on model fluxes after T helper 17 induction. (A) Numerical values of the parameter estimation results, showing error means for fitting the experimental data into the computational model. (B) Gradient analysis of parameters related to IL-21 reactions. (C to E) Fluxes were assessed in COPASI in the wild-type model (C, E) and the IL-21−/− model (D, F). Lines in panels C and D represent the reaction fluxes of all the reactions in the CD4+ T cell computational model. Fluxes in panels E and F are identified in the key. Download
IL-21 is required for the upregulation of Th1 and Th17 responses, and it modulates neutrophil recruitment in the gastric lamina propria during the chronic stages of H. pylori infection. (A) In silico time course experiment performed with an initial challenge of 5 × 107 CFU of H. pylori bacteria injected into the mathematical model, showing differences in numbers of gastric lamina propria CD4+ T cell subsets (A) and numbers of gastric lamina propria neutrophils (B) over time in both the wild-type and the IL-21-deficient model. Download
Ordinary differential equations (ODE) triggering activation and inhibition of regulatory and effector pathways in our CD4+ T cell model. Briefly, mass action and the Hill functions were used to reproduce CD4+ T cell behaviors in silico based on initial stimulation by external cytokines. Download
H. pylori-specific IgG1 and IgG2a are reduced in IL-21-deficient mice during H. pylori infection. Isotype antibodies specific for H. pylori (strain SS1) were quantitated by enzyme-linked immunosorbent assay (ELISA). Levels of H. pylori-specific IgG1 and IgG2a in sera from SS1-infected mice were measured at 3 months postinfection. Nine or 10 mice (independent serum samples) were used for each mouse group. Download
In silico approach for IL-21-knockout reaction. In order to create an in silico IL-21-null system, we downregulated the three reactions depicted here, therefore impairing the ability of the computational model to control and upregulate IL-21.
ACKNOWLEDGMENTS
We thank the NIH Consortium for providing IL-21+/− mice and Y. Kokoye at Vanderbilt Division of Animal Care for help breeding Helicobacter-free mice. We thank Jana Radin for technical assistance and Tim Cover, Jana Radin, and Mark Boothby for helpful discussions.
Flow cytometry experiments were performed in the VUMC Flow Cytometry Shared Resource. The VUMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (P30DK058404).
This work has been funded through Vanderbilt University Medical Center’s Digestive Disease Research Center supported by National Institutes of Health grants P30DK058404 (Pilot projects awarded to H.M.S.A. and D.O.-V.), R01DK053620 (to K.T.W.), and K01AT007324 (to R.C.), NIAID contract no. HHSN272201000056C to J.B.-R., and funds from the Nutritional Immunology and Molecular Medicine Laboratory. This work has also been funded by a VA Career Development Award (to H.M.S.A.) and Merit Review grants IBX000915A (to H.M.S.A.) and 1I01BX001453 (to K.T.W.) from the Office of Medical Research, Department of Veterans Affairs.
Footnotes
Citation Carbo A, Olivares-Villagómez D, Hontecillas R, Bassaganya-Riera J, Chaturvedi R, Piazuelo MB, Delgado A, Washington MK, Wilson KT, Algood HMS. 2014. Systems modeling of the role of IL-21 in the maintenance of effector CD4+ T cell responses during chronic Helicobacter pylori infection. mBio 5(4):e01243-14. doi:10.1128/mBio.01243-14.
REFERENCES
- 1. Algood HM, Cover TL. 2006. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin. Microbiol. Rev. 19:597–613. 10.1128/CMR.00006-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blaser MJ, Atherton JC. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Invest. 113:321–333. 10.1172/JCI20925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peek RM, Jr, Fiske C, Wilson KT. 2010. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol. Rev. 90:831–858. 10.1152/physrev.00039.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blaser MJ. 2008. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer. Prev. Res. (Phila.) 1:308–311. 10.1158/1940-6207.CAPR-08-0170 [DOI] [PubMed] [Google Scholar]
- 5. Vieth M, Masoud B, Meining A, Stolte M. 2000. Helicobacter pylori infection: protection against Barrett’s mucosa and neoplasia? Digestion 62:225–231. 10.1159/000007820 [DOI] [PubMed] [Google Scholar]
- 6. Vaezi MF, Falk GW, Peek RM, Vicari JJ, Goldblum JR, Perez-Perez GI, Rice TW, Blaser MJ, Richter JE. 2000. CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am. J. Gastroenterol. 95:2206–2211. 10.1111/j.1572-0241.2000.02305.x [DOI] [PubMed] [Google Scholar]
- 7. Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, Perez-Perez GI, Schoenberg JB, Stanford JL, Rotterdam H, West AB, Fraumeni JF., Jr. 1998. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 58:588–590 [PubMed] [Google Scholar]
- 8. Blaser MJ, Chen Y, Reibman J. 2008. Does Helicobacter pylori protect against asthma and allergy? Gut 57:561–567. 10.1136/gut.2007.133462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Blaser MJ. 2008. Helicobacter pylori colonization is inversely associated with childhood asthma. J. Infect. Dis. 198:553–560. 10.1086/590158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lang L. 2007. Childhood acquisition of Helicobacter pylori linked to reduced asthma and allergy risk. Gastroenterology 133:6. 10.1053/S0016-5085(07)01419-9 [DOI] [PubMed] [Google Scholar]
- 11. McCune A, Lane A, Murray L, Harvey I, Nair P, Donovan J, Harvey R. 2003. Reduced risk of atopic disorders in adults with Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 15:637–640. 10.1097/00042737-200306000-00010 [DOI] [PubMed] [Google Scholar]
- 12. Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Jr, Cover TL. 2007. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol. Med. Microbiol. 51:577–586. 10.1111/j.1348-0421.2007.tb03935.x [DOI] [PubMed] [Google Scholar]
- 13. Wilson KT, Crabtree JE. 2007. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 133:288–308. 10.1053/j.gastro.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 14. Malfertheiner P, Bazzoli F, Delchier JC, Celinski K, Giguere M, Riviere M, Megraud F, Study Pylera Group 2011. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 377:905–913 [DOI] [PubMed] [Google Scholar]
- 15. Carbo A, Bassaganya-Riera J, Pedragosa M, Viladomiu M, Marathe M, Eubank S, Wendelsdorf K, Bisset K, Hoops S, Deng X, Alam M, Kronsteiner B, Mei Y, Hontecillas R. 2013. Predictive computational modeling of the mucosal immune responses during Helicobacter pylori infection. PLoS One 8:e73365. 10.1371/journal.pone.0073365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karttunen R, Karttunen T, Ekre HP, MacDonald TT. 1995. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 36:341–345. 10.1136/gut.36.3.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haeberle HA, Kubin M, Bamford KB, Garofalo R, Graham DY, El-Zaatari F, Karttunen R, Crowe SE, Reyes VE, Ernst PB. 1997. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect. Immun. 65:4229–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482–492. 10.1016/S0016-5085(98)70531-1 [DOI] [PubMed] [Google Scholar]
- 19. Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964–5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sommer F, Faller G, Konturek P, Kirchner T, Hahn EG, Zeus J, Röllinghoff M, Lohoff M. 1998. Antrum- and corpus mucosa-infiltrating CD4(+) lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luzza F, Parrello T, Sebkova L, Pensabene L, Imeneo M, Mancuso M, La Vecchia AM, Monteleone G, Strisciuglio P, Pallone F. 2001. Expression of proinflammatory and Th1 but not Th2 cytokines is enhanced in gastric mucosa of Helicobacter pylori infected children. Dig. Liver Dis. 33:14–20. 10.1016/S1590-8658(01)80130-4 [DOI] [PubMed] [Google Scholar]
- 22. Itoh T, Yoshida M, Chiba T, Kita T, Wakatsuki Y. 2003. A coordinated cytotoxic effect of IFN-gamma and cross-reactive antibodies in the pathogenesis of Helicobacter pylori gastritis. Helicobacter 8:268–278. 10.1046/j.1523-5378.2003.00154.x [DOI] [PubMed] [Google Scholar]
- 23. Mizuno T, Ando T, Nobata K, Tsuzuki T, Maeda O, Watanabe O, Minami M, Ina K, Kusugami K, Peek RM, Goto H. 2005. Interleukin-17 levels in Helicobacter pylori-infected gastric mucosa and pathologic sequelae of colonization. World J. Gastroenterol. 11:6305–6311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caruso R, Fina D, Paoluzi OA, Del Vecchio Blanco G, Stolfi C, Rizzo A, Caprioli F, Sarra M, Andrei F, Fantini MC, MacDonald TT, Pallone F, Monteleone G. 2008. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur. J. Immunol. 38:470–478. 10.1002/eji.200737635 [DOI] [PubMed] [Google Scholar]
- 25. Sugimoto M, Ohno T, Graham DY, Yamaoka Y. 2009. Gastric mucosal interleukin-17 and -18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer Sci. 100:2152–2159. 10.1111/j.1349-7006.2009.01291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horvath DJ, Jr, Washington MK, Cope VA, Algood HM. 2012. IL-23 contributes to control of chronic Helicobacter pylori infection and the development of T helper responses in a mouse model. Front. Immunol. 3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Monteleone G, Pallone F, Macdonald TT. 2009. Interleukin-21 (IL-21)-mediated pathways in T cell-mediated disease. Cytokine Growth Factor Rev. 20:185–191. 10.1016/j.cytogfr.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 28. Algood HM, Allen SS, Washington MK, Peek RM, Jr, Miller GG, Cover TL. 2009. Regulation of gastric B cell recruitment is dependent on IL-17 receptor A signaling in a model of chronic bacterial infection. J. Immunol. 183:5837–5846. 10.4049/jimmunol.0901206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caruso R, Fina D, Peluso I, Fantini MC, Tosti C, Del Vecchio Blanco G, Paoluzi OA, Caprioli F, Andrei F, Stolfi C, Romano M, Ricci V, MacDonald TT, Pallone F, Monteleone G. 2007. IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. J. Immunol. 178:5957–5965. 10.4049/jimmunol.178.9.5957 [DOI] [PubMed] [Google Scholar]
- 30. Philipson CW, Bassaganya-Riera J, Viladomiu M, Pedragosa M, Guerrant RL, Roche JK, Hontecillas R. 2013. The role of peroxisome proliferator-activated receptor gamma in immune responses to enteroaggregative Escherichia coli infection. PLoS One 8:e57812. 10.1371/journal.pone.0057812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viladomiu M, Hontecillas R, Pedragosa M, Carbo A, Hoops S, Michalak P, Michalak K, Guerrant RL, Roche JK, Warren CA, Bassaganya-Riera J. 2012. Modeling the role of peroxisome proliferator-activated receptor gamma and microRNA-146 in mucosal immune responses to Clostridium difficile. PLoS One 7:e47525. 10.1371/journal.pone.0047525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carbo A, Hontecillas R, Kronsteiner B, Viladomiu M, Pedragosa M, Lu P, Philipson CW, Hoops S, Marathe M, Eubank S, Bisset K, Wendelsdorf K, Jarrah A, Mei Y, Bassaganya-Riera J. 2013. Systems modeling of molecular mechanisms controlling cytokine-driven CD4+ T cell differentiation and phenotype plasticity. PLoS Comput. Biol. 9:e1003027. 10.1371/journal.pcbi.1003027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huber M, Brüstle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Löw E, Lohoff M. 2008. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc. Natl. Acad. Sci. U. S. A. 105:20846–20851. 10.1073/pnas.0809077106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu J, Paul WE. 2010. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 238:247–262. 10.1111/j.1600-065X.2010.00951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deenick EK, Tangye SG. 2007. Autoimmunity: IL-21: a new player in Th17-cell differentiation. Immunol. Cell Biol. 85:503–505. 10.1038/sj.icb.7100114 [DOI] [PubMed] [Google Scholar]
- 36. Cope A, Le Friec G, Cardone J, Kemper C. 2011. The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol. 32:278–286. 10.1016/j.it.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 37. Anderson CF, Oukka M, Kuchroo VJ, Sacks D. 2007. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204:285–297. 10.1084/jem.20061886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. 2007. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283. 10.1084/jem.20062175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8:1390–1397. 10.1038/ni1539 [DOI] [PubMed] [Google Scholar]
- 40. Akhiani AA, Pappo J, Kabok Z, Schön K, Gao W, Franzén LE, Lycke N. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977–6984. 10.4049/jimmunol.169.12.6977 [DOI] [PubMed] [Google Scholar]
- 41. Eaton KA, Mefford M, Thevenot T. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456–7461. 10.4049/jimmunol.166.12.7456 [DOI] [PubMed] [Google Scholar]
- 42. Eaton KA, Benson LH, Haeger J, Gray BM. 2006. Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice. Infect. Immun. 74:4673–4684. 10.1128/IAI.01887-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, Caruso R, Tersigni R, Alessandroni L, Biancone L, Naccari GC, MacDonald TT, Pallone F. 2005. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology 128:687–694. 10.1053/j.gastro.2004.12.042 [DOI] [PubMed] [Google Scholar]
- 44. Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M, Pallone F, Macdonald TT, Monteleone G. 2008. Regulation of gut inflammation and Th17 cell response by interleukin-21. Gastroenterology 134:1038–1048. 10.1053/j.gastro.2008.01.041 [DOI] [PubMed] [Google Scholar]
- 45. Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. 2007. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 178:3822–3830. 10.4049/jimmunol.178.6.3822 [DOI] [PubMed] [Google Scholar]
- 46. Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, Senices M, Wu PW, Sibley B, Leathurby Y, Brown TP, Nickerson-Nutter C, Keith JC, Jr, Collins M. 2007. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 56:1152–1163. 10.1002/art.22452 [DOI] [PubMed] [Google Scholar]
- 47. Terrier B, Costedoat-Chalumeau N, Garrido M, Geri G, Rosenzwajg M, Musset L, Klatzmann D, Saadoun D, Cacoub P. 2012. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J. Rheumatol. 39:1819–1828. 10.3899/jrheum.120468 [DOI] [PubMed] [Google Scholar]
- 48. Horvath DJ, Jr, Radin JN, Cho SH, Washington MK, Algood HM. 2013. The interleukin-17 receptor B subunit is essential for the Th2 response to Helicobacter pylori, but not for control of bacterial burden. PLoS One 8:e60363. 10.1371/journal.pone.0060363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elias JM, Greene C. 1979. Modified Steiner method for the demonstration of Spirochetes in tissue. Am. J. Clin. Pathol. 71:109–111 [DOI] [PubMed] [Google Scholar]
- 50. Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. 2003. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124:762–777. 10.1016/S0016-5085(03)83849-0 [DOI] [PubMed] [Google Scholar]
- 51. Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O’Brien DP, Romero-Gallo J, Peek RM., Jr. 2005. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 102:10646–10651. 10.1073/pnas.0504927102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romero-Gallo J, Harris EJ, Krishna U, Washington MK, Perez-Perez GI, Peek RM., Jr. 2008. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab. Invest. 88:328–336. 10.1038/labinvest.3700719 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Creation of CD4+ T cell differentiation model, calibrations, and in silico experimentation addendum. In-depth explanation of the computational modeling approach taken to calibrate and simulate the CD4+ T cell differentiation model, as well as the strategy to create the in silico IL-21-null system. Download
Calibration process using the CD4+ T cell computational model to adjust dynamics to H. pylori-specific data. A top-down approach was used to calibrate IL-21-specific pathways with H. pylori infection data. Briefly, the dynamics of the CD4+ T cell computational model were adjusted by using a calibration database with data obtained from published repositories or generated in house. After the parameter estimation process was completed and the model fitted to the experimental data, computational simulations were performed. These first sets of simulations were published in reference 32. H. pylori-specific data regarding the expression of IL-17 and IFN-γ were used for further calibration of the model by running parameter estimations iteratively. Download
Computational fitting of computational model parameters from H. pylori-derived RT-PCR and protein data in COPASI. IFN-γ (A) and IL-17 (B) were fitted by COPASI using the Particle Swarm algorithm (2,000 iterations). The fitted value (dark blue dots) could reproduce the behavior of the measured value (red dots). The weighted error (green dots) is low, indicating that the fitting has been performed successfully. After completion, a new set of hypotheses regarding the role of IL-21 in Th1 and Th17 during H. pylori infection were generated and experimental animal studies were run in order to validate such computational predictions. Download
IL-21 is required for control of bacterial burden and enhances gastritis and Th1 and Th17 cytokine production in C57BL/6 mice. (A) The number of CFU was calibrated to the weight of the tissue, and CFU/gram from tissues harvested between 2 and 3 months postinfection are presented. (B) Levels of acute and chronic inflammation in stomach tissue (in the corpus and antrum) harvested between 2 and 3 months postinfection were scored, and total inflammation was scored on a scale of 0 to 12. (C) Representative sections of the gastric mucosa are presented (×200). (D) Real-time RT-PCR was performed on stomach tissue of H. pylori-infected mice. Relative units of IL-17A and IFN-γ were measured. Relative units are normalized using the relative expression calibrated to the level of expression in uninfected wild-type mice, with GAPDH as the endogenous control. Data are representative of 2 independent experiments. *, P < 0.05; **, P < 0.01. Download
H. pylori-infected IL-21−/− mice express reduced levels of proinflammatory cytokines and chemokines during chronic infection. Gastric protein levels were measured in the stomach tissue using a Milliplex assay at 2 and 3 months postinfection. Protein levels are reported as picograms of protein per microgram of total tissue. (A to C) Levels of IFN-γ-induced chemokines (RANTES, IP-10, and MIP-1β) (A), IL-17A-induced chemokine (KC) (B), and proinflammatory cytokines IL-1β and TNF-α (C) are reduced at the protein level in H. pylori-infected IL-21−/− mice. Real-time RT-PCR was performed on stomach tissue of H. pylori-infected mice. (D) Relative units of IL-1β and TNF-α were measured. Relative units are normalized using the relative expression calibrated to the expression level in uninfected wild-type mice, with GAPDH as the endogenous control. Data are representative of 2 independent experiments. *, P < 0.05; **P < 0.01; ***, P ≤ 0.001. Abundances of IL-12p40, G-CSF, GM-CSF, and MCP-1 are not affected by IL-21 deficiency during H. pylori infection (data not shown). Download
Parameter estimation results and differential reactions on model fluxes after T helper 17 induction. (A) Numerical values of the parameter estimation results, showing error means for fitting the experimental data into the computational model. (B) Gradient analysis of parameters related to IL-21 reactions. (C to E) Fluxes were assessed in COPASI in the wild-type model (C, E) and the IL-21−/− model (D, F). Lines in panels C and D represent the reaction fluxes of all the reactions in the CD4+ T cell computational model. Fluxes in panels E and F are identified in the key. Download
IL-21 is required for the upregulation of Th1 and Th17 responses, and it modulates neutrophil recruitment in the gastric lamina propria during the chronic stages of H. pylori infection. (A) In silico time course experiment performed with an initial challenge of 5 × 107 CFU of H. pylori bacteria injected into the mathematical model, showing differences in numbers of gastric lamina propria CD4+ T cell subsets (A) and numbers of gastric lamina propria neutrophils (B) over time in both the wild-type and the IL-21-deficient model. Download
Ordinary differential equations (ODE) triggering activation and inhibition of regulatory and effector pathways in our CD4+ T cell model. Briefly, mass action and the Hill functions were used to reproduce CD4+ T cell behaviors in silico based on initial stimulation by external cytokines. Download
H. pylori-specific IgG1 and IgG2a are reduced in IL-21-deficient mice during H. pylori infection. Isotype antibodies specific for H. pylori (strain SS1) were quantitated by enzyme-linked immunosorbent assay (ELISA). Levels of H. pylori-specific IgG1 and IgG2a in sera from SS1-infected mice were measured at 3 months postinfection. Nine or 10 mice (independent serum samples) were used for each mouse group. Download
In silico approach for IL-21-knockout reaction. In order to create an in silico IL-21-null system, we downregulated the three reactions depicted here, therefore impairing the ability of the computational model to control and upregulate IL-21.