Abstract
Introduction:
Metallothioneins (MTs) are a group of low-molecular weight, cysteine-rich proteins. In general, MT is known to modulate three fundamental processes: (1) the release of gaseous mediators such as hydroxyl radical or nitric oxide, (2) apoptosis and (3) the binding and exchange of heavy metals such as zinc, cadmium or copper. Previous studies have shown a positive correlation between the expression of MT with invasion, metastasis and poor prognosis in various cancers. Most of the previous studies primarily used immunohistochemistry to analyze localization of MT in renal cell carcinoma (RCC). No information is available on the gene expression of MT2A isoform in different types and grades of RCC.
Materials and Methods:
In the present study, total RNA was isolated from 38 histopathologically confirmed cases of RCC of different types and grades. Corresponding adjacent normal renal parenchyma was taken as control. Real-time polymerase chain reaction (RT PCR) analysis was done for the MT2A gene expression using β-actin as an internal control. All statistical calculations were performed using SPSS software.
Results:
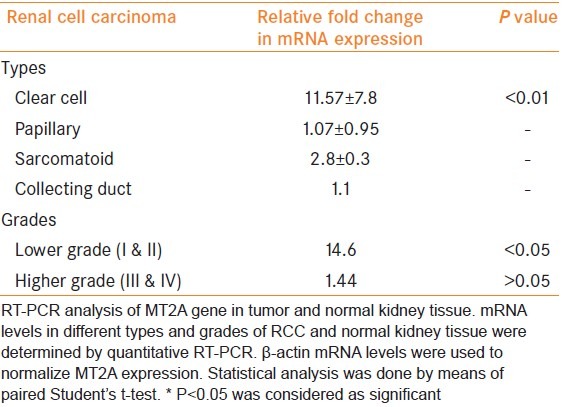
The MT2A gene expression was found to be significantly increased (P < 0.01) in clear cell RCC in comparison with the adjacent normal renal parenchyma. The expression of MT2A was two to three-fold higher in sarcomatoid RCC, whereas there was no change in papillary and collecting duct RCC. MT2A gene expression was significantly higher in lower grade (grades I and II, P < 0.05), while no change was observed in high-grade tumor (grade III and IV) in comparison to adjacent normal renal tissue.
Conclusion:
The first report of the expression of MT2A in different types and grades of RCC and also these data further support the role of MT2A in tumorigenesis.
Keywords: Clear cell carcinoma, grade, metallothionein, renal cell carcinoma, Real time polymerase chain reaction
INTRODUCTION
Metallothioneins (MTs) are a group of low-molecular weight, cysteine-rich intracellular proteins that are encoded by a family of genes containing at least 10 functional isoforms in humans.[1] MT is thought to be an important intracellular storage site for zinc, and possibly other essential trace elements.[2] MT1 and MT2 isoforms are usually expressed in low levels, but are inducible by a variety of metal ions, hormones, inflammatory cytokines and xenobiotics.[3] Previous studies have shown that MT has a role in the development of drug resistance in tumor cells.[4] This protein has properties like detoxification of heavy metals like mercury and cadmium, homeostasis of essential metals including copper and zinc and antioxidation against reactive oxygen species. MTs also have a role in the cellular repair process, cell proliferation, differentiation and carcinogenesis.[5] MT synthesis is regulated by the rate of MT gene transcription, which in turn is mediated by interactions between upstream regulatory DNA sequences and unidentified cellular factors.[6] Changes in gene structure such as amplification, methylation and cellular differentiation and development can alter MT expression.[7] The expression of MT has been demonstrated in various types of human tumors, including thyroid,[8] testicular germ cell tumor,[9] urinary bladder tumor,[10] salivary gland[11] and prostate tumor.[12] There is direct evidence that certain tumors have enhanced levels of MT, and that induction of these metallo-proteins is often associated with rapidly proliferating cells.[3]
Metallothionein as a potential biomarker is at the center of interest and its properties, function and behavior under various conditions are intensively studied.[13] New biological roles for these proteins have been identified, including those needed in the carcinogenic process. However, their use as a predictive marker remains controversial. Several reports have disclosed MT expression as a prognostic factor for tumor progression and drug resistance in a variety of malignancies, particularly breast, prostatic, ovarian, head and neck, non-small cell lung cancer, melanoma and soft tissue sarcoma.[14] Previous studies reported increased levels of MT1 and MT2 mRNA and protein expression in various human cancers, such as breast, kidney, lung, nasopharyngeal, ovarian, prostate, salivary gland, testicular, urinary bladder, cervical and endometrial cancers, as well as skin carcinoma and melanoma.[15] However, MT1 and MT2 are down-regulated in other types of tumors, e.g. hepatocellular,[16] gastric,[17] colorectal[18] and liver adenocarcinoma.[19]
Renal cell carcinoma (RCC) is the most common renal tumor, accounting for approximately 3% of adult malignancies and constituting 90% of all renal malignancies.[20] Previous studies have shown an increase in immunoreactivity in lower stage tumor, suggesting a role of MT in enhancing the proliferative response of tumor cells during their growth cycle.[3,4,21] A number of studies have also demonstrated altered MT2A protein expression in RCC by the immunostaining approach.[3] While most of these studies primarily used immunohistochemistry to analyze the localization and protein level of MT2A in tumor cells, no information is available on the gene expression of MT2A isoform in different grades and subtypes of RCC. In this study, we investigated the gene expression of MT2A isoforms in various subtypes and grades of RCC.
MATERIALS AND METHODS
Patients
The present study was approved by the institute ethics committee and has been performed in accordance with the ethical standards laid down in an appropriate version of the 2000 Declaration of Helsinki as well as the Declaration of Istanbul, 2009. Informed consent was obtained from all patients before conducting the study. Following nephrectomy, tissue samples were taken from the tumor and grossly normal renal parenchyma separately. The samples were snap-frozen in liquid nitrogen and stored at -80°C till further use. Grading of tumors was performed by the Fuhrman grading system.[22]
RNA isolation
Total RNA was isolated from tumorous and normal adjacent kidney tissue using the PureLinkTM RNA mini Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The concentration and purity of each sample were evaluated using a spectrophotometer at A260/A280. A total of 10 μg total RNA was treated with 1 U DNase I (Fermentas) for 30 min at 37°C and then heat-inactivated at 65°C for 10 min before reverse transcription to eliminate genomic DNA (gDNA) contamination.
cDNA preparations
For first-strand cDNA synthesis, 1 μg of treated RNA in a final volume of 20 μL was used to synthesize cDNA using a super scriptIII first strand synthesis system (Invitrogen). The mixture was incubated at 42°C for 1 h. RNA without DNase I treatment was used as a control to test gDNA contamination. After first strand synthesis, the reactions were heat-inactivated at 70°C for 5 min.
Real time polymerase chain reaction
Oligonucleotide primers were MT 2A, CTCCA AGTCCCAGCGAAC and GAGCAGTTGG GATCCATGG; β-actin, CGTGACATTAAGGAGAAGCTG and CTAGAAG CATTTGCGGTG GAC. Real time analysis was performed on a 7300 RT PCR system (Roche, Indianapolis, IN, USA) using the SYBR Green PCR Master Mix (TaKaRa, Madison, WI, USA). β-actin was used as an internal control. For MT2A and β-actin gene amplification, 1 μL of the synthesized first strand cDNA was used. The cycles were programmed for 95°C for a 2-min initial step, then 95°C for 30 s, 62°C for 30 s and 72°C for 30 s. Each assay included a no-template control and a cDNA template (in triplicate). The expression levels of MT2A were normalized to the β-actin transcript level and calculated to MT2A levels in normal renal parenchyma using the following equation:
Relative expression: 2-(sampleΔCt-ControlΔCt); where ΔCt = average Ct (MT2A) - averageΔCt(β-actin).
Specificity of the PCR products was confirmed by analyzing the dissociation curve.
Statistical analysis
Data were expressed as mean ± SD. Statistical analyses was performed based on an independent samples t-test to compare data. P value less than 0.05 is considered statistically significant.
RESULTS
Patients’ characteristics
A total of 38 cases of histopathologically proven RCC and corresponding controls were included in this study. Various characteristics of the patients included in this study are shown in Figure 1. The patients were in the age group of 35-76 years, with a mean age of 52.3 ± 13.8 years. Most of the patients were in their 5th to 6th decades of life [Figure 1a]. Of a total of 38 cases of RCC, 30 cases were of clear cell carcinoma, four cases were of papillary cell carcinoma, three cases were of sarcomatoid carcinoma and one case was of collecting duct carcinoma [Figure 1b]. The 30 cases of clear cell carcinoma included four cases of grade I, ninteen cases of grade II, five cases of grade III and two cases of grade IV [Figure 1c].
Figure 1.
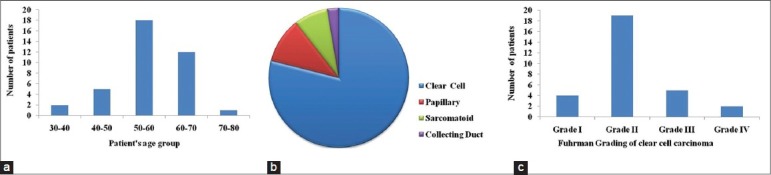
Patients’ characteristics. (a) Bar diagram showing the number of patients in the different age groups. (b) Pie diagram showing the number of patients in the different types of renal cell carcinoma. (c) Bar diagram showing the number of patients in the different grades of clear cell carcinoma
Relative quantification of MT gene expression
Figure 2 shows the quality of isolated RNA from different tissues. Quantitation of the expression profile of MT2A gene included the use of β-actin as a housekeeping gene for comparison. When compared with the corresponding normal renal parenchyma, quantitative relative expressions for MT2A were found to be significantly increased in clear cell carcinoma as compared with corresponding normal renal tissue [P < 0.01; Table 1], whereas the MT2A expression was two- to three-fold higher in sarcomatoid carcinoma compared with normal renal tissue [Table 1]. On the other hand, there was no change in the expression of MT2A in papillary and collecting duct carcinoma as compared with their corresponding normal tissues [Table 1]. Further, when the MT2A expression was determined in different grades of clear cell carcinoma, MT2A expression was found to be significantly increased in lower grade (P < 0.05 in grade I and grade II; Table 1), whereas no significant change was observed in the higher grades (grade II and grade IV) clear cell carcinoma [Table 1] when compared with adjacent normal renal tissue. Figure 3a shows the amplification curves and Figure 3b shows the melting peaks of different samples. A single melting peak shows the specific amplified product in each sample.
Figure 2.
Analysis of total RNA. Total RNA were isolated from renal cell carcinoma and adjacent normal tissue and visualized on 1% formaldehyde agarose gel after ethidium bromide staining. Two bands corresponding to 28S and 18S rRNA were visualized, which are the hallmark of good-quality RNA. Lane C represents control RNA, lane N represents total RNA from normal tissue and lane T represents total RNA from the corresponding tumor tissue
Table 1.
Relative fold change in mRNA expression of MT2A gene in different types and grades of renal cell carcinoma
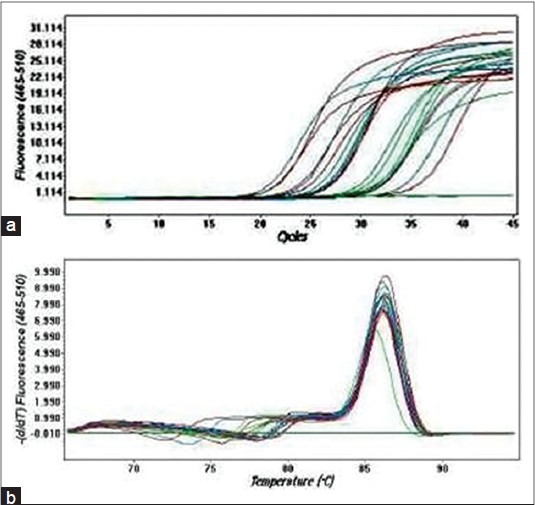
Figure 3.
Real time polymerase chain reaction analysis of metallothioneins 2A gene in tumor and normal kidney tissue. (a) SYBR Green fluorescence history versus cycle number of MT2A and & β-actin;-actin in normal and tumor cDNA. (b) Melting peaks of the different RT PCR amplification products
DISCUSSION
The primary goal of this study was to determine the expression profile of MT2A gene in different types and grades of RCC by RT PCR. In the present study, increased MT2A gene expression in lower grades (grade I and II) RCC indicates a possible role of MT2A in tumor growth and dedifferentiation. Our results are consistent with the previous studies.[4,23,24] There is no obvious explanation for the observation that lower grade tumors had increased MT2A gene expression relative to the other tumors in this study. However, one possible explanation is that the synthesis of MT may depend on the cell cycle, and it has been demonstrated that MT is preferentially expressed in S phase cells.[2,25] Notwithstanding, we observed difference in MT2A gene expression in various types of RCC but because of the small sample size in different subgroups of RCC, we could not draw any considerable conclusion related MT2A expression in various forms of RCC.
In conclusion, this is the first study on MT2A gene expression in different types and grades of RCC. However, further studies are required to understand the role of MT in renal cell carcinoma carcinogenesis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cherian MG, Jayasurya A, Bay BH. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat Res. 2003;533:201–9. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Cherian MG, Howell SB, Imura N, Klaassen CD, Koropatnick J, Lazo JS, et al. Role of metallothionein in carcinogenesis. Toxicol Appl Pharm. 1994;126:1–5. doi: 10.1006/taap.1994.1083. [DOI] [PubMed] [Google Scholar]
- 3.Mitropoulos D, Kyroudi-Voulgari A, Theocharis S, Serafetinides E, Moraitis E, Zervas A, et al. Prognostic significance of metallothionein expression in renal cell carcinoma. World J Surg Oncol. 2005;3:5. doi: 10.1186/1477-7819-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izawa JI, Moussa M, Cherian MG, Doig G, Chin JL. Metallothionein expression in renal cancer. Urology. 1998;52:767–72. doi: 10.1016/s0090-4295(98)00323-9. [DOI] [PubMed] [Google Scholar]
- 5.Cherian MG, Huang PC, Klaassen CD, Liu YP, Longfellow DG, Waalkes MP. National cancer institute workshop on the possible roles of Metallothionein in carcinogenesis. Cancer Res. 1993;53:922–5. [PubMed] [Google Scholar]
- 6.Kagi JH, Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–15. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- 7.Hamer DH. Metallothionein. Annu Rev Biochem. 1986;55:913–51. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 8.Nartey N, Cherian MG, Banerjee D. Immunohistochemical localization of metallothionein in human thyroid tumours. Am J Pathol. 1987;129:177–82. [PMC free article] [PubMed] [Google Scholar]
- 9.Kontozoglou TE, Banerjee D, Cherian MG. Immunohistochemical localization of metallothionein in human testicular embryonal carcinoma cells. Virchows Arch A Pathol Anat. 1989;415:545–9. doi: 10.1007/BF00718648. [DOI] [PubMed] [Google Scholar]
- 10.Bahnson RR, Banner BF, Ernstoff MS, Lazo JS, Cherian MG, Banerjee D, et al. Immunohistochemical localization of metallothionein in transitional cell carcinoma of the bladder. J Urol. 1991;146:1518–20. doi: 10.1016/s0022-5347(17)38155-7. [DOI] [PubMed] [Google Scholar]
- 11.Chauvin PJ, Cherian MG, Wysocki GP, Pringle G. A comparison of metallothionein expression in human normal and neoplastic salivary gland tissue using immunohistochemistry (abstract) Toxicologist. 1992;12:379. [Google Scholar]
- 12.Mousssa M, Kloth D, Peers G, Cherian MG, Frei JV, Chin JL. Metallothionein expression in prostate carcinoma: Correlation with Gleason grade, pathological stage, DNA content and serum level of prostate specific antigen. Clin Invest Med. 1997;20:371–80. [PubMed] [Google Scholar]
- 13.Ryvolova M, Hynek D, Skutkova H, Provaznik I, Kizek R. Structural changes in metallothionein isoforms revealed by capillary electrophoresis and Brdicka reaction. Electrophoresis. 2012;33:270–9. doi: 10.1002/elps.201100312. [DOI] [PubMed] [Google Scholar]
- 14.Eckschlager T, Adam V, Hrabeta J, Figova K, Kizek R. Metallothioneins and cancer. Curr Protein Pept Sci. 2009;10:360–75. doi: 10.2174/138920309788922243. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen MO, Larsen A, Stoltenberg M, Penkowa M. The role of metallothionein in oncogenesis and cancer prognosis. Prog Histochem Cytochem. 2009;44:29–64. doi: 10.1016/j.proghi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Huang GW, Yang LY. Metallothionein expression in hepatocellular carcinoma. World J Gastroenterol. 2002;8:650–3. doi: 10.3748/wjg.v8.i4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen AM, Van Duijn W, Kubben FJ, Griffioen G, Lamers CB, van Krieken JH, et al. Prognostic significance of metallothionein in human gastrointestinal cancer. Clin Cancer Res. 2002;8:1889–96. [PubMed] [Google Scholar]
- 18.Arriaga JM, Levy EM, Bravo AI, Bayo SM, Amat M, Aris M, et al. Metallothionein expression in colorectal cancer: Relevance of different isoforms for tumor progression and patient survival. Hum Pathol. 2012;43:197–208. doi: 10.1016/j.humpath.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Thirumoorthy N, Shyam Sunder A, Manisenthil Kumar KT, Senthil Kumar M, Ganesh G, Chatterjee M. A review of metallothionein isoforms and their role in pathophysiology. World J Surg Oncol. 2011;9:54. doi: 10.1186/1477-7819-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma U, Singh SK, Pal D, Khajuria R, Mandal AK, Prasad R. Implication of BBM lipid composition and fluidity in mitigated alkaline phosphatase activity in renal cell carcinoma. Mol Cell Biochem. 2012;369:287–93. doi: 10.1007/s11010-012-1391-y. [DOI] [PubMed] [Google Scholar]
- 21.Tuzel E, Kirkali Z, Yorukoglu K, Mungan MU, Sade M. Metallothionein expression in renal cell carcinoma: Subcellular localization and prognostic significance. J Urol. 2001;165:1710–3. [PubMed] [Google Scholar]
- 22.Fuhrman HA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen A, Jing Z, Mahoney PS, Davis R, Sikka SC, Agrawal KC, et al. In vivo gene expression profile analysis of Metallothionein in renal cell carcinoma. Cancer Lett. 2000;160:133–40. doi: 10.1016/s0304-3835(00)00534-6. [DOI] [PubMed] [Google Scholar]
- 24.Krizkova S, Fabrik I, Adam V, Hrabeta J, Eckschlager T, Kizek R. Metallothionein-a promising tool for cancer diagnostics. Bratisl Lek Listy. 2009;110:93–7. [PubMed] [Google Scholar]
- 25.Kondo Y, Rusnak JM, Hoyt DG, Settineri CE, Pitt BR, Lazo JS. Enhanced apoptosis in metallothionein null cells. Mol Pharmacol. 1997;52:195–01. doi: 10.1124/mol.52.2.195. [DOI] [PubMed] [Google Scholar]


