Abstract
Introduction and Objectives:
The benefits of robotic surgery when compared to standard laparoscopy have been well established, especially when it comes to reconstructive procedures. The application of robotic technology to laparoscopic pyeloplasty has reduced the steep learning curve associated with the procedure. Consequently, this has allowed surgeons who are less experienced with laparoscopy to offer this treatment to their patients, instead of referring them to centers of excellence. Robotic pyeloplasty has also proved useful for repairing secondary UPJO, a procedure which is considered extremely difficult using a conventional laparoscopic approach. Finally, the pursuit of scarless surgery has seen the development of laparoendoscopic single site (LESS) procedures. The application of robotics to LESS (R-LESS) has also reduced the difficulty in performing conventional LESS pyeloplasty. Herein we present a literature review with regards to robotic-assisted laparoscopic pyeloplasty. We also discuss the benefits of robotic surgery with regards to reconstruction of the lower urinary tract.
Materials and Methods:
A systematic literature review was performed using PubMed to identify relevant studies. There were no time restrictions applied to the search, but only studies in English were included. We utilized the following search terms: Ureteropelvic junction obstruction and laparoscopy; laparoscopic pyeloplasty; robotic pyeloplasty; robotic ureteric reimplantation; robotic ureteroneocystostomy; robotic boari flap; robotic psoas hitch.
Results:
There has been considerable experience in the literature with robotic pyeloplasty. Unfortunately, no prospective randomized studies have been conducted, however there are a number of meta analyses and systematic reviews. While there are no clear benefits when it comes to surgical and functional outcomes when compared to standard laparoscopic pyeloplasty, it is clear that robotics makes the operation easier to perform. There is also a benefit to the robotic approach when performing a redo-pyeloplasty. Robotic pyeloplasty has also been applied to the pediatric population, and there may be a benefit in older children while in very young patients, retroperitoneal open pyeloplasty is still the gold standard. In the field of single incision surgery R-LESS is technically easier to perform than conventional LESS. However, the design of the current robotic platform is not completely suited for this application, limiting its utility and often requiring a larger incision. Optimized R-LESS specific technology is awaited. What is clear, from a number of analyses, is that robotic pyeloplasty is considerably more expensive than the laparoscopic approach, largely due to costs of instrumentation and the capital expense of the robot. Until cheaper robotic technology is available, this technique will continue to be expensive, and a cost-benefit analysis must be undertaken by each hospital planning to undertake this surgery. Finally, the benefits of upper tract reconstruction apply equally to the lower tract although there is considerably less experience. However, there have been a number of studies demonstrating the technical feasibility of ureteral reimplantation.
Conclusions:
Robotic-assisted laparoscopic pyeloplasty is gaining popularity, likely due to the shorter learning curve, greater surgeon comfort, and easier intracorporeal suturing. This has allowed more surgeons to perform the procedure, improving accessibility. Robotic technology is also beneficial in the field of LESS. Nevertheless, the procedure still is not as cost-effective as the conventional laparoscopic approach, and until more affordable robotic technology is available, it will not be universally offered.
Keywords: Laparoendoscopic single site pyeloplasty, robotic pyeloplasty, ureteropelvic junction obstruction
INTRODUCTION
The widespread adoption of minimally invasive surgery by Urologists has greatly benefited patients. The merits of laparoscopic and robotic surgery have been well documented, and include less morbidity, less pain, and a faster convalescence.[1] Furthermore, with advancements in laparoscopic technology, the key steps of open surgical procedures can be duplicated, but in a minimally invasive fashion. Nowhere has this been more important than in the realm of urinary tract reconstruction, as strict adherence to surgical principles is the only way to achieve successful, lasting outcomes.
Ureteropelvic junction obstruction (UPJO) is the most common congenital anomaly of the ureter, with an incidence of 1 in 20,000 live births.[2] Historically, open dismembered pyeloplasty was the standard of care with a documented success rate of over 90%.[3] However, the increased morbidity and longer recovery times associated with the procedure[4] led to the pursuit of minimally invasive techniques. Endopyelotomy and balloon dilation have fulfilled the goals of being less morbid with a faster convalesce when compared to open surgery; however, the success rates have been reported as only 60-80%.[5] The first laparoscopic dismembered pyeloplasty was performed in 1993[6] and achieved the same success rate as the open procedure, but with minimal morbidity. Despite the technical challenges and steep learning curve, the technique was adopted as the standard of care for uncomplicated primary UPJO repairs. However, the operation remained mainly in the hands of expert surgeons at larger centers. The need for intracorporeal laparoscopic suturing has limited widespread adoption of the procedure.
The introduction of robotics has helped to reduce some of the challenges associated with laparoscopic UPJO repair, especially with regards to suturing. The “endo-wrist” technology and improved visualization have helped to reduce the steep learning curve associated with the procedure.[7] The first reported robotic pyeloplasty series was by Gettman in 2002[8] and since then there have been numerous others.[9] Additionally, there have been a number of comparative analyses comparing the conventional laparoscopic approach, with robotics.[10,11,12] Both the transperitoneal and retroperitoneal approaches have been explored,[13] as well as robotic pyeloplasty in the pediatric population.[14] Finally, recently there has been interest in the field of “scarless” surgery, and laparoendoscopic single-site pyeloplasty (LESS) has been described.[15]
The benefits of robotic surgery have also been applied to ureteral reconstruction and reimplantation. Although limited in number, there are several series describing robotic-assisted ureteral reimplantation, with and without psoas hitch/boari flap.[16,17,18]
The main objectives of this article are to provide a review of the available literature for both robotic-assisted laparoscopic pyeloplasty and ureteric reimplantation. Trends with regards to surgical technique and approach will be analyzed. We also will review the comparative series of robotic versus laparoscopic pyeloplasty, looking at surgical outcomes as well as cost analysis.
MATERIALS AND METHODS
A systematic literature review was performed using PubMed to identify relevant studies. There were no time restrictions applied to the search, but only studies in English were included. We utilized the following search terms: Ureteropelvic junction obstruction and laparoscopy; laparoscopic pyeloplasty; robotic pyeloplasty; robotic ureteric reimplantation; robotic ureteroneocystotomy; robotic boari flap; robotic psoas hitch.
RESULTS
Practice patterns: Adoption of robotic-assisted laparoscopic pyeloplasty
Data from the Nationwide Inpatient Sample Database has recently been used to establish practice patterns with regards to the utilization of robotic pyeloplasty in the USA. Sukumar et al.[19] included 29,456 patients in their analysis who underwent either open or minimally invasive pyeloplasty (laparoscopic or robotic) between 1998 and 2009. While only 15.3% of the patients underwent a minimally invasive pyeloplasty, the use of the technique increased dramatically from 2.4% to 55.3%. Unfortunately prior to 2008, there was no separate billing code for RALP, as it was the same as conventional laparoscopic pyeloplasty, so it was impossible to separate the two groups. However, in 2009 only 10.2% of all pyeloplasties performed were laparoscopic while 45.1% were RALP and 44.7% were open. Monn et al.[20] used the same database to analyze the trends in RALP utilization since the adoption of the separate billing code. The study period was from 2005 to 2010 and included 3,947 patients. Overall, 47.4% of the pyeloplasties performed were done robotically, and there was an increase in overall number of robotic pyeloplasties from 2008 to 2010 (P = 0.002). A summary of the largest robotic pyeloplasty series is included in Table 1.
Table 1.
Larger robotic-assisted laparoscopic pyeloplasty series
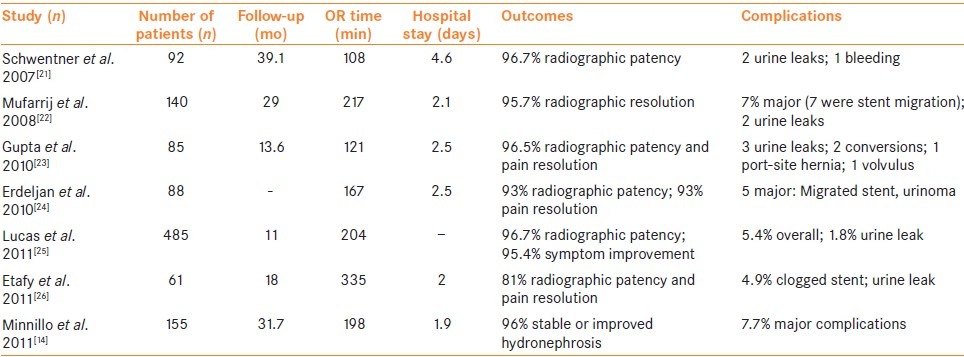
Approach: Transperitoneal vs. retroperitoneal
With regards to access for robotic-assisted pyeloplasty, the majority of reported series describe the transperitoneal technique. Retroperitoneal access as been extensively studied for standard laparoscopic pyeloplasty and provides rapid access to the UPJ with minimal dissection. Additional benefits include the limitation of a potential urinoma to the retroperitoneum and performing the procedure on an outpatient basis. Limitations include a smaller working space for suturing, and potential difficulty identifying a crossing vessel when present. Kaouk et al.[13] described their experience with 10 patients who underwent a robotic pyeloplasty via retroperitoneal access. All procedures were completed successfully without complications or the need for conversion. The authors concluded that the retroperitoneal approach was technically feasible, and outcomes (both surgical and functional) were comparable to the transperitoneal robotic technique. Cestari et al.[27] compared 36 patients who underwent retroperitoneal robotic pyeloplasty with 19 patients using the transperitoneal approach. Operative outcomes were similar between the two groups; however, the only two recurrences occurred in the retroperitioneal group, one of which was due to an unrecognized crossing vessel. In three cases, a crossing vessel was identified that was not seen on pre-operative imaging, and the UPJ was successfully transposed. It seems then that robotic-assisted pyeloplasty can successfully be performed by either approach, and extra care must be taken to identify crossing vessels with the retroperitoneal approach, as they may not be as evident as with the transperitoneal approach.
Approach: Retrocolic vs. transmesocolic
Traditionally during laparoscopic/robotic transperitoneal pyeloplasty, the initial step is to mobilize the colon medially along the white line of Toldt, in order to gain access to the retroperitoneum and expose the renal pelvis/ureter. By creating a window in the mesocolon adjacent to the hilum, total operative time could be reduced and bowel manipulation would be minimized. The first transmesocolic laparoscopic pyeloplasty was described by Nicol et al.[28] The technique was also performed robotically and described by Gupta et al. in 2009.[23] They performed transmesocolic pyeloplasty in 24 patients with a left sided UPJO with a mean operative time of 125.33 minutes. Twenty-three patients were followed for at least a year and demonstrated no recurrence of their obstruction. The authors did mention that the technique was difficult in patients with a high body mass index (BMI) with a thick mesentery.
Pediatric robotic-assisted laparoscopic pyeloplasty
Retroperitoneal access is the standard approach for an open pyeloplasty in children, and this concept was applied to pediatric robotic-assisted laparoscopic pyeloplasty. Olsen et al.[29] described the first series of retroperitoneoscopic robotic pyeloplasties in 13 children, and later published their expanded 5-year experience, which included 65 children. The median ages for the two studies were 6.7 and 7.9 years, respectively, with the youngest being 1.7 years old. Overall, the outcomes and complication rates were equivalent to the open and laparoscopic approaches. The authors also noted that total operative times were shorter than in two contemporary transperitoneal pediatric robotic pyeloplasty series.[30,31] The largest published series to date is by Minnillo et al.[14] from the Children's Hospital in Boston, which included 155 patients. In their study, 98% of the cases were done via the transperitoneal approach, which was based on surgeon preference. The mean operative time was 198.5 minutes, and the mean length of hospitalization was 1.96 days. The primary technical success rate at a mean follow up of 31.7 months was 96%. The authors also described their adoption of a standardized RALP program which was a collaboration between the surgeons, anesthesiologists, and OR nursing staff. They found that their OR times and efficiency improved over the study period. They concluded that within a pediatric urology training program successful collaboration can lead to shorter OR times and hospital stays, and achieve functional results comparable to the gold standard (open surgery). While these studies show that RALP is technically feasible by either a transperitoneal or a retroperitoneal approach, with results comparable to open surgery, the true benefit has yet to be realized. This is particularly true with regards to very small children, as often the open incision is very small, and there is no difference in morbidity. However, no prospective data exist comparing open surgery with RALP, and this question remains unanswered for now.
Secondary (“Redo”) robotic pyeloplasty
The management of a failed initial pyeloplasty repair, by whatever approach used, is technically challenging. Scarring, adhesions, and obliterated surgical planes make reconstruction difficult. For this reason, open surgery has been the gold standard in the past, albeit with higher morbidity. There have been a number of endourologic techniques employed in order to tackle this difficult clinical scenario, including balloon dilation and endopyelotomy, with varying success rates. For example, the documented success rate for endopyelotomy after a failed primary repair is between 60% and 70%, depending on the primary treatment modality.[32,33] Laparoscopic redo-pyeloplasty has been found to have higher success rates [>85%), but remains a difficult procedure.[34,35] It was has been postulated suggested that the benefits of robotic surgery would be provides advantageous significant advantages over standard laparoscopy for a minimally invasive option in these difficult salvage procedures. Niver et al.[36] retrospectively analyzed 19 patients who had a RALP for a secondary UPJO, and compared the outcomes with 97 patients who had a primary RALP. There were no significant differences in operative data (i.e., EBL, OR time, complications) or postoperative or radiographic outcomes. For patients in the primary UPJO group, 96.1% had radiographic resolution of their obstruction, as compared to 94.1% in the secondary UPJO group (P = 0.72). The authors concluded that RALP offers durable outcomes for the repair of secondary UPJO; however, the follow-up period was only 22.6 months and radiographic follow up was not standardized. Indeed it is our feeling that one of the great advantages of the robot over laparoscopy is the ease in performing minimally invasive secondary reconstructive surgery.
Robotic laparoendoscopic single-site surgery
The pursuit of “scarless” surgery has lead to the development of laparoendoscopic single-site surgery (LESS), which has found broad applications in urology. A number of extirpative and reconstructive procedures have been performed via a LESS approach, using different ports and laparoscopic instruments.[37] However, LESS is technically challenging due to issues with triangulation and instrument clashing. Intracorporeal suturing has been shown to be even more challenging than in standard laparoscopy.[38] Additionally, comparative studies have shown no significant benefits for LESS when compared to standard laparoscopy.[39] However, it has been postulated that patients undergoing pyeloplasty might be ideal candidates for LESS as they are usually young with benign pathology, and the procedure is non-extirpative, thereby not requiring a larger incision for specimen extraction. To overcome the challenges associated with standard LESS, the robotic platform has been applied (R-LESS). Despite the fact that the current generation robotic system was not designed for single site surgery, surgeons noticed that dissection and suturing was easier.[40] However, nevertheless instrument clashing remains an issue, and robotic LESS specific instrumentation is under development.[41]
Kaouk et al., described their early experience with R-LESS pyeloplasty[40] and since then there have been a number of other series using various access ports.[15,42,43] The unifying conclusion from all authors is that use of the robotic system helps to reduce the technical difficulty of LESS pyeloplasty and shortens the learning curve associated with the procedure. Olweny et al.[44] compared 10 patients who underwent conventional LESS (C-LESS) pyeloplasty with 10 patients who underwent R-LESS. Perioperative outcomes were analyzed including OR time, EBL, complications, morphine narcotic usage requirement, and length of stay in hospital (LOS). Cosmetic and long-term functional outcomes were not included in the analysis. There was no significant difference between R-LESS and C-LESS except for OR time, which was significantly longer for R-LESS (226 vs. 188 minutes, P = 0.007). Additionally, there were two conversions to standard laparoscopy in the C-LESS group as compared to none in the R-LESS group. Despite there being no clear advantage for R-LESS with regards to outcomes, the authors found the superior optics and endo-wrist technology of the robotic system beneficial. Cestari et al.[43] tested the feasibility and short-term perioperative outcomes of the daVinici single site surgery platform in nine patients with a UPJO. The system uses a novel single port access device with curved cannulas and robotic instruments. Additionally, the instruments are crossed at the abdominal wall to minimize clashing and improved triangulation. All cases were completely successfully without complication or conversion. However, the authors noted the main limitation of the system was the lack of articulation of the instruments, which is one of the principal advantages gained with the application of the robotic system to LESS.
Comparative series: Laparoscopic pyeloplasty vs. robotic pyeloplasty
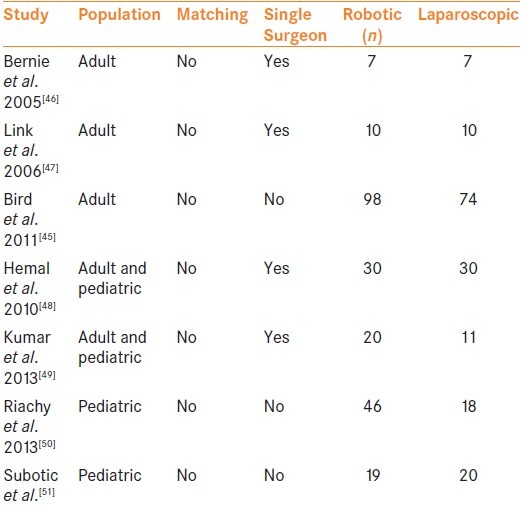
The benefits of robotics with regards to reconstructive surgery in urology have been well established, but the question remains as to whether they translate into superior patient outcomes. Unfortunately prospective randomized data comparing laparoscopic and robotic pyeloplasty does not exist, and the most insight has come from retrospective multi-center studies and a meta-analysis. Braga et al.[12] included eight studies in their meta-analysis and found that robotic pyeloplasty was associated with a significantly shorter hospital stay (WMD: –0.5 d; P < 0.01). There were no significant differences between the two approaches with regards to technical success or complications. However, despite the methodological quality, the analysis suffered from a low number of studies included, which were of low quality as they were mostly retrospective observational studies with small numbers of patients, and no randomized trials were included. Lucas et al.[25] have published the largest retrospective multi-center study to date comparing the two approaches. They included 759 cases (274 laparoscopic pyeloplasties with a mean follow up of 15 months and 465 robotic pyeloplasties with a mean follow up of 11 months, P < 0.001). Bivariate and multivariate analysis was performed to identify factors that were associated with a decreased freedom from secondary procedures. Laparoscopic pyeloplasty, previous endopyelotomy, and intraoperative crossing vessels were associated with decreased freedom from secondary procedures on bivariate analysis, with a 2-year freedom from secondary procedures of 87% for laparoscopic pyeloplasty vs. 95% for robotic pyeloplasty, 81% vs. 93% for patients with vs. without previous endopyelotomy and 88% vs. 95% for patients with vs. without intraoperative crossing vessels, respectively. However, on multivariate analysis, only previous endopyelotomy (HR 4.35) and intraoperative crossing vessels (HR 2.73) significantly impacted freedom from secondary procedures, and the approach (Laparoscopic Wvs. RALP) was no longer significant. Bird et al.[45] published their single center comparative analysis which included 172 cases (98 robotic and 74 laparoscopic pyeloplasties). They reported no difference in operative time, complication rates, and radiographic success rates at 6 months. Smaller comparative series are included in Table 2.
Table 2.
Robotic versus laparoscopic comparative series
The issue of cost effectiveness with regards to robotics remains a relevant issue, as increasingly more procedures are done using this approach. There have been several authors that have directly compared costs for robotic and laparoscopy pyeloplasty. Bhayani et al.[11] compared their early robotic pyeloplasties with a matched cohort of laparoscopic cases. The cost of equipment and capital depreciation for both procedures, as well as assessment of room set-up time, takedown time, and personnel was analyzed. Laparoscopic pyeloplasty was found to be more cost effective when OR times were kept lower than 338 minutes. They also found that for robotic pyeloplasty to be as cost effective as standard laparoscopy at their center, total in room time for the robotic procedure had to be <130 minutes, with a yearly case volume > 500. The group from University of Texas Southwestern Medical Center[52] developed a decision analysis model to compare the costs of each procedure. They found that despite shorter operative times and hospitalization, robotic pyeloplasty was more costly, with the main difference coming from the fixed cost of the robot and surgical supplies. According to their model, even if robotic pyeloplasties were performed on an outpatient basis or their volume was >1000 cases/year, laparoscopic pyeloplasty would still be more cost effective. One-way sensitivity analysis revealed that if robotic pyeloplasty could be performed in 96 minutes or less, it would be cost effective.
Robotic approach to the lower ureter: Ureteroneocystotomy ± Psoas hitch/boari flap
There is considerably less experience with regards to robotics and distal ureteric pathology which necessitates ureteroneocystostomy. A number of case reports emerged in 2007 describing robotic ureteral surgery for both benign and malignant conditions. Hemal and coworkers[17] described 18 distal ureteral procedures which included 5 ureteroneocystotomies (with distal ureteral excision and cuff of bladder resection) for urothelial malignancy with mean operating time of 190 minutes and mean EBL of 100 mL. They also repaired and reimplanted 8 megaureters with a mean OR time of 142.5 minutes and EBL of 50 mL. The remaining procedures consisted of one ureteroneocystostomy with a psoas hitch, two ureteroneocystotomies with vesicovaginal fistula repair, and two ureteroneocystotomies for ureteric injury during radical prostatectomy. There were no delayed urine leaks, and all procedures were technically successful. Patil et al.[16] performed a multi-institutional study of robotic ureteral reimplantation with psoas hitch. Twelve patients from three centers were included and underwent the procedure for a number of different indications including stricture disease due to stones (n = 6), injury during gynecologic surgery (n = 4), stricture after reimplant surgery (n = 1), and endometriosis (n = 1). The mean operative time was 208 minutes and EBL was 48 mL. All procedures were completed successfully without open conversion and there were no intraoperative or postoperative complications. After a mean follow up of 15.5 months, all patients were asymptomatic without clinical evidence of obstruction. The study was limited by the fact that the three centers had different post-operative management strategies, which affected LOS and catheterization/stent duration. Kozinn and colleagues[53] compared 10 patients who underwent a mid/distal robotic ureteric reconstruction with 10 patients who underwent an open reconstruction. Again, there was a variety of ureteric pathology including stricture from stone disease and iatrogenic injury. In the robotic group, four primary ureteroneocystotomies, four psoas hitches, and two Boari flaps were performed, as compared to the open group with six ureteroneocystotomies, three psoas hitches, and one Boari flap. Estimated blood loss (30.6 vs. 327.5 mL, P = 0.001) and length of hospital stay (2.4 vs. 5.1 d, P = 0.01) were significantly less in the robotic group, whereas OR time was longer (306.6 vs. 270.0 min, P = 0.316). There were no complications or conversions to open surgery in the robotic group. All patients in both groups had complete resolution on follow up, which was up to 24 months in the robotic group. We recently described the Cleveland Clinic experience, which is the largest series comparing robotic ureteroneocystotomy (RUNC) to open surgery (OUNC).[54] Twenty-five patients who underwent RUNC and 41 patients who underwent OUNC or at our institution between 2000 and 2010 were retrospectively analyzed. The OUNC procedures were associated with a shorter median operative time (200 vs. 279 min., P = 0.0008), whereas RUNC patients had a shorter hospital stay (median 3 vs. 5 days, P = 0.0004), less narcotic pain requirement (morphine equivalent, mg 104.6 vs. 290, P = 0.0001), and less estimated blood loss (100 vs. 150 mL, P = < 0.0002). There was no significant difference in the rate of reoperation between groups (RUNC 2/25 (7.6%) vs. OUNC 4/41 (9.7%) P = 0.8).
CONCLUSIONS
What is clear is that the gold standard for repair of a primary UPJO in adults involves a minimally invasive approach. Laparoscopic pyeloplasty has been the most widely utilized technique in the past, but with the well-documented advantages of robotic surgery with regards to suturing and learning curve reduction, we are seeing a change in the current surgical climate. While it is clear from the literature that a robotic pyeloplasty is easier to learn and perform, no distinct advantage with regards to clinical and functional outcomes has emerged. Nevertheless, the application of robotic technology has allowed more surgeons to offer this procedure to their patients, thus improving accessibility. In the past the laparoscopic technique was only performed by a few experienced surgeons, and patients would often have to travel to “centers of excellence.” Additionally, as more and more hospitals in the USA invest in a surgical robot, we are likely to see this trend continue. However, it is well known that the cost-effectiveness of robotic pyeloplasty is inferior, and this needs to be weighed against the benefits for both the surgeon and the patient. With the possible availability of more affordable robotic technology in the future this concern would be minimized. For pediatric pyeloplasty the benefits of robotics are also controversial. In very small patients, given the size of the incision, the retroperitoneal open approach still might be the most practical method.
Robotics does seem to provide a benefit for cases of secondary UPJO, as the enhanced vision and the precision offered by the “endowrist” technology may make approaching these demanding procedures less challenging when compared to conventional laparoscopy. Finally, “scarless” surgery performed with LESS technique has been demonstrated as feasible using robotic instrumentation and certainly easier to perform than conventional LESS, given the benefits of easier intracorporeal suturing and improved surgeon ergonomics. Nevertheless, there is no clear advantage demonstrated over conventional robotic pyeloplasty, and the current robotic platform is not optimally suited for LESS because of the large external profile. Several robotic LESS-specific platforms are currently under development, and this may lead to increased utilization of the technique. For now it seems reasonable to offer this approach to patients who are especially concerned with cosmetic results.
There has been less experience with distal ureteric reconstructive procedures; however, there are a number of series demonstrating the feasibility of ureteroneocystotomy ± psoas hitch/boari flap. Benefits in terms of hospital stay and narcotic requirements have been demonstrated when compared to open surgery. As the field of robotics continues to evolve and expand it is likely that more of these cases will be performed robotically.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bansal P, Gupta A, Mongha R, Narayan S, Das RK, Bera M, et al. Laparoscopic versus open pyeloplasty: Comparison of two surgical approaches-a single centre experience of three years. Indian J Surg. 2011;73:264–7. doi: 10.1007/s12262-011-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripp BM, Homsy YL. Neonatal hydronephrosis-the controversy and the management. Pediatr Nephrol. 1995;9:503–9. doi: 10.1007/BF00866741. [DOI] [PubMed] [Google Scholar]
- 3.Salem YH, Majd M, Rushton HG, Belman AB. Outcome analysis of pediatric pyeloplasty as a function of patient age, presentation and differential renal function. J Urol. 1995;154:1889–93. [PubMed] [Google Scholar]
- 4.Brooks JD, Kaoussi LR, Preminger GM, Schuessler WW, Moore RG. Comparison of Open and Endourologic Approaches to the Obstructed Ureteropelvic Junction. Urology. 1995;46:791–5. doi: 10.1016/S0090-4295(99)80345-8. [DOI] [PubMed] [Google Scholar]
- 5.Meretyk I, Meretyk S, Clayman RV. Endopyelotomy: Comparison of ureteroscopic retrograde and antegrade percutaneous techniques. J Urol. 1992;148:775–82. doi: 10.1016/s0022-5347(17)36717-4. [DOI] [PubMed] [Google Scholar]
- 6.Schuessler WW, Grune MT, Tecuanhuey LV, Preminger GM. Laparoscopic dismembered pyeloplasty. J Urol. 1993;150:1795–9. doi: 10.1016/s0022-5347(17)35898-6. [DOI] [PubMed] [Google Scholar]
- 7.Passerotti CC, Passerotti AM, Dall'Oglio MF, Leite KR, Nunes RL, Srougi M, et al. comparing the quality of the suture anastomosis and the learning curves associated with performing open, freehand, and robotic-assisted laparoscopic pyeloplasty in a swine animal model. J Am Coll Surg. 2009;208:576–86. doi: 10.1016/j.jamcollsurg.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Gettman MT, Neururer R, Bartsch G, Reinhard P. Anderson-Hynes Dismembered Pyeloplasty Performed Using the da Vinci Robotic System. Urology. 2002;60:509–13. doi: 10.1016/s0090-4295(02)01761-2. [DOI] [PubMed] [Google Scholar]
- 9.Sivaraman A, Leveillee RJ, Patel MB, Chauhan S, Bracho JE, Moore CR, et al. Robot-assisted laparoscopic dismembered pyeloplasty for ureteropelvic junction obstruction: A multi-institutional experience. Urology. 2012;79:351–5. doi: 10.1016/j.urology.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Gettman MT, Peschel R, Neururer R, Bartsch G. A comparison of laparoscopic pyeloplasty performed with the davinci robotic system versus standard laparoscopic techniques: Initial clinical results. Eur Urol. 2002;42:453–8. doi: 10.1016/s0302-2838(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 11.Bhayani SB, Link RE, Varkarakis JM, Kaoussi LR. Complete daVinci versus laparoscopic pyeloplasty: Cost analysis. J Endourol. 2005;19:327–32. doi: 10.1089/end.2005.19.327. [DOI] [PubMed] [Google Scholar]
- 12.Braga LHP, Pace K, DeMaria J, Lorenzo AJ. Systematic review and meta-analysis of robotic-assisted versus conventional laparoscopic pyeloplasty for patients with ureteropelvic junction obstruction: Effect on operative time, length of hospital stay, postoperative complications, and success rate. Eur Urol. 2009;56:848–58. doi: 10.1016/j.eururo.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 13.Kaouk JH, Hafron J, Parekattil S, Moinzadeh A, Stein R, Gill IS, et al. Is Retroperitoneal approach feasible for robotic dismembered pyeloplasty: Initial experience and long-term results. J Endourol. 2008;22:2153–60. doi: 10.1089/end.2008.0130. [DOI] [PubMed] [Google Scholar]
- 14.Minnillo BJ, Cruz JA, Sayao RH, Passerotti CC, Houck CS, Meier PM, et al. Long-Term experience and outcomes of robotic assisted laparoscopic pyeloplasty in children and young adults. J Urol. 2011;185:1455–60. doi: 10.1016/j.juro.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 15.Seideman CA, Tan YK, Faddegon S, Park SK, Best SL, Cadeddu JA, et al. Robot-assisted laparoendoscopic single-site pyeloplasty: Technique using the da vinci si robotic platform. J Endourol. 2012;26:971–4. doi: 10.1089/end.2011.0573. [DOI] [PubMed] [Google Scholar]
- 16.Patil NN, Mottrie A, Sundaram B, Patel VR. Robotic-assisted laparoscopic ureteral reimplantation with psoas hitch: A multi-institutional, multinational evaluation. Urology. 2008;72:47–50. doi: 10.1016/j.urology.2007.12.097. [DOI] [PubMed] [Google Scholar]
- 17.Hemal AK, Nayyar R, Gupta NP, Dorairajan LN. Experience with robot assisted laparoscopic surgery for upper and lower benign and malignant ureteral pathologies. Urology. 2010;76:1387–93. doi: 10.1016/j.urology.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Phillips EA, Wang DS. Current status of robot-assisted laparoscopic ureteral reimplantation and reconstruction. Curr Urol Rep. 2012;13:190–4. doi: 10.1007/s11934-012-0250-4. [DOI] [PubMed] [Google Scholar]
- 19.Sukumar S, Sun M, Karakiewicz PI, Friedman AA, Chun FK, Sammon J, et al. National trends and disparities in the use of minimally invasive adult pyeloplasty. J Urol. 2012;188:913–8. doi: 10.1016/j.juro.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Monn MF, Bahler CD, Schneider EB, Sundaram CP. Emerging trends in robotic pyeloplasty for the management of ureteropelvic junction obstruction in adults. J Urol. 2013;189:1352–7. doi: 10.1016/j.juro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Schwentner C, Pelzer A, Neururer R, Springer B, Horninger W, Bartsch G, et al. Robotic Anderson-Hynes pyeloplasty: 5-year experience of one centre. BJU Int. 2007;100:880–5. doi: 10.1111/j.1464-410X.2007.07032.x. [DOI] [PubMed] [Google Scholar]
- 22.Mufarrij PW, Woods M, Shah OD, Palese MA, Berger AD, Thomas R, et al. Robotic dismembered pyeloplasty: A 6-year, multi-institutional experience. J Urol. 2008;180:1391–6. doi: 10.1016/j.juro.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Gupta NP, Mukherjee S, Nayyar R, Hemal AK, Kumar R. Transmesocolic robot-assisted pyeloplasty: Single center experience. J Endourol. 2009;23:945–8. doi: 10.1089/end.2008.0430. [DOI] [PubMed] [Google Scholar]
- 24.Erdeljan P, Caumartin Y, Warren J, Nguan C, Nott L, Luke PP, et al. Robot-assisted pyeloplasty: Follow-up of first Canadian experience with comparison of outcomes between experienced and trainee surgeons. J Endourol. 2010;24:1447–50. doi: 10.1089/end.2009.0617. [DOI] [PubMed] [Google Scholar]
- 25.Lucas SM, Sundaram CP, Wolf JS, Leveillee RJ, Bird VG, Aziz M, et al. Factors that impact the outcome of minimally invasive pyeloplasty: Results of the multi-institutional laparoscopic and robotic pyeloplasty collaborative group. J Urol. 2012;187:522–7. doi: 10.1016/j.juro.2011.09.158. [DOI] [PubMed] [Google Scholar]
- 26.Etafy M, Pick D, Said S, Hsueh T, Kerbl D, Mucksavage P, et al. Robotic pyeloplasty: The University of California-Irvine experience. J Urol. 2011;185:2196–200. doi: 10.1016/j.juro.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 27.Cestari A, Buffi NM, Lista G, Sangalli M, Scapaticci E, Fabbri F, et al. Retroperitoneal and transperitoneal robot-assisted pyeloplasty in adults: Techniques and results. Eur Urol. 2010;58:711–8. doi: 10.1016/j.eururo.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Nicol DL, Smiters BM. Laparoscopic approach to the left kidney avoiding colonic mobilization. J Urol. 1994;152:1967–9. doi: 10.1016/s0022-5347(17)32280-2. [DOI] [PubMed] [Google Scholar]
- 29.Olsen LH, Jorgensen TM. Computer assisted pyeloplasty in children: The retroperitoneal approach. J Urol. 2004;171:2629–31. doi: 10.1097/01.ju.0000110655.38368.56. [DOI] [PubMed] [Google Scholar]
- 30.Lee RS, Retik AB, Borer JG, Peters CA. Pediatric robot assisted laparoscopic dismembered pyeloplasty: Comparison with a cohort of open surgery. J Urol. 2006;175:683–7. doi: 10.1016/S0022-5347(05)00183-7. [DOI] [PubMed] [Google Scholar]
- 31.Yee DS, Shanberg AM, Duel BP, Rodriguez E, Eichel L, Rajpoot D. Initial comparison of robotic-assisted laparoscopic versus open pyeloplasty in children. Urology. 2006;67:599–602. doi: 10.1016/j.urology.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Ng CS, Yost AJ, Streem SB. Management of failed primary intervention for ureteropelvic junction obstruction: 12-year, single-center experience. Urology. 2003;61:291–6. doi: 10.1016/s0090-4295(02)02160-x. [DOI] [PubMed] [Google Scholar]
- 33.Park J, Kim WS, Hong B, Park T, Park HK. Long-term outcome of secondary endopyelotomy after failed primary intervention for ureteropelvic junction obstruction. Int J Urol. 2008;15:490–4. doi: 10.1111/j.1442-2042.2008.02035.x. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro EY, Cho JS, Srinivasan A, Seideman CA, Huckabay CP, Andonian S, et al. Long-Term follow-up for salvage laparoscopic pyeloplasty after failed open pyeloplasty. Urology. 2009;73:115–8. doi: 10.1016/j.urology.2008.08.483. [DOI] [PubMed] [Google Scholar]
- 35.Basiri A, Behjati S, Zand S, Moghaddam SM. Laparoscopic pyeloplasty in secondary ureteropelvic junction obstruction after failed open surgery. J Endourol. 2007;21:1045–52. doi: 10.1089/end.2006.0414. [DOI] [PubMed] [Google Scholar]
- 36.Niver BE, Agalliu I, Bareket R, Mufarrij P, Shah O, Stifelman MD. Analysis of robotic-assisted laparoscopic pyleloplasty for primary versus secondary repair in 119 consecutive cases. Urology. 2012;79:689–94. doi: 10.1016/j.urology.2011.10.072. [DOI] [PubMed] [Google Scholar]
- 37.Kaouk JH, Autorino R, Kim FJ, Han DH, Lee SW, Yinghao S, et al. Laparoendoscopic single-site surgery in urology: Worldwide multi-institutional analysis of 1076 cases. Eur Urol. 2011;60:998–1005. doi: 10.1016/j.eururo.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Kaouk JH, Goel RK, Haber GP, Crouzet S, Desai MM, Gill IS. Single-port laparoscopic radical prostatectomy. Urology. 2008;72:1190–3. doi: 10.1016/j.urology.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Stein RJ, Berger AK, Brandina R, Patel NS, Canes D, Irwin BH, et al. Laparoendoscopic single-site pyeloplasty: A comparison with the standard laparoscopic technique. BJU Int. 2011;107:811–5. doi: 10.1111/j.1464-410X.2010.09558.x. [DOI] [PubMed] [Google Scholar]
- 40.Kaouk JH, Goel RK, Haber GP, Crouzet S, Stein RJ. Robotic single-port transumbilical surgery in humans: Initial report. BJU Int. 2009;103:366–9. doi: 10.1111/j.1464-410X.2008.07949.x. [DOI] [PubMed] [Google Scholar]
- 41.Haber GP, White MA, Autorino R, Escobar PF, Kroh MD, Chalikonda S, et al. Novel Robotic da Vinci Instruments for Laparoendoscopic Single-site Surgery. Urology. 2010;76:1279–82. doi: 10.1016/j.urology.2010.06.070. [DOI] [PubMed] [Google Scholar]
- 42.Stein RJ, White WM, Goel RK, Irwin BH, Haber GP, Kaouk JH. Robotic laparoendoscopic single-site surgery using gelport as the access platform. Eur Urol. 2010;57:132–7. doi: 10.1016/j.eururo.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 43.Cestari A, Buffi NM, Lista G, Lughezzani G, Larcher A, Lazzeri M, et al. Feasibility and preliminary clinical outcomes of robotic laparoendoscopic single-site pyeloplasty using a new single-port platform. Eur Urol. 2012;62:175–9. doi: 10.1016/j.eururo.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 44.Olweny EO, Park SK, Tan YK, Gurbuz C, Cadeddu JA, Best SL. Perioperative comparison of robotic assisted laparoendoscopic single-site pyeloplasty versus conventional less pyeloplasty. Eur Urol. 2012;61:410–4. doi: 10.1016/j.eururo.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Bird VG, Leveillee RJ, Eldefrawy A, Bracho J, Aziz MS. Comparison of robot-assisted versus conventional laparoscopic transperitoneal pyeloplasty for patients with ureteropelvic junction obstruction: A single-center study. Urology. 2011;77:730–4. doi: 10.1016/j.urology.2010.07.540. [DOI] [PubMed] [Google Scholar]
- 46.Bernie JE, Venkatesh R, Brown J, Gardner TA, Sundaram CP. Comparison of laparoscopic pyeloplasty with and without robotic assistance. JSLS. 2005;9:258–61. [PMC free article] [PubMed] [Google Scholar]
- 47.Link RE, Bhayani SB, Kavoussi LR. A prospective comparison of robotic and laparoscopic pyeloplasty. Ann Surg. 2006;243:486–91. doi: 10.1097/01.sla.0000205626.71982.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemal AK, Mukherjee S, Singh K. Laparoscopic pyeloplasty versus robotic pyeloplasty for ureteropelvic junction obstruction: A series of 60 cases performed by a single surgeon. Can J Urol. 2010;17:5012–6. [PubMed] [Google Scholar]
- 49.Kumar R, Nayak B. Robotic versus conventional laparoscopic pyeloplasty: A single surgeon concurrent cohort review. Indian J Urol. 2013;29:19–21. doi: 10.4103/0970-1591.109978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riachy E, Cost NG, Defoor WR, Reddy PP, Minevich EA, Noh PH. Pediatric standard and robot-assisted laparoscopic pyeloplasty: A comparative single institution study. J Urol. 2013;189:283–7. doi: 10.1016/j.juro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Subotic U, Rohard I, Weber DM, Gobet R, Moehrlen U, Gonzalez R. A minimal invasive surgical approach for children of all ages with ureteropelvic junction obstruction. J Pediatr Urol. 2012;8:354–8. doi: 10.1016/j.jpurol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Seideman CA, Sleeper JP, Lotan Y. Cost comparison of robot-assisted and laparoscopic pyeloplasty. J Endourol. 2012;26:1044–8. doi: 10.1089/end.2012.0026. [DOI] [PubMed] [Google Scholar]
- 53.Kozinn SI, Canes D, Sorcini A, Moinzadeh A. Robotic versus open distal ureteral reconstruction and reimplantation for benign stricture disease. J Endourol. 2012;26:147–51. doi: 10.1089/end.2011.0234. [DOI] [PubMed] [Google Scholar]
- 54.Isac W, Kaouk J, Altunrende F, Rizkala E, Autorino R, Hillyer SP, et al. Robot -assisted ureteroneocystostomy: Technique and comparative outcomes. J Endourol. 2013;27:318–23. doi: 10.1089/end.2012.0196. [DOI] [PubMed] [Google Scholar]


