Abstract
A lady of 52 years with painful bladder syndrome/interstitial cystitis (PBS/IC) presented with chronic pelvic pain, irritative voiding with sphincter dominance on urodynamics. 3 yrs of oral analgesics, antispasmodics and intravesical therapy was ineffective. We surmised her pain, and irritative voiding to be secondary to constant straining against a dysfunctional pelvic floor. We treated PBS/IC as a neuropathic phenomenon with a combination of neuromodulator medications and continuous caudal epidural analgesia to reduce the pain induced peripheral and central sensitisation. Botulinum toxin type A injection into pelvic floor muscles appeared to address their dysfuction. Clinical and urodynamics response was encouraging.
Keywords: Botox injection into pelvic floor muscles, caudal epidural analgesia, interstitial cystitis, painful bladder syndrome
INTRODUCTION
Painful bladder syndrome (PBS)/interstitial cystitis (IC) is characterized by suprapubic pain with bladder filling and increased daytime and nighttime frequency in the absence of proven urinary infection or other obvious pathology.[1] The role of the central nervous system and pelvic floor dysfunction (PFD) in its pathogenesis and clinical manifestations has now been recognized.[2] We report our case to demonstrate the possible role of peripheral and central sensitization as a cause of visceral pain in IC and neuromodulation as a therapeutic modality for the same.
CASE REPORT
Intravesical hydrodistension had been performed thrice without any effect. Pelvic and neurological examinations were normal. A lady of 52 years was referred to us by a urologist with a diagnosis of ICS for pain management. Intravesical hydrodistension had been performed thrice without any effect. The following treatment options were suggested to the patient and her relatives: Neuromodulation using pregabalin and amitriptyline and interventional therapies like continuous caudal epidural infusion (CCEI) of local anesthetics for 1 month to reduce central sensitization and botulinum toxin A injection into the pelvic floor muscles, including the urethral sphincter, to relieve the high-tone pelvic floor muscle spasm that has been theorized in IC.[2]
We usually start pregabalin with a dose of half a 75 mg capsule in women weighing less than 55 kg. In men and women weighing more than 55 kg, a single capsule of 75 mg pregabalin is prescribed. In the absence of side-effects like dizziness and excessive somnolence, the dose may be escalated to a twice-daily regimen after 5 days. After five more days, if the pain persists at a level greater than five on Numerical Rating Scale NRS, we increase the dose to three-times daily. The starting dose of amitryptyline used is 5 mg at night, which may be increased to 10 mg if the patient is still in pain after 5 days.
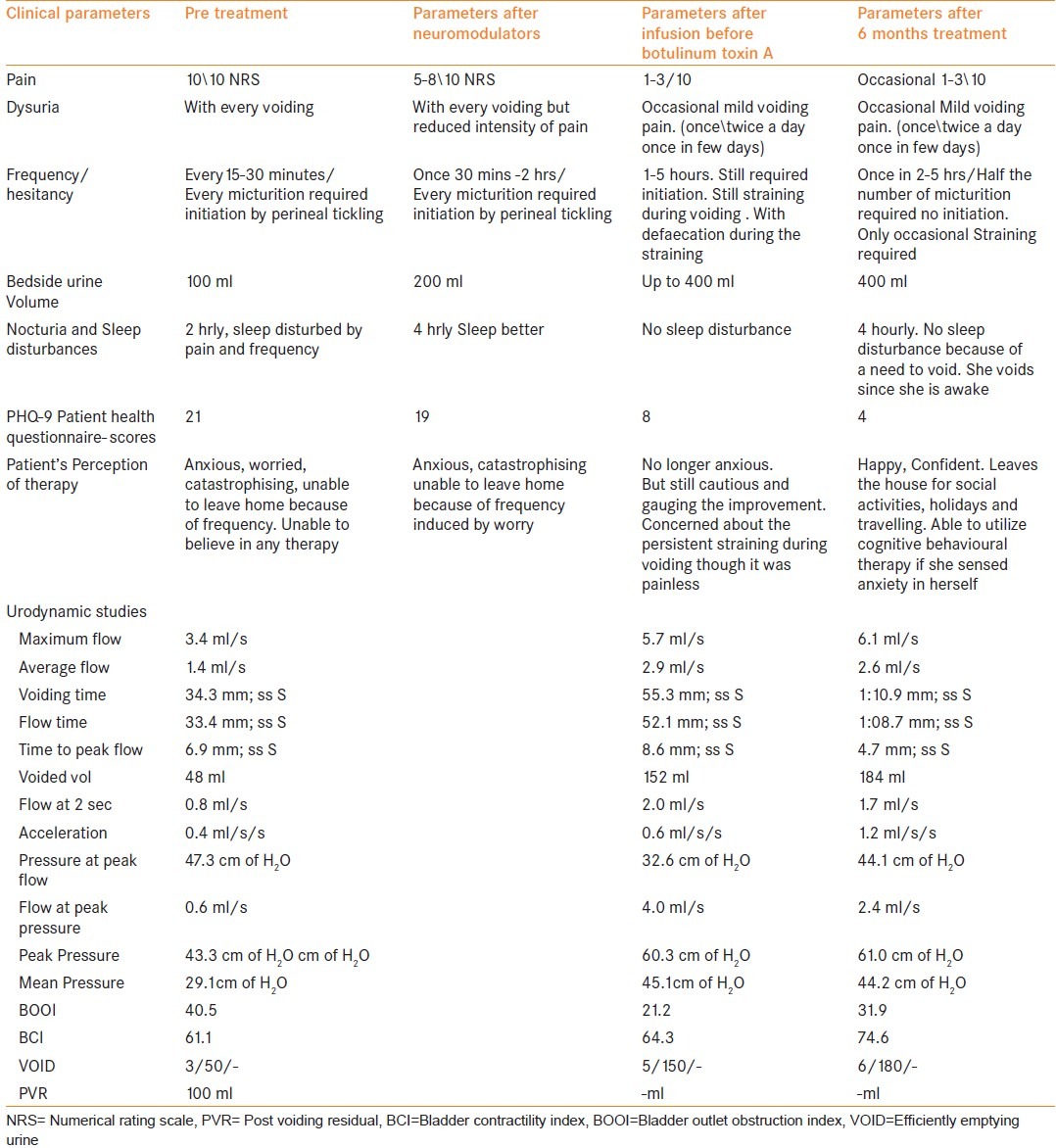
Pregabalin 37 mg was advised initially and amitriptyline 10 mg was added 1 week later according to our protocol. The patient was advised to monitor and chart her urinary frequency and volume at home. After 1 month of neuromodulators, she showed a favorable response [Table 1], but opted for CCEI and botulinum toxin A injection because her social life continued to revolve round the severe restraints posed by her frequent distressing attempts to void. Increase in voided urine volume to 500 mL was decided as an end point for terminating the CCEI.
Table 1.
Clinical findings and urodynamic parameters at various stages of treatment and at 6 months
The interventions were carried out in an operation theater with appropriate monitoring (oximeter, ECG, non-invasive blood pressure) and antibiotic cover with intravenous cefuroxime 750 mg. A high-frequency probe of S MSK (Sonosite Bothell USA) ultrasonography-guided 18 gauge Touhy needle was introduced into the sacral hiatus [Figure 1].
Figure 1.
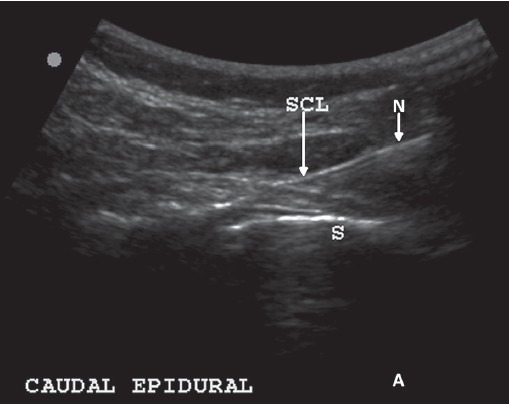
Needle entering the sacrococcygeal ligament - SCL (arrow)
The catheter was passed up to the second sacral segment and omnipaque dye spread was confirmed to be around the second to fourth sacral nerves with fluoroscopy [Figure 2]. The distal end of the catheter was tunneled superolaterally in the subcutaneous tissue through a 10-cm Touhy needle and connected to an infusion of 0.1% bupivacaine at a rate of 2 mL/h administered through an elastomeric pump (dosifusor Leventon, Spain) [Figure 3]. The patient was advised oral antibiotics and was instructed to follow-up on an alternate day basis and take care of the caudal epidural infusion and its discontinuation in case of urinary retention. Catheter dressing and pump refill were performed once in 5 days. Pelvic floor relaxation exercises were taught.
Figure 2.
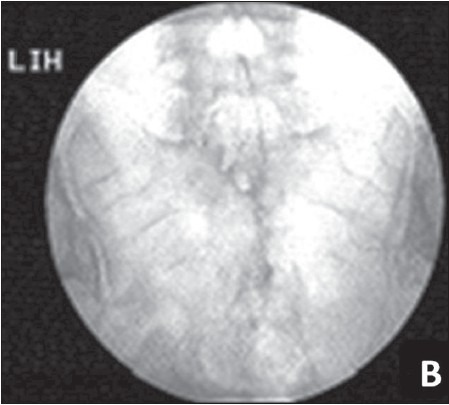
Spread of dye (omnipaque) in the caudal epidural space
Figure 3.
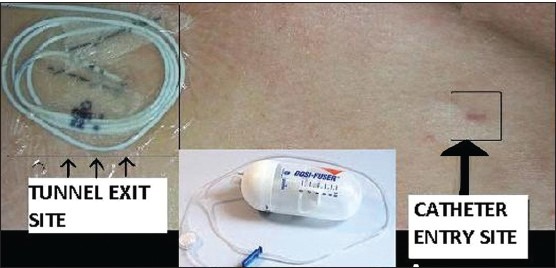
The tunneled caudal epidural catheter connected to the elastomeric pump (dosifusor)
After 2 weeks, the patient developed a fever of 100°F with mild leukocytosis for 2 days. The CCEI catheter was prematurely removed even though the catheter insertion and tunnel exit sites appeared normal, because it was placed in the central neuraxis. The catheter tip culture was negative. The fever resolved 1 day later. At this time, all parameters like pain, frequency (from 15-30 min to 2-4 hr) and urine volume (from 150 to 400 mL) had improved.
One week after CCEI termination, Botulinum toxin A injection was given intramuscularly into the perineal muscles (external urethral sphincter -70 units, 5 units each into the ischiocavernosus, bulbospongiosus and transverses perinei muscles on both sides under topical analgesia with lignocaine jelly). The patient continued to maintain the improved parameters after catheter removal and 15 days after botulinum toxin A, she reported effortless voiding. The improvement continued to be present at 1, 3 and 6 months of follow-up [Table 1], with continued treatment with pregabalin and nortryptyline at night and spasmoproxyvon as needed.
A urodynamic study 1 month after therapy showed an unequivocal improvement of the patient health questionnaire, bladder volume (100-400 mL), frequency and residual volume (100-0 mL), which was maintained 6 months later without any pain. The clinical details and findings of the urodynamics studies before and after treatment are shown in [Table 1].
DISCUSSION
PBS/IC is characterized by suprapubic pain associated with bladder filling and increased daytime and nighttime frequency in the absence of proven urinary infection or other obvious pathology.[1] The diagnosis of IC is by exclusion as there is no consensus on its etiology, diagnostic criteria, pathophysiology and treatment strategies.[3] The treatment of PBS has been recommended to aim at palliation and alleviation of symptoms.[4]
The management involves patient education, self-care (diet modification) and stress management as the first-line treatment. Physical therapy, oral medications (amitryptiline, cimetidine or hydroxyzine, pentosanpolysulfate), bladder instillations (DMSO, heparin or lidocaine) are considered if the first-line treatment fails. Treatment of Hunner's ulcers and hydrodistention (low pressure, short duration) are applied for refractory cases. The next line of management would involve neuromodulation (sacral or pudendal nerve block) and Cyclosposine A or Botulinum toxin injection (BTX-A) into the bladder. Ultimately, if all other forms of treatment fail, surgical intervention may be the last resort in the form of urinary diversion or augmentation cystoplasty.
However, there is an emerging consensus as to the central role of epithelial dysfunction, bladder sensory nerve up-regulation and mast cell activation in the genesis of IC. The importance of the central nervous system and PFD in the pathogenesis and clinical manifestations of IC is now recognized.[1,2,5]
We addressed central sensitivity and PFD in this patient to achieve a clinical and urodynamic improvement. We assumed that peripheral sensitization of bladder C fibers was triggered by the ongoing pain from neuroinflammation and straining against an unrelenting obstruction due to the pelvic floor neuromuscular dysfunction. Peripheral hyperexcitability of pain fibers led to persistent activation of the spinal cord neurons, which evolved to be centrally maintained. Progression to central sensitivity led to the development of a generalized irritable bladder that could not hold a normal volume of urine (reduced capacity) and developed irritative voiding symptoms.
The response of our patient to the neuromodulator pregabalin emphasized the contribution of neuropathic mechanisms in her symptoms. This was confirmed by resolution of pain, frequency, urgency and nocturia after CCEI, which attenuated the peripheral and central sensitization. However, the persistence of straining indicated that obstructive mechanisms from the dysfunctional pelvic floor muscles were still operational. We addressed this with pelvic floor botulinum toxin A injection rather than intravesical botulinum toxin A. This combination therapy addressed the two issues separately, with the belief that pelvic floor muscle spasm was the primary issue leading to peripheral and central sensitivity. This combination has been developed from our prior experience in the past decade in several patients.
The concept of peripheral and central sensitization in visceral pain has been reported.[5] Our report demonstrates that it is a possibility to be considered in a condition like IC where very few effective options are available for successful therapy. However, a study in a large number of patients would establish this surmise.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sant GR. Etiology, Pathogenesis, and Diagnosis of Interstitial Cystitis. Rev Urol. 2002;4(Suppl 1):S9–15. [PMC free article] [PubMed] [Google Scholar]
- 2.Lukban JC, Parkin JV, Holzberg AS, Caraballo R, Kellogg-Spadt S, Whitmore KE. Interstitial Cystitis And Pelvic Floor Dysfunction: A Comprehensive Review. Pain Med. 2001;2:60–8. doi: 10.1046/j.1526-4637.2001.002001060.x. [DOI] [PubMed] [Google Scholar]
- 3.Gousse AE. Current Investigation and treatment of Interstistial Cystitis. Curr Urol Rep. 2000 Oct;1:190–8. doi: 10.1007/s11934-000-0018-0. [DOI] [PubMed] [Google Scholar]
- 4.Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, Fitzgerald MP, Forrest JB. Diagnosis and treatment of Interststialcystistis/painful bladder syndrome AUA guidelines 2011. J Urol. 2011 Jun;185:2162–70. doi: 10.1016/j.juro.2011.03.064. doi: 10.1016/j.juro.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wesselmann U. Interstitial cystitis: A chronic visceral pain syndrome. Urology. 2001;57(5 Suppl 1):32–9. doi: 10.1016/s0090-4295(01)01123-2. [DOI] [PubMed] [Google Scholar]


