Abstract
The plasma protein Serum Amyloid P (SAP) reduces neutrophil adhesion, inhibits the differentiation of monocytes into fibroblast-like cells called fibrocytes, and promotes phagocytosis of cell debris by macrophages. Together, these effects of SAP reduce key aspects of inflammation and fibrosis, and SAP injections improve lung function in pulmonary fibrosis patients. SAP functions are mediated in part by Fcγ receptors, but the contribution of each Fcγ receptor is not fully understood. We found that amino acids Q55 and E126 in human SAP affect human fibrocyte differentiation and SAP binding to FcγRI. E126, K130 and Q128 affect neutrophil adhesion and SAP affinity for FcγRIIa. Q128 also affects phagocytosis by macrophages and SAP affinity for FcγRI. All the identified functionally significant amino acids in SAP form a binding site that is distinct from the previously described SAP-FcγRIIa binding site. Blocking FcγRI with an IgG blocking antibody reduces the SAP effect on fibrocyte differentiation, and ligating FcγRIIa with antibodies reduces neutrophil adhesion. Together, these results suggest that SAP binds to FcγRI on monocytes to inhibit fibrocyte differentiation, and binds to FcγRIIa on neutrophils to reduce neutrophil adhesion.
Introduction
Aberrant scar tissue formation is the hallmark of fibrosing diseases such as end-stage kidney disease, liver cirrhosis, pulmonary fibrosis, and congestive heart disease (1–3). The inappropriate scar tissue in fibrosis ultimately leads to organ failure and/or death. Fibrosing diseases are associated with 45% of deaths in the US, but despite their high prevalence, there are no FDA-approved therapies (1, 4).
Serum Amyloid P component (SAP) is a pentameric protein that belongs to the pentraxin family of evolutionarily conserved proteins. Pentraxins also include C-reactive protein (CRP) and the long pentraxin PTX-3 (5). SAP, CRP, and PTX-3 all have regulatory roles in the immune system (6–8). Injections of SAP inhibit inflammation and fibrosis in mouse models of pulmonary fibrosis, ischemic cardiac fibrosis, and renal fibrosis (9–12), and in a phase 1b clinical trial, SAP injections appear to improve lung function in pulmonary fibrosis patients (13).
At the onset of tissue damage and inflammation, neutrophils are recruited to the tissue in response to chemokines such as CXCL2 and CXCL8 to remove pathogens and/or cell debris via phagocytosis (14). This migration and activation of neutrophils is tightly regulated by factors expressed and secreted by endothelial cells, macrophages and other cell types (14). When this regulation is compromised, the elevated influx of neutrophils and recruitment of other immune cells by activated neutrophils can cause severe organ damage and fibrosis (14–16). SAP binds neutrophils to inhibit their spreading and adhesion to components of extracellular matrix and endothelial cells (12, 17). Injections of SAP decrease the infiltration of neutrophils into the lungs following bleomycin insult in mice (12). However, the mechanism for this function is not well understood.
Following neutrophil migration into the inflammation site, CD14+ monocytes enter and differentiate into macrophages and fibrocytes (3). Fibrocytes are CD45+ collagen I+ fibroblast-like cells that share characteristics of both hematopoietic and stromal cells (18). Fibrocytes are found in healing dermal wounds and some fibrotic lesions, and secrete collagen and enzymes which modify the extracellular matrix (3, 9, 10, 19, 20). SAP inhibits fibrocyte differentiation partly through a group of receptors called Fcγ receptors (11, 21–24). These receptors bind IgG and consist of FcγRI, FcγRIIa, FcγRIIb, FcγRIIIa, and FcγRIIIb (25). We have previously shown that FcγRI is one of the receptors responsible for the effect of SAP on fibrocyte differentiation in both humans and mice (21). SAP also binds the IgA receptor FcαRI (26).
In addition to modifying neutrophil adhesion and monocyte differentiation, SAP can also enhance phagocytosis of cell debris by professional phagocytes such as macrophages (24, 27). The SAP pentamer forms a flat disk, and binds to bacteria and cell debris on one surface, and to Fcγ receptors on the other surface, to promote phagocytosis by cells (24). Previous studies have implicated FcγRI as the key receptor for SAP-induced phagocytosis, but the precise role of each Fcγ receptor in this process is unclear (24, 27).
In this report, we examined how SAP interacts with Fcγ receptors to regulate different aspects of the immune system. We found that SAP inhibits fibrocyte differentiation and promotes phagocytosis by macrophages through FcγRI, while it reduces neutrophil adhesion via FcγRIIa. Using site-directed mutagenesis we determined that although the same site on SAP affects monocytes, macrophages, and neutrophils, it is possible to affect specific SAP functions without altering the other functions in an appreciable way. In addition, we identified a novel Fcγ receptor binding site that is distinct from the site previously identified in a co-crystal structure of SAP and FcγRIIa (23).
Materials and methods
PBMC and neutrophil isolation, cell culture, fibrocyte and macrophage differentiation
Human peripheral blood was collected into heparin tubes (BD Bioscience, San Jose, CA) from healthy adult volunteers who gave written consent and with specific approval from the Texas A&M University human subjects Institutional Review Board. Peripheral blood mononuclear cells (PBMC) were isolated from the blood using Ficoll-Paque Plus (GE Healthcare Biosciences, Piscataway, NJ), as described previously (28). PBMCs were cultured in Fibrolife (LifeLine Cell Technology, Walkersville, MD) defined serum-free medium (SFM) in the presence or absence of SAP variants as previously described (21). To determine the contribution of each Fcγ receptor to the SAP effect on fibrocyte differentiation, we incubated PBMCs with 5 μg/ml of F(ab′)2 fragments of either anti-FcγRI antibody clone 10.1 (mouse IgG1, Ancell, Bayport, MN), anti-FcγRII antibody clone 7.3 (mouse IgG1, Ancell), anti-FcγRIII antibody clone 3G8 (mouse IgG1, Ancell), or a mouse IgG1 isotype control (Ancell) in the presence and absence of SAP. Fibrocytes were identified and counted based on their elongated spindle-shaped morphology in five different 900 μm-diameter fields of view per well (28–30). PBMCs were incubated overnight in RPMI-1640 (Lonza, Allendale, NJ) with 10% fetal bovine serum (Caisson Laboratories, North Logan, UT) to generate macrophages, as described previously (24). Neutrophils were isolated from blood using Lympholyte-poly (Cedarlane Laboratories, Hornby, BC, Canada) following the manufacturer’s protocol and resuspended in 2% BSA (fraction V, A3059; Sigma-Aldrich) in RPMI-1640 (12, 31). HEK293 cells (Life Technologies, Grand Island, NY) were cultured in Freestyle (Life Technologies) medium following the manufacturer’s protocol. K562 cells (ATCC, Manassas, VA) were grown in RPMI-1640 with 10% fetal bovine serum (Caisson).
SAP variant expression, purification, size exclusion chromatography, and labeling
Starting with the previously described SAP expression vector (21), SAP variants were generated with a QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) following the manufacturer’s protocol, and the DNA sequences of the constructs were verified. SAP variants were expressed in HEK293 cells as described previously and then purified by affinity purification (21). Briefly, cell supernatants from the SAP-expressing HEK293 cells were clarified by centrifugation at 300 × g. 1 M CaCl2 was added to the supernatant to a final concentration of 2 mM, and the cell supernatant was then mixed with 1 ml of a 50% slurry of Sepharose Fast Flow (GE Healthcare BioSciences, Piscataway, NJ, USA) in 20 mM Tris, 140 mM NaCl, 2 mM CaCl2, pH 7.4 for 1 h. The Sepharose beads were collected and washed 3 times with 15 ml wash buffer (20 mM Tris, 300 mM NaCl, 2 mM CaCl2, pH 7.4). Bound protein was eluted overnight at 4°C with 20 mM Tris, 140 mM NaCl, 50 mM EDTA, pH 7.4. The eluted protein was then buffer exchanged into 20 mM sodium phosphate buffer pH 7.4 (21). The purified SAP was assayed by size exclusion chromatography using a Superose 12 (GE Healthcare Life Sciences, Piscataway, NJ) column on an AKTA chromatography system (GE Healthcare Life Sciences) as previously described (21). Purified SAP was labeled using Alexa Fluor 647-NHS (Life Technologies) following the manufacturer’s protocol.
Neutrophil adhesion assay, macrophage phagocytosis assay, and SAP binding to Zymosan A
Neutrophils were incubated with 80 nM (10 μg/ml) of wildtype (WT) or mutated SAP, and their binding to human plasma fibronectin (Trevigen, Gaithersburg, MD) was assessed as previously described (12). To determine the effect of Fcγ receptor ligation on neutrophil adhesion, neutrophils were incubated with 5 μg/ml of either anti-FcγRI antibody clone 10.1 (mouse IgG1, eBiosciences), anti-FcγRII antibody clone Clkm-5 (mouse IgG1, Millipore, Billerica, MA), anti-FcγRII antibody clone FUN-2 (mouse IgG1, Biolegend), anti-FcγRII antibody clone AT10 (mouse IgG1, Abcam, Cambridge, MA), anti-FcγRIII antibody clone 3G8 (mouse IgG1, Biolegend), or a mouse IgG1 isotype control (Biolegend). Phagocytosis of FITC-conjugated Zymosan A bio-particles (Life Technologies) by macrophages was assayed as described previously (24). To measure the binding of SAP to Zymosan A bio-particles, we first quenched the fluorescence of FITC-conjugated Zymosan A with 2% Trypan blue in PBS for 20 minutes and then incubated the quenched Zymosan A bio-particles with 240 nM (30 μg/ml) of WT SAP or mutant SAP in 20 mM Tris, 140 mM NaCl, 2 mM CaCl2 for 1 hour. The bio-particles were then washed, and bound SAP was detected by staining with anti-SAP antibody clone 5.4A (Millipore) and goat anti-mouse Alexa Fluor-647 (Life Technologies) on an Accuri C6 flow cytometer (BD Bioscience).
SAP affinity assays and receptor expression
FcγRI and Fcε common γ-chain (FcRγ) cDNA (PSI:Biology-materials repository, Tempe, AZ) (32, 33) were ligated into the pCMV6-AC-His vector (OriGene, Rockville, MD) and then transfected into HEK293 cells using jetPRIME (Polyplus, New York, NY) following the manufacturer’s protocol. FcγRIIIb plasmid was obtained from the PSI:Biology-materials repository and transfected into HEK293 cells. K562 cells, which express FcγRIIa, were used to measure the affinity of SAP for FcγRIIa (34). The binding of fluorescently labeled WT SAP or mutant SAP to cells was then measured as previously described using an Accuri C6 flow cytometer (24). When measuring SAP binding to HEK293 cells expressing FcγRI or FcγRIIIb, mock transfected HEK293 cells were used to estimate the non-specific binding. K562, HEK293, FcγRI+ HEK293, and FcγRIIa+ HEK293 cells were stained for FcγRI (Cone 10.1, eBiosciences), FcγRII (Clone FUN-2, Biolegend), and FcγRIII (Clone 3G8, Biolegend) to determine the expression of the indicated receptor by flow cytometry (29). Leukocytes stained for CD3 (Biolegend), CD14 (Biolegend), CD15 (Biolegend), CD19 (Biolegend), CD45 (Biolegend), FcγRI (Clone 10.1, eBiosciences), FcγRII (Clone FUN-2, Biolegend), and FcγRIII (Clone 3G8, Biolegend) were assayed by flow cytometry to determine the presence of different immune cell populations as previously described (29, 30, 35).
Statistical analysis
Data was analyzed by ANOVA (with Dunnett’s post test) or t-test when appropriate using Prism software (GraphPad software, San Diego, CA). Data were fit to the appropriate model of binding as determined by F-tests. Normality was tested using Shapiro-Wilk and D’Agostino-Pearson omnibus tests when applicable.
Results
Identification of SAP amino acids that affect neutrophil adhesion
We previously made site-directed mutations of human SAP at amino acids that interact with human FcγRIIa in a SAP-FcγRIIa co-crystal structure (23), and observed that changes to these amino acids had no significant effect on the ability of SAP to inhibit fibrocyte differentiation (21). To better understand the interaction of SAP with Fcγ receptors, we compared the amino acid sequence of human SAP to the related pentraxin CRP. SAP and CRP have 51% sequence identity and similar crystal structures, but have different affinities for Fcγ receptors and different roles in the immune system (6, 11, 23, 27). Thus, the sequence differences can be used to identify structurally and functionally significant amino acids. Of the amino acids that were different between SAP and CRP, we mutated only the ones that were exposed on the surface of SAP (23). E153, which is at the interface between SAP monomers, was also mutated in an attempt to destabilize the pentameric protein and introduce functional defects. We then expressed all the generated SAP variants in HEK293 cells. All the SAP variants eluted at 10–12 ml from a Superose 12 size exclusion chromatography column, indicating the absence of aggregates larger than pentamers, and the absence of free monomers (Table SI and Fig 1A). We subsequently tested the ability of these variants to decrease neutrophil adhesion, inhibit fibrocyte differentiation, and promote phagocytosis. In addition, we examined the binding of these variants to Fcγ receptors.
Figure 1. Some SAP variants have an altered effect on neutrophil adhesion to human fibronectin.
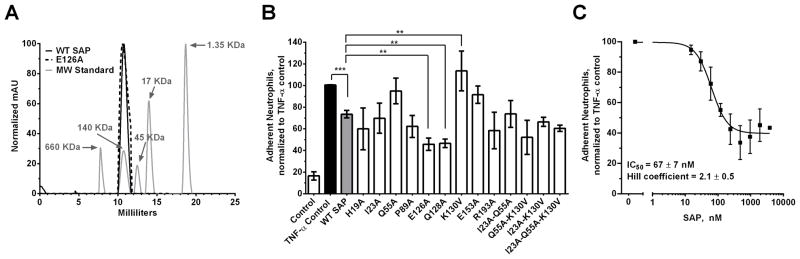
A) A representative plot indicating that both WT SAP and K87A variant are pentameric. B) Human neutrophils were incubated with 80 nM (10 μg/ml) of the indicated SAP variants. Following the initial incubation with SAP, the neutrophils were transferred to a fibronectin coated plate and were then activated by the addition of TNF-α. After 30 minutes, the non-adherent neutrophils were removed and the remaining cells were stained and counted. C) Human neutrophils were incubated with increasing concentrations of WT SAP to estimate the IC50 of SAP for reducing neutrophil adhesion. The data were fit to a sigmoidal dose response curve with variable Hill coefficient. Values are adherent neutrophils normalized to the TNF-α control ± SEM, n=3–7. ** represents p<0.01 and *** represents <0.001 by t-test.
We first examined the ability of our SAP variants to reduce neutrophil adhesion to human fibronectin. We screened 29 SAP variants for their ability to reduce neutrophil adhesion and then based on our preliminary data focused on 13 of the examined variants (data not shown). These 13 SAP variants were screened at 80 nM (10μg/mL) (Fig. 1B). We chose this concentration because it was close to the IC50 (67 ± 7 nM) of SAP for reducing neutrophil adhesion (Fig. 1C) and hence allowed us to detect both increases and decreases in the SAP effect on neutrophils. Following our screen, we observed that SAP variants E126A and Q128A had increased inhibitory effect on neutrophil adhesion compared to WT SAP (Fig. 1B). SAP variant K130V conversely had a significantly reduced inhibitory effect on neutrophil adhesion (Fig. 1B).
Identification of SAP amino acids that affect fibrocyte differentiation
Since SAP inhibits fibrocyte differentiation, we also screened the 29 SAP variants for their ability to inhibit the differentiation of monocytes into fibrocytes (Table SI) (21, 28, 29, 36). Using PBMCs from a variety of donors, we observed 1200 to 3100 fibrocytes per 105 PBMCs. Because of this variability, fibrocyte counts were normalized to the no-SAP control, as described previously (21, 28, 37). WT SAP inhibited fibrocyte differentiation with an IC50 of 2.9 ± 0.3 nM, similar to previously published data (21, 36). 25 out of the 29 variants tested did not significantly alter the ability of SAP to inhibit fibrocyte differentiation (Table SI). Compared to wildtype SAP, variants Q55A and K130V were more effective at inhibiting fibrocyte differentiation whereas variants E153A and E126A had reduced activity (Fig. 2 and Table SI). In addition, we observed significant changes in the Hill coefficient of SAP variants V68A and Q128A compared to WT SAP (Table SI). This change could be due to alterations in SAP variant binding to Fcγ receptors and/or how these variants activate the receptors.
Figure 2. Some SAP variants have an altered effect on human fibrocyte differentiation.
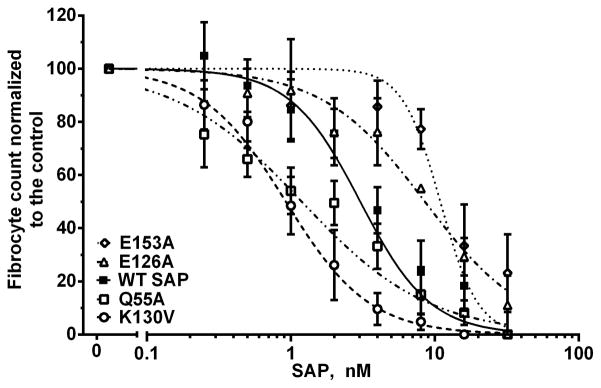
PBMCs were incubated for 5 days in the presence of WT SAP or SAP variants. Compared to WT SAP, the SAP variants K130V and Q55A were more effective inhibitors of fibrocyte differentiation, whereas E126A and E153A had reduced activity. Values are fibrocyte count normalized to the no-SAP control ± SEM, n=3–5. The data were fit to sigmoidal dose response curves with variable Hill coefficients. The absence of error bars indicates that the error was smaller than the plot symbol.
Identification of SAP amino acids that affect phagocytosis
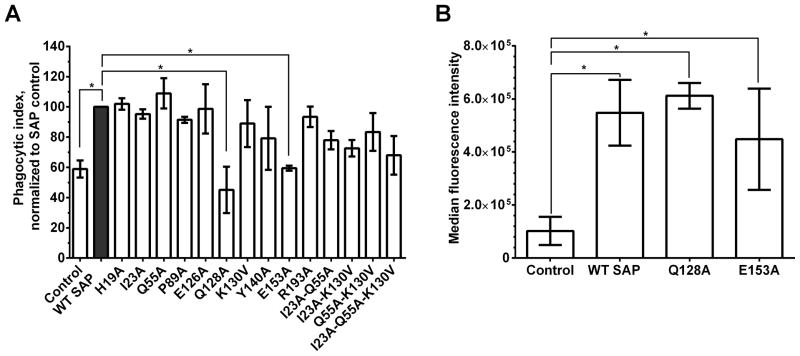
SAP enhances phagocytosis of pathogens and cell debris through Fcγ receptors (24, 27). However, the exact receptor and amino acids involved are unknown. We first screened the 29 SAP variants for their ability to enhance phagocytosis and then based on our preliminary data focused on 13 variants (data not shown). The 13 SAP variants were screened at 240 nM (30μg/mL, physiological concentration in the human plasma) for their ability to promote phagocytosis of Zymosan A bio-particles by macrophages (Fig 3A). 11 of the SAP variants examined did not significantly alter the ability of SAP to enhance phagocytosis (Fig 3A). Compared to WT SAP, SAP variants Q128A and E153A had significantly reduced ability to promote phagocytosis by macrophages (Fig 3A). We then measured the binding of WT, Q128A and E153A SAP to Zymosan A bio-particles to determine if these SAP variants had deficiencies in binding the bio-particles. Compared to WT SAP, we found no statistically significant differences in the binding of Q128A or E153A to Zymosan A (Fig 3B). Together, this indicates that SAP variants Q128A and E153A have defects in binding and/or activating Fcγ receptors to promote phagocytosis of Zymosan A.
Figure 3. Amino acids Q128 and E153 on SAP are necessary for SAP-mediated phagocytosis.
A) FITC-labeled Zymosan A bio-particles were incubated in the presence of WT SAP or SAP variants and then added to monocyte-derived macrophages. After 1 hour, the free bio-particles were removed and the number of phagocytized particles was counted. The phagocytic index was estimated as the number of bio-particles engulfed by 100 macrophages. Values are means of the phagocytic index normalized to the WT SAP ± SEM, n=3–6. B) The binding of WT, Q128A, and E153A SAP to Zymonan A was detected using an anti-SAP antibody and flow cytometry. Values are mean ± SEM, n=3. * represents p<0.05 by t-test.
SAP binds to endogenous FcγRI and FcγRIIa on immune cells
Much of the work done on SAP binding to Fcγ receptors has focused on SAP binding to recombinant Fcγ receptors or to receptors expressed on non-human cells such as COS-7 and NIH-3T3 (11, 23, 24). This is problematic because the affinity of Fcγ receptors for their ligands is sensitive to the receptor glycosylation state and the presence of intracellular signaling proteins (38–41). Therefore to identify functionally significant receptors in the SAP response, we examined the binding of SAP to endogenous Fcγ receptors on human immune cells and to receptors expressed on the human-derived cell line HEK293. We fluorescently labeled WT SAP (SAP-f) and then measured the binding to different peripheral blood cell populations as identified by their flow characteristics and receptor expression (Fig. S1). We tested the activity of SAP-f on neutrophils, monocytes, and macrophages and observed no functional defects compared to unlabeled SAP (Fig. S2). When SAP-f was incubated with leukocytes, we observed no binding to the lymphocyte population (Fig 4A). Since B cells (~5 % of lymphocytes) express FcγRIIb (25), and NK cells (~5–10% of lymphocytes) express FcγRIIIa (25), this suggests that SAP does not bind to these receptors under our experimental conditions. However, SAP-f did bind to monocytes and neutrophils (Fig. 4A). Monocytes express FcγRI, FcγRIIa, and some FcγRIIIa (Fig S1) (12, 30). This indicates that SAP could be binding to any or all of the Fcγ receptors on monocytes. As NK cells express FcγRIIIa, and we did not detect binding of SAP-f to NK cells, this suggests that SAP binds to FcγRI and/or FcγRIIa on monocytes. Neutrophils express FcγRIIa and FcγRIIIb (Fig S1) (12, 30). When we incubated SAP-f with FcγRIIIb+ HEK293 cells, we did not detect any appreciable binding although the receptor was functional as determined by human IgG binding (Fig. 4B and C and Fig. S3). This then suggests that SAP binds to FcγRIIa on neutrophils. We observed more SAP biding to monocytes than to neutrophils, and we hypothesize that this is likely due to the presence of FcγRI on monocytes (Fig. 4A). Together, our data indicates that SAP-f binds to endogenous FcγRI and FcγRIIa on monocytes and neutrophils but not to FcγRIIb, FcγRIIIa, and FcγRIIIb (Fig. 4).
Figure 4. Alexa Fluor 647-labeled SAP (SAP-f) binds to monocytes and neutrophils.
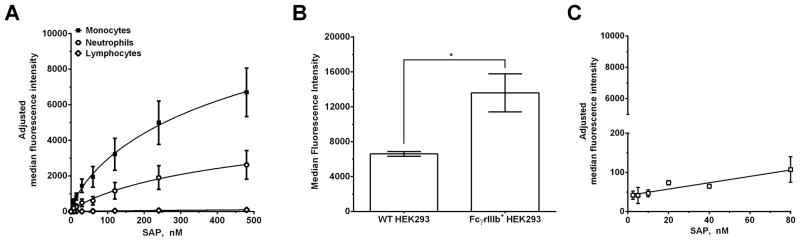
A) SAP-f was incubated with isolated leukocytes and then subjected to flow cytometry. Neutrophils, monocytes, and lymphocytes were identified based on forward scatter and side scatter as in Figure S2. Autofluorescence values were subtracted from the total binding values. Curves are fits of the resulting data to models of one-site binding with variable Hill coefficient. B) WT HEK293 cells and HEK293 cells expressing FcγRIIIb were incubated with 20 μg/ml of human IgG-Alexa Fluor 488 and then subjected to flow cytometry to determine if the expressed receptor is functional and therefore binds IgG. Values are mean ± SEM, n=3. * represents p<0.05 by t-test. C) HEK293 cells expressing FcγRIIIb were incubated with SAP-f and then subjected to flow cytometry to measure SAP binding. Mock transfected cells were used to estimate the non-specific binding, which was then subtracted from the total binding. Values are adjusted median fluorescence intensity ± SEM, n=3. The data were fit to a line. The absence of error bars indicates that the error was smaller than the plot symbol.
SAP binds to FcγRI and FcγRIIa on HEK293 cells
Following our initial binding assays using human immune cells, we investigated the binding of our SAP variants to FcγRI and FcγRIIa. Of the 29 SAP variants, we chose 6 that had altered functions as measured by neutrophil adhesion assays, fibrocyte differentiation assays, and macrophage phagocytosis assays. The 6 SAP variants were fluorescently labeled and then incubated with K562 cells to measure the binding to FcγRIIa. The only known receptor that binds SAP on the surface of K562 cells is FcγRIIa ((34) and Fig S3). WT SAP bound to FcγRIIa with a Kd of 19.7 ± 3.4 nM (Fig 5A and Table 1). Previous measurements of the Kd for SAP binding to FcγRIIa range from 0.29 nM to 1.4 μM (11, 23, 24). As previously described, these inconsistencies are most likely caused by the method of receptor expression and how the Kd was estimated (38). Of the 6 SAP variants tested, compared to WT SAP, E126A, Q128A, and K130V had significant differences in their binding to FcγRIIa (Fig 5A and Table I). These changes in affinity correlate with the ability of these SAP variants to reduce neutrophil adhesion. Variants E126A and Q128A have a higher affinity for FcγRIIa and have an increased inhibitory effect on neutrophil adhesion (Table I and Fig. 1). Conversely, variant K130V has a decreased affinity for FcγRIIa and has a reduced inhibitory effect on neutrophil adhesion to fibronectin (Table I and Fig. 1). In addition, variant E126A has a Hill coefficient of 3.3 ± 0.5, indicating cooperativity in SAP E126A-FcγRIIa binding. This cooperativity is absent from the other SAP variants as their Hill coefficient is not significantly different from the 1.2 ± 0.2 we measured for WT SAP. One possible explanation for this increased Hill coefficient is self-aggregation of SAP E126A following binding to cells. This would then manifest as an increase in the maximal binding (Bmax) of SAP E126A to cells. However, the SAP E126A Bmax was 69.7 ± 10.8 % of WT SAP (mean ± SEM, p not significant by t-test), indicating that SAP E126A was not aggregating on the surface of cells.
Figure 5. SAP variant binding to FcγRIIa and FcγRI.
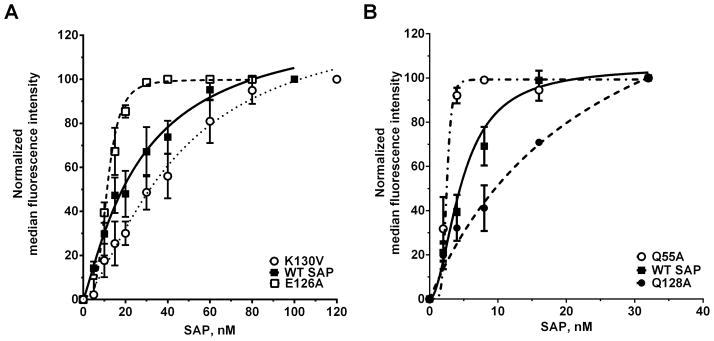
A) K562 cells, which express FcγRIIa, were incubated with fluorescently-labeled SAP variants. The cells were then washed and the binding of the labeled SAP to the cells was measured by flow cytometry. B) HEK293 cells expressing FcγRI were incubated with fluorescently-labeled SAP variants and then binding was measured by flow cytometry. Mock transfected cells were used to estimate the non-specific binding. Median fluorescne intensity values were normalized to the intensity value of the highest SAP concentration. Values are normalized mean ± SEM, n=3–5. Curves are fits to models of one-site binding with variable Hill coefficient. The absence of error bars indicates that the error was smaller than the plot symbol.
Table I. Binding of SAP variants to FcγRI and FcγRIIa.
HEK293 cells expressing FcγRI were incubated with Alexa Fluor 647-labeled SAP variants. The cells were then washed and the binding of the labeled SAP to the cells was measured by flow cytometry. Mock transfected cells were used to estimate the non-specific binding. K562 cells were used to measure the binding of SAP variants to FcγRIIa.
| SAP Variants | FcγRI Kd (nM ± SEM) | Hill coefficient | FcγRIIa Kd (nM ± SEM) | Hill coefficient |
|---|---|---|---|---|
| WT SAP | 4.6 ± 0.8 | 2.1 ± 0.6 | 19.7 ± 3.4 | 1.2 ± 0.2 |
| I23G | 9.8 ± 5.8 | 1.1 ± 0.4 | 25.2 ± 9.8 | 1.5 ± 0.4 |
| Q55A | 2.6 ± 0.1 *** | 6.7 ± 0.2 *** | 22.3 ± 3.3 | 1.7 ± 0.2 |
| E126A | 36.9 ± 2.8*** | 0.9 ± 0.1 | 11.9 ± 0.6 * | 3.3 ± 0.5 * |
| Q128A | 24.7 ± 6.0 * | 0.9 ± 0.1* | 11.9 ± 2.1 * | 2.3 ± 0.6 |
| K130V | 4.5 ± 0.7 | 3.7 ± 1.9 | 43.7 ± 8.7 * | 1.4 ± 0.4 |
| E153A | 5.3 ± 0.6 | 3.2 ± 0.9 | 27.8 ± 1.4 | 4.7 ± 0.9 |
Values are mean ± SEM, n=3–6.
* indicates p < 0.05 and *** indicates p < 0.001 by t-test when compared to the corresponding wildtype (WT) control.
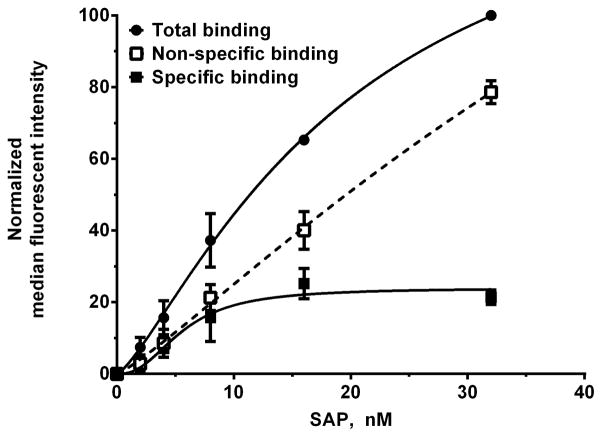
To measure the binding of our 6 SAP variants to FcγRI, we used FcγRI+ HEK293 cells (Fig S3). The FcγRI+ HEK293 cells were co-transfected with FcRγ, as this intracellular protein is necessary for FcγRI localization to the cell membrane (42). The mock transfected cells were used to estimate the non-specific binding (Fig. 6). WT SAP bound to FcγRI with a Kd of 4.6 ± 0.8 nM and a Hill coefficient of 2.1 ± 0.6 (Table I). This affinity matches the previously published Kd of 4.3 nM (11). The Hill coefficient of greater than 1 for the SAP-FcγRI interaction suggests cooperativity, and could indicate FcγRI receptor-receptor interactions. Compared to WT SAP, variants Q55A, E126A, and Q128A had significant changes in their affinity for FcγRI (Fig 5B and Table I). However, we did not observe any significant changes in the Bmax values of Q55A (105 ± 36.7 % of WT SAP), E126A (123.9 ± 45.2), and E128A (110.9 ± 14.4) when binding to FcγRI. All the changes in affinity observed for Q55A, E126A, E128A correlate with the ability of these variants to inhibit fibrocyte differentiation. Variant Q55A has a higher affinity for FcγRI and increased inhibitory effect on fibrocyte differentiation (Table I and Table SI). SAP variant E126A has decreased affinity for FcγRI and has reduced inhibitory effect on fibrocyte differentiation (Table I and Table SI). The decrease in affinity of Q128A variant for FcγRI does not alter the IC50 of SAP for fibrocyte differentiation but it does abrogate enhancement of phagocytosis by macrophages (Fig 3A and Table SI). Additionally, the decrease in affinity of SAP Q128A for FcγRI significantly increases the Hill coefficient from 1.8 ± 0.1 in WT SAP to 2.7 ± 0.3 in our fibrocyte differentiation assay (Table SI). This indicates that although the IC50 is not altered by this mutation, Q128 still plays a role in inhibition of fibrocyte differentiation by SAP. Together, our data indicates that SAP binds to FcγRIIa on neutrophils to reduce neutrophil adhesion and to FcγRI on monocytes to inhibit fibrocyte differentiation.
Figure 6. Estimating the specific binding of WT SAP to FcγRI.
HEK293 cells expressing FcγRI were incubated with Alexa Fluor 647-labeled WT SAP (SAP-f) as in Table 1. The cells were then washed and the binding of SAP-f to the cells was measured by flow cytometry. The specific binding was estimated by subtracting the binding of SAP-f to mock transfected cells from FcγRI expressing cells. The values were normalized to the median fluorescence intensity of SAP total binding at 32 nM. Values are mean ± SEM, n=3. The absence of error bars indicates that the error was smaller than the plot symbol.
FcγRIIa and FcgRIIIb ligation reduces neutrophil adhesion
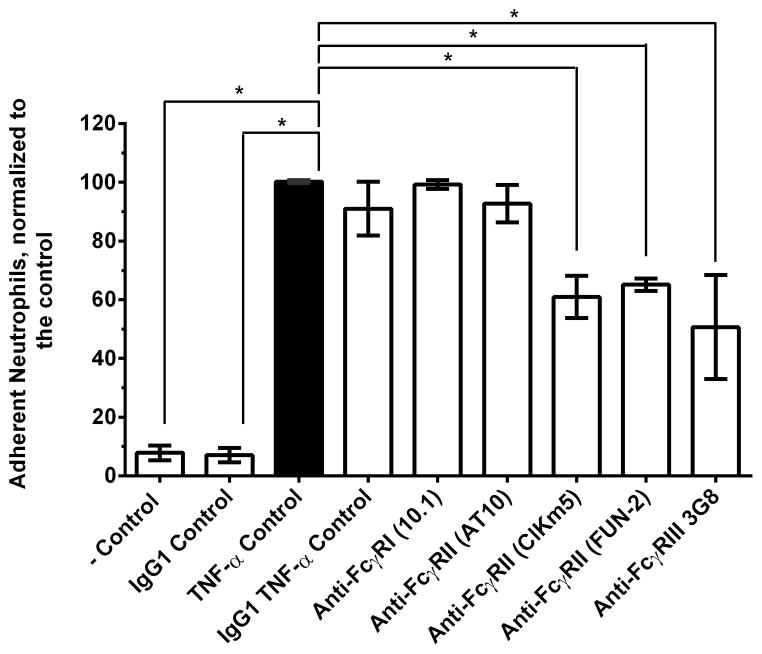
Our analysis of SAP variant binding to FcγRI and FcγRIIa indicates the significance of FcγRIIa in SAP reduction of neutrophil adhesion. To corroborate this finding, we investigated the effect of antibody-mediated ligation of FcγRI, FcγRIIa, and FcγRIIIb on neutrophil adhesion. Mouse IgG1 and the anti-FcγRI antibody clone 10.1 had no effect on neutrophil adhesion (Fig. 7). However, 2 of 3 anti-FcγRIIa antibodies and an anti-FcγRIIIb antibody tested decreased neutrophil adhesion (Fig. 7). The anti-FcγRII antibody clone AT10 is a blocking antibody and reduces IgG binding to FcγRII, but in our assay it had no effect on neutrophil adhesion. Together, this indicates that SAP binds FcγRIIa to decrease neutrophil adhesion, and that FcγRIIIb ligation by antibodies can also reduce neutrophil adhesion to fibronectin.
Figure 7. Ligating FcγRIIa and FcγRIIIb by antibodies decreases neutrophil adhesion.
Neutrophils were incubated with the indicated anti-Fcγ receptor antibodies to assess the effect of Fcγ receptor ligation on neutrophil adhesion to fibronectin as described in Figure 1. Values are adherent neutrophils normalized to the TNF-α control, mean ± SEM, n=3. * indicates p<0.05 by t-test.
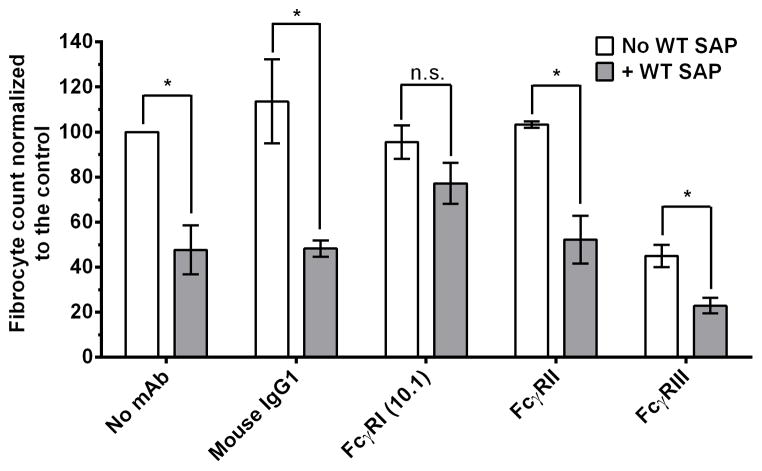
Blocking FcγRI using antibodies abrogates SAP inhibition of fibrocyte differentiation
SAP variant binding to FcγRI and the functional data on fibrocyte differentiation suggests a critical role for FcγRI in mediating the SAP effect on fibrocytes. To test this hypothesis, we incubated PBMCs with F(ab′)2 fragments of anti-FcγRI, anti-FcγRII, and anti-FcγRIII antibodies in the presence or absence of WT SAP. The anti-FcγRII and anti-FcγRIII antibodies used in this experiment do not discriminate between the different FcγRII or FcγRIII isoforms (43). However, they do block IgG binding to all FcγRII or FcγRIII receptors (43). Mouse IgG1, anti-FcγRII, and anti-FcγRIII antibodies had no effect on the inhibitory effect of SAP on fibrocyte differentiation (Fig. 8). However, the F(ab′)2 fragment of anti-FcγRI antibody clone 10.1 reduced the ability of SAP to inhibit fibrocyte differentiation (Fig. 8). This indicates that SAP binds to FcγRI to inhibit fibrocyte differentiation.
Figure 8. FcγRI blocking antibodies reduce the effect of SAP on fibrocyte differentiation.
PBMCs were incubated with F(ab′)2 fragments of anti-Fcγ receptor antibodies in the presence or absence of WT SAP to determine their effect on SAP-mediated inhibition of fibrocyte differentiation. Values are fibrocyte count normalized to the control ± SEM, n=4. * represent p <0.05 by t-test; n.s. indicates not significant.
Identification of a novel Fcγ receptor binding site on SAP
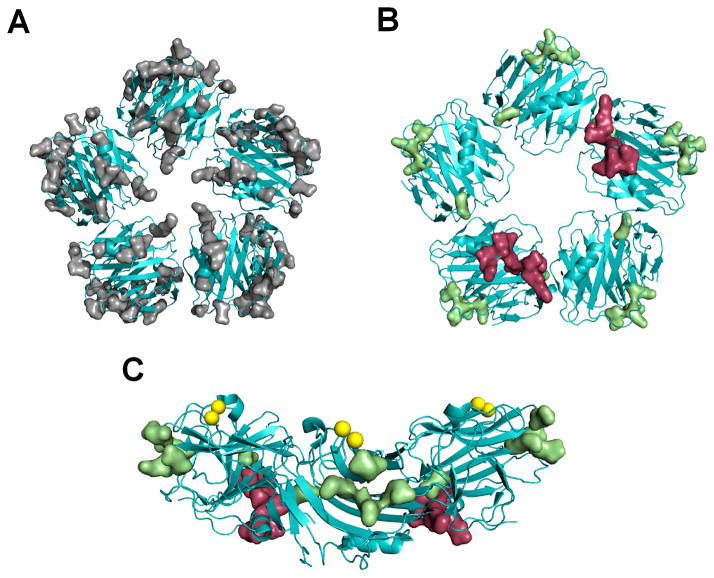
Following our functional assays, we mapped all the mutated amino acids onto the SAP structure (Fig 9A). Excluding amino acid E153, all the functionally significant amino acids form a distinct binding site on the surface of SAP (Fig 9B and 9C). This novel binding site is different from the previously identified FcγRIIa binding site (Fig 9B and (24)). The position of this novel binding site on SAP may allow for the binding of multiple Fcγ receptors.
Figure 9. Identification of a novel Fcγ receptor binding site on SAP.
A) Mutated amino acid residues are indicated by molecular surface representation (gray) on the SAP structure. B) The functionally significant amino acid residues (green) are distinct from the previously identified FcγRIIa binding site (red). C) When E153 is excluded; the remaining functionally significant amino acid residues form a distinct binding site. The yellow spheres represent the two calciums bound to SAP.
Discussion
The pentraxin Serum Amyloid P (SAP) is an anti-fibrotic agent that inhibits aberrant scar tissue formation by regulating neutrophils, monocytes, and macrophages (11, 12, 24, 44, 45). All SAP functions appear to be mediated partly through Fcγ receptors (11, 21, 46). As there are multiple Fcγ receptors on neutrophils, monocytes, and macrophages, we determined how each receptor contributed to different SAP function. We found through site-directed mutagenesis that SAP binds to FcγRI on monocytes to inhibit fibrocyte differentiation and to FcγRIIa on neutrophils to reduce adhesion to fibronectin. In addition, we identified a novel Fcγ receptor binding site on SAP.
Mutations in SAP that affect binding to FcγRIIa significantly change the ability of SAP to reduce neutrophil adhesion to fibronectin. Similar to SAP, ligating FcγRIIa by anti-FcγRII antibodies decreases neutrophil adhesion. This suggests that SAP binds to FcγRIIa to decrease cell adhesion. The activation of FcγRIIa results in the phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) in the cytosolic region of this receptor (47). ITAM phosphorylation is implicated in inside-out signaling and regulation of adhesion molecules (47). This then suggests that FcγRIIa activation can reduce adhesion of neutrophils to fibronectin by regulating adhesion molecules on neutrophils. Additionally, ligating FcγRIIIb by an anti-FcγRIII antibody reduces neutrophil adhesion to fibronectin, suggesting that ligands of FcγRIIIb such as immunoglobulin could also regulate neutrophil adhesion to fibronectin.
Mutations in SAP that affect binding to FcγRI significantly alter the ability of SAP to inhibit fibrocyte differentiation. In addition, blocking FcγRI with an IgG blocking antibody reduces the SAP effect on fibrocyte differentiation. Together, this suggests that although there are multiple Fcγ receptors on monocytes, SAP activates FcγRI to inhibit fibrocyte differentiation. This is in agreement with our previous results where we observed that siRNA knock down of FcγRI in humans results in decreased inhibitory effect of SAP on fibrocyte differentiation (21), and that cross-linking FcγRI with antibodies can mimic the inhibitory effect of SAP on fibrocyte differentiation (48). Here, we have identified a FcγRI binding site on each SAP monomer, suggesting that SAP can cross-link multiple Fcγ receptors (Fig 9). Together, this suggests a role for FcγRI cross-linking in SAP inhibition of fibrocyte differentiation.
SAP appears to promote phagocytosis of bio-particles such as Zymosan A through FcγRI (Table I and (27). However, not all SAP variants with alteration in FcγRI binding have defects in phagocytosis. For instance, variant E126A has a ~ 10 fold reduction in affinity for FcγRI and defects in inhibiting fibrocyte differentiation, but has no deficiencies in promoting phagocytosis. This suggests that a SAP opsonized bio-particle activates FcγRI to promote phagocytosis in a manner that is distinct from how SAP activates FcγRI to inhibit fibrocyte formation. This is supported by the fact that FcγRI mediated phagocytosis is Syk-kinase dependent but inhibition of fibrocyte differentiation by SAP is Syk-kinase independent (46, 49–51). It is also possible that SAP binding to FcγRI is sufficient to promote phagocytosis irrespective of changes in SAP-FcγRI affinity. Alternatively, it is feasible that Q128A and E153A modulate Zymosan A phagocytosis by altering macrophage activation. However, SAP opsonized Zymosan A particles were incubated with macrophages for a short time (60 minutes), which would not allow for significant alteration in macrophage activation and phenotype.
In surface plasmon resonance experiments, SAP binds to all of the Fcγ receptors (11, 23). However, we observed that SAP only binds to endogenous FcγRI and FcγRIIa on immune cells. This inconsistency can be explained by the differences in the glycosylation state of the receptors and/or the lack of some intracellular signaling components (38). FcγRIIIa is a highly glycosylated receptor in humans. Modifying FcγRIIIa glycosylation changes its affinity for IgG and perhaps SAP (38). FcγRI and FcγRIIIa in humans interact with an intracellular protein called Fcε common γ-chain (FcRγ) (38, 42). The absence of FcRγ alters the affinity of FcγRI and FcγRIIIa for IgG in humans (38, 42). This can potentially alter SAP binding to FcγRI and FcγRIIIa. Together, this suggests that the SAP affinity for Fcγ receptors is dependent on the modification of these Fc receptors and the interactions they make prior to binding SAP.
Our findings indicate that it is possible to mimic specific SAP functions by targeting particular Fcγ receptors (Fig 10). For instance, activation of FcγRIIa by antibodies or small molecules could be used to decrease neutrophil adhesion and hence reduce neutrophil accumulation in lungs of patients suffering from acute respiratory distress syndrome or cystic fibrosis. Similarly, blocking SAP binding to FcγRI might promote fibrocyte differentiation and wound healing. In addition, our results suggest that altering the SAP sequence could improve its ability to inhibit fibrocyte differentiation and/or reduce neutrophil adhesion. This could lead to the development of a more potent SAP anti-fibrotic.
Figure 10. A model of the SAP effect on monocytes and neutrophils.
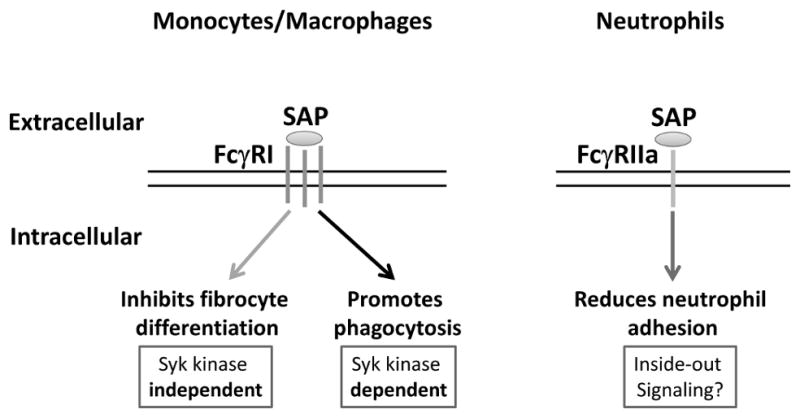
SAP cross-links FcγRI to inhibit fibrocyte differentiation in a Syk kinase independent manner. SAP also binds FcγRI to promote phagocytosis of zymosan A by macrophages. However, this pathway is Syk kinase dependent. SAP binds to FcγRIIa which then phosphorylates the ITAM domain of FcγRIIa. This then regulates adhesion molecules on the surface of neutrophils and decrease neutrophil adhesion.
Supplementary Material
Acknowledgments
We thank Jeffrey R. Crawford for his guidance and constructive discussion. We also thank the staff at Beutel student health center for drawing blood from volunteers. Additionally, we would like to thank the PSI:Biology-materials repository for providing us with FcγRI, FcγRIIIb, and Fcε common γ-chain plasmids.
Funding: This work was supported by NIH grant HL 083029.
References
- 1.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.du Bois RM, Nathan SD, Richeldi L, Schwarz MI, Noble PW. Idiopathic pulmonary fibrosis: lung function is a clinically meaningful endpoint for phase III trials. Am J Respir Crit Care Med. 2012;186:712–715. doi: 10.1164/rccm.201206-1010PP. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Valentino S, Gentile S, Inforzato A, Bottazzi B, Garlanda C. The long pentraxin PTX3: a paradigm for humoral pattern recognition molecules. Ann N Y Acad Sci. 2013;1285:1–14. doi: 10.1111/nyas.12043. [DOI] [PubMed] [Google Scholar]
- 6.Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res. 2011;343:237–249. doi: 10.1007/s00441-010-1018-0. [DOI] [PubMed] [Google Scholar]
- 7.Deban L, Bottazzi B, Garlanda C, de la Torre YM, Mantovani A. Pentraxins: multifunctional proteins at the interface of innate immunity and inflammation. BioFactors. 2009;35:138–145. doi: 10.1002/biof.21. [DOI] [PubMed] [Google Scholar]
- 8.Peisajovich A, Marnell L, Mold C, Du Clos TW. C-reactive protein at the interface between innate immunity and inflammation. Expert review of clinical immunology. 2008;4:379–390. doi: 10.1586/1744666X.4.3.379. [DOI] [PubMed] [Google Scholar]
- 9.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castaño AP, Lin SL, Surowy T, Nowlin BT, Turlapati SA, Patel T, Singh A, Li S, Lupher ML, Jr, Duffield JS. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maharjan AS, Roife D, Brazill D, Gomer RH. Serum amyloid P inhibits granulocyte adhesion. Fibrogenesis Tissue Repair. 2013;6:2. doi: 10.1186/1755-1536-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillingh MR, van den Blink B, Moerland M, van Dongen MG, Levi M, Kleinjan A, Wijsenbeek MS, Lupher ML, Jr, Harper DM, Getsy JA, Hoogsteden HC, Burggraaf J. Recombinant human serum amyloid P in healthy volunteers and patients with pulmonary fibrosis. Pulm Pharmacol Ther. 2013;26:672–676. doi: 10.1016/j.pupt.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 15.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 16.Takemasa A, Ishii Y, Fukuda T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. The European respiratory journal. 2012;40:1475–1482. doi: 10.1183/09031936.00127011. [DOI] [PubMed] [Google Scholar]
- 17.Stibenz D, Gräfe M, Debus N, Hasbach M, Bahr I, Graf K, Fleck E, Thanabalasingam U, Bührer C. Binding of human serum amyloid P componentto L-selectin. European journal of immunology. 2006;36:446–456. doi: 10.1002/eji.200425360. [DOI] [PubMed] [Google Scholar]
- 18.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Molecular medicine. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 19.Gomer RH, Pilling D, Kauvar LM, Ellsworth S, Ronkainen SD, Roife D, Davis SC. A serum amyloid P-binding hydrogel speeds healing of partial thickness wounds in pigs. Wound Repair Regen. 2009;17:397–404. doi: 10.1111/j.1524-475X.2009.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8:145–150. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- 21.Crawford JR, Pilling D, Gomer RH. FcgammaRI mediates serum amyloid P inhibition of fibrocyte differentiation. J Leukoc Biol. 2012;92:699–711. doi: 10.1189/jlb.0112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci U S A. 2008;105:10179–10184. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharadwaj D, Mold C, Markham E, Du Clos TW. Serum amyloid P component binds to Fc gamma receptors and opsonizes particles for phagocytosis. J Immunol. 2001;166:6735–6741. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- 25.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nature reviews Immunology. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Marjon KD, Marnell LL, Wang R, Mold C, Du Clos TW, Sun P. Recognition and functional activation of the human IgA receptor (FcalphaRI) by C-reactive protein. Proc Natl Acad Sci U S A. 2011;108:4974–4979. doi: 10.1073/pnas.1018369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mold C, Gresham HD, Du Clos TW. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J Immunol. 2001;166:1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- 28.Pilling D, Vakil V, Gomer RH. Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods. 2009;351:62–70. doi: 10.1016/j.jim.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox N, Pilling D, Gomer RH. NaCl potentiates human fibrocyte differentiation. PloS one. 2012;7:e45674. doi: 10.1371/journal.pone.0045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PloS one. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herlihy SE, Pilling D, Maharjan AS, Gomer RH. Dipeptidyl peptidase IV is a human and murine neutrophil chemorepellent. J Immunol. 2013;190:6468–6477. doi: 10.4049/jimmunol.1202583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cormier CY, Park JG, Fiacco M, Steel J, Hunter P, Kramer J, Singla R, LaBaer J. PSI: Biology-materials repository: a biologist’s resource for protein expression plasmids. Journal of structural and functional genomics. 2011;12:55–62. doi: 10.1007/s10969-011-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witt AE, Hines LM, Collins NL, Hu Y, Gunawardane RN, Moreira D, Raphael J, Jepson D, Koundinya M, Rolfs A, Taron B, Isakoff SJ, Brugge JS, LaBaer J. Functional proteomics approach to investigate the biological activities of cDNAs implicated in breast cancer. J Proteome Res. 2006;5:599–610. doi: 10.1021/pr050395r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bang R, Marnell L, Mold C, Stein MP, Clos KT, Chivington-Buck C, Clos TW. Analysis of binding sites in human C-reactive protein for Fc{gamma}RI, Fc{gamma}RIIA, and C1q by site-directed mutagenesis. J Biol Chem. 2005;280:25095–25102. doi: 10.1074/jbc.M504782200. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Arguelles A, Perez-Romano B. Immunophenotypic analysis of peripheral blood lymphocytes. In: Paul Robinson J, et al., editors. Current protocols in cytometry. Unit 6. Chapter 6. 2001. p. 5. [DOI] [PubMed] [Google Scholar]
- 36.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford JR, Pilling D, Gomer RH. Improved serum-free culture conditions for spleen-derived murine fibrocytes. Journal of immunological methods. 2010;363:9–20. doi: 10.1016/j.jim.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 39.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. 2012;1253:170–180. doi: 10.1111/j.1749-6632.2011.06305.x. [DOI] [PubMed] [Google Scholar]
- 40.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maxwell KF, Powell MS, Hulett MD, Barton Pa, McKenzie IF, Garrett TP, Hogarth PM. Crystal structure of the human leukocyte Fc receptor, Fc gammaRIIa. Nature structural biology. 1999;6:437–442. doi: 10.1038/8241. [DOI] [PubMed] [Google Scholar]
- 42.van Vugt MJ, Heijnen AF, Capel PJ, Park SY, Ra C, Saito T, Verbeek JS, van de Winkel JG. FcR gamma-chain is essential for both surface expression and function of human Fc gamma RI (CD64) in vivo. Blood. 1996;87:3593–3599. [PubMed] [Google Scholar]
- 43.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 44.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Xu W, Xiong S. Macrophage differentiation and polarization via phosphatidylinositol 3-kinase/Akt-ERK signaling pathway conferred by serum amyloid P component. J Immunol. 2011;187:1764–1777. doi: 10.4049/jimmunol.1002315. [DOI] [PubMed] [Google Scholar]
- 46.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79:1242–1251. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes fied the factor in serum, which inhibits fibrocyte (SAP) Journal of leukocyte biology. 2006;79 doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MK, Pan XQ, Huang ZY, Hunter S, Hwang PH, Indik ZK, Schreiber AD. Fc gamma receptors differ in their structural requirements for interaction with the tyrosine kinase Syk in the initial steps of signaling for phagocytosis. Clinical immunology. 2001;98:125–132. doi: 10.1006/clim.2000.4955. [DOI] [PubMed] [Google Scholar]
- 50.Huang ZY, Hunter S, Kim MK, Chien P, Worth RG, Indik ZK, Schreiber AD. The monocyte Fcgamma receptors FcgammaRI/gamma and FcgammaRIIA differ in their interaction with Syk and with Src-related tyrosine kinases. J Leukoc Biol. 2004;76:491–499. doi: 10.1189/jlb.1103562. [DOI] [PubMed] [Google Scholar]
- 51.Hunter S, Sato N, Kim MK, Huang ZY, Chu DH, Park JG, Schreiber AD. Structural requirements of Syk kinase for Fcgamma receptor-mediated phagocytosis. Experimental hematology. 1999;27:875–884. doi: 10.1016/s0301-472x(99)00025-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.