Abstract
Exosomes, nano-vesicles naturally released from living cells, have been well recognized to play critical roles in mediating cell-to-cell communication. Given that diabetic hearts exhibit insufficient angiogenesis, it is significant to test whether diabetic cardiomyocyte-derived exosomes possess any capacity in regulating angiogenesis. In this study, we first observed that both proliferation and migration of mouse cardiac endothelial cells (MCECs) were inhibited when co-cultured with cardiomyocytes isolated from adult Goto-Kakizaki (GK) rats, a commonly used animal model of type 2 diabetes. However, GK-myocyte-mediated anti-angiogenic effects were negated upon addition of GW4869, an inhibitor of exosome formation/release, into the co-cultures. Next, exosomes were purified from the myocyte culture supernatants by differential centrifugation. While exosomes derived from GK myocytes (GK-exosomes) displayed similar size and molecular markers (CD63 and CD81) to those originated from the control Wistar rat myocytes (WT-exosomes), their regulatory role in angiogenesis is opposite. We observed that the MCEC proliferation, migration and tube-like formation were inhibited by GK-exosomes, but were promoted by WT-exosomes. Mechanistically, we found that GK-exosomes encapsulated higher levels of miR-320 and lower levels of miR-126 compared to WT-exosomes. Furthermore, GK-exosomes were effectively taken up by MCECs and delivered miR-320. In addition, transportation of miR-320 from myocytes to MCECs could be blocked by GW4869. Importantly, the exosomal miR-320 functionally down-regulated its target genes (IGF-1, Hsp20 and Ets2) in recipient MCECs, and overexpression of miR-320 inhibited MCEC migration and tube formation. GK exosome-mediated inhibitory effects on angiogenesis were removed by knockdown of miR-320. Together, these data indicate that cardiomyocytes exert an anti-angiogenic function in type 2 diabetic rats through exosomal transfer of miR-320 into endothelial cells. Thus, our study provides a novel mechanism underlying diabetes mellitus-induced myocardial vascular deficiency which may be caused by secretion of anti-angiogenic exosomes from cardiomyocyes.
Keywords: Type 2 diabetes, Myocardial angiogenesis, Exosomes, miR-320, Cardiomyocytes
1. Introduction
Diabetes mellitus is a group of metabolic diseases characterized by high blood glucose either due to lack of (type 1) or resistance to (type 2) insulin [1]. Early in the course of diabetes, high glucose levels in the bloodstream can lead to endothelial dysfunction and microvascular rarefaction [2,3]. Actually, insufficient myocardial angiogenesis is the major manifestation of diabetes-caused ischemic cardiovascular disease [4,5]. Recent studies by Yan et al. [6] reported that the capillary/myofiber ratio and the arteriolar size were more severely diminished in type 2 diabetes compared to that in type 1 diabetes, leading to delayed and less effective blood flow recovery. In addition, exogenous VEGF rescues impaired blood flow in type 1 diabetic NOD mice; however, it is not sufficient to enhance type 2 diabetic revascularization [7,8]. Together, these observations indicate the severity of impaired neovascularization in type 2 diabetes. Therefore, a better understanding of the mechanisms underlying diabetes mellitus-caused microvascular rarefaction is urgently needed to improve the therapeutic outcome.
Within the mammalian heart, it is well recognized that endothelial cells play a critical role in cardiomyocyte survival and myocardial contraction [9]. However, in response to stress conditions (i.e. hyperglycemia), whether cardiomyocytes have an ability to affect endothelial cell function remains largely unknown. Recently, exosomes, small membrane vesicles secreted by various cell types, have gained much attention for their multiple roles in mediating cell-to-cell communication, cell survival, immune responses and angiogenesis [10–13]. We and others have observed that exosomes derived from cardiomyocytes harbor a variety of mRNAs, miRNAs and proteins, which may be transferred to the adjacent endothelial cells and modulate their function [14–16]. Currently, several miRNAs including miR-126, miR-320, and miR-503 have been shown to regulate endothelial function and angiogenesis in diabetes [17–20]. Of interest, the levels of miR-320 are elevated in cardiac microvascular cells collected from type 2 diabetic rats [17]. Controversially, recent studies by Feng et al. [20] showed that miR-320 was downregulated in endothelial cells cultured in high glucose conditions. Given that miR-320 is encapsulated in peripheral blood exosomes [21], we hypothesized that increased miR-320 in diabetic myocardial endothothelial cells may have originated from other cell types through exosomes.
In this study, we focused on determining whether cardiomyocytes either collected from type 2 diabetic rats or treated with high levels of glucose impaired angiogenesis through the exosomal transfer of miR-320 into endothelial cells. The goal of our study was to characterize the function of diabetic cardiomyocyte-derived exosomes and their ability to modulate myocardial angiogenesis. Here, we report that miR-320 was enriched in exosomes collected from the Goto-Kakizaki rat cardiomyocytes and high glucose-treated cardiomyocytes. Such exosomes inhibited endothelial cell proliferation, migration and tube-like formation. Mechanistically, diabetic cardiomyocyte-derived exosomes were absorbed by endothelial cells, leading to increased levels of miR-320 and decreased expression of IGF-1, Hsp20 and Ets2. These data expand our knowledge of an important role for cardiomyocyte-derived exosomes in the diabetes mellitus-caused impairment of myocardial angiogenesis.
2. Methods and materials
2.1. Animals, adult rat cardiomyocytes and cardiac endothelial cells
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the NIH, and were approved by the University of Cincinnati Animal Care and Use Committee (Animal Welfare Assurance Number: A3295-01). Goto-Kakizaki (GK) rats (10–11 weeks of age, male) with higher fasting and post-prandial blood glucose levels and age-matched normal male Wistar rats were purchased from Charles River Laboratories (www.criver.com). These rats were used for isolation and culture of primary cardiomyocytes, as described previously [22]. Briefly, cardiomyocytes were seeded into dishes pre-coated with mouse laminin (10 μg/ml) for 2 h at room temperature, followed by infection with Ad.rno-miR-320-off (#mr5309, Abm Inc.) for 48 h to knock down endogenous miR-320. Ad.miR-off (#m010, Abm Inc.) was used as a control. Culture supernatants were then collected for isolation of exosomes. A mouse cardiac endothelial cell line (MCEC) was initially developed in Dr. Weksler’s lab [23]. We purchased it from CELLutions Biosystems Inc. MCECs were cultured in DMEM with 10 mM penicillin/streptomycin (Gibco), 10 mM HEPES (Sigma) and 5% FBS in 0.2% gelatin-coated plates. For the exosome collection and function assays, exosome-depleted FBS (Syetem Biosciences Inc.) was used in the cell culture.
2.2. Cardiomyocytes/endothelial cells co-culture
Cell co-culture was performed using a double-chamber co-culture system in which MCECs were cultured on collagen membranes in the upper chamber and cardiomyocytes were cultured in the lower chamber pre-coated with mouse laminin (10 μg/ml). For assaying proliferation of the upper chamber MCECs, the same amount of MCECs (5 × 10 [4] cells/well) were plated in the upper chamber with a 0.4 μm pore size membrane (12-well insert, BD Biosciences) separating the upper and lower chamber cells. After 16 h co-culture, the upper chamber MCECs were taken out and put in a new well with 200 μl of fresh medium containing 20 μl MTS dye, and the absorbance of the dye solution was measured at OD490 nm, which correlates with the number of viable endothelial cells (CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit, Promega). To determine cell migration, we used a Boyden chamber trans-well system (BD Biosciences) with the upper chamber having an 8 μm-pore size membrane, in which MCECs were co-cultured (4 × 10 [4] cells/well). After 12 h of incubation, cells were fixed and stained with hematoxylin (Sigma-Aldrich) for 10 min and the upper surface of transwell chambers was wiped with a cotton swab. Migrating cells were counted in five random microscopic fields (×200).
For high glucose treatment, co-cultured cells (Wistar rat cardiomyocytes/MCECs) were exposed to 25 mM D-glucose (simulating hyperglycemia, HG) for 24 h. 5 mM D-glucose (simulating euglycemia, LG) was used as control. Then, upper-chamber MCECs were collected for measuring miR-320 levels. For blocking of exosome release from co-cultured cardiomyocytes, these cells were treated with GW-4869 (10 μM, Sigma-Aldrich) for 12 h, as previously described [24].
2.3. Isolation and characterizations of exosomes
Exosomes were isolated according to the method described before [14]. Briefly, supernatants from cultured cardiomyocytes were collected on ice and centrifuged at 10,000 g for 30 min to remove any cells and cellular debris, then supernatants were transferred to a fresh tube, filtered through the 0.22 μm membrane and centrifuged at 120,000 g for 2 h at 4 °C. The isolated exosomal pellet was washed once with sterile PBS and resuspended in 500 μl of PBS. Alternatively, the culture supernatants were first concentrated from 50 ml to 1 ml using an Amicon Ultra filter (Millipore, Billerica, MA) with a 100,000 molecular weight cutoff. Subsequently, the concentrated supernatants were used to isolate exosomes with an ExoQuick kit (System Biosciences), per the manufacturer’s instructions.
The quality of exosomes was confirmed by dynamic light scattering using a particle and molecular size analyzer (Zetasizer Nano ZS, Malvern Instruments) according to the manufacturer’s instructions. The quantity of exosomes was determined by the Micro-BCA assay (Pierce, Rockford, IL) for measurement of total protein. In addition, acetylcholinesterase activity, which reflects the amount of cell membrane present, was used to indirectly determine the quantity of exosomes, as previously described [24]. All samples were measured in triplicate. The value represents the acetylcholinesterase activity after 30 min of incubation.
Electron microscopy was done per the approach of Malik et al. [25]. Exosomes were ultracentrifuged to generate a pellet as part of the final step of isolation. A drop of purified exosome pellet was allowed to settle on a gold-coated grid, blotted, fixed in 1% glutaraldehyde, washed for 2 min in double-distilled water, incubated in uranyl oxylate for 5 min. Subsequently, it was incubated in three separate drops of methyl cellulose with uranyl acetate for 5 min in the first two drops and 10 min in the last drop, and finally removed from methyl cellulose–uranyl acetate by slow-drag on edge on filter paper. Exosomes were visualized by standard transmission electron microscopy with a Philips CM120 microscope.
2.4. Western blot analysis
Total protein was extracted from exosomes, or exosome-treated endothelial cells with procedures as described in detail elsewhere [22]. Equal amounts of protein were subject to SDS-PAGE. Binding of the primary antibody was detected by peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Amersham Pharmacia), and bands were quantified with densitometry. The sources of antibodies and dilutions used were as follows: rabbit anti-CD63 (sc-15363, 1:500 dilution), rabbit anti-CD81 (sc-9158, 1:400 dilution), and rabbit anti-IGF-1 (sc-9013, 1:200 dilution) (Santa Cruz). Ets2 mouse monoclonal antibody (clone 1H4) was purchased from Origene Inc. (1:2000 dilution). A primary antibody against Hsp20 was ordered from Research Diagnostics Inc. (1:5000 dilution). Either α-actin or β-actin (1:1000 dilution, Sigma-Aldrich) was used as an internal control.
2.5. Measurement of miRNA levels by stem-loop quantitative RT-PCR
Total RNA was isolated from exosomes and exosome-treated endothelial cells as well as their respective controls, using a miRNeasy Mini kit (Qiagen) according to the manufacturer’s protocol. The concentration of RNA was determined by a NanoDrop ND-1000 Spectrophotometer (NanoDrop Tech., Rockland, DE). A stem-loop reverse-transcription was performed using the SuperScript™ III First-Strand Synthesis SuperMix (Invitrogen). Quantitative real-time PCR (qRT-PCR) was run in triplicate in a GeneAmp PCR 9700 Thermocycler (Applied Biosystems), using iQ™ SYBR Green Supermix (Bio-Rad). U6 was used as an internal control for qRT-PCR of total RNA from endothelial cells. Considering that U6 RNA may not be encased within exosomes, we used the Caenorhabdits elegans (C. elegans) miR-39 miRNA mimic (Qiagene, #219610) as a spike-in control. Briefly, when total exosomal RNA was isolated and quantified, 0.1 pmol cel-miR-39 was immediately added per μg exosomal RNA. The PCR condition was 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Relative expression was calculated using the comparative threshold cycle (Ct) method, as previously described [26]. The following primers for rno-miR-320: stem-loop RT primer: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTA TTCG CACTGGATACGACTCGC CC; PCR forward primer: 5′-GCAGAGAAAA GCTGGGT TGAG. The following primers for rno-miR-126, stem-loop RT primer: 5′-GTC GTATCCA GTGCAGGGTCCGAGGTATT CGCACTGGATAC GAC CGCATT; PCR forward primer: 5′-GCAGAGTCGTACCGTGA GTAA. The following primers for cel-miR-39: stem-loop RT primer: 5′-GTCG TATCCAGTG CA GGGTCCGAGGTATTC GCACTGGATACGAC CAAGCTG; PCR forward primer: 5′-GCAGAGAGTGGCCC ACATTTAG. All PCR reverse primers for these miRNAs were the same as 5′-GTGCA GGGTCCGAGGT-3′. The RT and reverse primer for U6: 5′-GTGCA GGGTCCGAGGT-3′; the forward primer for U6: 5′-CTCGCTTCGGCAGCACA-3′.
2.6. Exosome labeling
Exosomes were labeled using DiD (1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethylindodi-carbocyanine, 4-chlorobenzenesulfonate salt; Biotium, Inc.) in a 1:200 dilution, according to the manufacturer’s instructions. Briefly, the exosomal pellet was resuspended in 1 ml DiD solution and incubated for 10 min. After ultracentrifugation at 100,000 g for 70 min at 4 °C, the exosomal pellet was washed once in PBS, followed by centrifugation for 90 min at 150,000 g to remove free dye. Then the pellet was re-suspended in 500 μl DMEM supplemented with 10% exosome-depleted FBS (System Biosciences Inc.). Labeled exosomes (Exo-Did) were 50-fold diluted and added to mouse cardiac endothelial cells (MCECs) for 4 h. Then, exosome-Did-treated MCECs were fixed and stained with a FITC-labeled CD31 antibody (dilution at 1:100, Abbiotec Inc.). Uptake assays were performed under fluorescence microscopy.
2.7. Endothelial cell proliferation and migration/wound healing assay
The assessment of MCEC proliferation was performed using a MTS assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit, Promega), per the manufacturer’s instructions. MCECs were seeded into 96-well plates at an initial density of 5 × 103 cells/well. After 2 h, exosomes were added at various doses (5 μg/ml, 10 μg/ml and 20 μg/ml) for 24 h. A curve of cell proliferation was constructed by measuring cell growth with a microplate reader at 490 nm. The wound healing assay was employed to measure MCEC migration. Cells were seeded in 6-well plates at a density of 5 × 105 cells/well and allowed to attach overnight. When cells came to confluence, the scratches were generated in the center of the well using the extremity of a 200-μl sterile pipette tip. Then, the cells were treated with exosomes (20 μg/ml) for 8 h and photographed with the microscope. The distance between the cell fronts was measured using Image-Pro plus 5.1.
2.8. Capillary-like tube formation assay on matrigel
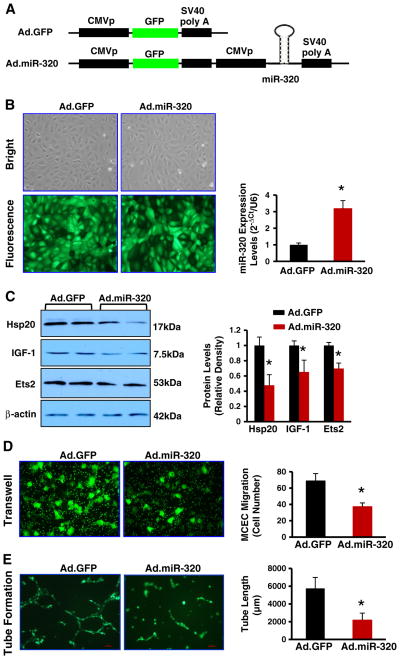
The formation of capillary-like structures was assessed in a 12-well plate using a growth factor-reduced Matrigel (BD Biosciences), per the manufacturer’s instructions. For this procedure, MCECs were treated with exosomes (20 μg/ml) for 6 h. Subsequently, MCECs were collected and seeded (3 × 104 cells/well) on top of Matrigel (400 μl/well). After 6 h, cells were fixed in 70% ethanol and photos were taken under an inverted light microscope at 200× magnification. Total capillary tube lengths and tube branch points were quantified using the software Image-Pro plus 5.1. Five independent fields were assessed for each well, and the average number of tubes was calculated. For adenovirus vector-mediated overexpression of miR-320 in MCECs, Ad.miR-320 generated as described previously [27] was infected MCECs at a MOI of 10. Ad.GFP was used as a control. Twenty-four hours later, MCECs were collected for determination of the miR-320 levels by qRT-PCR and the protein levels of miR-320 targets by Western-blotting. In parallel, Ad.miR-320-infected MCECs were harvested for performing trans-well and tube-formation assays, as described above.
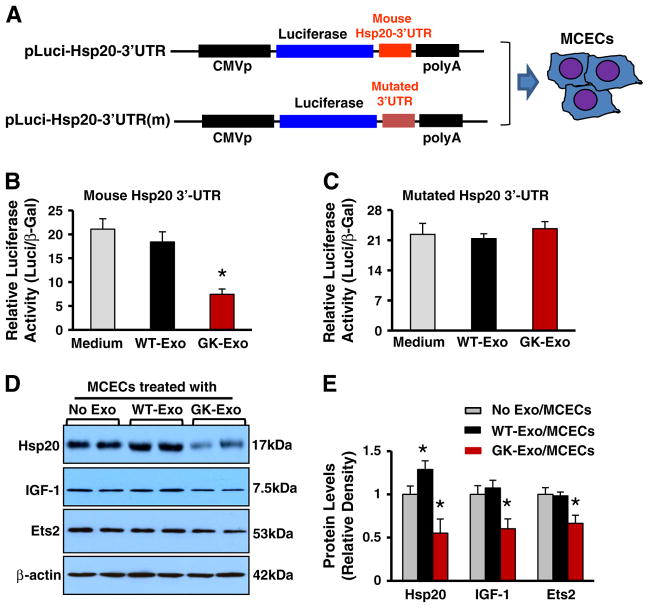
2.9. Luciferase reporter assay for miR-320-targeting Hsp20 3′-UTRs
The luciferase reporter plasmid which includes mouse Hsp20 3′-UTR or mutated Hsp20 3′-UTR was constructed previously [27]. MCECs were co-transfected in 12-well plates using Lipofectamine 2000 reagent (Invitrogen) according to the protocol of the manufacturer, with 0.4 μg of the Hsp20-UTR luciferase reporter vector and 0.08 μg of the control vector pMIR-REPORT (Ambion, Inc.). For each well, 10 μg/ml WT-exosomes or GK-exosomes were added. Cell lysates were prepared 48 h later, and luciferase activity was measured, using a Monolight 3010 luminometer (Pharmingen), and expressed as relative light units using a luciferase assay kit (Promega). β-galactosidase activity was measured with a commercially available kit (Promega). 3′-UTR activity of each construct was expressed as the ratio of luciferase/β-galactosidase activity.
2.10. Statistical analysis
Data are presented as mean ± SD of at least 3 independent experiments. Analysis of variance followed by Newman–Keuls test was performed for multi-group comparisons. Comparisons were made by Student’s t test as appropriate. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Proliferation and migration of endothelial cells are suppressed when co-cultured with cardiomyocytes from type 2 diabetic rats
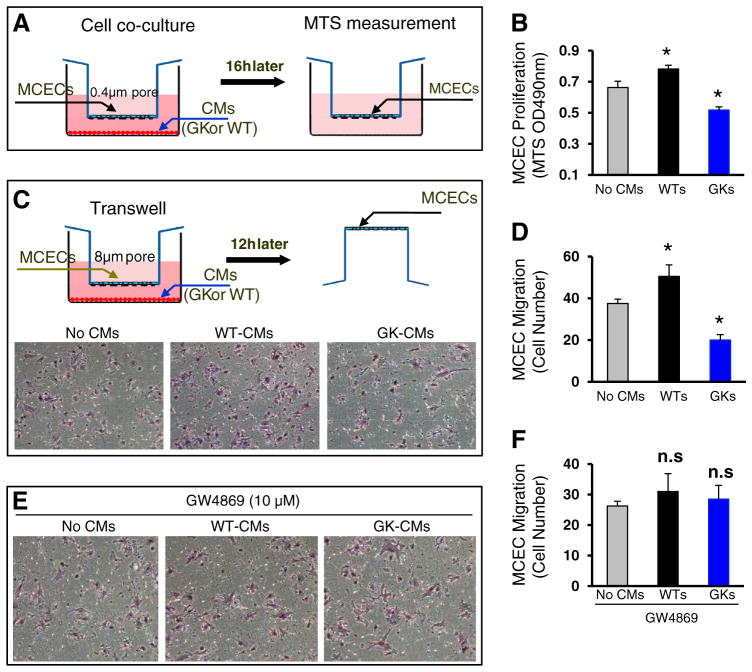
Numerous studies have elucidated the mechanisms underlying the impairment of myocardial vessel growth in diabetes, i.e. reduction of VEGF-A levels, increases of inflammation and accumulation of advanced glycation end products in endothelial cells [2–4]. Interestingly, all of these observations focused on endothelial cells themselves. Whether cardiomyocytes, however, make any contributions to such an impaired angiogenesis in diabetic hearts remains elusive. To this end, we co-cultured a cardiac endothelial cell line (MCECs) with cardiomyocytes (CMs) isolated from the male Goto-Kakizaki (GK) rat, the most widely used animal model of type 2 diabetes [28]. Cardiomyocytes isolated from the age-matched male Wistar (WT) rat were used as controls. After co-culturing overnight, we measured MCEC proliferation by MTS incorporation (Fig. 1A), and observed that the proliferation was significantly inhibited in those endothelial cells co-cultured with GK cardiomyocytes, whereas it was greatly promoted in those cells co-cultured with WT myocytes, compared to those co-cultured without myocytes (Fig. 1B, n = 4, p < 0.05). Similarly, using a Boyden chamber trans-well system (Fig. 1C), we observed that the number of migrated endothelial cells was reduced by 47%, when co-cultured with GK myocytes, whereas it was increased by 35%, when co-cultured with WT myocytes, compared with controls (Fig. 1C/D, n = 4, p < 0.01). Together, these results provide compelling evidence that cardiomyocytes from diabetic hearts elicit negative effects on angiogenesis, suggesting a feedback communication between cardiomyocytes and endothelial cells within the diabetic myocardium.
Fig. 1.
Proliferation and migration of mouse cardiac endothelial cells (MCECs) are inhibited when co-cultured with cardiomyocytes isolated from GK rats, whereas they are promoted when co-cultured with Wistar (WT) rat cardiomyocytes. (A) A scheme of cell co-culture system in which cardiomyocytes were cultured in the lower chamber of a 12-well plate pre-coated with mouse laminin (10 μg/ml) and MCECs were cultured in the upper chamber of a 12-well insert. (B) GK myocytes inhibited MCEC proliferation, which was promoted by WT myocytes. (C) A scheme of the transwell experiment to evaluate endothelial cell migration. Representative endothelial cells which were trans-welled when co-cultured with WT-cardiomyocytes or GK cardiomyocytes. The quantitative results of endothelial cells migrated are shown in (D). (E/F) Cardiomyocyte-mediated regulatory effects on EC migration were negated upon addition of GW4869. n = 4 wells for each group, and similar results were observed in three additional, independent experiments; *p < 0.05 vs. no cardiomyocyes (CMs).
Recently, emerging evidence indicates that exosomes, small membrane vesicles of endocytic origin secreted by most cell types, possess the capacity of either pro- or anti-angiogenesis [13]. Therefore, we next treated MCEC/cardiomyocyte co-cultures with GW-4869 (10 μM), an inhibitor of exosome biogenesis and release. We observed that cardiomyocyte-induced regulatory effects on MCEC migration were negated in the presence of GW-4869 (Fig. 1E/F). These data suggest that cardiomyocyte-derived exosomes may play a pro- or anti-angiogenic role in the heart, depending on the patho-physiological conditions.
3.2. Characterizations of exosomes collected from type 2 diabetic rat cardiomyocytes
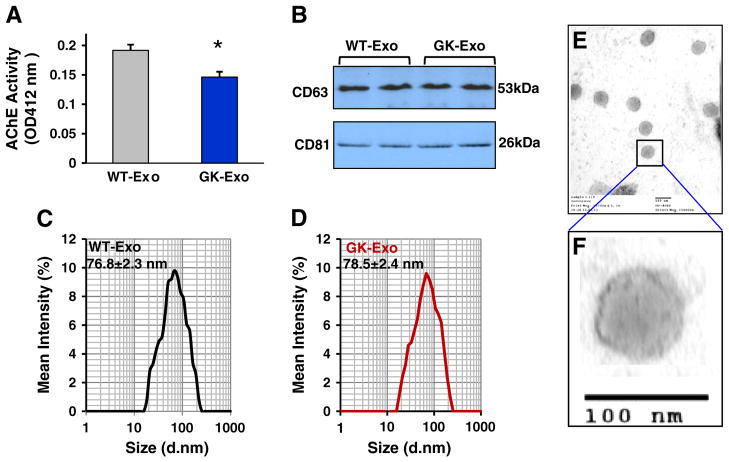
To explore the possible role of diabetic cardiomyocyte-derived exosomes in angiogenesis, we first quantified the amount of exosomes released from GK and WT cardiomyocytes by measuring the activity of acetylcholinesterase (AChE), an enzyme that is specific to these vesicles [29]. Our results showed that the amount of exosomes secreted from GK myocytes (Exo-GK) was less than those of WT myocytes (Exo-WT) (Fig. 2A). Nevertheless, when equal amounts of exosomal protein were loaded for detecting the levels of CD63 and CD81, two molecular markers of exosomes, we observed that neither was altered between GK-exosomes and WT-exosomes (Fig. 2B). Furthermore, the size of exosomes secreted from GK myocytes (78.5 ± 2.4 nm) was similar to that of WT-exosomes (76.8 ± 2.3 nm), which was measured with dynamic light scattering and electron microscopy (Figs. 2C–F). Collectively, these data indicate that diabetic conditions may inhibit the exosome release from cardiomyocytes, but do not alter their size and molecular markers.
Fig. 2.
Characterization of exosomes released from WT- and GK-cardiomyocytes. (A) The activity of acetycholinesterase (AChE) was reduced in GK-exosomes collected from the same amount of cardiomyocytes, compared with controls (n = 4 rats, *p < 0.05). (B) The levels of CD63 and CD81 were similar between GK-exosomes and WT-exosomes (100 μg of exosomal protein was loaded for Western-blotting). (C/D) The exosome size was measured using a Zetasizer Nano instrument (n = 4 independent experiments). (E/F) WT-exosomes were examined under electron microscopy.
3.3. Type 2 diabetic rat cardiomyocyte-derived exosomes inhibit endothelial cell proliferation, migration and tube formation
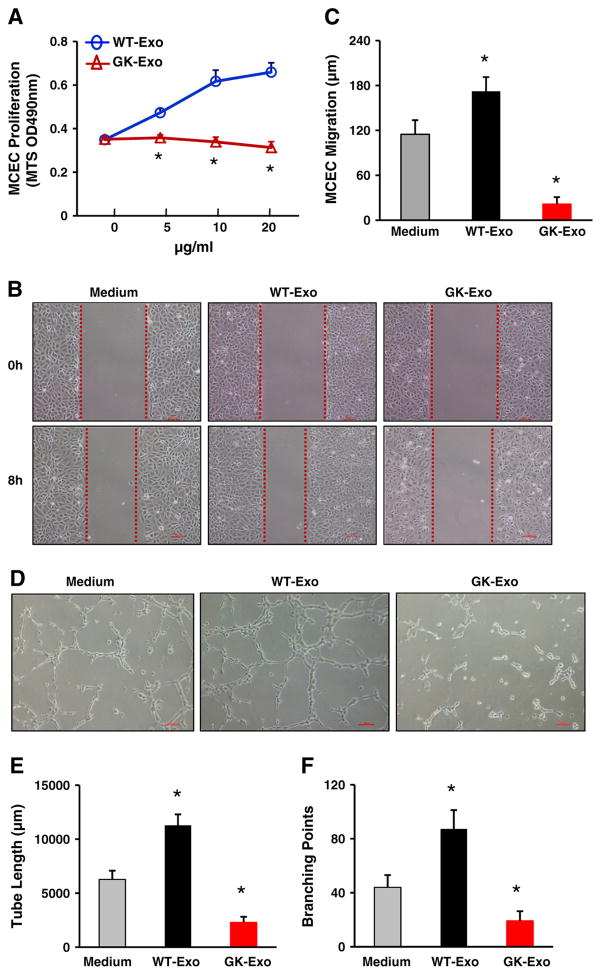
Next, we determined the effects of diabetic myocyte-derived exosomes on EC proliferation, migration and tube formation. Cardiac endothelial cells were cultured and incubated with different doses of exosomes collected from GK or WT cardiomyocytes. The MTS-incorporation assay showed that GK-exosomes inhibited endothelial cell growth, whereas WT-exosomes promoted endothelial cell proliferation in a dose-dependent manner (Fig. 3A). The scratch assay further demonstrated that WT-exosomes enhanced endothelial cell migration and GK-exosomes, however, limited the migratory capacity of endothelial cells, compared to the medium control (Fig. 3B/C). In addition, the tube formation assay on Matrigel (Fig. 3D) revealed that the cumulative capillary tube length was reduced by 63 ± 8% in GK-exosome-treated cells, whereas tube length was increased by 80 ± 9% in WT-exosome-treated samples, compared to medium controls (Fig. 3E). Consistently, the number of tube branch points in the GK-exosome-treated group (19.2 ± 3.1) was remarkably less than that of the controls (44.0 ±5.2), and was significantly increased in the WT-exosome group (87.2 ± 7.5) (Fig. 3F). Put together, these data suggest that GK myocyte-derived exosomes impair angiogenesis, and WT cardiomyocyte-derived exosomes, in contrast, promote angiogenesis.
Fig. 3.
Effects of GK-exosomes on angiogenesis. (A) GK-exosomes significantly inhibited MCEC proliferation in a dose-dependent manner (n = 6 wells, *p < 0.05 vs. WT-exosomes, similar results were observed in three independent experiments). (B/C) GK-exosomes (20 μg/ml) attenuated MCEC migration and (D–F) tube-like formation. Conversely, WT-exosomes(20 μg/ml) exhibited pro-angiogenic effects (B–F). Total capillary tube length (E) and tube branch points (F) were measured by analytical software Image-Pro Plus 5.1. Five independent fields were assessed for each well (n = 3 wells for each group). All values were expressed as means ± SD; *p < 0.05 vs. medium controls. Similar results were observed in three additional, independent experiments.
3.4. Cardiomyocyte-derived exosome uptake and transfer of miR-320 to endothelial cells
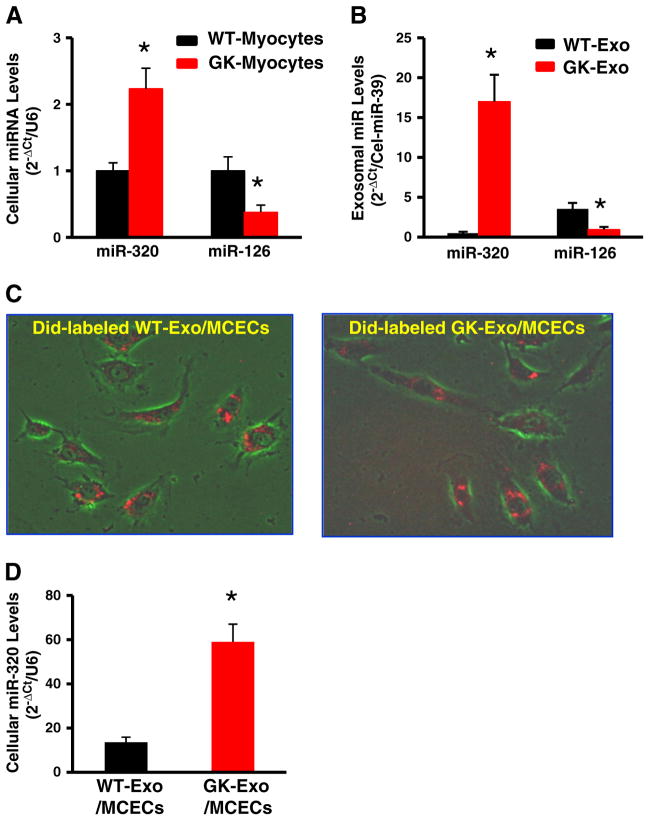
Accumulating evidence has shown that exosomes contain donor-cell microRNAs (miRs, miRNAs) which may affect the phenotype/behavior of the recipient cells. In this regard, we investigated which miRNAs were packaged in cardiomyocyte-derived exosomes. After performing a comparable analysis between published exosomal miRNAs and angiogenesis/diabetes-related miRNAs, we narrowed down and focused on miR-320 (anti-angiogenic miR) and miR-126 (an-giogenic miR). The quantitative RT-PCR assay revealed that GK cardiomyocytes contained higher levels of miR-320 and lower levels of miR-126 than WT cardiomyocytes (Fig. 4A). Importantly, these differences of miR-320 and miR-126 levels exhibited in cardiomyocytes were more dramatic in myocyte-derived exosomes (Fig. 4B). We then tested whether cardiomyocyte-derived exosomes could enter endothelial cells. Exosomes purified from the supernatants of cultured cardiomyocytes were labeled with the red fluorescent membrane dye DiD and added to MCECs. Six hours later, the majority of endothelial cells acquired the red dye-labeled cardiomyocyte exosomes and importantly, both GK-exosomes and WT-exosomes were distributed similarly in the cytoplasm of endothelial cells (Fig. 4C). Accordingly, the miR-320 levels were increased by 4.3-fold in MCECs incubated with GK-exosomes, compared to WT-exosome-treated cells (Fig. 4D). Notably, the levels of miR-126 were similar between these two sources of exosome-treated MCECs. This may be interpreted as levels of endogenous miR-126 in endothelial cells are too high to distinguish its subtle increase caused by exosomes. Collectively, these results indicate that exosomes derived from GK-cardiomyocytes can effectively enter and thereby transfer miR-320 to endothelial cells.
Fig. 4.
Cardiomyocyte-exosomes uptake and deliver miR-320 in endothelial cells. (A) The levels of miR-320 and miR-126 were examined in (A) cardiomyocytes and (B) myocyte-derived exosomes. (C) Red fluorescent dye DiD-labeled WT-exosomes and GK-exosomes were uptaken and distributed in the cytoplasm of MCECs. (D) The miR-320 levels were dramatically increased in MCECs upon exposure to GK-exosomes. U6 RNA was used as an internal control for qRT-PCR of cellular RNA analysis. C. elegans miR-39 was used as spike-in control for qRT-PCR of exosomal RNA analysis. (n = 5, *p < 0.01 vs. WT-exosomes.)
3.5. Cardiomyocyte exosomal miR-320 regulates its target genes in recipient endothelial cells
Recently, we showed that miR-320 directly targets Hsp20 in cardiomyocytes [27]. To test whether cardiomyocyte exosomal miR-320 is functional in recipient endothelial cells, we performed a luciferase reporter assay. We transfected MCECs with a construct containing the 3′-UTR of mouse Hsp20 gene fused downstream to the luciferase coding sequence (Fig. 5A), followed by addition of WT-exosomes or GK-exosomes for 48 h. We observed that GK-exosomes strongly inhibited the luciferase activity in MCECs transfected with the reporter construct containing the 3′UTR of mouse Hsp20 gene, whereas no effect was observed in MCECs with a construct containing the mutated 3′UTR of mouse Hsp20 gene, compared with controls (Fig. 5B/C). Accordingly, Western-blotting assay showed that the protein levels of Hsp20 were reduced by 45% in MCECs treated with GK-exosomes, compared with no exosome-treated ECs (Figs. 5D/E). It is important to note here that the levels of Hsp20 protein were increased by 29% in WT-exosome-treated MCECs, compared with ECs in the absence of exosomes (Figs. 5D/E). This is consistent with our previous reports showing that healthy myocyte-derived exosomes encase a large amount of Hsp20 [14].
Fig. 5.
Cardiomyocyte exosomal miR-320 functionally down-regulates its target genes in recipient endothelial cells. (A) Diagrams of plasmid construction in which mouse Hsp20 3′-UTR or its mutation was inserted downstream of the luciferase cDNA. (B/C) Dual luciferase activity assay in MCECs treated with WT-exosomes or GK exosomes. (D/E) Western-blotting assay revealed that the protein levels of Hsp20, IGF-1, and Ets2 were significantly reduced in GK-exosome-treated MCECs. n = 4 independent experiments; *p < 0.05 vs. exosome-null-treated MCECs.
In addition, miR-320 has been shown to repress the expression of several proangiogenic factors such as IGF-1 [17] a nd Ets2 (a transcription factor required for endothelial cell survival) [30]. Therefore, we tested whether the protein levels of IGF-1 and Ets2 were altered in GK-exosome-treated MCECs. Western-blotting analysis showed that GK-exosomes significantly reduced the IGF-1 and Ets2 protein levels in MCECs, compared with control MCECs (Figs. 5D/E). Collectively, these results indicate that cardiomyocyte miR-320 via exosomal transport is functional to regulate its target gene expression in endothelial cells.
3.6. Overexpression of miR-320 inhibits endothelial cell migration and tube formation
To further examine the consequence of increased miR-320 expression in endothelial cells, we infected MCECs with Ad.miR-320 or control Ad.GFP (Figs. 6A/B). Results of qRT-PCR showed that miR-320 levels were increased by 3.2-fold in Ad.miR-320-infected cells, compared with Ad.GFP-cells (Fig. 6B). Western-blotting analysis revealed that overexpression of miR-320 significantly reduced protein levels of Hsp20, IGF-1 and Ets2, compared to controls (Fig. 6C). Importantly, we observed that the migration of endothelial cells and the tube-like formation were greatly inhibited in miR-320-overexpressing MCECs, related to Ad.GFP-infected cells (Figs. 6D/E). Together, these results validate that elevation of miR-320 could inhibit angiogenesis.
Fig. 6.
Overexpression of miR-320 inhibits MCEC migration and tube formation. (A) Diagrams of recombinant adenoviral vectors (Ad.GFP and Ad.miR-320). (B) MCECs were infected with Ad.GFP or Ad.miR-320, and the miR-320 levels were measured with qRT-PCR. U6 RNA was used as an internal control. (C) The protein levels of Hsp20, IGF-1 and Ets2 were significantly reduced in Ad.miR-320-cells, compared with Ad.GFP-cells. Overexpression of miR-320 greatly inhibited MCEC migration (D) and tube formation (E). *p < 0.05 vs. Ad.GFP, n = 3 independent experiments.
3.7. Transportation of miR-320 from cardiomyocytes to endothelial cells is dependent on exosomes
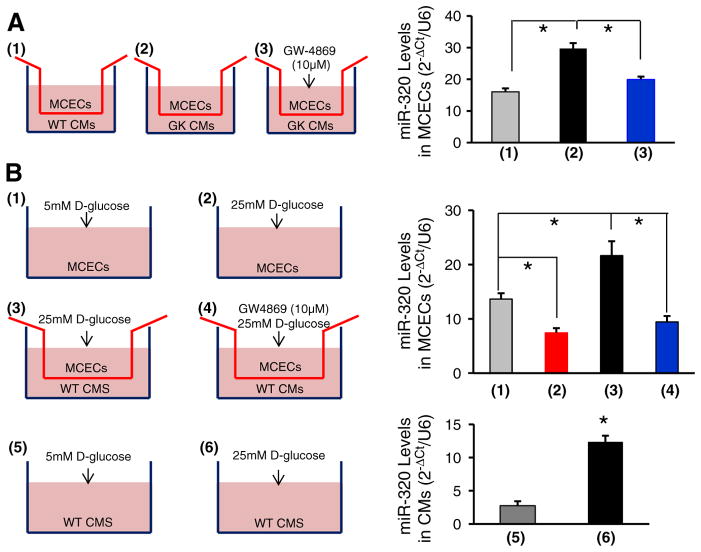
We next tested whether delivery of cardiomyocyte miR-320 to endothelial cells is dependent on exosomes. Co-cultures of MCECs/cardiomyocytes were treated with GW-4869 (10 μM), an inhibitor for the exosome formation and release [24]. We observed that GK-myocyte-mediated elevation of miR-320 in MCECs was significantly reduced upon addition of GW-4869 (Fig. 7A). These data suggest that transportation of miR-320 from cardiomyocytes to endothelial cells is dependent on exosomes.
Fig. 7.
Transportation of miR-320 from cardiomyocytes into MCECs is blocked by the exosome inhibitor GW4869. (A) Schemes of MCECs co-cultured with: (1) WT-myocytes, (2) GK-myocytes and (3) GW4869-treated GK-myocytes. Twenty-four hours later, MCECs were collected for the determination of miR-320 levels by qRT-PCR. Addition of GW4869 prevented the increase of miR-320 in MCECs co-cultured with GK-myocytes (*p < 0.05 vs. controls as indicated). Similar results were observed in three additional, independent experiments. (B) Schemes of MCECs: (1) cultured in the presence of 5 mM D-glucose, (2) in the presence of 25 mM D-glucose, (3) co-cultured with WT-cardiomyocytes in the presence of 25 mM D-glucose and (4) co-cultured with WT-cardiomyocytes in the presence of 25 mM D-glucose plus GW4869; schemes of WT cardiomyocytes cultured in the presence of 5 mM D-glucose (5) and in the presence of 25 mM D-glucose (6). The levels of miR-320 were reduced in MCECs under high glucose conditions (2), whereas its levels were remarkably increased when co-cultured with high-glucose-treated cardiomyocytes (3). Such an increase of miR-320 levels may have originated from cardiomyocytes, as miR-320 levels were increased in WT cardiomyocytes upon high-glucose treatment (6), and addition of the exosome inhibitor GW4869 blocked the transfer of miR-320 from cardiomyocytes into MCECs (4). (*p < 0.05 vs. controls as indicated). Similar results were observed in three additional, independent experiments.
In addition, we isolated cardiomyocytes from healthy Wistar (WT) rats and co-cultured with MCECs, followed by the treatment with 25 mM of D-glucose (simulating hyperglycemia, a key initiating factor for endothelial cell damage in diabetes) or 25 mM of D-glucose plus 10 μM of GW-4869 (Fig. 7B). In parallel, MCECs treated with 25 mM of D-glucose or 5 mM of D-glucose (simulating a normal level of glucose in the blood) were used as controls. After 24 h, MCECs were collected to measure the levels of miR-320. RT-PCR analysis results showed that high levels of glucose caused a significant reduction of miR-320 in endothelial cells, compared to low-glucose conditions (Fig. 7B1/2). This is consistent with a recent report by Feng & Chakrabarti [20] showing that miR-320 was down-regulated by ~60% in human umbilical vein endothelial cells (HUVECs) upon exposure to 25 mM of D-glucose for 24 h. However, when co-cultured with cardiomyocytes, the levels of miR-320 were markedly elevated in MCECs upon high-glucose conditions (Fig. 7B3), and the increase of miR-320 was attenuated by addition of GW-4869 (Fig. 7B4). We further observed that miR-320 levels were increased by 4.5-fold in high glucose-treated WT cardiomyocytes, compared with controls (Fig. 7B5/6). Collectively, these data suggest that elevation of miR-320 in diabetic myocardial endothelial cells observed by Wang et al. [17] might have originated from cardiomyocytes via exosomal transportation, which eventually contributes to microvascular rarefaction in diabetic hearts.
3.8. GK exosome-mediated inhibitory effects on angiogenesis are removed by knockdown of miR-320
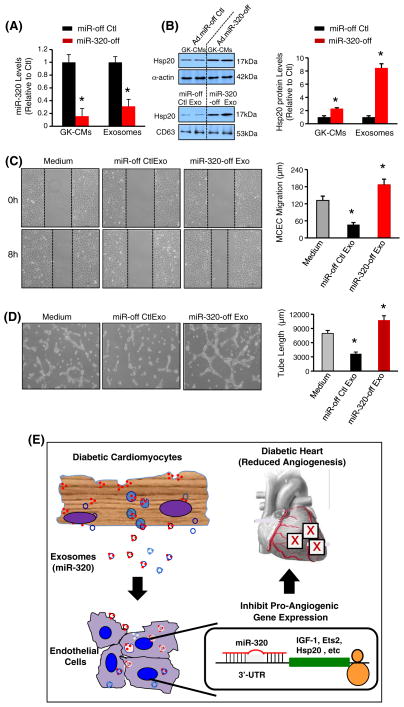
To determine whether anti-angiogenic effects of GK-cardiomyocyte-derived exosomes are associated with miR-320, we knocked down miR-320 levels in GK cardiomyocytes by infection with an inhibitory rno-miR-320 adenovirus (Ad.miR-miR-320-off). Ad.miR-off was used as a control. RT-PCR results showed that miR-320 levels were reduced by 84% in Ad.miR-320-off-infected GK myocytes, and decreased by 68% in exosomes collected form their supernatants, compared with controls, respectively (Fig. 8A). Accordingly, protein levels of Hsp20, one of miR-320 targets, were increased by 2.3-fold in miR-320-off GK-myocytes, and by 8.5-fold in their released exosomes (Fig. 8B). We next added miR-320-off exosomes or control exosomes to MCECs and performed migration and tube formation analysis. Our results showed that miR-320-reduced exosomes actually promoted MCEC migration and tube formation, compared to control exosome-treated group (Figs. 8C/D). This may be ascribed to increased angiogenic factors (i.e. Hsp20) in miR-320-reduced exosomes. Therefore, these data indicate that exosomes and exosomal miR-320 may play critical roles in mediating anti-angiogenic effects of GK-cardiomyocytes.
Fig. 8.
Effects of miR-320-reduced exosomes collected form miR-320-off GK cardiomyocytes on angiogenesis. (A) Infection of GK-cardiomyocytes with Ad.miR-320-off dramatically reduced miR-320 levels in cardiomyocytes and their released exosomes. (B) Protein levels of Hsp20 were remarkably increased in Ad.miR-320-off-infected GK myocytes and their released exosomes. n = 4, *p < 0.05 vs. controls indicated. miR-320-off-exosomes (20 μg/ml) significantly promoted (C) MCEC migration and (D) tube-like formation, compared with miR-off control exosomes. Total capillary tube length was measured as described in Fig. 3. All values were expressed as means ± SD; *p < 0.05 vs. medium controls. Similar results were observed in three additional, independent experiments. (E) Proposed scheme for cardiomyocyte-mediated anti-angiogenesis through exosomal miR-320 in diabetic hearts.
4. Discussion
In the present study, we have observed that cardiomyocytes isolated from the type 2 diabetic rats elicited inhibitory effects on angiogenesis through the exosomal transfer of miR-320 into endothelial cells (Fig. 8E). Our results provide a novel molecular mechanism underlying the diabetes-caused impairment of myocardial angiogenesis, that is, an anti-angiogenic signal from cardiomyocytes via exosomes, but not from endothelial cells themselves.
Diabetes mellitus (DM) is a chronic metabolic disease characterized by the presence of hyperglycemia, which can lead to many complications (i.e. nephropathy, retinopathy, neuropathy, impaired wound healing and accelerated atherosclerosis) [1–5]. However, there are two types of angiogenic conditions that co-exist in different tissues during diabetes: defective angiogenesis in peripheral limbs and myocardium; while excessive angiogenesis in the retina and kidney [4]. Despite a number of factors (i.e. cytokines, oxidative stress, advanced glycation end products and others) may be involved in this aberrant angiogenesis [2–4], the local microenvironment of each organ definitely plays a critical role in the angiogenic process. Indeed, our data indicate that type 2 diabetic GK rat-derived cardiomyocytes secrete miR-320-enriched exosomes, which can be taken up by endothelial cells and inhibit myocardial angiogenesis (Fig. 8E). Importantly, transportation of miR-320 from cardiomyocytes to endothelial cells is dependent on exosomes, indicating their pivotal role in local cell-cell communication. miR-320 has been predicted to target multiple angiogenesis-related genes, such as Flk-1, VEGF-c, IGF-1, IGF-1R, FGFs, Hsp20 and Ets2. In particular, IGF-1, Hsp20 and Ets2 have been confirmed to be bona fide targets of miR-320 in cardiomyocytes [27] and endothelial cells [17,30]. The present study showed that cardiomyocyte exosome-delivered miR-320 appeared functional in endothelial cells, as the miR-320 target genes (Hsp20, IGF-1 and Ets2) were downregulated upon exposure to GK-myocyte exosomes, which resulted in decreased proliferation, reduced migration and tube-like formation of endothelial cells. Hence, miR-320 that is transferred from cardiomyocytes into neighboring endothelial cells can be one of the extracellular signaling molecules that can mediate anti-angiogenesis in diabetic hearts. Given that exosomes also encapsulate other specific proteins or miRNAs reflective of parental cells [9–13], it is plausible that other exosomal contents could be delivered to endothelial cells and thereby contribute to the impaired myocardial angiogenesis in diabetes.
Distinct from the anti-angiogenic effects of GK-myocyte exosomes (GK-exosomes), healthy cardiomyocyte-derived exosomes (WT-exosomes) promote endothelial cell proliferation, migration and tube-like formation. Importantly, while these two sources of exosomes contain both pro-angiogenic miR-126 and anti-angiogenic miR-320, there is a difference in the amount of the miRs included. WT-exosomes incorporate higher levels of miR-126 and lower levels of miR-320 than those in GK-exosomes, which may partially lead to enhanced angiogenesis. Recently, we observed that WT-exosomes incorporated a significant amount of Hsp20, which activates the VEGFR2 signaling pathway in endothelial cells and promotes angiogenesis [14]. Thus, healthy cardiomyocytes may manipulate the local environment through exosomal miRNAs/proteins to maintain normal myocardial angiogenesis. By contrast, hyperglycemic conditions may trigger cardiomyocytes to sort a specific set of miRNAs/proteins into exosomes, which communicate with endothelial cells and exert an anti-angiogenic function in the myocardium. In this regard, the composition of exosomal miRNAs/proteins may be dynamic and dependent on physiological/pathological conditions of cells/tissues. Accordingly, exosomal miRNAs/proteins may serve as useful biomarkers for diagnosis of human disease.
Considering that cardiomyocytes release “harmful” exosomes during diabetes, it should be a potential target for diabetes therapy to impair the secretion of exosomes from myocytes. Actually, blockage of exosome secretion from cardiomyocytes by the chemical inhibitor GW4869 remarkably impeded the transfer of miR-320, a critical anti-angiogenic miRNA, into co-cultured endothelial cells. In addition, as a compensatory mechanism, diabetic cardiomyocytes did reduce the secretion of exosomes (Fig. 2A). Nonetheless, the clinical application of an exosome inhibitor would be limited, as it may interfere with normal/beneficial cell–cell communication mediated by exosomes. Therefore, the ideal strategy to improve angiogenesis in diabetic hearts may be to use biotechnologically engineered-exosomes to incorporate large amounts of angio-miRs (i.e. miR-126) or angio-proteins (i.e. Hsp20, Ets2), and this needs to be further explored in future studies. The major limitation for this study is that we did not validate if miR-320-enriched exosomes released from diabetic cardiomyocytes would impair myocardial angiogenesis in vivo. Such an issue could be addressed using an inducible cardiac-specific overexpression of miR-320 animal model upon diabetic conditions [i.e. streptozotocin (STZ)-induced diabetes]. Also, it would be important to address whether cardiac-specific deletion of miR-320 could improve myocardial angiogenesis in diabetes mellitus.
In summary, our present findings demonstrate a previously unrecognized mechanism underlying the impairment of myocardial angiogenesis in diabetes. Exosomes released from diabetic cardiomyocytes inhibit endothelial cell proliferation, migration and tube-like formation through the transfer of miR-320, a well-known anti-angiogenic miRNA, into endothelial cells. Our study may pave a new way for the treatment of diabetes-caused aberrant angiogenesis.
Acknowledgments
Funding sources
This study was supported by NIH grants 2R01HL-087861 (G.-C. Fan), R01HL-102314 and K02HL-098956 (J. Chang).
Footnotes
Conflict of interest disclosures
None.
References
- 1.Raslova K. An update on the treatment of type 1 and type 2 diabetes mellitus: focus on insulin detemir, a long-acting human insulin analog. Vasc Health Risk Manag. 2010;6:399–410. doi: 10.2147/vhrm.s10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev. 2005;1:59–63. doi: 10.2174/1573399052952550. [DOI] [PubMed] [Google Scholar]
- 3.Cohen G, Riahi Y, Alpert E, Gruzman A, Sasson S. The roles of hyperglycaemia and oxidative stress in the rise and collapse of the natural protective mechanism against vascular endothelial cell dysfunction in diabetes. Arch Physiol Biochem. 2007;113:259–67. doi: 10.1080/13813450701783513. [DOI] [PubMed] [Google Scholar]
- 4.Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92:1037–45. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Heather LC, Clarke K. Metabolism, hypoxia and the diabetic heart. J Mol Cell Cardiol. 2011;50:598–605. doi: 10.1016/j.yjmcc.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Yan J, Tie G, Park B, Yan Y, Nowicki PT, Messina LM. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg. 2009;50:1412–22. doi: 10.1016/j.jvs.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–63. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons M. Angiogenesis, arteriogenesis, and diabetes: paradigm reassessed? J Am Coll Cardiol. 2005;46:835–7. doi: 10.1016/j.jacc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9(6):871–81. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 1820;2012:940–8. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes function in pro- and anti-angiogenesis. Curr Angiogenesis. 2013;2:54–9. doi: 10.2174/22115528113020020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS ONE. 2012;7(3):e32765. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldenström A, Gennebäck N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS ONE. 2012;7(4):e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, et al. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol. 2013;304:H954–65. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2009;36:181–8. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 18.Caporali A, Meloni M, Völlenkle C, Bonci D, Sala-Newby GB, Addis R, et al. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–91. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 19.Mocharla P, Briand S, Giannotti G, Dörries C, Jakob P, Paneni F, et al. AngiomiR-126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood. 2013;121:226–36. doi: 10.1182/blood-2012-01-407106. [DOI] [PubMed] [Google Scholar]
- 20.Feng B, Chakrabarti S. miR-320 regulates glucose-induced gene expression in diabetes. ISRN Endocrinol. 2012:1–6. doi: 10.5402/2012/549875. http://dx.doi.org/10.5402/2012/549875. [DOI] [PMC free article] [PubMed]
- 21.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Zingarelli B, O’Connor M, Zhang P, Adeyemo A, Kranias EG, et al. Overexpression of Hsp20 prevents endotoxin-induced myocardial dysfunction and apoptosis via inhibition of NF-kappaB activation. J Mol Cell Cardiol. 2009;47:382–90. doi: 10.1016/j.yjmcc.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-1beta to alter beta-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J. 2007;21:1831–43. doi: 10.1096/fj.06-7557com. [DOI] [PubMed] [Google Scholar]
- 24.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, et al. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol. 2013;304:H954–65. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 27.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–66. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portha B, Giroix MH, Tourrel-Cuzin C, Le-Stunff H, Movassat J. The GK rat: a prototype for the study of non-overweight type 2 diabetes. Methods Mol Biol. 2012;933:125–59. doi: 10.1007/978-1-62703-068-7_9. [DOI] [PubMed] [Google Scholar]
- 29.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol. 2011;14:159–67. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]