Abstract
Background
Working memory impairments are commonly found in Attention Deficit/Hyperactivity Disorder (AD/HD) and often improve with psychostimulant treatment. Little is known about how these medications affect the function of frontoparietal brain regions engaged for working memory. This study used functional magnetic resonance imaging (fMRI) to examine medication-related changes in brain activation and functional connectivity in AD/HD.
Methods
Eighteen AD/HD-Combined subtype youths (ages 11-17) twice completed a Sternberg working memory fMRI task in a randomized, double-blind, placebo-controlled design. Medications were individualized as patients' standard, clinically-effective psychostimulant (e.g. methylphenidate or dextroamphetamine/amphetamine combination) dose. Brain activity and functional connectivity were characterized using group independent component analysis (ICA). SPM5 repeated-measures t tests compared AD/HD patients' network engagement and regional functional connectivity on and off medication.
Results
ICA identified six frontoparietal networks/components with hemodynamic responses to Encoding/Maintenance or Retrieval phases of the Sternberg fMRI task. On medication, three of these networks significantly increased activation. Functional connectivity analyses found medication led to recruitment of additional brain regions that were not engaged into the networks when participants were on placebo. Also, medication strengthened connectivity of some frontoparietal regions. Many connectivity changes were directly related to improved working memory reaction time. Overall, there was strong evidence for regional functional connectivity changes following medication in structures previously implicated as abnormal in AD/HD, such as anterior cingulate, ventrolateral prefrontal cortex, and precuneus.
Conclusions
Stimulant medication has widespread effects on the functional connectivity of frontoparietal brain networks, which might be a mechanism that underlies their beneficial effects on working memory performance.
Keywords: ADHD, adolescents, fMRI, working memory, Sternberg, stimulant
Many children with Attention Deficit/Hyperactivity Disorder (AD/HD) (1) have problems with working memory (2, 3), typically defined as the ability to temporarily hold and manipulate information mentally in the absence of stimuli (4). Although not present in all AD/HD patients (5), a meta-analysis found medium effect sizes for AD/HD impairments in both verbal (d=0.55) and spatial (d=0.63) working memory (3). Working memory performance is directly linked to brain activity and to catecholaminergic regulation (6, 7), which are proposed pathophysiological components of AD/HD (8). Brain regions engaged during working memory include dorso/ventrolateral prefrontal cortex, posterior parietal cortex, anterior and mid-cingulate, inferior temporal lobe, basal ganglia, thalamus and cerebellum (9). Neuroimaging studies of working memory have found significantly less activation of ventrolateral prefrontal cortex (10, 11), cerebellar and occipital regions (12) in adults with AD/HD compared to controls. Similar studies of AD/HD youths are inconsistent. Sheridan et al. found no differences between working memory-elicited brain activity in AD/HD girls and controls, although ventrolateral prefrontal cortex activity was related to better behavioral performance in AD/HD patients (13). More recently, Kobel et al. found precentral, superior/inferior parietal lobule, and cerebellar activation deficits in AD/HD boys on an N-back task (14). These findings link AD/HD to ventrolateral prefrontal and parietal lobe abnormalities during working memory tasks, though additional investigation could clarify incongruent findings.
Psychostimulant medications improve many AD/HD cognitive impairments (15-17), but their effect on working memory is unclear. A review found that stimulants improved working memory performance in AD/HD within roughly half of 40 placebo-controlled studies (17), which suggests that its efficacy might depend on AD/HD clinical characteristics (e.g., DSM-IV subtype) or type of working memory task used. Both positron emission tomography (PET) studies of brain metabolism (18-21) and functional magnetic resonance imaging (fMRI) studies of working memory (14, 22) have produced mixed results for specific regional increases or decreases of activation following acute drug treatment. Discrepancies among studies illustrate the need to further explore how stimulant medications alter working memory brain function in AD/HD.
An interesting possibility is that stimulants might enhance working memory ability through better coordination of information processing across the nodes of widely distributed, yet functionally connected networks. Functional connectivity among brain regions can be inferred when distal regions show strongly correlated temporal patterns of fMRI-measured signal change (23). Functional connectivity studies have shown that working memory brain regions are organized into distinct functional networks (24-27). In particular, frontal and parietal lobe brain regions comprise neural circuits that are engaged for working memory regardless of information encoding or retrieval demands (28-32). In a pilot study of five AD/HD adolescent girls, Sheridan et al. found that lateral prefrontal cortex functional connectivity was altered by stimulant treatment (22). Drug administration increased functional connectivity with middle frontal gyrus and cerebellar vermis, but resulted in less middle frontal gyrus connectivity with striatum, temporal/parietal junction, and numerous other cortical and paralimbic regions. The only other comparable study in AD/HD children found methylphenidate increased frontoparietal, frontostriatal, and frontocerebellar connectivity during a sustained attention task (33).
The study objective was to assess stimulant medication effects on frontoparietal network activity and functional connectivity in AD/HD. We focused on frontoparietal networks because of their frequently demonstrated importance to both working memory encoding/maintenance and retrieval (28), which we dissociated using a Sternberg working memory fMRI task. We employed independent component analysis (ICA), as it is ideally suited to characterize multiple discrete networks, examine their profiles of task-related activation, and test for regional functional connectivity differences while on/off medications. We hypothesized that psychostimulant medications would alter network engagement (activation) and change regional functional connectivity. We wished to ascertain whether medications increased strength of functional connectivity within already-active brain regions, or increased the connectivity of frontoparietal network regions with other brain areas (including those known to be dysfunctional in AD/HD, e.g., cingulate, striatum, cerebellum (34, 35)). The former would suggest that medications specifically facilitate communication between the key functionally-specialized nodes of the network, while the latter would suggest that the systems-level influence of effective AD/HD psychostimulant treatment is to improve large-scale distributed network communication. We also predicted medications would improve Sternberg task performance, and conducted supplemental tests of relationships between performance and regional connectivity.
Methods
Participants and Clinical Characterization
Eighteen children/adolescents with DSM-IV 314.01 AD/HD-Combined subtype (1) (mean age=14.6; range=11-17; 83% male) of normal IQ were recruited via physician referral and community advertisements. Potential participants were excluded for history of learning disability, neurological illness, lost consciousness >30 minutes or significant medical conditions. AD/HD and other psychiatric diagnoses were evaluated using the Schedule for Affective Disorders and Schizophrenia (K-SADS-PL) (36). Participants with comorbid psychiatric/substance diagnoses other than Oppositional Defiant Disorder (ODD) were excluded (only 1 participant had ODD). All were regularly treated with methylphenidate or dextroamphetamine/amphetamine combination prescribed by personal physicians, at doses judged to be clinically effective by each family. Participants/legal guardians gave written informed assent/consent before study participation using procedures approved by Hartford Hospital's Institutional Review Board. Participants received monetary compensation for their time. Sample demographic and clinical characteristics are reported in Supplemental Table 1.
Medication and Placebo Control Procedures
The study used a double-blind, randomized, placebo-controlled design. A “washout” period was used to ensure active drug had been eliminated. 48 hours prior to MRI sessions, participants began randomized consumption of opaque capsules (to prevent visual inspection of contents) prepared by a local pharmacy. This ensured that participants received their normal drug regimen (or placebo) for assessment. Because AD/HD patients often can readily ascertain whether or not they are medicated, participants were informed they might receive a lower dose of their normal medication to mitigate expectancy effects on performance, when in reality they received either their normally-prescribed medication dose(s) or placebo. Debriefing revealed all experimental procedures.
fMRI Task
The Sternberg Item Recognition paradigm (37) was chosen because most Sternberg behavioral studies find deficits in AD/HD (38, 39) or show test gains with AD/HD medications (40, 41), typically in reaction time. The 7-minute task required subjects to memorize a list of consonants, maintain them in memory, then differentiate target letters from foils. During each Encoding period, participants saw each single letter sequentially for 1.5 sec, with a 1 sec inter-stimulus interval. After a 9 sec Maintenance period (during which participants silently rehearsed each consonant set), target or foil letters were presented for Retrieval (2.5 sec with a 500 msec inter-stimulus interval). They were instructed to make an index finger button-press for items in the immediately-preceding list (targets) or a middle finger button-press for any non-seen letters (foils). Trial/condition onsets were constructed such that fMRI modeling could separate hemodynamic change of the Encoding/Maintenance phases from the Retrieval phase. The task included different memory loads (4, 5, or 6 letters) (Supplemental Table 2) (42). Participants practiced the task prior to the fMRI to ensure understanding and ability. fMRI sessions were 6 weeks apart on average.
Imaging Parameters and Processing
Imaging used a Siemens Allegra 3T MRI at the Olin Neuropsychiatry Research Center. Localizer images were acquired to prescribe functional image volumes. The echo planar image (EPI) gradient-echo pulse sequence sensitive to endogenous BOLD signal (TR/TE 1860/27 ms, flip angle 70°, 3.44×3.44 mm in-plane resolution, 5 mm effective slice thickness, 36 slices) effectively covered the entire brain in 1.86 seconds. Head motion was restricted using a custom-built cushion inside the head coil. 226 time points were collected. The initial five images were discarded to avoid T1 saturation effects.
Functional images were reconstructed offline and each timeseries was separately realigned (43). Average head displacement was ≤ 3.32 mm. There was no correlation between movement and task conditions (average r ≤ .08 across motion parameters). Because the ICA technique is relatively robust to sporadic, rapid head motions (typically separating such signal changes from brain activation within “junk” components), occasional, rare head motion beyond a 1-voxel cutoff was deemed acceptable. Average per voxel signal-to-noise ratio (SNR) values did not differ between the medicated/unmedicated fMRI sessions. Image timeseries underwent slice timing acquisition correction, and a mean volume was used to determine parameters for spatial normalization into Montreal Neurological Institute space, which were applied to all participant volumes. Normalized images were smoothed with an 8 mm FWHM Gaussian filter.
Independent Component Analysis
ICA is a whole brain, data-driven multivariate analysis method that identifies distinct groups of brain regions with the same temporal pattern of hemodynamic signal change. Analysis included intensity normalization, two principal component analyses (PCA), concatenated data reduction stages (44, 45), and estimation of independent components using an algorithm that minimizes the mutual information of the network outputs (46). The final ICA rotation was performed on the group of participants' aggregate data and produced spatial maps and timecourses that represented both the spatial and temporal characteristics of each component's ‘functionally-connected network’. This group solution was used to back-reconstruct single-subject time courses and spatial maps from the raw data using methods that accurately preserved participant-to-participant variability (i.e., GICA3 (47)). The resulting single-subject timecourse amplitudes were then calibrated using raw data so that they reflected percent fMRI signal change (44). The ICA methods are available in a Group ICA of fMRI Toolbox (GIFT v1.3h) implemented in Matlab (http://icatb.sourceforge.net). Data dimensionality (number of components) was estimated using the minimum description length (MDL) criteria tool in GIFT, which suggested that ∼40 components were present in the data (48). ICA solution reliability was assessed using ICASSO (http://www.cis.hut.fi/projects/ica/icasso) where only components consistently identified across 100 separate FastICA estimations were examined (Iq coefficients >.90).
As in previous work (49-51), the R2 association between each component's spatial map absolute values and a priori probabilistic masks of brain tissue (MNI templates) identified which components should be retained for further analysis. We discarded components with high correlation to white matter or cerebral spinal fluid (CSF) as they largely reflected eyeball movements, head motion, and cardiac-induced pulsatile artifact. Of the remaining components, we retained only components that depicted brain activity within frontal lobe regions, parietal lobe regions, or both in either hemisphere.
Examination of Component Temporal Dynamics
Task engagement analysis involved parameterizing the timecourses to provide estimates of the association between component timecourse and experimental design. The Sternberg Encoding/Maintenance and Retrieval conditions were represented by convolving trial onsets with a hemodynamic response model. This is analogous to a conventional “SPM-type” analysis of “activation,” with greater β-weights typically representing greater amplitude of distributed networks' general hemodynamic responses. β-weights showing the relationship of each component to experimental conditions were examined to determine to which task conditions each was activated (one-sample t tests against zero). Figures depicting ICA timecourse data averaged over either Encoding/Maintenance or Retrieval (i.e., event-related averages) were constructed for visualization of task-associated positive and negative signal change patterns.
Visualization of Spatial Components
Each set of participant spatial maps (18 maps for each component) was entered into SPM5 voxelwise one-sample t tests in order to visualize which brain regions were part of each network. Significance was evaluated using p < .001 whole-brain family-wise error correction (52). Component spatial structure was visualized by color-coded component maps projected to cortical surface renderings (53).
Hypothesis Testing
Task Engagement (“Activation”)
β-weights representing the fit of ICA components with canonical hemodynamic models of the Sternberg task conditions were examined using 12 repeated-measures two-sided t tests (i.e., one for each of the 6 components, separately for Encoding/Maintenance and Retrieval conditions). Multiple comparisons corrections were made using False Discovery Rate methods (54).
Functional Connectivity
Tests for differences in degree of regional functional connectivity were done by comparing ICA-generated spatial maps for each subject between medication and placebo fMRI sessions using a series of SPM5 repeated-measures t test models. Clusterwise statistical inference was used (p<.01 entry-level). Effects are noted if they met either corrected or uncorrected p<.05 levels.
fMRI Task and Neuropsychological Behavioral Analyses
Sternberg task behavioral data included reaction time to targets, reaction time to foils, and accuracy for Retrieval trials. These were evaluated in three multivariate working memory load (4, 5, or 6) × medication status (placebo or medication) repeated-measures ANOVA models with Greehouse-Geiser corrections to determine the effect of medications on task performance. Post hoc univariate effects were examined to determine the most important contributions to any significant multivariate effect. Supplemental SPM5 correlation analyses (without multiple comparisons correction) examined the linear relationship of change in mean target reaction time with change in regional functional connectivity across the brain for medication versus placebo (SPM5 small volume correction within 6 mm radius spheres at coordinates of peak medication versus placebo difference).
Participants underwent a short battery of neuropsychological tasks performed outside the scanner, which were examined using a series of repeated-measures t tests to compare medication and placebo performance (Supplemental Table 1).
Results
Frontoparietal Networks' Structure
ICA identified 6 frontoparietal components, each representing unique networks. Supplemental Table 3 lists brain regions comprising each (Components A-F), including x,y,z coordinates and t score of peak regional connectivity within discrete regions. When networks included brain regions that showed negative BOLD signal change relative to other network nodes, these are noted by negative t score values for each regional peak.
Medication Effects on Network Task Engagement
Table 1 lists mean β-weights from one-sample t tests that test whether or not each component was engaged during Sternberg task conditions. Components B and C were engaged during Encoding/Maintenance trials for both placebo (β=-0.47, p=0.01; β=-0.41, p=0.009) and medication (β=-0.40, p=0.02; β=-0.26, p=0.08, i.e., “trend”) sessions. Component A was engaged during Encoding/Maintenance only when participants were medicated (β=0.31, p=0.009). A similar picture of more networks being engaged during medication sessions emerged for the Retrieval condition as well. Components B (β=0.54, p=0.005) and F (β=-0.36, p=0.02) and C (β=-0.23, p=0.09, trend) were engaged during placebo Retrieval trials. However, when participants were medicated, Components B-E were engaged for Retrieval (β=0.67, p=0.0003; β=0.22, p=0.04; β=0.20, p=0.02; β=0.22, p=0.03; A at a trend level; β=0.20, p=0.06). Only Component F showed no evidence for task-induced change in activity amplitude.
Table 1.
The association of ICA-estimated BOLD signal timecourse with the SPM model of hemodynamic response to Encoding/Maintenance and Retrieval phases of the fMRI Sternberg working memory paradigm in AD/HD adolescents during Placebo and standard Medication study conditions. Columns list average β-weights and significance levels.
| Encoding/Maintenance | Retrieval | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Placebo | Medication | Placebo | Medication | |||||
|
| ||||||||
| Mean β | p | Mean β | p | Mean β | p | Mean β | p | |
| Component A | -0.13 | ns | 0.31 | .009 | 0.10 | ns | 0.20 | .06 |
| Component B | -0.47 | .01 | -0.40 | .02 | 0.54 | .005 | 0.67 | .00003 |
| Component C | -0.41 | .009 | -0.26 | .08 | -0.23 | .09 | 0.22 | .04 |
| Component D | -0.02 | ns | 0.02 | ns | 0.26 | ns | 0.20 | .02 |
| Component E | -0.09 | ns | 0.23 | ns | -0.27 | ns | 0.22 | .03 |
| Component F | -0.10 | ns | 0.16 | ns | -0.36 | .02 | -0.10 | ns |
Repeated-measures t tests of the hypothesis that networks would be more greatly engaged when AD/HD participants were medicated found activity in Component A significantly increased during Encoding/Maintenance during medicated sessions (mean β change=-0.13 to 0.31). Two components increased engagement between placebo and medication sessions during Retrieval: Component C (mean β change=-0.23 to 0.22) and Component E (mean β change=-0.27 to 0.22). Figure 1 illustrates these results with component timecourse averages over Encoding/Maintenance or Retrieval trials. Component A failed to engage for Encoding/Maintenance (and actually reverses engagement to ‘deactivate’) during placebo, but shows a typical event-related response to Encoding/Maintenance trials when AD/HD participants are medicated. Component C did not engage the network during the expected peak of activation to Retrieval trials during placebo, but medication produced a more consistent profile of BOLD signal change to task. Component E shows a sharp spike of activation at the end of Retrieval trials on-medication.
Figure 1.
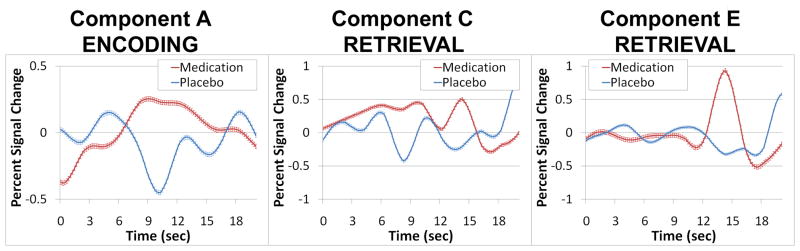
Significant differences in the amplitude of BOLD signal change during Encoding/Maintenance or Retrieval trials of the Sternberg fMRI task between medication and placebo conditions. These activity increases following medication survive corrections for multiple comparisons using False Discovery Rate (q<.05).
Medication Effects of Regional Functional Connectivity
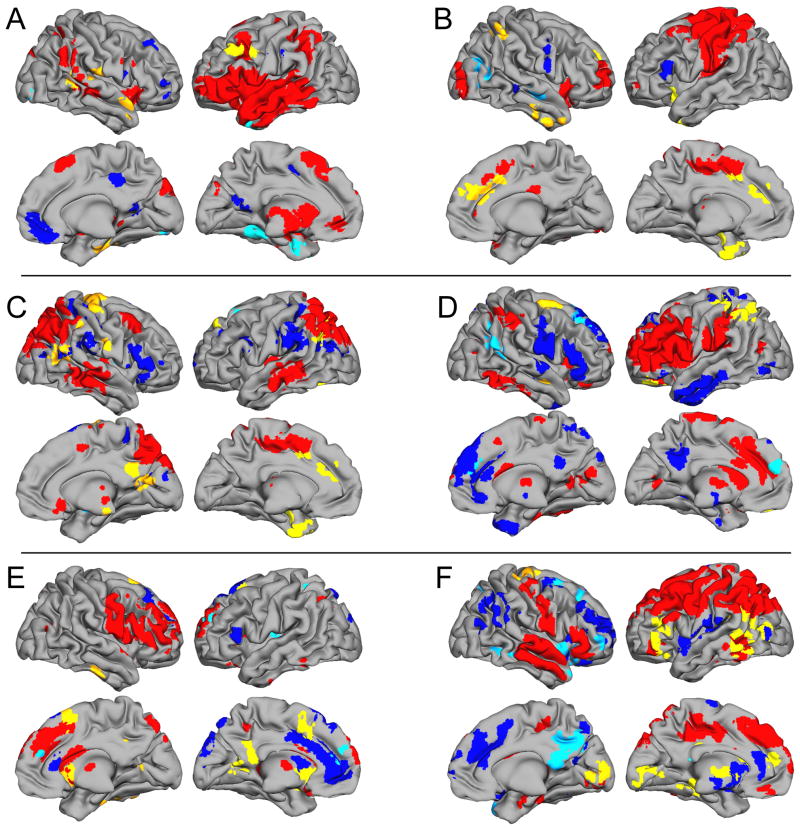
Results of 6 SPM5 repeated-measures t tests on spatial maps representing regional functional connectivity are listed in Table 2 and depicted in Figure 2. Table 2 lists all clusterwise differences among medication versus placebo fMRI sessions for each component. The left column shows Medication > Placebo, while the right shows Medication < Placebo. t statistics surviving clusterwise multiple comparisons are noted. The strongest evidence for changes in regional functional connectivity (clusterwise-corrected) was found for regions that were not part of the networks when on placebo, but became significantly integrated while medicated. Across the different networks, “newly-engaged” structures that survived multiple comparisons corrections include different postcentral gyrus regions (Components A and C), anterior cingulate (Component B), precuneus/posterior cingulate (Component C), medial frontal gyrus (Component E), and cuneus/lingual gyri (Component F). In contrast, only two components showed functional connectivity modulation of already-engaged frontal or parietal lobe regions (D-inferior parietal lobule and F-inferior frontal gyrus). If uncorrected significance levels are considered, the evidence for regional connectivity changes is far more extensive. Nearly every component showed additional recruitment of brain regions not traditionally thought of as part of frontoparietal working memory networks. All these effects ranged in size from d=1.97 to 3.13, representing considerably large medication effects (55).
Table 2.
Results of exploratory analyses to determine how regularly-prescribed psychostimulant medication affects AD/HD regional functional connectivity strength within each network. For each network, regions where Medication > Placebo and Medication < Placebo are listed, along with peak regional x, y, z coordinates. All regions reported were significant at an uncorrected p < .05 clusterwise level of significance (voxelwise entry-level threshold uncorrected p < .01). For each region, exploratory evidence for significant correlation (voxelwise p<.05 uncorrected) between medication-related improvement in target identification reaction time during Sternberg Retrieval and functional connectivity in an 8mm radius sphere around each voxel of peak medication versus placebo difference is listed. Reported values include the t score and p value. Negative t scores for brain regions where Medication > Placebo indicate that improvements were related to enhanced functional connectivity, whereas positive t scores reflect the same relationship for brain regions where regional connectivity for Medication < Placebo.
| Medication > Placebo | Medication < Placebo | ||||||
|---|---|---|---|---|---|---|---|
| Component A) | Peak x, y, z | t | Target RT Correlation t; p | Peak x, y, z | t | Target RT Correlation t; p | |
| Left middle/inferior frontal gyri (BA 9) | -51, 24 ,39 | 6.45 | -2.99; .004 | Posterior cingulate/precuneus | 0,-63,15 | 3.67 | ns |
| Right postcentral gyrus (BA 43) | 54,-15,15 | 4.43* | -2.13; .024 | Left parahippocampal gyrus/hippocampus | -27,-3,30 | 4.47 | -2.77; .007 |
| Right fusiform/parahippocampal gyri | 36,-24,-27 | 4.43 | -1.86; .040 | Left fusiform/ parahippocampal gyri | -33,-45,-9 | 4.26 | 1.78, .046 |
| Right superior/middle temporal gyri (BA 38/21) | 63,0,12 | 3.32 | 1.82; .043 | Right middle/inferior occipital gyri | 30,-90,-6 | 4.17 | 2.32; .017 |
| Right superior/transverse temporal gyri (BA 41/22) | 66,24,15 | 3.24 | ns | ||||
| Right anterior cerebellum | 18,-33,-33 | 4.71 | ns | ||||
| Component B) | |||||||
| Left inferior frontal gyrus/insula (BA 47/13) | -30,15,-12 | 6.30 | ns | Right middle/superior temporal gyri | 54,-12,-9 | 4.88 | 2.11; .025 |
| Anterior cingulate/medial frontal gyri (BA 24/32) | 0,39,30 | 5.04* | -3.39; .002 | Right middle temporal/occipital gyri | 42,-75,24 | 4.00 | -1.81; .044 |
| Right inferior parietal lobule (BA 40) | 48,-45,51 | 3.93 | -2.04; .028 | ||||
| Left uncus/fusiform gyrus | -21,0,-36 | 4.77 | ns | ||||
| Inferior temporal/fusiform gyri (BA 20) | 51,12,-39 | 4.55 | ns | ||||
| Left parahippocampal gyrus/amygdala | -27,-9,-12 | 3.93 | ns | ||||
| Component C) | |||||||
| Right inferior parietal lobule/supramarginal gyrus | 33,-24,36 | 4.84 | -3.37; .002 | Left superior frontal gyrus (BA 8/6) | -18,21-54 | 3.88 | ns |
| Medial frontal gyrus (BA 6/8) | -9,39,42 | 4.75 | ns | Right putamen | 30,3,0 | 3.77 | ns |
| Right precentral/postcentral gyri | 39,24,69 | 5.77* | ns | ||||
| Left angular gyrus (BA 39) | -48,-66,30 | 4.91 | -1.80; .045 | ||||
| Precuneus/posterior cingulate | 9,-63,12 | 4.34* | -1.96; .033 | ||||
| Left precuneus | -15,-84,36 | 3.64 | -2.30; .017 | ||||
| Right superior/middle temporal gyri (post) | 54,-60,21 | 5.34 | -1.89; .038 | ||||
| Component D) | |||||||
| Left superior/middle frontal gyri (BA 11/47) | -18,42,-18 | 5.23 | ns | Right superior/middle frontal gyri (BA 8/9) | 24,42,39 | 4.32 | -3.82; .0008 |
| Right middle frontal gyrus | 30,-6,66 | 3.67 | 2.39; .015 | Medial frontal gyrus/anterior cingulate | 0,54,24 | 3.74 | -2.21; .021 |
| Left inferior parietal lobule/postcentral gyrus | -51,-45,51 | 4.16* | ns | Left angular/supramarginal gyri (BA 39) | 48,-57,18 | 5.74 | ns |
| Right superior temporal gyrus | 45,0,-18 | 3.94 | ns | ||||
| Right superior frontal gyrus | 27,36,15 | 3.75 | ns | ||||
| Component E) | |||||||
| Cingulate/superior frontal/medial frontal gyrus (BA 6) | 12,18,66 | 4.67* | ns | ||||
| Posterior cingulate/precuneus | -3,-51,33 | 3.76 | ns | Left superior frontal gyrus (BA 10) | -21,54,30 | 4.69 | 2.15; .023 |
| Left posterior cingulate | -21,-57,0 | 3.53 | 2.94; .005 | Medial frontal gyrus (BA 9) | -3,45,18 | 3.77 | 2.59; .010 |
| Right inferior temporal/fusiform gyri | 42,-18,-30 | 3.37 | ns | Left insula (BA 13) | -36,-12,15 | 3.55 | 3.82; .0008 |
| Right caudate | 18,6,3 | 4.40 | 3.21; .0007 | Left precuneus/superior parietal lobule | -21,-51,51 | 3.97 | 1.73; .050 |
| Left caudate | -15,18,-6 | 4.26 | 1.82; .043 | ||||
| Right cerebellum | 45,-48,-30 | 4.92 | -2.49; .012 | ||||
| Left cerebellum | -12,-90,-27 | 4.78 | 2.30; .017 | ||||
| Anterior cerebellum | 6,-51,-24 | 4.07 | ns | ||||
| Component F) | |||||||
| Left inferior frontal gyrus (BA 46/47) | -39,36,-15 | 5.37* | -1.68; .054 | Right superior frontal gyrus | 15,33,45 | 5.36 | 1.98; .032 |
| Left supramarginal/angular/middle temporal gyri | -54,-48,3 | 5.53* | ns | Right middle/inferior frontal gyri (BA 10) | 42,57,3 | 4.68 | -1.95; .034 |
| Medial frontal gyrus | -12,48,15 | 4.99 | -1.79; .045 | Right middle/superior frontal gyri (BA 6) | 21,3,63 | 4.60 | 2.20; .021 |
| Right precentral/postcentral gyri | 27,-15,72 | 4.16 | -5.11; .00005 | Right insula/inferior frontal gyrus/superior temporal gyrus (BA 47/38) | 42,15,-9 | 3.97 | 1.75; .049 |
| Cingulate gyrus | -9,-30,-27 | 3.98 | 3.47; .002 | Right insula/superior temporal gyrus | 48,-9,3 | 3.33 | ns |
| Left parahippocampus/hippocampus | -24,-30,-6 | 4.95 | -2.09; .026 | Precuneus/posterior cingulate | 9,-69,36 | 5.38* | ns |
| Cuneus/lingual gyri | -12,-90,6 | 5.71* | -2.59; .010 | Right middle temporal gyrus (BA 37) | 51,-51,-9 | 3.54 | ns |
| Thalamus | -6,-3,3 | 3.65 | -2.22; .021 | ||||
AD/HD brain regions that had differences between medicated and unmedicated states in strength of functional connectivity after correcting for clusterwise multiple comparisons (p < .05).
Figure 2.
Inflated brain renderings showing network structure for each frontoparietal component and effects of medication on brain functional connectivity. Areas in red and blue show regions of positive- or negative-going BOLD signal change within each functionally-connected network (thresholded at False Discovery Rate q<.001). Other colors depict all regional effects of psychostimulant medication on functional connectivity (p<.05 clusterwise uncorrected). Yellow represents Medication > Placebo while turquoise shows Medication < Placebo.
Behavioral Data
None of the various non-working memory neuropsychological tasks showed improvement with stimulant treatment (Supplemental Table 1). All these performances were in the average normative range.
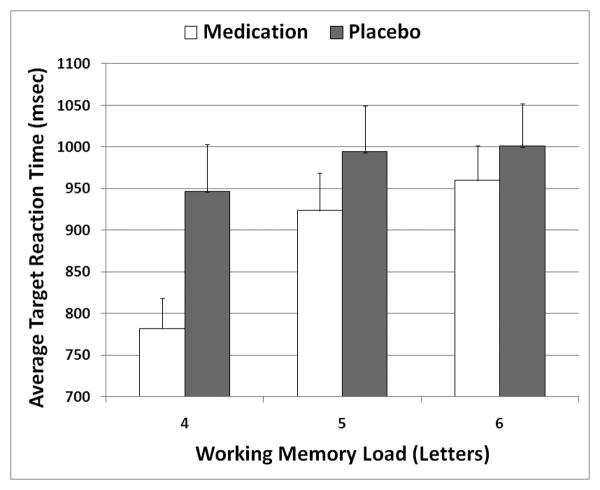
Increasing load increased target reaction time (F2,34=8.571, p=.002) and foil reaction time (F2,34=7.077, p=.003), but accuracy differences were only significant at a trend level (F2,34=2.978, p=.065). Supplemental correlations explored possible speed-accuracy tradeoff effects, but failed to find relationships between medication-related reaction time and accuracy changes at any load. Only target reaction time showed the expected pattern of consistently linearly-increasing response time with more difficulty (Figure 3). Accuracy and foil reaction time had a mixed relationship with task difficulty (Supplemental Table 1). Medication reduced target (F1,17=4.437, p=.050) and foil reaction times (F1,17=8.123, p=.011), in each case improving performance following medication. Task load × medication interaction tests showed there was a significant effect only for target reaction time (F2,34=4.101, p=.027), with largest gains at the lowest load.
Figure 3.
Effects of working memory load and psychostimulant medication on reaction time to target stimuli during Retrieval trials of the Sternberg fMRI task.
Of brain regions having significant relationships with reaction time (Table 2), exploratory analyses of the relationship between target reaction time change and functional connectivity change found that nearly all associations were linked to reductions in how long AD/HD participants mentally searched working memory storage. Only Component D (whose activation was not affected by medication) showed the opposite pattern. Target reaction time was related to several regions where evidence for medication-induced connectivity changes survived clusterwise corrections (Component A–right postcentral gyrus; Component B–anterior cingulate; Component C–precuneus/posterior cingulate; Component F–left inferior frontal gyrus and cuneus/lingual). However, associations between connectivity and reaction time were found for most frontal and parietal lobe regions (Components A-F), and the caudate and cerebellum (Component E), with large effect sizes ranging from d=0.84-2.55, average=1.21.
Discussion
This study shows that regularly-prescribed, clinically-effective stimulant medications alter AD/HD brain activity during a Sternberg working memory fMRI task by increasing the magnitude of some frontoparietal networks' activity and changing regional functional connectivity across the brain, not just between frontal and parietal lobe structures. Improved target identification reaction time was significantly associated with many medication-induced regional functional connectivity changes. The value in examining whole brain connectivity of several distinct frontoparietal networks is the potential to make conclusions about systems-level effects of psychostimulant medications on AD/HD brain function. Although some evidence was found indicating that medications enhanced functional connectivity between working memory-specialized frontal and parietal lobe regions, the majority of evidence indicated that medications increased functional connectivity of key frontoparietal networks with other brain structures. We saw several examples where brain regions that did not appear to be integrated into working memory circuits during placebo were engaged when participants took their medications. Insofar as increased connectivity reflects enhanced inter-regional communication, this indicates that the net effect of psychostimulants is to facilitate neurotransmission through long-distance connections between widespread brain regions. Many of these putatively “re-connected” brain regions (e.g., anterior cingulate, lateral prefrontal cortex, caudate and cerebellum) have been shown in previous fMRI/PET studies to be dysfunctional in AD/HD (34, 35). Although the predominant effect of medications was to increase connectivity, evidence for connectivity decreases also was found. Some of these “reductions” (Table 2; Figure 2) actually indicate greater connectivity (i.e., more negative connectivity values for “deactivating” regions). However, others might reflect shifting of regional engagement between networks (e.g., anterior cingulate from Component E to C, posterior cingulate from Component F to C, temporal cortex from Component A to B). Future research should seek to validate such medication-induced shifts and link them to clinical correlates.
Consistent with previous reports (40, 41), stimulant medication did not improve Sternberg behavioral accuracy, but AD/HD participants had faster response times while medicated indicating decreased working memory storage “search” time. The greatest behavioral effect was at the lowest load, whereas medicated performance at the highest load resembled unmedicated performance. These gains could not be simply due to motor response facilitation which would have produced equivalent gains at every load. Functional connectivity within nearly two-thirds of the brain regions whose connectivity was modulated by psychostimulants was directly related to improved target probe identification time, supporting the role of widespread AD/HD brain functional connectivity in working memory.
The left dorso/ventrolateral prefrontal/parietal network (Component A) was not significantly active during placebo, but increased activity during Encoding/Maintenance following medication (Figure 2). Reduced ventrolateral prefrontal activation was found in two previous adult AD/HD fMRI working memory studies (10, 12) and in AD/HD children (14). Stimulant treatment increases ventrolateral prefrontal cortex activation in AD/HD on other paradigms (33, 56-58), with evidence for a predominant left-lateralized effect (59). Given this frequent finding, left ventrolateral prefrontal cortex might represent a general target of psychostimulant effects, particularly as evidence links treatment to normalized AD/HD cortical thickness in this area (60). Both methylphenidate and amphetamine increase extracellular catecholamine availability (61, 62), and prefrontal cortex activity can be modulated by dopamine and norepinephrine through D1/D5 or α2A receptor abnormalities or synaptic neurotransmitter levels (6, 63-67). In our study, medication increased the integration of left dorso/ventrolateral prefrontal cortex with left inferior parietal lobe regions (Components D and F). Although 83% of the sample took methylphenidate, inclusion of three amphetamine-treated patients limits inferences about cellular mechanisms, which differ by drug at typical therapeutic levels (presynaptic dopamine transporter (DAT) and norepinephrine transporter (NET) blockade versus dopamine release stimulation) (61, 62). It would be fruitful to learn which specific cellular mechanisms mediate each stimulant's effects on prefrontal connectivity in AD/HD, as this might be tied to genetic profile.
Burgess et al. (68) found better working memory performance was correlated with increased activation in inferior parietal lobule, temporal and frontal cortex, and the motor areas in AD/HD. Our study shows that medications not only increased activity, but also functional connectivity in many of those areas. Ventrolateral/parietal network engagement (activity) increased during both Encoding/Maintenance and Retrieval (Figure 1; Component A), along with specific increasing of regional functional connectivity in dorsolateral prefrontal cortex (Figure 2a). Timecourse analysis of Component E (Figure 1) indicates that medication increased engagement of this right dorso/ventrolateral prefrontal/putamen network near or after the termination of Retrieval trials, suggesting a role in task disengagement or preparation for the next Encoding trial. Finally, bilateral dorsal anterior cingulate was recruited into Component B. The importance of cingulate dysfunction in AD/HD has often been studied (69, 70). Our findings suggest that better functional integration of this area could be facilitating comparisons of probe stimuli to working memory representations through the cingulate's role in conflict detection and performance monitoring/adjustment (71, 72).
We found evidence for medication-induced reductions in prefrontal cortex functional connectivity with other brain regions, some of which overlapped those reported by Sheridan et al. (22). Discrepancies might be related to that study's fMRI paradigm choice, its smaller sample size, or the presence of other psychotropic medication use or unreported psychiatric comorbidity in their sample. Alternatively, ICA whole brain analysis allowed us to avoid averaging across bilateral dorsolateral prefrontal regions-of-interest as done by Sheridan et al. (22). The lack of prominent striatal connectivity changes might reflect the fact this was a working memory, not motor inhibition paradigm. There is some evidence that indicates long-term stimulant treatment might affect brain structure (60, 73, 74). As all our participants had medication histories and served as their own controls, this issue was not a major limitation but results should be confirmed in medication-naïve AD/HD adolescents. However, it is unlikely that fMRI-measured connectivity changed due to medication effects across the relatively short ∼6-week test-retest interval. Study strengths include rigorous frontoparietal functional connectivity assessment, placebo-control design, 48-hour medication washout, and sampling restriction to AD/HD-Combined subtype without significant psychiatric comorbidities.
In conclusion, psychostimulant doses that result in clinical benefit alter regional brain functional connectivity during working memory in frontoparietal networks. The study supports the possibility that therapeutic effects of psychostimulant medications are achieved by re-engaging brain regions that normally participate in functionally-specialized networks (24-27), particularly those known to be impaired in AD/HD (34, 35). Given that some of the targets of medications observed in this study coincide with areas of deficits in AD/HD reported in previous research, future studies should confirm medications are acting to normalize working memory activation and connectivity deficits in AD/HD to levels comparable to healthy controls (10-12).
Supplementary Material
Acknowledgments
This study was funded by a grant from Hartford Hospital (PI Stevens) and was supported in part by K23 MH070036 (PI Stevens) and R01 MH080956 (PI Stevens). Appreciation is extended to Sandra Navarro who aided data collection.
Footnotes
Financial Disclosures: Ms. Wong and Dr. Stevens have no biomedical financial interests or potential conflicts of interest.
References
- 1.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 2.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 3.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 5.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- 7.Ellis KA, Nathan PJ. The pharmacology of human working memory. Int J Neuropsychopharmacol. 2001;4:299–313. doi: 10.1017/S1461145701002541. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 9.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valera EM, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, et al. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry. 167:86–94. doi: 10.1176/appi.ajp.2009.09020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlis AC, Bahne CG, Jacob CP, Herrmann MJ, Fallgatter AJ. Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: a functional near-infrared spectroscopy (fNIRS) study. J Psychiatr Res. 2008;42:1060–1067. doi: 10.1016/j.jpsychires.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Wolf RC, Plichta MM, Sambataro F, Fallgatter AJ, Jacob C, Lesch KP, et al. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:2252–2266. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheridan MA, Hinshaw S, D'Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- 14.Kobel M, Bechtel N, Weber P, Specht K, Klarhofer M, Scheffler K, et al. Effects of methylphenidate on working memory functioning in children with attention deficit/hyperactivity disorder. Eur J Paediatr Neurol. 2009;13:516–523. doi: 10.1016/j.ejpn.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer SV, Hawk LW, Jr, Richards JB, Shiels K, Pelham WE, Jr, Waxmonsky JG. Stimulant treatment reduces lapses in attention among children with ADHD: the effects of methylphenidate on intra-individual response time distributions. J Abnorm Child Psychol. 2009;37:805–816. doi: 10.1007/s10802-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2006;30:1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Ernst M, Zametkin AJ, Matochik JA, Liebenauer L, Fitzgerald GA, Cohen RM. Effects of intravenous dextroamphetamine on brain metabolism in adults with attention-deficit hyperactivity disorder (ADHD). Preliminary findings. Psychopharmacol Bull. 1994;30:219–225. [PubMed] [Google Scholar]
- 19.Matochik JA, Nordahl TE, Gross M, Semple WE, King AC, Cohen RM, et al. Effects of acute stimulant medication on cerebral metabolism in adults with hyperactivity. Neuropsychopharmacology. 1993;8:377–386. doi: 10.1038/npp.1993.38. [DOI] [PubMed] [Google Scholar]
- 20.Schweitzer JB, Lee DO, Hanford RB, Tagamets MA, Hoffman JM, Grafton ST, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28:967–973. doi: 10.1038/sj.npp.1300110. [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer JB, Lee DO, Hanford RB, Zink CF, Ely TD, Tagamets MA, et al. Effect of methylphenidate on executive functioning in adults with attention-deficit/hyperactivity disorder: normalization of behavior but not related brain activity. Biol Psychiatry. 2004;56:597–606. doi: 10.1016/j.biopsych.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan MA, Hinshaw S, D'Esposito M. Stimulant medication and prefrontal functional connectivity during working memory in ADHD: a preliminary report. J Atten Disord. 2010;14:69–78. doi: 10.1177/1087054709347444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friston K. Beyond phrenology: what can neuroimaging tell us about distributed circuitry? Annu Rev Neurosci. 2002;25:221–250. doi: 10.1146/annurev.neuro.25.112701.142846. [DOI] [PubMed] [Google Scholar]
- 24.Gazzaley A, Rissman J, D'Esposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- 25.Babiloni C, Babiloni F, Carducci F, Cincotti F, Vecchio F, Cola B, et al. Functional frontoparietal connectivity during short-term memory as revealed by high-resolution EEG coherence analysis. Behav Neurosci. 2004;118:687–697. doi: 10.1037/0735-7044.118.4.687. [DOI] [PubMed] [Google Scholar]
- 26.Curtis CE, Sun FT, Miller LM, D'Esposito M. Coherence between fMRI time-series distinguishes two spatial working memory networks. Neuroimage. 2005;26:177–183. doi: 10.1016/j.neuroimage.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Miller BT, Deouell LY, Dam C, Knight RT, D'Esposito M. Spatio-temporal dynamics of neural mechanisms underlying component operations in working memory. Brain Res. 2008;1206:61–75. doi: 10.1016/j.brainres.2008.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 29.D'Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37:1303–1315. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 30.D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- 31.Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. Neuroimage. 2007;35:904–915. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 35.Cherkasova MV, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: Beyond the frontostriatal circuitry. Candian Journal of Psychiatry. 2009;54:651–661. doi: 10.1177/070674370905401002. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 38.Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD. Methylphenidate reduces abnormalities of stimulus classification in adolescents with attention deficit disorder. J Abnorm Psychol. 1992;101:130–138. doi: 10.1037//0021-843x.101.1.130. [DOI] [PubMed] [Google Scholar]
- 39.Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD, Strauss J. Clinical and cognitive effects of methylphenidate on children with attention deficit disorder as a function of aggression/oppositionality and age. J Abnorm Psychol. 1994;103:206–221. doi: 10.1037//0021-843x.103.2.206. [DOI] [PubMed] [Google Scholar]
- 40.Krusch DA, Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD, Strauss J. Methylphenidate slows reactions of children with attention deficit disorder during and after an error. J Abnorm Child Psychol. 1996;24:633–650. doi: 10.1007/BF01670104. [DOI] [PubMed] [Google Scholar]
- 41.Conners CK, Casat CD, Gualtieri CT, Weller E, Reader M, Reiss A, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996;35:1314–1321. doi: 10.1097/00004583-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Johnson MR, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, et al. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry. 2006;60:11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- 44.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmithorst VJ, Holland SK. Comparison of three methods for generating group statistical inferences from independent component analysis of functional magnetic resonance imaging data. J Magn Reson Imaging. 2004;19:365–368. doi: 10.1002/jmri.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 47.Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adlali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping. 2010 doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28:1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Hum Brain Mapp. 2007;28:394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Brain network dynamics during error commission. Hum Brain Mapp. 2009;30:24–37. doi: 10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 53.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 54.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 55.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 1990. [Google Scholar]
- 56.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sci. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- 59.Rubia K, Halari R, Mohammad AM, Taylor E, Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70:255–262. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, et al. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bidwell LC, McClernon FJ, Kollins SH. Cognitive enhancers for the treatment of ADHD. Pharmacol Biochem Behav. 99:262–274. doi: 10.1016/j.pbb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 63.Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, et al. Differential Sensitivity to Psychostimulants Across Prefrontal Cognitive Tasks: Differential Involvement of Noradrenergic alpha(1)- and alpha(2)-Receptors. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e101–111. doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64:626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilens TE. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2008;28:S46–53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- 67.Prince J. Catecholamine dysfunction in attention-deficit/hyperactivity disorder: an update. J Clin Psychopharmacol. 2008;28:S39–45. doi: 10.1097/JCP.0b013e318174f92a. [DOI] [PubMed] [Google Scholar]
- 68.Burgess GC, Depue BE, Ruzic L, Willcutt EG, Du YP, Banich MT. Attentional control activation relates to working memory in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67:632–640. doi: 10.1016/j.biopsych.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 70.Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, et al. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- 71.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 72.Ridderinkhof RK, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 73.Sobel LJ, Bansal R, Maia TV, Sanchez J, Mazzone L, Durkin K, et al. Basal ganglia surface morphology and the effects of stimulant medications in youth with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167:977–986. doi: 10.1176/appi.ajp.2010.09091259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bledsoe J, Semrud-Clikeman M, Pliszka SR. A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naive children with attention-deficit/hyperactivity disorder combined type. Biol Psychiatry. 2009;65:620–624. doi: 10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.