Abstract
Decitabine improves overall survival in myelodysplastic syndromes (MDS). This retrospective analysis of 2 decitabine studies (N=183) compared patients with baseline marrow blasts ≥20% (RAEB-t group, acute myeloid leukemia [AML] by WHO criteria) and <20% (MDS group). There was no significant difference in overall response between RAEB-t (n=26) and MDS patients (n=157). Patients with RAEB-t MDS may benefit from decitabine therapy.
Background
After the World Health Organization’s definition of acute myeloid leukemia changed to ≥20% blasts, International Working Group (IWG) response criteria for myelodysplasia were updated. This retrospective analysis evaluated response to decitabine using updated IWG criteria in patients pooled from 2 decitabine trials.
Materials and Methods
Outcomes for patients with myelodysplastic syndromes (MDS) with baseline marrow blasts ≥20% and <30% (RAEB-t group) and <20% (MDS group) were compared.
Results
RAEB-t patients (n=26) had significantly shorter time from diagnosis (7.3 vs 18.3 months), higher International Prognostic Scoring System risk (77% vs 16% high-risk patients), and lower median baseline platelet count (62.3 vs 112.7 ×103/μL) versus MDS patients (n=157), yet no significant difference in overall response rates (15.4% vs 28.0%). MDS patients had better duration of response (9.9 vs 5 months; P = .024) and overall survival (16.6 vs 9.0 months, P = .021) compared with RAEB-t patients.
Conclusion
Decitabine is active in and may benefit patients with >20% blasts (RAEB-t).
Keywords: acute myeloid leukemia, myelodysplastic syndromes, survival, RAEB-t, FAB classification
INTRODUCTION
Myelodysplastic syndromes (MDS) have 5 French-American-British (FAB) classification system subtypes: refractory anemia (RA; <5% bone marrow blasts); RA with ringed sideroblasts (RARS; <5% blasts plus >15% ringed sideroblasts); RA with excess of blasts (RAEB; 5%–20% blasts); RAEB in transformation (RAEB-t; 21%–30% blasts); and chronic myelomonocytic leukemia (CMML; 5%–20% blasts plus >1 × 103 monocytes/μL blood).1
Acute myeloid leukemia (AML) may be de novo or result from progression of MDS.2 According to FAB classification, patients with >30% blasts have AML,1 but the World Health Organization (WHO) considers ≥20% blasts as AML.3 The International Working Group (IWG) response criteria for myelodysplasia, updated in 2006, define AML using the WHO classification.4 The National Comprehensive Cancer Network (NCCN) endorses both FAB and WHO systems; thus, RAEB-t may be diagnosed as MDS or AML.1 The IWG asserts that patients with RAEB-t may benefit from treatment with low-intensity or MDS-specific protocols if they meet study inclusion criteria.4
The DNA hypomethylating agent decitabine is indicated for treatment of MDS, including previously treated and untreated de novo and secondary MDS of all FAB subtypes and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System (IPSS) groups.5 Although patients with RAEB-t have responded to decitabine,6,7 responses may differ from those of patients with MDS with <20% marrow blasts, as patients with RAEB-t have poorer prognoses.1
This analysis compared baseline characteristics, overall response rates (ORR), and overall survival (OS) in decitabine-treated patients previously characterized as having MDS with baseline marrow blasts ≥20% (RAEB-t MDS), now classified as AML, versus patients with baseline marrow blasts <20% to determine the activity of decitabine in this RAEB-t/AML patient population.
MATERIALS AND METHODS
Study design
This was a retrospective analysis of data pooled from 2 multicenter trials of decitabine. Study D-0007 (NCT00043381) was a phase III, open-label, randomized comparison of supportive care with or without decitabine in patients aged ≥18 years with de novo or secondary intermediate-risk or high-risk MDS, including chronic myelomonocytic leukemia, with white blood cell count <12,000/μL and IPSS score >0.5. Decitabine 15 mg/m2 was given as an intravenous (IV) infusion every 8 hours for 3 consecutive days, every 6 weeks. The original analysis evaluated decitabine responses with the 2000 IWG response criteria.6 Study DACO-020 (NCT00260065) was a phase II, open-label, nonrandomized, single-arm study in patients aged ≥18 years with de novo or secondary MDS of any FAB subtype. Decitabine 20 mg/m2 was given as an IV infusion daily for 5 consecutive days, every 4 weeks. The original analysis used 2000 and 2006 IWG response criteria.7
Patients
Inclusion criteria were common to both trials and included: Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2. Excluded patients had AML (defined as >30% blasts) or other malignancy; had received any investigational agent within 30 days prior to study; or had concurrent autoimmune hemolytic anemia, thrombocytopenia, or infection.
This pooled analysis comprised patients from each trial who received ≥1 dose of decitabine and had available baseline bone marrow blast data. Patients were stratified by baseline marrow blasts: ≥20% and <30% (RAEB-t) or <20% (MDS).
Study evaluations
Response categories were evaluated using IWG 2006 criteria: ORR (complete response [CR] + partial response [PR] + marrow CR [mCR]); hematologic improvement (HI); overall improvement rate (OIR; CR + mCR + PR + HI); stable disease (SD) rate; and progressive disease (PD) rate. Overall survival and tolerability were also assessed.
Statistical analysis
Baseline characteristics were compared between groups using Fisher’s exact test for categorical variables and t test for continuous variables. ORR, OIR, and clinical responses were compared between groups with Fisher’s exact test. Using the log-rank test, OS, time to initial improvement, time to initial response, duration of improvement, and duration of response were compared between groups. Logistic regression was performed to assess the relationship between baseline marrow blast category and treatment response.
RESULTS
This retrospective analysis included 183 patients—85 from Study D-0007 and 98 from Study DACO-020. Demographic and baseline characteristics of the pooled population, categorized as either RAEB-t or MDS by baseline marrow blasts, were similar between groups, with exceptions of time from diagnosis, IPSS risk, and median platelet count (Table I). RAEB-t patients (n=26) had a significantly shorter time from diagnosis (7.3 vs 18.3 months; P = .0002), higher IPSS risk (77% vs 16% high-risk patients), and lower median baseline platelet count (62.3 vs 112.7 ×103/μL) versus MDS patients (n=157). The median number of cycles of decitabine administered was 3 for Study D-0007 and 5 for Study DACO-020.
Table I.
Demographic and baseline characteristics of decitabine-treated patients: pooled analysis
| Characteristic | RAEB-t* (n=26) | MDS*† (n=157) | P value |
|---|---|---|---|
| Mean age, y (SD) | 72 (9) | 70 (9) | NS |
|
| |||
| Sex, n (%) | NS | ||
| Female | 6 (23) | 50 (32) | |
| Male | 20 (77) | 107 (68) | |
|
| |||
| FAB classification, n (%) | <.0001 | ||
| CMML | — | 16 (10) | |
| RA | — | 30 (19) | |
| RAEB | 6 (23) | 84 (54) | |
| RAEB-t | 20 (77) | 3 (2) | |
| RARS | — | 24 (15) | |
|
| |||
| Marrow blast 20%–30%, n (%)‡ | 23 (89) | — | <.0001 |
|
| |||
| Marrow blast >30%, n (%)§ | 3 (12) | — | .003 |
|
| |||
| Median marrow blasts, % (range) | 24.0 (20–60) | 6.5 (0–19) | <.0001 |
|
| |||
| IPSS cytogenetic status, n (%) | NS | ||
| Good | 13 (50) | 77 (49) | |
| Intermediate | 2 (8) | 23 (15) | |
| Poor | 10 (39) | 46 (29) | |
| Unknown | 1 (4) | 11 (7) | |
|
| |||
| IPSS classification, n (%) | <.0001 | ||
| High risk | 20 (77) | 25 (16) | |
| Intermediate-2 | 5 (19) | 53 (34) | |
| Intermediate-1 | 1 (4) | 78 (50) | |
| Low risk | — | 1 (1) | |
|
| |||
| Mean time from diagnosis, months (SD) | 7.3 (9.5) | 18.3 (27) | .0002 |
|
| |||
| Type of MDS, n (%) | NS | ||
| De novo | 20 (77) | 141 (90) | |
| Secondary | 6 (23) | 16 (10) | |
|
| |||
| Mean white blood cells, 103/μL (SD) | 3.9 (8.4) | 4.2 (5.4) | NS |
|
| |||
| Mean hemoglobin, g/dL (SD) | 9.5 (1.4) | 10.6 (9.9) | NS |
|
| |||
| Mean platelets, 103/μL (SD) | 62.3 (52.8) | 112.7 (129.1) | .001 |
|
| |||
| Red blood cell transfusion status, n (%) | NS | ||
| Dependent | 17 (65) | 112 (71) | |
| Independent | 9 (35) | 45 (29) | |
|
| |||
| Platelet transfusion status, n (%) | NS | ||
| Dependent | 7 (27) | 26 (17) | |
| Independent | 19 (73) | 131 (83) | |
AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; FAB, French-American-British; MDS, myelodysplastic syndromes; NS, not significant; RA, refractory anemia; RAEB, refractory anemia with excess of blasts; RAEB-t, refractory anemia with excess of blasts in transformation; RARS, refractory anemia with ringed sideroblasts; SD, standard deviation.
Percentages may sum to >100% due to rounding.
MDS patient population includes all patients except those with RAEB-t.
Six patients who were categorized as RAEB by FAB classification were found to have marrow blasts ≥20%.
Three patients who were categorized as RAEB-t by FAB classification were found to have marrow blasts >30%. One patient was not classified as having AML by the investigator, and 2 patients had RAEB.
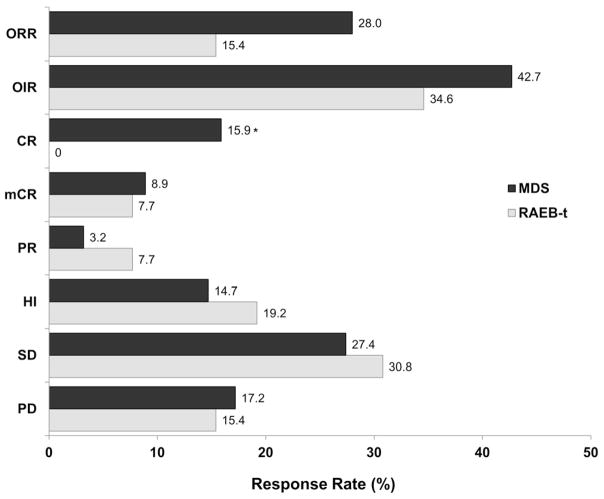
Despite the differences in patient profiles in each group, ORR, OIR, PR, mCR, HI, SD, and PD were not significantly different the RAEB-t and MDS groups (Figure 1). Only CR differed significantly between groups (P = .028). Among responders, time to initial improvement (CR, mCR, PR, or HI; RAEB-t: n=9, median 43 days; MDS: n=67, median 50 days; P = .348) and initial response (CR, PR, or mCR; RAEB-t: n=4, median 121 days; MDS: n=44, median 88 days; P = .122) did not differ significantly between groups. However, duration of improvement (RAEB-t: n=9, median 204 days; MDS: n=67, median 296 days; P = .006) and duration of response (RAEB-t: n=4, median 152 days; MDS: n=44, median 302 days; P = .024) were significantly longer in the MDS group. Time to disease progression did not differ significantly between groups (RAEB-t: n=23, median 392 days; MDS: n=157, median not reached). Median OS was significantly shorter for RAEB-t patients (n=26; 275 days; 95% CI: 157, 431) versus MDS patients (n=157; 506 days; 95% CI: 411, 596; P = .021).
Figure 1. Pooled analysis of decitabine response rates in patients with RAEB-t and in patients with myelodysplastic syndromes (MDS).
Significant differences in response rates between the RAEB-t and MDS groups were observed only for complete response (P = .028).
CR, complete response; HI, hematologic improvement; mCR, marrow complete response; OIR, overall improvement rate; ORR, overall response rate; PD, progressive disease; PR, partial response; RAEB-t, refractory anemia with excess of blasts in transformation; SD, stable disease.
RAEB-t was associated with a lower likelihood for achieving a response (CR, PR, or MCR). Using logistic regression analysis, the odds ratio for achieving a response was 0.467 (95% confidence interval: 0.152, 1.432) for patients in the RAEB-t group versus the MDS group.
The incidence of adverse events was similar in both groups. The most common treatment-related adverse events in each group were thrombocytopenia (RAEB-t, 62%; MDS, 44%), neutropenia (RAEB-t, 54%; MDS, 54%), and anemia (RAEB-t, 31%; MDS, 32%). The most common treatment-related serious adverse events occurring in >4% of patients in any group were febrile neutropenia (RAEB-t, 15%; MDS, 13%), pneumonia (RAEB-t, 12%; MDS, 7%), and neutropenia (RAEB-t, 4%; MDS, 7%).
DISCUSSION
Compared with patients with other forms of MDS, patients with RAEB-t had lower platelet counts and higher IPSS risk at baseline, suggesting they had more advanced disease, yet overall responses to decitabine treatment were not significantly different between the 2 groups. In higher-risk MDS patients who received 3 different decitabine regimens, CR ranged from 21% to 39% versus 15.9% in MDS patients in the pooled population.8 Difference in CR may be a function of differing dosage regimens between the 2 analyses. These data support those of another study in higher-risk MDS patients who received the same 3 decitabine regimens, in which CR was 35%.9 Furthermore, in a study investigating the use of hypomethylating agents (azacitidine or decitabine) as initial therapy in patients with AML or high-risk MDS, CR was 41%, again in a range similar to that of the data reported in our analysis,10 and in a recent phase III study of decitabine in older (≥65 years) patients with newly diagnosed AML, CR was 16%.11
In comparison, a retrospective subanalysis of phase III data from MDS patients evaluated survival with azacitidine (n=55) versus conventional care (best standard of care, low-dose cytarabine, or intensive chemotherapy; n=58).12,13 Patients originally classified as having RAEB-t (≥20% blasts) were retrospectively recoded as having AML, according to updated WHO criteria.13 Median OS was 25 months for azacitidine versus 16 months for conventional care (P = .005), but no survival advantage was found compared with low-dose cytarabine (median OS, 25 vs 17 months; P = .08). Median OS for patients receiving conventional care was unusually high, suggesting patient selection favoring improved survival.
The results of this analysis should be interpreted in the context of its limitations. These include its retrospective nature and the fact that it is a pooled analysis from 2 studies using different dosage regimens of decitabine. Moreover, the MDS subgroup was heterogeneous in that it comprised patients with a range of baseline marrow blasts (from 0% to 19%). Patients with such a range of blasts may have had different clinical characteristics and outcomes when considered separately, so it may not have been ideal to compare this heterogenous MDS group with the RAEB-t group. In addition, 3 patients (12%) with >30% blasts (AML) were included in the RAEB-t group, potentially adversely affecting outcomes.
CONCLUSION
RAEB-t patients demonstrated clinical responses to decitabine therapy similar to those of MDS patients, indicating that patients with RAEB-t MDS benefit from decitabine therapy and should not be excluded from treatment based on their marrow blast status. Treatment should be individualized according to patient characteristics.
CLINICAL PRACTICE POINTS.
Decitabine, a hypomethylating agent indicated for the treatment of myelodysplastic syndromes (MDS) in the US, improves overall survival and reduces the risk of progression to acute myeloid leukemia (AML) in patients with MDS.
After the World Health Organization (WHO) definition of AML changed to ≥20% baseline marrow blasts (French-American-British [FAB] classification RAEB in transformation [RAEB-t]), the International Working Group (IWG) response criteria for MDS were updated in 2006.
This retrospective analysis of 2 decitabine studies (N=183) compared patients with baseline marrow blasts ≥20% (RAEB-t group; AML by WHO criteria) and <20% (MDS group).
At baseline, RAEB-t patients (n=26) had significantly shorter time from diagnosis (7.3 vs 18.3 months), higher International Prognostic Scoring System risk (77% vs 16% high-risk patients), and lower median platelet count (62.3 vs 112.7 ×103/mL) versus MDS patients (n=157), yet no significant difference in overall response rates (15.4% vs 28.0%) was noted.
Thus, patients with RAEB-t MDS may benefit from decitabine therapy and should not be excluded from treatment based on their marrow blast status; treatment should be individualized according to patient characteristics.
Acknowledgments
The authors wish to thank Yvonne E. Yarker, PhD, CMPP, of Peloton Advantage for providing medical writing and editorial assistance during the development of this manuscript.
Footnotes
ClinicalTrials.gov Identifiers: NCT00043381 and NCT00260065
Scientific Section Designation: Clinical Trials and Observations
Contributions: E.J., J.C., and H.K. designed the study. E.J., J.C., F.R., A.M., G.G-M., H.K., and S.O’B. performed the research. G.G-M. and T.K. contributed vital new reagents or analytical tools. A.M. and H.K. collected data. A.T. performed statistical analysis. J.C., A.M., G.G-M., A.T., and H.K. performed data analysis and interpretation. E.J., F.R., A.M., T.K., H.K., M.A.W., and A.T. developed the manuscript. E.J., J.C., F.R., G.G-M., T.K., H.K., S.O’B., M.A.W., and A.T. were responsible for manuscript review and revisions. E.J., J.C., F.R., A.M., G.G-M., T.K., H.K., S.O’B., M.A.W., and A.T. provided final approval of the manuscript.
Funding disclosure: This study was supported by research funding from Eisai Inc. Medical writing, editing, and graphics assistance were provided by Peloton Advantage and were funded by Eisai Inc.
CONFLICT OF INTEREST
H.K. has received research grants from Eisai Inc. T.K. has received research grants from GlaxoSmithKline. A.T. is an employee of Eisai Inc. J.C. has served on a speaker’s bureau and has received honoraria and research grants from Ariad, Bristol-Myers Squibb, Chemgenex, and Novartis. F.R. has served as a remunerated consultant for Sunesis and Genzyme, has received honoraria from Bristol-Myers Squibb, Celgene, Genzyme, Novartis, and Sunesis, and has received research grants from Bristol-Myers Squibb, Bayer-Onyx, Celgene, EMD Serono, Incyte, Merck, Serono, and Sanofi. A.M., E.J., S.O’B, M.A.W., and G.G-M. declare no competing financial interests. The authors received no honoraria or other form of financial support related to the development of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Myelodysplastic Syndromes, v2.2011. National Comprehensive Cancer Network; [Accessed March 2, 2011]. NCCN Clinical Practice Guidelines on Oncology. Available at: www.nccn.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 5.Dacogen (decitabine) [prescribing information] Woodcliff Lake, NJ: Eisai Inc; 2010. [Google Scholar]
- 6.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 7.Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842–3848. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian HM, O’Brien S, Huang X, et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience. Cancer. 2007;109:1133–1137. doi: 10.1002/cncr.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravandi F, Issa JP, Garcia-Manero G, et al. Superior outcome with hypomethylating therapy in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome and chromosome 5 and 7 abnormalities. Cancer. 2009;115:5746–5751. doi: 10.1002/cncr.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]