Abstract
The characterization of microbes, such as of opportunists and pathogens (e.g. methicillin resistant Staphylococcus aureus [MRSA]), in indoor air is important for understanding disease transmission from person-to-person. Common genera found in the human skin microbiome include Micrococcus and Staphylococcus, but there only a limited number of tests to differentiate these genera and/or species. Both genera are believed to be released into indoor air from the shedding of human skin and are morphologically difficult to distinguish. In the current work, after the extraction of proteins from micrococci and the separation of these proteins on one dimensional electrophoretic gels, tryptic peptides were analyzed by MALDI TOF MS and the mass profiles compared with those of a reference strain (ATCC 4698). The results confirmed that all strains were consistent in identity with Micrococcus luteus.
Keywords: Indoor air, Peptide markers, MALDI-TOF MS, Mass spectrometry, Micrococcus
1. Introduction
In indoor air, bacteria are derived from building occupants and the environment. The characterization of airborne bacteria is important for understanding disease transmission from person-to-person. Unfortunately, building monitoring is generally limited to total colony forming units (CFUs). It is extremely time-consuming for all of the diverse colony types in indoor air to be characterized by conventional means and is rarely undertaken. Common genera found in the human skin microbiome include Staphylococcus and Micrococcus [1,2]; these organisms are believed to be released into indoor air [3]. Staphylococcus and Micrococcus are difficult to distinguish morphologically (both being observed as tetrads). Clinical samples have been reported to rarely contain micrococci (~ 4% of isolates are micrococci and 96% staphylococci) [4]. However, ~ two thirds of the environmental isolates in studies by us and others were micrococci [5,6]. Thus, for environmental samples there is a greater need for the characterization of micrococci. Of the five known Micrococcus species, M. luteus is the only one whose primary habitat is human skin [7–9]; M. lylae is only occasionally isolated from this organ [7].
Previously, we developed an approach for discriminating Micrococcus from Staphylococcus using MALDI TOF MS (matrix assisted laser desorption time-of-flight mass spectrometry), applying the Bruker Biotyper software and reference library (Bruker Daltonics, Bremen, Germany) and standard physiological tests. MALDI TOF MS for the analysis of bacterial proteins from bacteria isolated from clinical samples has shown 65.7% to 98.8% accuracy for the identification to the genus level, and 31.8% to 94.2% to species [10]. In MALDI TOF MS, mass profiles are produced from isolated bacterial colonies. Colonies are sampled from bacterial culture plates, dried directly on a MALDI plate with the ionization matrix and then subjected to MALDI TOF MS analysis. The resultant protein profiles are not attributed to known proteins which complicate extrapolation of information from one laboratory to another.
Users alerted the ATCC that ATCC 9341 displayed characteristics that were quite distinct from another M. luteus reference strain (ATCC 4698) [11]. This encouraged the ATCC and collaborators to perform a study to characterize these two strains involving 16S rDNA sequencing. Indeed, ATCC 4698 was shown to be Micrococcus luteus, whereas ATCC 9341 had been designated to be a different genus (Kokuria) within the Micrococcaceae family [11]. This encouraged us to further characterize our environmental isolates and use ATCC 4698 as the reference strain. Since members of the Micrococcaceae are not considered to be significant human pathogens, only a limited number of strains are found in current databases.
Isolation of proteins by gel electrophoresis allows one to focus on specific protein bands and compare the relatedness of proteins from different strains by MALDI TOF MS. Additionally, MALDI TOF-TOF MS-MS allows for peptide sequencing and identification of the protein [12,13]. This is not the case for the more widely used “direct” MALDI TOF MS profiling (e.g., the Bruker system). Homologous proteins in closely related bacteria can provide information on species identification (e.g., heat shock proteins [14], ribosomal proteins [15], and outer membrane proteins [16]).
With the continuing expansion in genomic datasets of bacteria it has been possible to identify an increasingly larger number of microorganisms. Utilization of MALDI TOF-TOF MS-MS sequencing of ions generated from tryptic digests of proteins provides the peptide sequence for comparison. This approach does not require any previous knowledge of the bacterial species. In this paper, tryptic peptides analyzed by MALDI TOF MS profiles allowed the characterization of the species identity of environmental isolates. MALDI TOF-TOF MS-MS was used to identify proteins from these peptides. Protein identification definitively categorized Micrococcus from environmental isolates and also distinguished them from staphylococcal species isolated from the same environment.
2. Methods
2.1. Air sampling
Air samples were collected in occupied and unoccupied rooms in a suburban elementary school in Columbia, SC, using an N6 Single Stage, Viable Impactor (Thermo Fisher Scientific, Inc., Waltham, MA), which has a nominal flow rate of 28.3 l/min. Sheep blood agar [SBA] plates were used with the viable impactor and subsequently incubated at 37 ˚C for 24–48 h. Individual β-hemolytic colonies were re-streaked several times until pure cultures were obtained. Gram-positive cocci occurring in tetrads or clusters are predominantly staphylococci and micrococci, and were selected for further characterization [3].
2.2. Strains
ASO3 C5, ASO3 C6, ASO3 C10, ASO3 C15, ASO3 C17, ASO3 C24, ASO3 C45, ASO3 C46, ASO3 C55, ASO3 C68, ASUNO5 2W, ASUNO5 3W, ASUNO15 C10, ASO15 C31, ASUNO2-15, C1White, ASUNO2-15 C3Y, ASUNO2-15 C4Y, ASUNO2-15 C5Y, ASUNO2-15 C7Y, ASUNO2-15 C9Y, ASUNO2-15 C10Y, ASUNO2-15 C12Y Reference strains: M. luteus ATCC 4698, M. luteus ATCC 49732, S. aureus ATCC 31240, S. hominis ATCC 27844. S. warneri (ATCC 49454).
2.3. Bacterial culturing and protein extraction
Bacteria were grown on nutrient agar and incubated at 37 °C for 24 h. Colonies were removed from plates and placed in 2 ml sterile, screw-top microcentrifuge tubes with 1 ml of protein extraction buffer (0.1 M NaCl, 50 mM Tris HCl, 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). The microcentrifuge tubes were weighed before and after bacteria were added to determine total wet weight of the bacteria processed. Samples were placed in a FastPrep®-24 (MP Biomedicals, Solon, OH) for 6 ms X 30 sec with 5 min on ice between each cycle for a total of 6 cycles. The samples were then centrifuged at 4 °C for 1 h at 10,000 ×g. The supernatant was removed and placed at −70 °C for two freeze-thaw cycles to eliminate DNA. The supernatant containing the total protein extract was normalized to 100 mg/ml wet weight of bacteria and stored at −70 °C until used.
2.4. Fermentation of glucose and glycerol
Purple Agar base (Difco Manual) was prepared and 0.5% glucose or glycerol was added. The media was autoclaved for 15 min. Glycerol media was poured into petri dishes and glucose media was placed in 5 ml tubes. For testing, bacteria were streaked from Nutrient agar after 24 h of growth on to glycerol plates and incubated at 37 °C for up to 72 h. Plates were examined every 24 h for acid production. Glucose tubes were inoculated from Nutrient agar after 24 h of growth and the media covered with sterile mineral oil. The tubes were incubated at 37 °C for up to 72 h. Tubes were examined for acid production every 24 h. Previous testing revealed three Micrococcus strains to have weak positive fermentation patterns (glycerol one; glucose 2 others). This finding was confirmed for two of the strains (ASO3-C10 and ASO3-C17; both were weakly positive for glucose metabolism, which is inconsistent with their being M. luteus. However, this was not confirmed for the other strain (AS03-C6) and does illustrate the subjectivity of reading color reactions.
2.5. Protein separation
Prior to electrophoresis samples were vortexed, and 20 µl of supernatant were added to 20 µl of 2× loading buffer (4% SDS, 10% 2-mercaptoethanol, 20% glycerol, 0.004% bromophenol blue, 0.125 M tris-HCl), and the sample was boiled at 100 °C for 5 min and centrifuged for 5 s. Samples were subjected to electrophoresis in 5% Criterion Tris-HCl gels (Bio-Rad) until the bromophenol blue was at the very bottom of the gel. The running buffer consisted of 192 mM glycine, 25 mM Tris and 0.1% SDS. The gels were washed 3 times in double de-ionized water for 5 min each and stained with Gel-Code Blue Stain reagent (Pierce, Rockford, IL) for 1 h. Gels were stored covered in double de-ionized water at 4 °C until the bands were excised.
2.6. Peptide preparation
Bands of interest were excised from Coomassie blue-stained gels and de-stained with 25 mM ammonium bicarbonate in 50% acetonitrile/50% water. The gel was dehydrated by addition of absolute acetonitrile (covering each gel piece) for 5 min. The acetonitrile was removed and the gel rehydrated with 100 mM ammonium bicarbonate (10 min). Then, the gel was dehydrated with acetonitrile (5 min), the acetonitrile removed and the sample dried in a Speed-Vac for 2–3 min. The sample was rehydrated with 10 mM dithiothreitol (DTT) and incubated for 30 min at room temperature (22–24 °C), then alkylated with 50 mM iodoacetamide for 30 min at room temperature, and then washed in 100 mM ammonium bicarbonate. Once the iodoacetamide had been removed, the sample went through 3 further rounds of dehydration-rehydration (100% acetonitrile- 100 mM ammonium bicarbonate). After the final dehydration, the sample was dried in a Speed-Vac. The gel spot was rehydrated (with a 12.5 ng/ml solution of trypsin) in ice-cold 50 mM ammonium bicarbonate. The sample was kept on ice for 45 min. The excess trypsin solution was removed and replaced with 50 mM ammonium bicarbonate, and then placed at 37 °C for 15 h. The trypsin digestion was stopped by the addition of 5% formic acid. Distilled de-ionized water (100 µl) was then added, and the tube vortexed gently. The sample was incubated at room temperature for 10 min and then centrifuged for 5 min at 3,000 xg. The supernatant was removed and placed in another tube with 5 µl of extraction solution (50% acetonitrile and 5% formic acid). The gel spot was extracted 3 more times with 50 µl of extraction solution and allowed to incubate for 15 min after each extraction. The extracts were combined and placed in a Speed-Vac and evaporated down to 20 µl. The peptide solution was passed through a C18 Spin column; the directions for the Protea C18 kit were followed, allowing for the removal of metal ions from samples (Protea Biosciences, Morgantown,WV).
2.7. MS and MS-MS analysis
Peptide extracts were sandwiched between 2 layers of matrix. A layer of matrix was dried on the plate (1 µl). Then the sample (1 µl of the above peptide digest) was placed on the matrix and dried. Finally 0.5 µl of matrix was added and dried. The matrix was α-cyano-4-hydroxy-cinnamic acid (α-CHCA, 10 mg/ml in 70% acetonitrile/30% 0.1% TFA) (Fluka). For external calibration in the protein mass range, angiotensin I and angiotensin II standards were used.
MALDI TOF MS was performed using a Bruker Ultraflex II instrument. MALDI TOF mass spectra were obtained in reflector mode with an acceleration voltage of 25 kV and a pulse ion extraction time of 20 nsec. The mass range for MS was generally between 800–2700 m/z. The program, Mascot (http://www.matrixscience.com), was utilized in these identifications.
MALDI TOF-TOF MS-MS analysis was performed (in the positive ion mode) at the MUSC Proteomics Center using an Applied Biosystems 4700 MALDI MS-MS. MALDI TOF TOF MS-MS was performed on abundant ions observed in MALDI TOF spectra. Peptide sequences were obtained from product ion spectra which are generated for peptide sequences (predicted from the DNA code of sequenced genomes). The experimental peptide spectra are compared to these virtual peptide spectra in the NCBI database and the best matches provided with computer-assistance.
3. Results and discussion
The strains selected here for study were identified previously as members of the genus Micrococcus (using fermentation tests and direct MALDI TOF profiles), but their species identification was less certain. MALDI TOF MS profiling identified all 22 isolates as Micrococcus luteus. However, two of the strains (ASO3-C10 and ASO3-C17 were weakly positive for metabolism of glucose, which is inconsistent with the known characteristics of M. luteus. The identification scores of these strains were in the range of “secure genus identification and probable species identification” according to the Bruker Biotyper software and reference library (Bruker Daltonics, Bremen, Germany), which contains more than 3,200 entries. The assigned scores ranged from 2.00 to 2.299. All of the strains fell within this scoring category. The MALDI TOF MS profiles were compared with data available in a database of reference strains, in order to determine the identification of unknown samples. When the work was performed (2009–2010), the Bruker database contained only one reference of Micrococcus luteus ATCC 4698 [6].
Physiological tests were performed on gram-positive cocci occurring as quads. Aerobic acid production from glycerol and anaerobic acid production from glucose are two simple tests to distinguish Micrococcus from Staphylococcus [17]. In the present study, acid production from glycerol and glucose was also assessed. M. luteus species are negative for both glycerol and glucose utilization. Two of the 22 environmental isolates produced acid from glucose, and none produced acid from glycerol. As noted above, strains ASO3-C10 and ASO3-C17 were weakly positive for glucose metabolism. These results are consistent with ASO3-C10 and ASO3-C17 being M. lylae. However, Kocuria varians also produces acid from glucose but not glycerol (5).
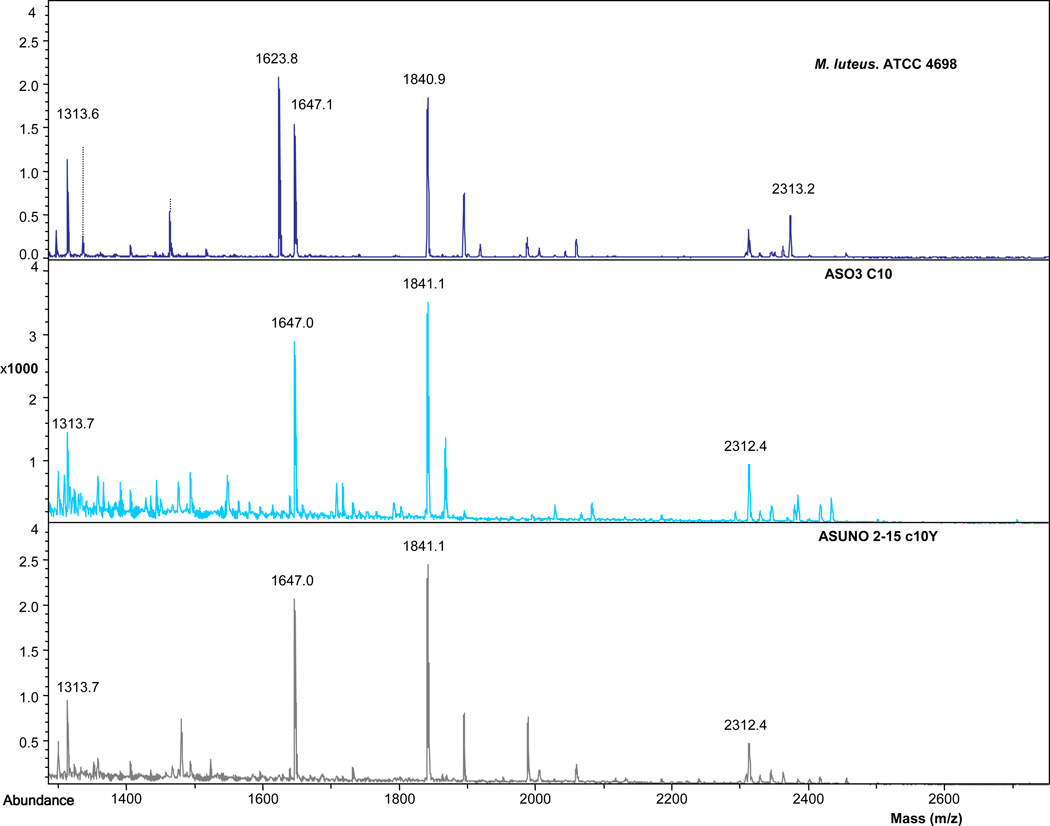
In order to confirm and extend the genus and species characterization of the environmental isolates, protein extracts were separated on one dimensional SDS PAGE gels. M. luteus ATCC 4698 (and M. luteus ATCC 49732) were used as reference strains. Selected gel bands were excised from electrophoretic bands and digested with trypsin. The MALDI TOF spectra were very similar among all environmental isolates (including the 2 strains [ASO3-C10 and ASO3-C17] that displayed aberrant fermentation) and the ATCC reference strains (ATCC 4698), suggesting their identity as M. luteus (see Table 1 for list of experimental ions). However, the mass spectrum of ATCC 4698 was the only strain to additionally display the prominent m/z 1623.9 peak. The mass 1623.9 in the other reference was not present in M. luteus ATCC 49732. Mass 1623.9 was presumably generated by mutation (K)GVLDVQGVEYEIFR(L) (ATCC 4698) to (K)GVLDVKGAEYEIFR(L) (SK 58). The presence of the additional lysine (SK58) generates an additional trypsin cleavage site, producing two peptides. These results are consistent with M. luteus not being a homogenous species. It has been proposed that M. luteus has 3 biovars [9] based on 16S rRNA sequence comparisons.
Table 1.
Characteristic masses determined by MALDI TOF MS for tryptic digests of aconitate hydratase derived from environmental and reference strains of Micrococcus
| Species/Strain | 1313.6 | 1623.8 | 1840.9 | 2313.5 | 2013.1 |
|---|---|---|---|---|---|
| Micrococus | |||||
| ATCC 4698 | (+) | (+) | (+) | (+) | ND |
| ATCC 49732 | (+) | ND | (+) | (+) | ND |
| ASO3 c5 | (+) | ND | (+) | (+) | ND |
| ASO3 c6 | (+) | ND | (+) | (+) | ND |
| ASO3 c10 | (+) | ND | (+) | (+) | ND |
| Uno c10Y | (+) | ND | (+) | (+) | ND |
| ASO3 c 45 | (+) | ND | (+) | ND | ND |
| ASO3 c31 | (+) | ND | (+) | (+) | ND |
| ASO3 c 17 | (+) | ND | (+) | (+) | ND |
| ASO3 c 19 | ND | ND | (+) | ND | ND |
| ASO15 c 31 | ND | ND | (+) | ND | ND |
| ASO3 c 15 | ND | ND | (+) | (+) | ND |
| Asuno 2 w | (+) | ND | (+) | (+) | ND |
| Staphylococci | |||||
| S. hominis | ND | ND | ND | ND | (+) |
| S. warneri | ND | ND | ND | ND | (+) |
| S. aureus | ND | ND | ND | ND | (+) |
An abundant 100 kDa-band was selected for use in MADLI TOF MS identification. Previous work [12] identified a similar sized band in staphylococcal species isolated from indoor air as aconitate hydratase. The identification of a unique aconitate hydratase sequence in Micrococcus could provide an additional means for the unequivocal identification of the two genera without the need for physiological tests. Batches of protein extracts from environmental strains were analyzed, together with two ATCC M. luteus reference strains (ATCC 4698 [the most frequently used reference standard] and ATCC 49732). S. warneri (ATCC 49454), S. aureus (ATCC 31240) and S. hominis (ATCC 27844) were used as additional control strains.
MALDI TOF MS (for species identification), coupled to MALDI TOF-TOF MS-MS analysis (for protein identification), is a powerful tool for the analysis of tryptic peptides derived from an unknown protein. Signature sequences in proteins can be detected, either through the presence of an amino acid substitution(s) or specific deletions or insertions [12].
The ~100 kDa bands detected in all individual environmental isolates and reference strains were digested with trypsin and the mass spectra of tryptic peptides were compared to evaluate similarities and differences. MALDI TOF MS peptide profiles verified that all of the environmental strains had similar mass spectra for this protein, with an abundant and unique peptide being located at 1840.9 m/z (see Fig. 1). Using the NCBI database within the MASCOT search engine, commonly used for identifying proteins based on mass spectral data, the ~100 kDa protein was identified as aconitate hydratase. Each environmental isolate was analyzed a minimum of twice, followed by processing the data using MASCOT. In total, 75 MALDI TOF MS analyses were subjected to MASCOT searches on the 22 environmental isolates. Although the spectra were remarkably similar, only 3 of the 75 mass spectra were identified as aconitate hydratase with a MOWSE score, but the value was less than the identification confidence level. Previously, it was noted that MASCOT only identified aconitate hydratase in some of the environmentally isolated staphylococcal strains [6], consistent with the results presented here for Micrococcus.
Fig. 1.
Mass spectra of an ATCC strain (M. luteus 4698) and two representative environmental isolates. The major peaks were found in all strains. The prominent 1623.9 m/z peak was found only in the ATCC 4698 strain of M. luteus.
Examination of mass spectra of the environmental isolates showed a similar pattern to Micrococcus luteus ATCC 49732. All environmental isolates and both Micrococcus luteus ATCC reference strains had an abundant ion at 1840.9 m/z (Table 1). They also shared masses 1313.6, 1647.1, 1840.9, 2312.1 but not mass 2013.1 (Table 1). Experimentally, mass 1647.1 was noted to be present in all M. luteus strains (including ATCC 4698 and 49732), but only appeared in virtual digests from the sequences derived from the genomes for SK 58 but not from ATCC 4698. None of the Staphylococcus strains displayed the mass 1840.9, and all Staphylococcus had an abundant m/z of at 2013.1 (Table 1) as recorded previously [2].
In order to determine whether the tryptic peptides isolated from environmental samples matched those of Micrococcus luteus, the aconitate hydratase protein sequence was downloaded from the genomic sequence of Micrococcus luteus ATCC 4698 and SK58 (www.ncbi.nlm.nih.gov/bioproject), and a virtual digest of the sequence was performed with Protein Prospector software (prospector.ucsf.edu/prospector). This software takes a known protein sequence and virtually cuts the protein with trypsin. Selecting the type of instrument used in the software, in this case MALDI TOF MS, generates a list of all possible m/z values, which could be present in the mass spectra. Mass spectral peak lists (virtual spectra) obtained with the aid of Protein Prospector were empirically compared with experimental spectra of two M. luteus ATCC strains, S. hominis ATCC 27844, and 9 environmental samples.
The genomic sequences of two strains of Micrococcus, M. luteus ATCC 4698 (also referred to as NCTC 2665) and M. luteus SK58 (2011), are currently present in the GenBank database. These two strains can be separated based on the BLAST distance tree results for both 16S rDNA and aconitate hydratase sequence. In order to verify that the aconitate hydratase sequences generate unique mass spectra from closely related genera, the aconitate hydratase sequences from the most closely related strains, based on the BLAST genomic search, were virtually digested in Protein Prospector and the ions compared. The virtual digest demonstrated that M. luteus ATCC 4698 is the only strain that would generate a mass of 1623.8 (see Table 2).
Table 2.
Comparison of predicted masses (virtual digest of aconite hydratase) of members in the Micrococcacae
| Species Strain |
M. luteus ATCC 4698 |
M. luteus SK48 |
Kocuria rhizophila DC2201 |
Brevibacterium linens BL2 |
Actinomyces urogenitalis DSM 1543 |
Mobiluncus curtisii ATCC 35241 |
Dermacoccus Ellin185 |
|---|---|---|---|---|---|---|---|
| Mass | |||||||
| 1314.4 | 1314.4 | - | - | - | - | - | |
| 1624.84 | - | - | - | - | - | - | |
| 1842.1 | 1842.1 | - | - | - | - | - | |
| 2313.55 | 2313.55 | - | - | - | - | - |
Abundant and characteristic masses recorded in MALD TOF MS were selected for further analysis utilizing MALDI TOF-TOF MS-MS. MALDI TOF TOF MS-MS spectra provide an amino acid sequence that allows the identification of the peptide. The MS-MS spectrum provides a breakdown of the peptide with individual masses that correspond to the most probable amino acid sequence. Based on this amino acid sequence, it is possible to verify the peptide producing the ion for a protein of interest. MS-MS spectra, when processed through databases, such as MASCOT, cannot always identify these proteins. Databases, such as NCBI, may not contain enough examples of species variation, particularly for organisms that are not associated with pathogenesis.
The utilization of tryptic peptides for the identification of uncharacterized environmental bacteria requires caution and empirical interpretation of mass spectral data. Programs, such as Protein Prospector, provide virtual digests linked to given MS-MS parent ions. Comparison of amino acid sequence ions in virtual fragmentation with experimental MS-MS spectra are invaluable for the comprehensive analysis of the data. As an example, ATCC 4698 on virtual analysis of a 16-mer (mass 1313.6) produced predominantly y ions. Of these 16 possible ions, 10 of 16 were observed. For the b ions, three were observed in the experimental spectra. The predicted sequence from the experimental MS-MS analysis was IDTPGEAEYYR, which was identical to the sequence generated for virtual MS-MS. Thus, almost complete coverage was observed for this peptide as a component of aconitate hydratase (ATCC 4698 and an environmental isolate).
4. Conclusions
Characterizing the microbial composition of indoor air is important for a better understanding of disease transmission in a human population. The natural flora found on human skin contains abundant levels of Micrococcus and Staphylococcus, which are shed into the air supply of indoor environments. These environmentally-derived organisms have been consistently difficult to classify. Within the micrococci, there is at least one well known example of a reference strain being mis-categorized [11], which led to an inaccurate reference standard for Food and Drug Administration (FDA)-mandated testing. In environmental indoor air samples, it was found previously that over two thirds of the environmental isolates were micrococci (Bruker Biotyping), creating a need for greater accuracy in characterization (6). Characterization of fermentation characteristics and MALDI TOF profiles allows that categorization of environmental Micrococcus isolates to the genus level. Tryptic peptide analysis (e.g., aconitate hydratase) by MALDI TOF MS profiles provides other criteria for a confirmation of the species identity for environmental isolates. MALDI TOF-TOF MS-MS can be used to identify proteins from these peptides. It has been reported that M. luteus may include more than one biovar, based on 16S rDNA sequence data [9]. Comparisons of mass spectral data of tryptic peptides also show a clear difference between the profiles of the two deposited M. luteus (ATCC 4698 and 49732), although the environmental strains were remarkably similar. Further investigations are warranted to determine whether these two ATCC strains and possibly others (displaying an amino acid substitution in aconitate hydratase) are distinct biovars or species within the genus Micrococcus.
Acknowledgements
Support for this work was provided by the National Science Foundation (grant no. 0959427; John Rose, Karen Fox and Alvin Fox). Jennifer Kooken was supported by a Sloan Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kloos WE, Musselwhite MS. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol. 1975;30:381–385. doi: 10.1128/am.30.3.381-395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noble WC. Skin Microbiology: Coming of Age. J. Med Microbiol. 1984;17:1–12. doi: 10.1099/00222615-17-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Fox A, Harley W, Feigley C, Salzberg D, Sebastian A, Larsson L. Increased levels of bacterial markers and CO2 in occupied school rooms. J Environ Monit. 2003;5:246–252. doi: 10.1039/b212341j. [DOI] [PubMed] [Google Scholar]
- 4.Baker J. Comparison of various methods for differentiation of staphylococci and micrococci. J Clin Microbiol. 1984;19:875. doi: 10.1128/jcm.19.6.875-879.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lis D, Pacha J, Idzik J. Methicillin resistance of airborne coagulase-negative staphylococci in homes of persons having contact with a hospital environment. Am J Infect Contr. 2009;37:177–182. doi: 10.1016/j.ajic.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Fox K, Fox A, Elssner T, Feigley C, Salzberg D. MALDI TOF Mass spectometry speciation of staphylococci and their discrimination from micrococci isolated from in indoor air of school rooms. J Environ Monit. 2010;12:917–923. doi: 10.1039/b925250a. [DOI] [PubMed] [Google Scholar]
- 7.Kloos WE, Tornabene T, Schleifer K. Isolation and characterization of micrococci from human skin, including two new species: Micrococcus lylae and Micrococcus kristinae . Int J Syst Bacteriol. 1974;24:79–101. [Google Scholar]
- 8.Stackebrandt E, Koch C, Gvozdiak O, Schumann P. Taxonomic dissection of the genus Micrococcus: Kocuria gen. nov., Nesterenkonia gen. nov., Kytococcus gen. nov., Dermacoccus gen. nov., and Micrococcus Cohn 1872 gen. emend. Int J Syst Bacteriol. 1995;45:682–692. doi: 10.1099/00207713-45-4-682. [DOI] [PubMed] [Google Scholar]
- 9.Wieser M, Denner EB, Kämpfer P, Schumann P, Tindall B, Steiner U, Vybiral D, Lubitz W, Maszenan AM, Patel BK, Seviour RJ, Radax C, Busse HJ. Emended descriptions of the genus Micrococcus, Micrococcus luteus (Cohn 1872) and Micrococcus lylae (Kloos et al. 1974) Int J Syst Evol Microbiol. 2002;52:629–637. doi: 10.1099/00207713-52-2-629. [DOI] [PubMed] [Google Scholar]
- 10.Carbonnelle E, Mesquita C, Bille E, Day N, Dauphin B, Beretti J-L, Ferroni A, Gutmann L, Nassif X. MALDI-TOF mass spectrometry tools for bacterial identification in clinical microbiology laboratory. Clin Biochem. 2011;44:104–109. doi: 10.1016/j.clinbiochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Tang JS, Gillevet PM. Reclassification of ATCC 9341 from Micrococcus luteus to Kocuria rhizophila . Int J Syst Evol Microbiol. 2003;53:995–997. doi: 10.1099/ijs.0.02372-0. [DOI] [PubMed] [Google Scholar]
- 12.Fox K, Fox A, Rose J, Walla M. Speciation of coagulase negative staphylococci, isolated from indoor air, using SDS-PAGE gel bands of expressed proteins followed by MALDI TOF MS and MALDI TOF-TOF MS-MS analysis of tryptic peptides. J Microbiol Meth. 2011;84:243–250. doi: 10.1016/j.mimet.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Gupta RS. Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes. Microbiol Mol Biol Rev. 1998;62:1435–1491. doi: 10.1128/mmbr.62.4.1435-1491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L, Li W, Dong X. Species identification of genus Bifidobacterium based on partial HSP60 gene sequences and proposal of Bifidobacterium thermacidophilum subsp. porcinum subsp. nov. Int J Syst Evol Microbiol. 2003;53 doi: 10.1099/ijs.0.02617-0. 1619-16162. [DOI] [PubMed] [Google Scholar]
- 15.Shinichi Kawamoto, Kozo Ochi. Comparative ribosomal protein (L1 I and L30) sequence analyses of several streptomyces spp. commonly used in genetic studies. Int J Syst Bacteriol. 1998;48:597–600. doi: 10.1099/00207713-48-2-597. [DOI] [PubMed] [Google Scholar]
- 16.Fournier PE, Roux V, Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Bacteriol. 1998;48:839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- 17.Davis GHG, Hoyling B. Observations on anaerobic glucose utilization tests in Staphlyococcus-Micrococcus identification. Applied Microbiol. 1975;30:381–395. [Google Scholar]