Abstract
Establishment of a functional immune system has important implications for health and disease, yet questions remain regarding the mechanism, location, and timing of development of myeloid and lymphoid cell compartments. The goal of this study was to characterize the ontogeny of the myeloid-lymphoid system in rhesus monkeys to enhance current knowledge of the developmental sequence of B cell (CD20, CD79), T cell (CD3, CD4, CD8, FoxP3), dendritic cell (CD205), and macrophage (CD68) lineages in the fetus and infant. Immunohistochemical assessments addressed the temporal and spatial expression of select phenotypic markers in the developing liver, thymus, spleen, lymph nodes, gut-associated lymphoid tissue (GALT), and bone marrow with antibodies known to cross-react with rhesus cells. CD3 was the earliest lymphoid marker identified in the first trimester thymus and, to a lesser extent, in the spleen. T cell markers were also expressed mid-gestation on cells of the liver, spleen, thymus, and in Peyer’s patches of the small and large intestine, and where CCR5 expression was noted. A myeloid marker, CD68, was found on hepatic cells near blood islands in the late first trimester. B cell markers were observed mid-second trimester in the liver, spleen, thymus, lymph nodes, bone marrow spaces, and occasionally in GALT. By the late third trimester and postnatally, secondary follicles with germinal centers were present in the thymus, spleen, and lymph nodes. These results suggest that immune ontogeny in monkeys is similar in temporal and anatomical sequence when compared to humans, providing important insights for translational studies.
Keywords: Fetus, Infant, Monkey, Immune System, Ontogeny
INTRODUCTION
The vertebrate immune system is essential to defend against a multitude of pathogens including viruses, bacteria, fungi, and parasites. Cells of the immune system are also responsible for destruction of deviant cells such as tumor cells and for the establishment of tolerance, the ability to distinguish “self” from “non-self”. Dysfunction of the immune system has been reported to result in more than 80 chronic, life-threatening diseases known collectively as autoimmune disorders (Rose, 2002). Together, these disorders affect nearly 24 million Americans with an estimated annual direct health care costs in the range of $100 billion (AARDA, 2011). Patients with other medical conditions including cancer, HIV disease and AIDS, other infectious diseases, and metabolic disorders depend on modulation of the immune system for effective treatments (Pardoll, 2012). Given the importance of the immune system in health and disease, studies to enhance understanding of the ontogeny of critical immune cell populations are important for providing clues on the induction of tolerance for cell and organ transplantation, and to facilitate the development of novel treatment strategies when immune modulation is needed.
Animal models are important for basic discovery, for the advancement of translational research, and for studies on the immune system that cannot be easily addressed in humans. Nonhuman primates share many developmental similarities with humans and are an important preclinical model to test novel therapies for the treatment of human diseases (Bontrop, 2001; Lee et al., 2001; Tarantal et al., 2001; Donahue and Dunbar, 2001; Donahue et al., 2005). In addition to genetic, anatomical, physiologic, developmental, and reproductive similarities (Tanimura and Tanioka, 1975; Tarantal and Gargosky, 1995; Lee et al., 2001; Tarantal, 2005: Gibbs et al., 2007; Batchelder et al., 2010; Herring et al., 2013), nonhuman primates are susceptible to many of the same infectious (Lackner and Veazey, 2007; Gardner and Luciw, 2008; Adams Waldorf et al., 2011) and metabolic diseases (Hansen and Bodkin, 1986; Bauer et al., 2011; Bremer et al., 2011) as humans. Monkeys are widely used as preclinical models for human bone marrow transplantation and stem cell gene therapy, and have substantial advantages when compared to other species (Berenson et al., 1988; Tarantal, 2005; Trobridge and Kiem, 2010; Tarantal and Nakayama, 2011; Tarantal and Skarlatos, 2012). Even in the context of “humanized” mouse models, long-term engraftment of human cells has been difficult to assess and inter-species differences in homing receptors and immune modulators (e.g., cytokines) create an environment of uncertain relevance to the human clinical setting (Horn et al., 2003; Mestas and Hughes, 2004; Mezquita et al., 2008; Sykes, 2009). Studies also suggest that the histology and time course for allograft rejection in monkeys parallels humans because of similarities in major histocompatibility complex (MHC) genes and immune ontogeny, while tolerance is much easier to achieve in mice (Cowan et al., 2001; Trobridge and Kiem, 2010; Gibbons and Spencer, 2011).
Establishment of a functional immune system has been characterized in mice and humans as a multi-stage process that occurs in a unique, coordinated, sequential, and temporal sequence. Hematopoietic ontogeny is a complex process incorporating multiple sites during development including the yolk sac, the aorta-gonad-mesonephros (AGM) region, liver, placenta, and bone marrow (Medvinsky et al., 2011). Both primitive and definitive hematopoietic stem cells (HSC) have been proposed to develop during ontogeny, although the initial mesodermal precursors and site(s) for definitive HSC remain unclear. Studies have shown that early mesodermal precursors first migrate to the yolk sac to initiate primitive erythropoiesis, and some investigations suggest that the yolk sac may be the only source of primitive HSC (Mikkola and Orkin, 2006). The AGM has been proposed as the site for definitive HSC development, although other sources have been considered that may aid in seeding the liver. An orderly migration of definitive HSC to the fetal liver results in the liver taking on the role of hematopoiesis (Medvinsky et al., 2011). From the fetal liver, hematopoietic cells migrate to the thymus, spleen, lymph nodes, and gut-associated lymphoid tissue (GALT), where additional expansion, differentiation, and maturation occurs (Holt and Jones, 2000; Holsapple et al., 2003; Ygberg and Nilsson, 2011). Pools of progenitor cells are formed and expanded in the bone marrow and spleen for myeloerythroid lineages, in the bone marrow for B cell lineages, and in the thymus for T cell lineages. Many questions remain unanswered regarding the mechanism(s), location(s), and timing of development of these various myeloid and lymphoid cell compartments.
The purpose of this study was to characterize the ontogeny of the myeloid-lymphoid system in developing organs of rhesus monkeys in order to enhance current knowledge of the developmental sequence of B cell (CD20, CD79), T cell (CD3, CD4, CD8, FoxP3), dendritic cell (CD205), and macrophage (CD68) populations. CCR5, a chemokine receptor important in transmission of HIV (Choe et al., 1996; Deng et al., 1996; Dragic et al., 1996), was also included. The insights provided herein will aid in advancing translational research in this clinically relevant primate model, and allow further characterization of similarities and differences with mouse and human ontogeny as it relates to health and disease.
MATERIALS AND METHODS
Animals
All animal procedures were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis, and were consistent with the requirements of the Animal Welfare Act. Activities related to animal care including diet and housing were performed as per standard operating procedures at the California National Primate Research Center. Normally cycling, healthy adult female rhesus monkeys (Macaca mulatta) that were disease-free and from the time-mated colony with a history of prior pregnancy were bred and identified as pregnant according to established methods (Tarantal et al., 2005) to obtain fetal specimens. Fetal tissues were collected by hysterotomy using established protocols (Tarantal et al., 2001), and tissues from postnatal animals were obtained at scheduled tissue harvests. Specimens were placed in 10% buffered formalin, embedded in paraffin, and sectioned at 5–6 μm for immunohistochemical analyses. Tissues included were the thymus, liver, spleen, axillary and mesenteric lymph nodes, small intestine (ileum, jejunum), colon, and bone marrow from first trimester (N=3), second trimester (N=3), and third trimester (N=3) fetuses, and infants at 3 months postnatal age (N=3).
Immunohistochemistry
Immunohistochemistry was performed with the EnVision™+ System-HRP for use with mouse/rabbit primary antibodies following the manufacturer’s recommendations (DAKO, Carpinteria, CA). Briefly, formalin-fixed sections were deparaffinized in xylene (three times for 5 min), rehydrated in a graded series of ethanol (100%, 90%, 70%, and 50%) twice for 2 min and washed in 1X phosphate-buffered saline (PBS). Antigen retrieval was performed on all sections (Decloaking Chamber, Biocare Medical, Concord, CA) using antibody-specific solutions: DIVA decloaker (Biocare Medical) for CD3 and CD205; EDTA decloaker (Biocare Medical) for CD4, CD8, CD68, and FoxP3; citrate buffer pH 6.0 (Life Technologies, Grand Island, NY) for CD20 and CCR5; and Reveal Decloaker (Biocare Medical) for CD79. After antigen retrieval, sections were washed twice in 1X PBS for 5 min each and incubated with antibodies specific for CCR5 (1:100, mouse clone CTC5, R&D Systems, Minneapolis, MN), CD3 (1:50, rabbit polyclonal, DAKO), CD4 (1:25, mouse clone BC/1F6, Abcam, Cambridge, MA), CD8 (1:200, rabbit polyclonal, Abcam), CD20 (1:200, CD20cy mouse clone L26, DAKO), CD68 (1:50, mouse clone KP1, Life Technologies), CD79 (1:50, CD79a mouse clone HM47/A9, Biocare Medical), CD205 (1:25, mouse clone PN-15, Santa Cruz Biotechnology, Santa Cruz, CA), and FoxP3 (1:500, rabbit polyclonal, Abcam). All of these antibodies are known to cross-react with rhesus cells and tissues and formed the basis for selection. Hydrogen peroxidase blocking was performed after primary antibody incubation for CD3, CD4, CD8, CD68, CD205, and CCR5 to enhance staining intensity. For the remaining antibodies used, the hydrogen peroxidase blocking was applied before the antigen retrieval process. Sections were washed twice in 1X PBS for 5 min each and incubated with horseradish peroxidase (HRP)-labeled polymer for 30 min at room temperature in the dark. Sections were then washed twice in 1X PBS for 5 min each and incubated with diaminobenzidine (DAB) substrate-chromogen solution for 2–4 min, with specific times optimized for each antibody. The substrate-chromogenic reaction was stopped by washing the sections in distilled water once for 5 min. After counterstaining with hematoxylin for 1 min, sections were dehydrated in a graded series of ethanol (50%, 70%, 90%, and 100%) twice for 2 min and xylene for 5 min prior to mounting and coverslip placement.
All antibodies were incubated at room temperature for 60 min except CD8, which was incubated at room temperature for 20 min and FoxP3 which was incubated at 4°C overnight. Negative controls consisted of rabbit serum (Invitrogen) for all the antibodies that were rabbit polyclonals (CD3, CD8, FoxP3) at a concentration that matched the individual primary antibody concentration. Specific IgG isotypes were utilized as negative controls for monoclonal antibodies. For each marker, all age groups and tissues were completed in a single run to ensure consistency. The presence of specific staining with appropriate cell-to-cell heterogeneity within a given run served as an internal positive control.
Microscopy
Sections of tissue were imaged using an Olympus BX61 microscope with an Olympus DP72 color camera (Olympus, Center Valley, PA) and images were collected with the 10x, 20x, and 40x non-oil objectives using MicroSuite™ software (Olympus America Inc., Melville, NY).
RESULTS
Observations of ontogeny in the rhesus monkey fetus and infant were completed for each of the tissues as described below. Representative images of a subset of findings are shown in the respective figures identified in the text.
Liver
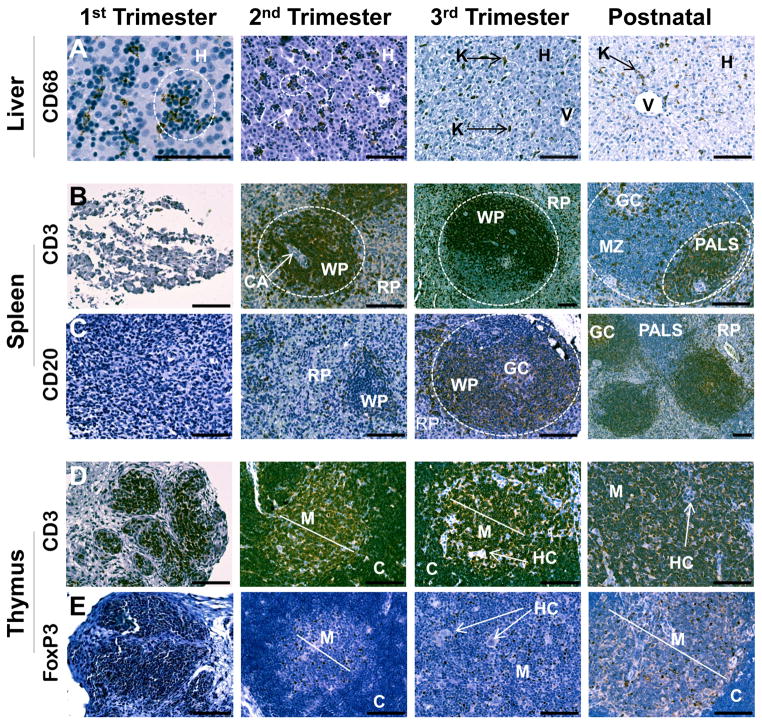
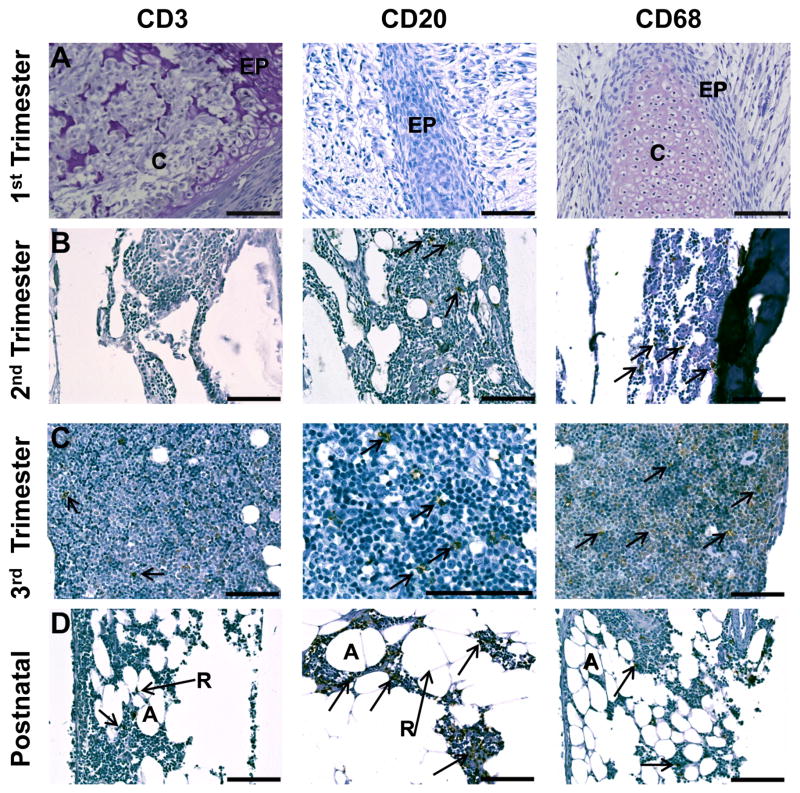
In the late first trimester, small clusters of erythroblasts with large round nuclei were notable in blood islands throughout the developing liver parenchyma (Fig. 1A). Cells staining for the macrophage marker selected, CD68, were observed only in blood islands, with no positive cells noted among surrounding hepatocytes. The number of blood islands and the frequency of CD68+ cells were greatest in the early second trimester, and decreased in frequency as gestation progressed. By the late second trimester, individual CD68+ cells with irregular polygonal morphology were occasionally observed in locations not associated with blood islands. In the late third trimester, blood islands were no longer present in the fetal liver and infrequent CD68+ cells with morphology typical of Kupffer cells were found distributed throughout the hepatic parenchyma. CD68+ cells were rarely noted in the early postnatal liver and were typically located near venules when present.
Figure 1. Rhesus monkey liver, spleen, and thymus.
A. CD68+ cells were only found within blood islands (examples circled) in the liver in the first trimester, but were also observed within hepatocytes (H) in the second trimester. Near term, blood islands were no longer present and infrequent CD68+ cells with morphology typical of Kupffer cells (K) were noted. In postnatal liver, CD68+ cells were most frequently found near venules (V). B. CD3+ cells were noted occasionally in the splenic mesenchyme in the first trimester. By the second trimester, organization of early follicles (dotted circle) with white pulp (WP) and red pulp (RP) regions were evident. Central arterioles (CA) were surrounded by CD3+ cells in developing periarteriolar lymphatic sheaths (PALS). CD3+ cells were concentrated in the PALS in postnatal follicles with a few positive cells noted in germinal centers (GC) and marginal zones (MZ). C. CD20+ cells were not observed until the second trimester in the spleen and were usually associated with white pulp follicles (dotted circle) by the third trimester. CD20+ cells were concentrated in the germinal center (GC) and marginal zone of the white pulp at 3 months of age. D. Developing lobules of the thymus were composed of CD3+ cells in the first trimester. Cortical (C) and medullary (M) regions were evident from the second trimester onward with nearly all cortical cells expressing CD3. The medulla contained CD3+ cells and unstained thymic epithelial cells. Small clusters of epithelial cells in Hassall’s corpuscles (HC) were noted from the early second trimester and increased in frequency with advancing gestation. E. FoxP3 expression was similar to CD3 in the first trimester with strong positive staining of nearly all thymic lobular cells. Expression of FoxP3 was restricted to a few cells in the medulla from the second trimester and onward. All images 20x except first trimester CD68 (40x), third trimester CD3 (10x), and postnatal CD20 (10x). Calibration bar = 100 μm.
Cells expressing CD4 were not observed until late in gestation when rare, individual positive cells were noted near the portal triad. In contrast, cells expressing the T cell markers, CD3 or CD8, were noted in the late second trimester in locations near the blood islands and in the late third trimester, with the portal triad or by hepatic venules. Cells positive for the B cell marker, CD20, were not observed until near term and were found as individual cells seen infrequently among the hepatocytes. In contrast, cells positive for the B cell marker, CD79, were observed more frequently beginning late in the second trimester and were more concentrated near hepatic venules or portal triads. CD205+ cells with a dendritic morphology were not observed in the liver in any of the gestational ages assessed or at three months postnatal age.
Spleen
The splenic mesenchyme was loosely organized in the late first trimester (Fig. 1B), with rare cells positive for CD3. Organization of red and white pulp regions was evident beginning in the second trimester, with appearance of clusters of cells around central arterioles as early periarteriolar lymphatic sheaths. Nearly 100% of the cells surrounding the central arterioles were CD3+, with less frequent CD4+ cells, regions of CD8+ cells near white pulp borders, and a few individual cells staining positive for the regulatory T cell (Treg) marker, FoxP3, throughout the parenchyma. Individual cells staining positive for each of these T cell markers were noted in the red pulp. Cells expressing the B cell markers CD20 or CD79 were not observed until early in the second trimester. When noted, these cells were located on the outer edges of the white pulp, in the developing follicles, and as individual cells throughout the red pulp parenchyma (Fig. 1C). Near term and thereafter, strong staining for CD20 or CD79 was noted in the germinal center of white pulp follicles. Cells expressing the macrophage marker, CD68, were first observed during the mid-second trimester, increased in frequency with advancing gestation, and were noted primarily, but not exclusively, in the red pulp. Cells expressing the dendritic marker, CD205, were first evident in the mid-second trimester in both white and red pulp. CD205+ cells declined in frequency thereafter and were found in the marginal zone of the follicles at three months postnatal age.
Thymus
The first trimester thymus contained lobules of densely packed CD3+ thymocytes with large nuclei embedded in a loose mesenchyme (Fig. 1D). The early second trimester thymus was noted with distinct lobules, each with a septum and visible demarcations of cortical and medullary regions. Hassall’s corpuscles were first observed in the early second trimester and increased in frequency as gestation progressed. Nearly all cells in the cortex in the second trimester thymus stained intensely for CD3, a pattern that continued throughout the third trimester and into the postnatal period. CD3+ cells were also observed in the medulla from the second trimester onward, although the frequency and intensity of staining was less than that noted in the cortex. CD4+ or CD8+ cells were first noted in the early second trimester in the outer cortex, medulla, and interlobular septal regions. This pattern continued throughout development, with the frequency of cells positive for CD4 or CD8 increasing in the medulla with advancing gestation. FoxP3 expression was similar to CD3 in the first trimester with strong positive staining of nearly all thymocytes (Fig. 1E). With the development of cortical and medullary regions in the second trimester, FoxP3+ cells were observed most frequently in the medulla for the remainder of gestation and at three months postnatal age.
In contrast to expression patterns of T cell markers, expression of B cell markers (CD20 or CD79) was observed initially in the second trimester, was lower in frequency, and was restricted to the corticomedullary junction, with rare cells in the medulla. The macrophage marker, CD68, was noted on occasional positive cells in the medulla in the second trimester, and increased in frequency with advancing gestation in both the medulla and cortex. The dendritic cell marker, CD205, was not expressed in the first trimester but was found occasionally in late second trimester medulla. In the third trimester and continuing after birth, this marker was also found on individual cells in the cortex.
Axillary and Mesenteric Lymph Nodes
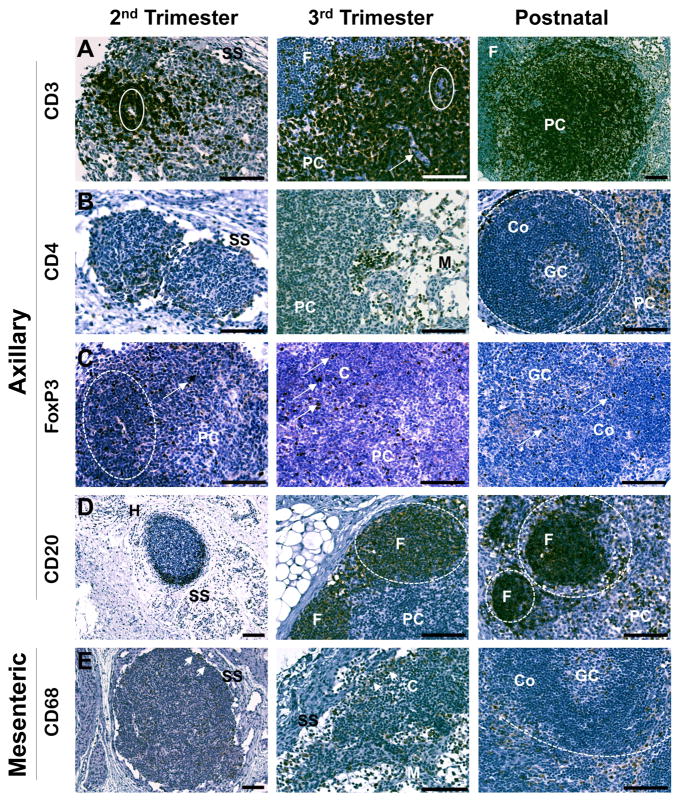
Given size limitations in early gestation, the axillary and mesenteric lymph nodes were assessed beginning in the second trimester. The cellular compartment of lymph nodes was similar in axillary and mesenteric locations, with no significant differences found as a function of time during development. Early second trimester axillary and mesenteric lymph nodes consisted of condensed regions of densely packed cells surrounded by developing subcapsular sinuses. Small trabeculae and high endothelial venules were evident. Within the developing lymph node, individual cells near the subcapsular sinus or small central clusters of cells stained positive for CD3, CD4, CD8, or FoxP3 (Fig. 2). Of the T cell markers studied, CD3+ cells were noted with greatest frequency at each developmental age. Organization of cells into distinct cortical and medullary regions was not evident before the late second trimester, at which time staining for each T cell marker was greatest in the paracortex, and as small clusters of cells in the medullary cords. Distinct follicular regions were evident by the late third trimester with secondary follicles containing germinal centers surrounded by coronal cells that were evident by three months postnatal age. Individual positive cells for each T cell marker were also noted in the germinal centers of the follicles and occasionally in the surrounding cortical coronal cells. CD4+ cells were noted near and within the medullary sinuses.
Figure 2. Rhesus monkey axillary and mesenteric lymph nodes.
A. In the second trimester, lymph nodes were surrounded by subcapsular sinuses. CD3 was strongly expressed in cells surrounding venules (solid ovals). As lymph nodes developed, CD3 expression was noted on nearly all cells of the paracortex (PC) except trabeculae (arrow), and less frequently in the follicle (F). B. CD4 was expressed near the subcapsular sinus (SS), and with follicle maturation was noted most frequently in the paracortex (PC) and near the medullary sinuses (M). Follicles (dotted circles) were present in the third trimester, with progression to secondary follicles containing germinal centers (GC) surrounded by coronal regions (Co) by 3 months postnatal age. C. Cells expressing FoxP3 were found as individual or small clusters of cells (arrows) in the cortical parenchyma (C), and were also noted with greater frequency in the paracortex (PC). With follicular maturation, frequency of CD68+ cells decreased and individual positive cells were noted in germinal centers (GC) and the surrounding corona (Co) of the follicles after birth. D. Expression of CD20 was concentrated near subcapsular sinuses (SS) opposite the hilum (H) of the developing lymph nodes. Individual CD20+ cells were also found throughout the developing parenchyma. Beginning with the third trimester most CD20+ cells were localized in developing follicles (F) (dotted circles). After birth, positive cells were also noted infrequently in the paracortex (PC). E. CD68+ cells were found along the border of the developing subcapsular sinus (SS), and localized to the cortical margins (C) of maturing follicles and medullary regions as development progressed. After birth, positive cells were less frequent and, when observed, were located in the medulla or the germinal centers (GC) of cortical (Co) follicles (dotted circle). All images 20x except second trimester CD20 and CD68 (10x), and postnatal CD3 (10x). Calibration bar = 100 μm.
The B cell markers, CD20 or CD79 (Fig. 2D), were expressed on cells found near the periphery of the developing lymph nodes in the early second trimester and were concentrated opposite the hilum. As the cortex developed, cells positive for B cell markers were localized near the subcapsular sinus and in the cortex, with strong staining in the developing primary follicles by the third trimester. Cells of the corona surrounding the germinal center stained intensely for CD20 or CD79 in differentiated follicles observed after birth.
The dendritic cell marker, CD205, was noted on occasional cells in the cortical region in the second trimester and increased with frequency as gestation advanced. CD205+ cells were found in the cortex, including the germinal centers and paracortex, after birth. The macrophage marker, CD68 (Fig. 2E), was noted in cells lining the subcapsular sinus and on occasional individual cells found throughout the developing lymph nodes in the second trimester. In the third trimester, CD68+ cells were observed frequently along the subcapsular sinus, in the developing medullary cords and sinuses, and occasionally in the paracortex. After birth, CD68+ cells were concentrated in the medulla and the medullary sinuses, with infrequent cells noted in the cortex including the germinal centers and trabeculae separating follicles.
Jejunum, Ileum, and Colon
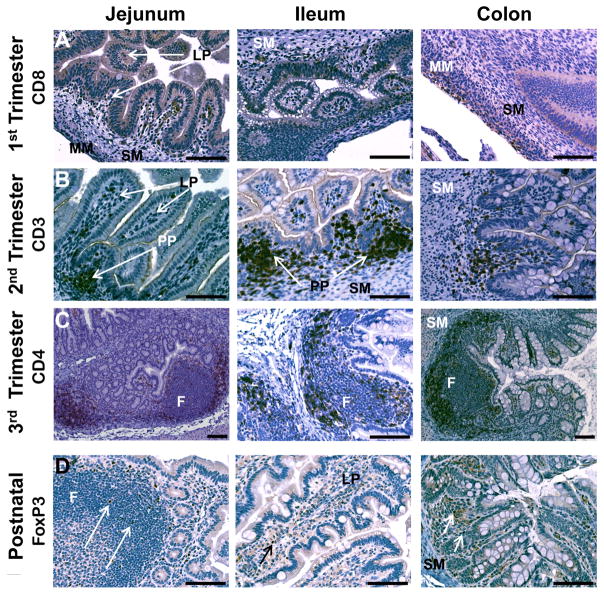
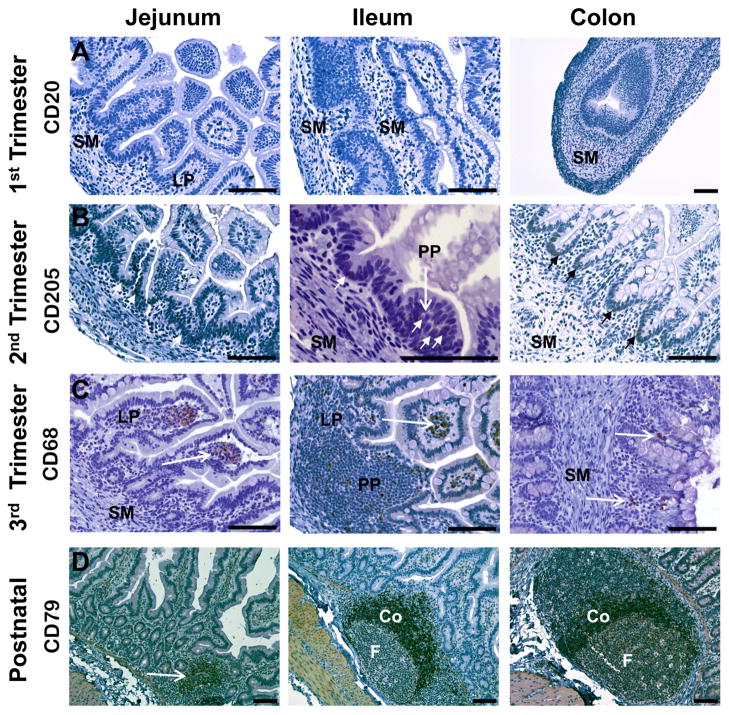
Late first trimester jejunum and ileum contained developing villi with pseudostratified columnar epithelium in deeper epithelial folds bordered by loosely organized cells of the submucosa. Tips of developing villi were composed of a lamina propria core with tall columnar surface epithelium. Many villi were contiguous with neighboring villi and limited luminal space was noted. A few clusters of dense cells were noted within the submucosa just below the epithelial layer. The anatomical morphology of the jejunum was more developed than that of the ileum and, in comparison, the colonic epithelium remained relatively undifferentiated without apparent villus formation. Of the T cell markers tested, only CD8 expression was observed, with positive cells located in the lamina propria of taller villi and as scattered cells in the submucosa (Fig. 3). In the colon, CD8+ cells were observed within the early muscularis layers. Cells expressing the markers selected for B cells (CD20, CD79), macrophages (CD68), and dendritic cells (CD205) were not found (Fig. 4).
Figure 3. Ontogeny of T lymphocytes in rhesus monkey jejunum, ileum, and colon.
A. CD8 was expressed in the lamina propria (LP) of elongated villi and in the submucosa (SM) in first trimester jejunum. CD8+ cells were also located within the surface epithelium in the ileum. Muscularis mucosa (MM). B. In the second trimester, CD3+ cells were located in the lamina propria (LP), developing Peyer’s patches (PP), and submucosa (SM). C. Mature follicles (F) were evident within Peyer’s patches in the third trimester. CD4+ cells were found with greater frequency in the interfollicular regions than within the follicle center. Submucosa (SM). D. FoxP3+ cells were found as individual cells within Peyer’s patches (arrows), submucosa (SM), and lamina propria (LP). All images 20x except third trimester jejunum and colon (10x). Calibration bar = 100 μm.
Figure 4. Ontogeny of B lymphocytes, macrophages, and dendritic cells in rhesus monkey jejunum, ileum, and colon.
A. Cells expressing B cell markers CD20 or CD79 (not shown) were not found in the submucosa (SM), lamina propria (LP), or surface epithelium in the late first trimester. B. Cells expressing the dendritic marker, CD205 (small arrows), were noted within the pseudostratified surface epithelium between the villi and within Peyer’s patches (PP) in the second trimester. Submucosa (SM). C. Cells expressing CD68 (macrophage marker) (arrows) were more abundant in the third trimester jejunum and ileum, were located in the tips of the lamina propria (LP), and as individual cells in Peyer’s patches (PP). Submucosa (SM). D. Postnatal Peyer’s patches contained distinct follicles (F) with a majority of cells CD20+ (not shown) or CD79+ (arrow). Dense CD20+ cells were found in the coronal region (Co) between the germinal center of the follicles and the surface epithelium. All images 20x except first trimester colon (10x), second trimester ileum (40x), and postnatal jejunum, ileum, and colon (10x). Calibration bar = 100 μm.
By the mid-second trimester, significant villus formation was noted in the jejunum, ileum, and colon. Villi of the jejunum were more developed and elongated, tended to be separate from neighboring villi, and more luminal space was evident when compared to the ileum and colon. Goblet cells were occasionally observed in the villus epithelium of the jejunum; these cells increased in frequency in the ileum and were observed with greatest frequency in the colon. Deep crypts of villi in the ileum and colon contained pseudostratified columnar epithelium but the majority of the villus surface was covered with tall columnar epithelium. Developing Peyer’s patches were found as dense clusters of cells in the submucosa below the luminal epithelium (Fig. 4). In the lamina propria of the jejunum and ileum, cells positive for CD3, CD4, or CD8 were noted, with strong expression of these markers in the developing Peyer’s patches. Cells expressing these markers were noted in similar locations in the colon but were less abundant. A few, individual FoxP3+ cells were observed in the lamina propria, submucosa, and muscularis layers of all three tissues. The Peyer’s patches also contained individual, rare cells expressing the B cell markers, CD20 or CD79, early in the second trimester, with the frequency of such cells increasing with advancing gestation. Cells expressing the dendritic marker, CD205, were noted by the late second trimester within the pseudostratified surface epithelium between villi and within Peyer’s patches by the late second trimester. Cells expressing the macrophage marker, CD68, were noted only rarely until late in the second trimester, when these cells were also found in developing Peyer’s patches.
Early third trimester tissues contained well-developed villi with distinctive Peyer’s patches in the submucosa, which was bordered by defined layers of circular and longitudinal smooth muscle. CD3+, CD4+, or CD8+ cells were located with greater frequency within the lamina propria and were occasionally observed among the tall columnar cells of the villus epithelium. Colonic glands were first observed early in the third trimester, were more abundant near term, and were composed of cells positive for CD8 by the late third trimester. CD8 was also strongly expressed in cells of developing lymphoid tissue in the Peyer’s patches similar to CD3 and CD4, although to a lesser degree (Fig. 4). Some Peyer’s patch regions contained primary follicles bordered by cells positive for CD3, CD4, or CD8 in locations ipsilateral to the overlying dome epithelium. Cells positive for FoxP3 were observed with greater frequency than in the second trimester and were found as individual cells within Peyer’s patches, submucosa, and the lamina propria. Distinct follicular structures in Peyer’s patches were composed of cells expressing the B cell markers, CD20 or CD79 (Fig. 4). These markers were observed only occasionally in the lamina propria or Peyer’s patch interfollicular regions. Cells expressing the macrophage marker, CD68, were found in the tips of the lamina propria, just below the tall columnar epithelium of the luminal surface and as occasional, individual cells in the Peyer’s patches. Similar to the late second trimester, CD205+ cells continued to be observed within the surface epithelium of deep crypts and glands near term.
Occasional CD3+ cells were located within the surface epithelium of the villi at three months postnatal age. Mature follicles were present in Peyer’s patches in jejunum, ileum, and colon at this age. The follicles were larger and more abundant in the jejunum than in the ileum and colon, with strong CD3 expression on cells in the intrafollicular regions. CD4+ or CD8+ cells were also found in the intrafollicular submucosa. In addition, many cells of the lamina propria were positive for these markers. Within the follicles, individual scattered cells were positive for CD3, CD4, or CD8 in the germinal centers, with sporadic positive cells in the surrounding coronal regions. FoxP3 was expressed on individual, infrequent cells located in germinal centers and the surrounding corona of Peyer’s patch follicles, in the lamina propria, and in the submucosa (Fig. 4). In the colon, cells positive for CD3, CD4, or CD8 were located in the intrafollicular regions of Peyer’s patches and in the submucosa surrounding the glands of the colon. FoxP3+ cells were located within the submucosa and associated with the endothelium of the muscularis layers. In contrast to T cell locations, the B cell markers, CD20 or CD79, were found on nearly all cells of the follicles (Fig. 4), with strong staining observed in the dense coronal cells surrounding germinal centers. B cells expressing these markers were also found occasionally in the interfollicular regions of Peyer’s patches and in the lamina propria. Cells expressing CD68 were most abundant in the lamina propria of villi tips and in the germinal centers of Peyer’s patch follicles. Cells positive for the dendritic marker, CD205, were located within the deep crypt epithelium and in the germinal centers of follicles.
Sequential sections of second trimester, third trimester, or postnatal gut tissues were stained with CD4 or CCR5 to assess regions of potential co-expression of these selected markers. As noted previously, CD4 expression increased in the ileum, jejunum, and colon from the second to the third trimesters, and was observed primarily in the submucosa and Peyer’s patches. In adjacent sections, CCR5+ cells were noted in similar locations as CD4+ cells, suggesting the possibility of CD4+CCR5+ cells in these locations both before and after birth (Fig. 5). Expression of CCR5 was more abundant than CD4 in the submucosa, lamina propria, and Peyer’s patches, and was also noted in some cells of the villous epithelium.
Figure 5. Expression of CD4 and CCR5 in the developing colon of rhesus monkeys.
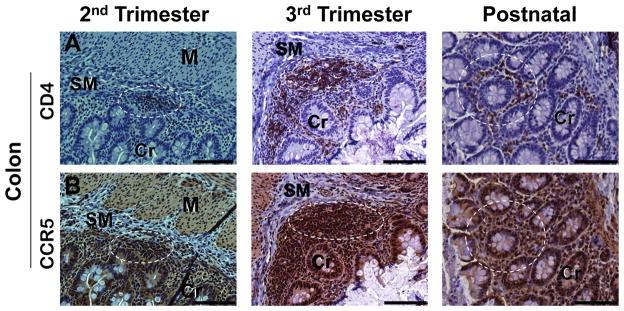
A. Cells expressing CD4 were found in developing Peyer’s patches, submucosa (SM), and lamina propria surrounding the crypts (Cr). CD4+ cells were not observed in the muscularis (MM). B. Cells expressing CCR5 were observed in the epithelium, Peyer’s patches, submucosa (SM), and lamina propria at mid-gestation and at later gestational ages. In sequential sections, CCR5+ cells were noted in locations where CD4+ cells were found (representative regions highlighted with dotted circles). All images 20x. Calibration bar = 100 μm.
Bone Marrow
The developing long bones present in the late first trimester contained large regions of hyaline cartilage with epiphyseal plates including reserve, proliferating, and hypertrophic cartilage regions. There was some evidence of endochondral ossification and small, primitive marrow spaces with no indication of positive staining for the markers tested (Fig. 6). The B cell markers, CD20 or CD79, were found on individual cells in the marrow space as was the macrophage marker, CD68. Cells positive for T cell markers (CD3, CD4, CD8, or FoxP3) or the dendritic cell marker, CD205, were not observed. With growth and ossification in the third trimester, the medullary cavity increased in size and contained hematopoietic cells and a few adipose and reticular cells. Many cells were positive for CD20, CD79, or CD68. A few cells stained positive for CD3. In comparison to prenatal bone marrow, the postnatal bone marrow contained numerous adipocytes and reticular cells. Of the T cell markers, only CD3 was noted. In comparison to the late third trimester, CD68+ cells were less frequent in the marrow cavity, while CD20+ or CD79+ cells were found with greater frequency.
Figure 6. Lymphoid and myeloid ontogeny in rhesus monkey bone marrow.
A. Developing long bones were composed largely of cartilage (C) and epiphyseal plates (EP) in the first trimester. B. Medullary cavities in the second trimester contained individual cells positive for CD20 (arrows) or CD68 (arrows), but not CD3. C. CD68 was noted with greater frequency than CD3 or CD20 in the third trimester marrow cavities. Arrows denote representative positive cells within CD3 or CD20 panels. D. Postnatal long bones at 3 months contained adipose (A) and reticular cells (R) with modest expression of CD3 and CD68 and frequent expression of CD20. All images 20x except third trimester CD20 (40x). Calibration bar = 100 μm.
DISCUSSION
In this study, we have conducted a comprehensive analysis of lymphoid and myeloid lineage development in the fetal and infant rhesus monkey. The structural development of the liver and thymus was more advanced in the first trimester than that of the spleen, axillary and mesenteric lymph nodes, GALT, and bone marrow. Of the antigens tested, CD3 was the earliest lymphoid marker expressed and was found in the first trimester thymus and, to a lesser extent, spleen. The myeloid marker, CD68, was found on cells near the blood islands in the liver in the late first trimester. B cell markers were first observed mid-second trimester in the liver, spleen, thymus, lymph nodes, bone marrow spaces, and occasionally in GALT. T cell markers were also expressed at this time during gestation on cells of the liver, spleen, thymus, and in Peyer’s patches of the jejunum, ileum, and colon. In contrast, T cell markers selected were not observed in the bone marrow medullary spaces at any gestational age and only staining for CD3 was noted at three months postnatal age in this location. CCR5, an essential T cell co-receptor for HIV, was abundant in the submucosa and lamina propria of the gut and in Peyer’s patches in the second and third trimesters, and co-localized with CD4+ cells. Follicles were evident in the spleen and thymus in the second trimester but were not observed in lymph nodes or Peyer’s patches until the early third trimester. By the late third trimester, secondary follicles with germinal centers were present in the spleen, thymus, and lymph nodes. Cells expressing the dendritic marker, CD205, were noted in the spleen, thymus, and axillary and mesenteric lymph nodes in the mid-second trimester but not until the third trimester in GALT, and were not observed in the liver or bone marrow cavities in this study.
Macrophages are a key component of the immune response, serving as a member of the innate immune system to eliminate pathogens by receptor-mediated phagocytosis or to stimulate adaptive immune responses as antigen-presenting cells. CD68, a macrophage marker and low-density lipoprotein receptor, was the earliest myeloid marker to be expressed in the developing first trimester liver, was noted in the thymus and spleen by the early second trimester, and was found in the tips of the lamina propria and Peyer’s patches of the gastrointestinal (GI) tract by the late second trimester. Kupffer cells represented the largest population of resident macrophages in the liver, with a phagocytic subset of these cells identified by CD68 (Klein et al., 2007; Kinoshita et al., 2011). Studies of the ontogeny of these cells in mice have shown that Kupffer cells proliferate and renew independently from bone marrow (Klein et al, 2007) and without the transcription factor Myb (Schulz et al., 2012) suggesting a potential bone marrow or yolk sac origin in this species. While the origin of Kupffer cells in nonhuman primates has not been studied, the observation here of CD68+ cells in the first trimester liver is interesting, as positive cells were not noted in developing bone, and marrow hematopoiesis is not yet established at this gestational age. Additional studies at earlier gestational ages and with additional markers may further define macrophage emergence and clarify Kupffer cell origin.
Studies on immune system development in humans provide evidence that fetal T cell compartments are ontologically and functionally distinct from adult T cell compartments (Byrne et al., 1994; Zhao et al., 2002). Fetal T cells, previously thought to be functionally immature, were found to be highly reactive when depleted of CD4+CD25high Tregs (Michaëlsson et al., 2006), and fetal naïve CD4+ T cells, compared with adult naïve CD4+ T cells, were shown to preferentially express FoxP3 when activated (Mold et al., 2008). The concept of a “layered” developmental sequence suggests that early fetal T cells are primed to differentiate into Tregs for active suppression by promoting a tolerogenic response to antigens encountered in utero, while adult T cells are more likely to engage in immunoreactive responses to foreign antigens (Mold et al., 2010; 2011). Of the T cell markers included in this study, CD3 was most strongly expressed in the thymus at all gestational ages investigated. In the spleen, CD3 expression was noted more frequently at mid-gestation than at three months postnatal age. Expression of CD4, CD8, or FoxP3 (a Treg marker) was more abundant in mid-gestation than after birth in the thymus and spleen. This pattern was not noted in the axillary and mesenteric lymph nodes or in GALT, where the T cell markers typically increased in frequency from the second trimester into the postnatal period. Additional studies involving multiple phenotypic markers and functional analysis are needed to determine the role these cells may play in the development of tolerance in nonhuman primates. Understanding the mechanisms by which tolerance is induced in utero and the corresponding effects on the early postnatal immune system is vital to developing optimal strategies for preventing transmission of pandemic diseases such as HIV and hepatitis C from mother to child (Babik et al., 2011). In these and other infectious diseases, differences in immune ontogeny between human and rodent models necessitate studies in nonhuman primates where disease pathogenesis more closely mimics humans. Transmission of simian immunodeficiency virus (SIV) in monkeys occurs by the same routes (e.g., in utero, breast-feeding) as HIV infection in humans and with similar disease progression (Abel, 2009). A rapid and irreversible CD4+ T cell loss in the GI tract has been noted in infected infants, comparable to findings in humans, and may reflect a carryover of the fetal immune environment skewed toward tolerance and thus primed for suppression by Tregs (Miller et al., 2005; Abel et al., 2006; Hartigan-O’Connor et al., 2007) and/or an otherwise altered susceptibility of intestinal CD4+ T cells for infection and destruction by SIV. FoxP3 was observed more frequently in the third trimester than after birth in the GI tract in this study, also suggesting a potential immune suppressive environment at this stage of development in rhesus monkeys.
Identification of cell populations important for mother-to-child transmission of HIV is an important step in the development of preventive or therapeutic strategies aimed at elimination of vertical transmission. The infant rhesus monkey intestinal epithelium was previously shown to contain a population of proliferating CD4+ T cells with an activated phenotype with implications for HIV transmission (Wang et al., 2007; 2010). CCR5, a chemokine receptor and HIV co-receptor was shown in this study to be widely expressed in the GI epithelium of monkeys in the second and third trimesters and postnatally. Although CD4+CCR5+ double staining was not performed, single staining of sequential sections showed an overlap in expression and strongly supports the presence of CD4+CCR5+ T cells from mid-gestation onward in the rhesus monkey. In humans, the infant GI mucosa was recently shown to contain a population of CD4+CCR5+ cells highly susceptible to HIV (Bunders et al., 2013). CD68+ cells, another potential target for HIV, were also noted in CCR5+ locations suggesting the presence of CD68+CCR5+ cells. Additional studies to explore these populations of cells in the rhesus monkey model before and after birth will be important for further development of new treatments targeting these age groups.
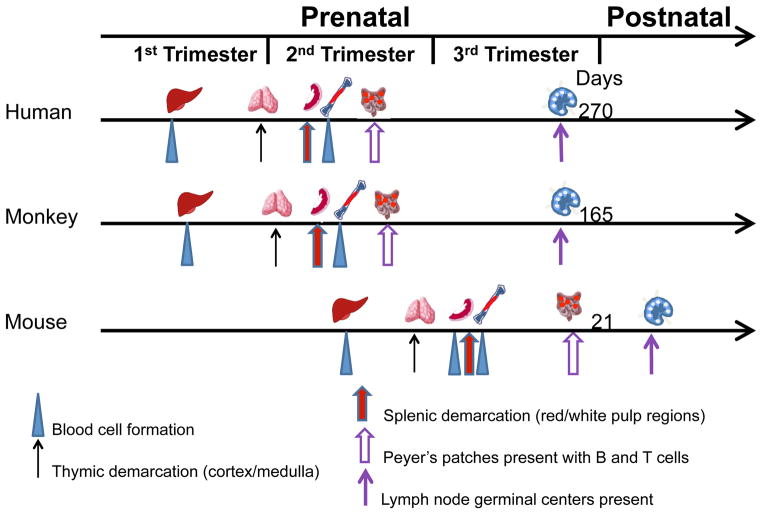
Differences in immune ontogeny between humans and rodents are well documented and highlight crucial disparities that may influence therapeutic outcomes for the developing fetus and infant (Mold and McCune, 2012). The results presented here, together with other reports (Makori et al., 2003), suggest immune ontogeny in the monkey is similar in temporal and anatomical sequence to human development (Fig. 6). The fetal liver assumes a major role in hematopoiesis beginning in the mid-first trimester in humans (reviewed in Holt and Jones, 2000; Holsapple et al., 2003) and in monkeys (Sharma et al., 1991; Tarantal, unpublished) but not until mid-gestation in mice (reviewed in Holsapple et al., 2003). Similarly, hematopoietic progenitors seed the thymus at the end of the first trimester in humans (Jotereau et al., 1987; Haynes et al., 1988; Ygberg et al., 2012) and monkeys, but not until mid-gestation in mice (Douagi et al., 2000; Pearse et al., 2006; Gordon et al., 2011; Rossi et al., 2006). Hassall’s corpuscles, believed to be involved in development of central tolerance by converting self-reactive thymocytes into Tregs (Watanabe et al., 2005) were noted in monkeys beginning in the second trimester, as previously observed in humans, but are poorly developed in rodents even after birth (Farr et al., 2002). Developing human (Cupedo et al., 2004; Ygberg and Nilsson, 2012) and monkey lymph nodes contained B and T lymphocytes by mid-gestation, with follicles present by the late second trimester and germinal centers noted before birth. In contrast, the mouse lymph node anlagen remains poorly organized at birth with follicle development occurring in the early postnatal period (Cupedo et al., 2004; Drayton et al., 2006). The appearance of B and T lymphocytes in Peyer’s patches of developing intestinal tissue occurs at E18.5 in mice (Adachi et al., 1997; Eberl et al., 2009), much later in gestation than humans (Braegger et al., 1992; Holsapple et al 2003; Ygberg and Nilsson, 2012) and rhesus monkeys. In addition to differences in temporal ontological development, humans and rodents appear to have very different responses to acute inflammatory stressors. Thus, the human transcriptional response to a variety of these stressors is surprisingly conserved, while mouse models of the same stressors show little or no correlation with the genes affected, the signaling pathways involved, or the timing of the response (Seok et al., 2013).
The developing fetus may be exposed to a variety of potential antigenic stimulants including maternal cells, infectious microbes or particles, auto-antigens, or allergenic proteins and molecules. Appropriate responses, or the lack thereof, likely affect the success of the pregnancy and establish patterns that carry forward to affect the health of the infant, and perhaps even throughout adult life. Studies of immune ontogeny are essential as new therapeutic strategies are developed for the fetus and infant, and may suggest new approaches to ensure adult health and to protect against diseases across the lifespan. The insights into myeloid and lymphoid development in the rhesus monkey provided here will aid in accelerating future translational studies in this important preclinical primate model system of human health and disease.
Figure 7. Temporal sequence of immune ontogeny in human, monkey, and mouse.
The liver is the major site of hematopoiesis in human and nonhuman primates from the mid-first trimester until bone marrow hematopoiesis is established in the mid-second trimester. Thymic and splenic demarcation is completed by mid-gestation followed by the presence of B and T cells within Peyer’s patches prior to the third trimester. Lymph node morphogenesis including formation of germinal centers is completed prior to birth in both species. In contrast, the mouse immune system is immature at birth with developmental events beginning in mid-gestation and with little or no development of Peyer’s patches and lymph nodes until after birth.
Acknowledgments
Grant sponsor: NIH grant numbers AI084109, AI090677, and OD011107
These studies were supported by grants from the National Institutes of Health (#AI084109 and #AI090677) and from the Bill and Melinda Gates Foundation (#OPP1024421) (to J.M.M.), and the California National Primate Research Center base grant (#OD011107).
LITERATURE CITED
- AARDA. The Cost Burden of Autoimmune Disease: The Latest Front in the War on Healthcare Spending, 2011. AARDA.org. 2011 Available at: http://www.aarda.org/pdf/cbad.pdf.
- Abel K. The rhesus macaque pediatric SIV infection model – a valuable tool in understanding infant HIV pathogenesis and for designing pediatric HIV prevention strategies. Curr HIV Res. 2009;7:2–11. doi: 10.2174/157016209787048528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel K, Pahar B, Van Rompay KK, Fritts L, Sin C, Schmidt K, Colon R, McChesney M, Marthas ML. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J Virol. 2006;80:6357–6367. doi: 10.1128/JVI.02240-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Yoshida H, Kataoka H, Nishikawa S-I. Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol. 1997;9:507–514. doi: 10.1093/intimm/9.4.507. [DOI] [PubMed] [Google Scholar]
- Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG. 2011;118:136–144. doi: 10.1111/j.1471-0528.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babik JM, Cohan D, Monto A, Hartigan-O’Connor DJ, McCune JM. The human fetal immune response to hepatitis C virus exposure in utero. J Inf Dis. 2011;203:196–206. doi: 10.1093/infdis/jiq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelder CA, Lee CCI, Martinez ML, Tarantal AF. Ontogeny of the kidney and renal developmental markers in the rhesus monkey (Macaca mulatta) Anat Rec. 2010;293:1971–1983. doi: 10.1002/ar.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer SA, Arndt TP, Leslie KE, Pearl DL, Turner PV. Obesity in rhesus and cynomolgus macaques: A comparative review of the condition and its implications for research. Comp Med. 2011;61:514–526. [PMC free article] [PubMed] [Google Scholar]
- Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, Bernstein ID. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988;81:951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontrop RE. Non-human primates: essential partners in biomedical research. Immunol Rev. 2001;183:5–9. doi: 10.1034/j.1600-065x.2001.1830101.x. [DOI] [PubMed] [Google Scholar]
- Braegger CP, Spencer J, MacDonald TT. Ontogenetic aspects of the intestinal immune system in man. Int J Clin Lab Res. 1992;22:1–4. [PubMed] [Google Scholar]
- Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel PJ. Fructose-fed rhesus monkeys: A nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2011;4:243–252. doi: 10.1111/j.1752-8062.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunders MJ, van der Loos CM, Klarenbeek PL, van Hamme JL, Boer K, Wilde JCH, van Lier RAW, Kootstra N, Pals ST, Kuijpers TW. Memory CD4+CCR5+ T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood. 2012;120:4383–4390. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Stankovic AK, Cooper MD. A novel subpopulation of primate T cells in the human fetus. J Immunol. 1994;152:3098–3106. [PubMed] [Google Scholar]
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Cowan MJ, Chou S-H, Tarantal AF. Tolerance induction post in utero stem cell transplantation. In: Holzgreve W, Lessl M, editors. Stem Cells from Cord Blood, In Utero Stem Cell Development, and Transplantation-Inclusive Gene Therapy; Ernst Schering Research Foundation Workshop #33; New York: Springer-Verlag; 2001. pp. 145–196. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Lund FE, Ngo VN, Randall TD, Jansen W, Greuter MJ, de Waal-Malefyt R, Kraal G, Cyster JG, Mebius RE. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CSC chemokine ligand 13. J Immunol. 2004;173:4889–4896. doi: 10.4049/jimmunol.173.8.4889. [DOI] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Djaldetti M, Bessler H, Rifkind RA. Hematopoiesis in the embryonic mouse spleen: An electron microscopic study. Blood. 1972;39:826–841. [PubMed] [Google Scholar]
- Donahue RE, Dunbar CE. Update on the use of nonhuman primate models for preclinical testing of approaches targeting hematopoietic cells. Hum Gene Ther. 2001;12:607–617. doi: 10.1089/104303401300057289. [DOI] [PubMed] [Google Scholar]
- Donahue RE, Kuramoto K, Dunbar CE. Large animal models for stem and progenitor cell analysis. Curr Protoc Immunol. 2005;69:22A.1.1–22A.1.29. doi: 10.1002/0471142735.im22a01s69. [DOI] [PubMed] [Google Scholar]
- Douagi I, André I, Ferraz J-C, Cumano A. Characterization of T cell precursor activity in the murine fetal thymus: evidence for an input of T cell precursors between days 12 and 14 of gestation. Eur J Immunol. 2000;30:2201–2210. doi: 10.1002/1521-4141(2000)30:8<2201::AID-IMMU2201>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CCR5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nature Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009;2:478–485. doi: 10.1038/mi.2009.114. [DOI] [PubMed] [Google Scholar]
- Farr AG, Dooley JL, Erickson M. Organization of thymic medullary epithelial heterogeneity: implications for mechanisms of epithelial differentiation. Immunol Rev. 2002;189:20–27. doi: 10.1034/j.1600-065x.2002.18903.x. [DOI] [PubMed] [Google Scholar]
- Gardner MB, Luciw PA. Macaque models of human infectious disease. ILAR. 2008;49:220–255. doi: 10.1093/ilar.49.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Gibbons DL, Spencer J. Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal Immunol. 2011;4:148–157. doi: 10.1038/mi.2010.85. [DOI] [PubMed] [Google Scholar]
- Gordon J, Manley NR. Mechanisms of thymus organogenesis and morphogenesis. Development. 2011;138:3865–3878. doi: 10.1242/dev.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BD, Bodkin NL. Heterogeneity of insulin responses: phases leading to type 2 (non-insulin-dependent) diabetes mellitus in the rhesus monkey. Diabetologia. 1986;29:713–719. doi: 10.1007/BF00870281. [DOI] [PubMed] [Google Scholar]
- Hartigan-O’Connor DJ, Abel K, McCune JM. Suppression of SIV-specific CD4+ T cells by infant but not adult macaque regulatory T cells: implications for SIV disease progression. J Exp Med. 2007;204:2679–2692. doi: 10.1084/jem.20071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Martin ME, Kay HH, Kurtzberg J. Early events in human T cell ontogeny. J Exp Med. 1988;168:1061–1080. doi: 10.1084/jem.168.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring MJ, Avdalovic MV, Quesenberry CL, Putney LF, Tyler NK, Ventimiglia FF, St George JA, Hyde DM. Accelerated structural decrements in the aging female rhesus macaque lung compared with males. Am J Physio Lung Cell Molec Physiol. 2013;304:L125–L134. doi: 10.1152/ajplung.00226.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsapple MP, West LJ, Landreth KS. Species comparison of anatomical and functional immune system development. Birth Defects Res B Dev Reprod Toxicol. 2003;68:321–334. doi: 10.1002/bdrb.10035. [DOI] [PubMed] [Google Scholar]
- Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- Hoorweg K, Cupedo T. Development of human lymph nodes and Peyer’s patches. Semin Immunol. 2008;20:164–170. doi: 10.1016/j.smim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Horn PA, Thomasson BM, Wood BL, Andrews RG, Morris JC, Kiem HP. Distinct hematopoietic stem/progenitor cells are responsible for repopulation in NOD/SCID mice compared with nonhuman primates. Blood. 2003;102:4329–4335. doi: 10.1182/blood-2003-01-0082. [DOI] [PubMed] [Google Scholar]
- Jotereau F, Heuze F, Salomon-Vie V, Gascan H. Cell kinetics in the fetal mouse thymus: Precursor cell input, proliferation, and emigration. J Immunol. 1987;138:1026–1030. [PubMed] [Google Scholar]
- Jung C, Hugot J-P, Barreau F. Peyer’s patches: The immune sensors of the intestine. Int J Inflamm. 2010 doi: 10.406/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Uchida T, Sato A, Nakashima M, Nakashima H, Shono S, Habu Y, Miyazaki H, Hiroi S, Seki S. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J Hepatol. 2010;53:903–910. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]
- Klein I, Cornejo JC, Polakos NK, Beena J, Wuensch SA, Topham DJ, Pierce RH, Crispe IN. Kupffer cell heterogeneity: functional properties of bone marrow-derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner AA, Veazey RW. Current concepts in AIDS pathogenesis: Insights from the SIV/macaque model. Annu Rev Med. 2007;58:461–476. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- Lee CI, Goldstein O, Han VKM, Tarantal AF. IGF-II and IGF binding protein (IGFBP-1, IGFBP-3) gene expression in fetal rhesus monkey tissues during the second and third trimesters. Pediatr Res. 2001;49:379–387. doi: 10.1203/00006450-200103000-00012. [DOI] [PubMed] [Google Scholar]
- MacDonald TT, Spencer J. Ontogeny of the gut-associated lymphoid system in man. Acta Paediatr Suppl. 1994;395:3–5. doi: 10.1111/j.1651-2227.1994.tb13219.x. [DOI] [PubMed] [Google Scholar]
- Makori N, Tarantal AF, Lü FX, Rourke T, Marthas ML, McChesney MB, Hendrickx AG, Miller CJ. Functional and morphological development of lymphoid tissues and immune regulatory and effector function in rhesus monkeys. Clin Diagn Lab Immunol. 2003;10:140–153. doi: 10.1128/CDLI.10.1.140-153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Mezquita P, Beard BC, Kiem H-P. NOD/SCID repopulating cells contribute only to short-term repopulation in the baboon. Gene Ther. 2008;15:1460–1462. doi: 10.1038/gt.2008.108. [DOI] [PubMed] [Google Scholar]
- Michaëlsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Orkin SA. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3344. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, McCune JM. At the crossroads between tolerance and aggression: Revisiting the “layered immune system” hypothesis. Chimerism. 2011;2:35–41. doi: 10.4161/chim.2.2.16329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, McCune JM. Immunological tolerance during fetal development: From mouse to man. Adv Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond DG. The ontogeny and organization of the lymphoid system. J Invest Dermatol. 1985;85:2s–9s. doi: 10.1111/1523-1747.ep12275397. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:242–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse G. Normal structure, function and histology of the thymus. Tox Pathol. 2006;34:504–514. doi: 10.1080/01926230600865549. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Hyde DM. The non-human primate as a model for studying COPD and asthma. Pulm Pharmacol Ther. 2008;21:755–766. doi: 10.1016/j.pupt.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Rose NR. In: Morton CC, Fagan T, editors. An immunology primer; Proceedings from Sex Differences in Immunology and Autoimmunity; Washington: Society for Women’s Health Research; 2002. pp. 7–9. [Google Scholar]
- Rossi S, Jenkinson WE, Anderson G, Jeninson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 2006;44:988–991. doi: 10.1038/nature04813. [DOI] [PubMed] [Google Scholar]
- Russell GJ, Bhan AK, Winter HS. The distribution of T and B lymphocyte populations and MHC class II expression in human fetal and postnatal intestine. Pediatr Res. 1990;27:239–244. doi: 10.1203/00006450-199003000-00007. [DOI] [PubMed] [Google Scholar]
- Satoh T, Sakurai E, Tada H, Masuda T. Ontogeny of reticular framework of white pulp and marginal zone in human spleen: Immunohistochemical studies of fetal spleens from the 17th to the 40th week of gestation. Cell Tissue Res. 2009;336:287–297. doi: 10.1007/s00441-009-0757-2. [DOI] [PubMed] [Google Scholar]
- Schulz C, Perdiguero EG, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SEW, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells in dependent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Karak A, Sharma DN, Aggarwal S. Hemopoietic stem-cell differentiation in fetal liver, spleen and thymus of rhesus monkeys. Med Oncol Tumor Pharmacother. 1991;8:113–144. doi: 10.1007/BF02988863. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. The unique value of primate models in translational research. Am J Primatol. 2009;72:715–721. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- Spencer J, Dillon SB, Isaacson PG, MacDonald TT. T cell subclasses in fetal human ileum. Clin Exp Immunol. 1986a;65:553–558. [PMC free article] [PubMed] [Google Scholar]
- Spencer J, MacDonald TT, Finn T, Isaacson PG. The development of gut associated lymphoid tissue in the terminal ileum of fetal human intestine. Clin Exp Immunol. 1986b;64:536–543. [PMC free article] [PubMed] [Google Scholar]
- Sykes M. Hematopoietic cell transplantation for tolerance induction: Animal models to clinical trials. Transplantation. 2009;87:309–316. doi: 10.1097/TP.0b013e31819535c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantal AF. Ultrasound imaging in rhesus (Macaca mulatta) and long-tailed (Macaca fascicularis) macaques: Reproductive and research applications. In: Wolfe-Coote S, editor. The Laboratory Primate. Amsterdam: Elsevier; 2005. pp. 317–351. [Google Scholar]
- Tarantal AF, Han VKM, Cochrum KC, Mok A, daSilva M, Matsell DG. Fetal rhesus monkey model of obstructive renal dysplasia. Kidney Int. 2001;59:446–456. doi: 10.1046/j.1523-1755.2001.059002446.x. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Nakayama KH. Use of large animal and nonhuman primate models for cell therapy and tissue engineering. In: Bernstein HS, editor. Tissue Engineering in Regenerative Medicine, Stem Cell Biology and Regenerative Medicine series. New York: Humana Press; 2011. pp. 393–413. [Google Scholar]
- Tarantal AF, Skarlatos SI. Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood Diseases: an NHLBI resource for the gene therapy community. Hum Gene Ther. 2012;23:1130–1135. doi: 10.1089/hum.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Kiem H-P. Large animal models of hematopoietic stem cell gene therapy. Gene Ther. 2010;17:939–948. doi: 10.1038/gt.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, Veazey RS. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood. 2007;109:1174–1181. doi: 10.1182/blood-2006-04-015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu H, Pahar B, Alvarez X, Green LC, Dufour J, Moroney-Rasmussen T, Lackner AA, Veazey RS. Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood. 2010;116:4168–4174. doi: 10.1182/blood-2010-03-273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Wang Y-H, Lee HK, Ito T, Wang Y-H, Cao W, Liu Y-J. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- Weitkamp J-H, Rudzinski E, Koyama T, Correa H, Matta P, Alberty B, Polk D-P. Ontogeny of FoxP3+ regulatory T cells in the postnatal human small intestinal and large intestinal lamina propria. Pediatr Dev Pathol. 2009;12:443–440. doi: 10.2350/08-09-0533.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ygberg S, Nilsson A. The developing immune system – from foetus to toddler. Acta Paediatr. 2012;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dai Z-P, LVP, Gao X-M. Phenotypic and functional analysis of human T lymphocytes in early second and third-trimester fetuses. Clin Exp Immunol. 2002;129:302–308. doi: 10.1046/j.1365-2249.2002.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]