Abstract
Background
Apparent treatment resistant hypertension (aTRH) is defined as uncontrolled hypertension despite the use of ≥3 antihypertensive medication classes or controlled hypertension while treated with ≥ 4 antihypertensive medication classes. We evaluated the association of aTRH with incident stroke, coronary heart disease (CHD) and all-cause mortality.
Methods
Participants from the population-based REasons for Geographic And Racial Differences in Stroke (REGARDS) Study treated for hypertension with aTRH (n=2,043) and without aTRH (n=12,479) were included. aTRH was further categorized as controlled aTRH (≥ 4 medication classes and controlled hypertension) and uncontrolled aTRH (≥ 3 medication classes and uncontrolled hypertension).
Results
Over a median of 5.9, 4.4, and 6.0 years of follow-up the multivariable adjusted hazard ratio for stroke, CHD, and all-cause mortality associated with aTRH versus no aTRH was 1.25 (0.94 – 1.65), 1.69 (1.27 – 2.24), and 1.29 (1.14 – 1.46), respectively. Compared to controlled aTRH, uncontrolled aTRH was associated with CHD (HR=2.33; 95% CI 1.21 – 4.48), but not stroke or mortality. Comparing controlled aTRH to no aTRH, risk of stroke, CHD and all-cause mortality was not elevated.
Conclusion
aTRH was associated with an increased risk for coronary heart disease and all-cause mortality.
Keywords: resistant hypertension, outcomes, severe hypertension, antihypertensives
Introduction
Treatment resistant hypertension (TRH) is defined as uncontrolled hypertension despite the use of ≥ 3 antihypertensive medication classes or controlled hypertension while treated with ≥ 4 antihypertensive medication classes 1. Although the definition of TRH is widely accepted and commonly applied in research, the term apparent TRH (aTRH) has been used for population-based studies unable to exclude cases of pseudoresistance 2. Using data from the National Health And Nutrition Examination Survey (NHANES) 2005-2008 Egan and colleagues estimated 11.8% of hypertensive US adults have aTRH2. aTRH prevalence estimates >10% among persons with hypertension have been reported in several other studies 3-5.
Hypertension is a major, modifiable risk factor for stroke, coronary heart disease (CHD) and all-cause mortality 6-8. Cross-sectional studies have found that, among those with hypertension, persons with aTRH have an increased burden of cardiovascular disease (CVD) risk factors and a higher 10-year Framingham coronary heart disease (CHD) risk score 2-4. However, few data are available from prospective studies on the risk for CVD among people with aTRH. The goal of the current study was to determine whether aTRH is associated with an increased risk for CVD. To do so, we evaluated the risk for stroke, CHD and all-cause mortality among 2,043 REasons for Geographic And Racial Differences in Stroke (REGARDS) participants with aTRH relative to 12,479 REGARDS participants with controlled hypertension treated with < 4 antihypertensive medication classes or uncontrolled hypertension treated with 1 or 2 antihypertensive medication classes. aTRH can be stratified into two subgroups including those with controlled hypertension on ≥4 antihypertensive medication classes (controlled aTRH) and uncontrolled hypertension on ≥ 3 antihypertensive medication classes (uncontrolled aTRH) 1. Given the association between level of blood pressure (BP) while on antihypertensive treatment and CVD, we also evaluated the risk for stroke, CHD and all-cause mortality among REGARDS participants with uncontrolled versus controlled aTRH 9,10.
Methods
Study Population
The design of the REGARDS study has been described previously 11. Briefly, adults ≥ 45 years of age from all 48 continental US states and the District of Columbia were included. A total of 30,239 individuals were enrolled into the study between January 2003 and October 2007. By design, the study oversampled blacks and residents of the “stroke belt” and “stroke buckle” regions of the US. The “stroke buckle” was defined as coastal North Carolina, South Carolina, and Georgia and the “stroke belt” as the remainder of North Carolina, South Carolina, and Georgia as well as Alabama, Mississippi, Tennessee, Arkansas and Louisiana. We restricted the current analysis to REGARDS participants who were treated for hypertension as determined by both pill bottle review and self-reported use of antihypertensive medication (n=14,811). We excluded participants without valid BP measurements at baseline (n=45) or missing follow up data (n=244). After these exclusions, 14,522 REGARDS participants who were treated for hypertension were included in the analysis of all-cause mortality (Supplemental Figure 1). Of this group 1,342 participants reported a history of stroke and were excluded from the analysis of incident stroke and 3,347 participants had a history of CHD and were excluded from the analysis of incident CHD. The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided informed consent.
Data Collected at Baseline
Computer-assisted telephone interviews administered by trained staff were used to collect information on participants’ age, race, sex, smoking status, education, physical activity, alcohol consumption, prior physician diagnosed co-morbid conditions (e.g., stroke and myocardial infarction), medication adherence, and age when first diagnosed with hypertension 12. Medication adherence was assessed by the 4-item Morisky Medication Adherence Scale (MMAS). MMAS scores have a range from 0 to 4 with higher scores indicating worse adherence 12. Duration of hypertension was calculated as the age at diagnosis subtracted from age at the baseline examination. Subsequent to the interview, trained health professionals conducted in-home examinations that included weight, height, BP measurements, an electrocardiogram (ECG), the collection of blood and a spot urine sample, plus a review of prescription and over the counter medications used over the prior 2 week period. C-reactive protein (hsCRP) was determined by particle enhanced immunonephelometry using the BNII nephelometer (N High Sensitivity CRP; Dade Behring, Deerfield, IL). Total and high-density lipoprotein (HDL) cholesterol and glucose were measured by colorimetric reflectance spectrophotometry using the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics, New Brunswick, NJ). Medication names were recorded and subsequently coded into drug classes. Information on medication dose was not recorded. History of CHD at baseline was defined by a self-reported history or ECG evidence of myocardial infarction (MI) or a self-reported history of a revascularization procedure. History of stroke was defined on the basis of self-report. Diabetes was defined as a serum glucose ≥126 mg/dL for participants who had fasted prior to their blood draw, serum glucose ≥200 mg/dL for those who had not fasted, self-report of a prior diagnosis of diabetes while not pregnant, or current use of insulin or oral hypoglycemic medications. Using isotope-dilution mass spectrometry (IDMS)–traceable serum creatinine, estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation 13. Low eGFR was defined as levels < 60 ml/min/1.73 m2. Albuminuria was defined as being present for individuals with a urinary albumin to urinary creatinine ratio ≥ 30 mg/g.
Blood Pressure measurement and definition of aTRH
During the in-home examination, BP was measured two times using aneroid sphygmomanometers following a standardized protocol by trained examiners. Participants were asked to sit for five minutes with both feet on the floor prior to the first BP measurement and there was a 30 second rest between measurements. Based on the average of the two measurements, uncontrolled hypertension was defined as systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg. Controlled hypertension was defined as SBP < 140 mm Hg and DBP < 90 mm Hg. Antihypertensive medication classes were defined as those listed in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) 14. Single-pill combinations were classified into individual medication classes. aTRH was defined as uncontrolled hypertension on ≥ 3 antihypertensive medication classes (uncontrolled aTRH) or controlled hypertension on ≥ 4 antihypertensive medication classes (controlled aTRH) 1. No aTRH was defined as controlled hypertension on < 4 classes of antihypertensive medication or uncontrolled hypertension on 1 or 2 classes of antihypertensive medication.
Data collected during follow-up
Living participants or their proxies were contacted every 6 months via telephone to assess new-onset stroke, CHD events and all-cause mortality. Medical records were retrieved for stroke and CHD-related hospitalizations and deaths. We studied the following outcomes in the current analyses.
Stroke
Stroke was assessed via self-report of a possible stroke/transient ischemic attack, a positive response to the stroke symptoms on the Questionnaire for Verifying Stroke-Free Status resulting in hospitalization during follow-up, or death related to stroke 15. When potential events were reported, hospital charts were retrieved for adjudication. Stroke was confirmed by a panel of neurologists according to the World Health Organization (WHO) definition 16. Events not meeting the WHO definition but characterized by symptoms lasting <24 hours with neuroimaging consistent with acute infarct or hemorrhage were classified as clinical strokes. Additionally, medical records in the last year of life, death certificates and autopsy reports were collected and reviewed to determine if the death was stroke related following guidelines described. This analysis included WHO–defined as well as clinical stroke cases 17. Data on incident stroke was available through September 30, 2011.
Coronary Heart Disease
The occurrence of CHD events, defined as nonfatal MI or CHD death, were also assessed during the follow-up telephone interviews. After report of a hospitalization or death that potentially could be related to CHD, medical records were retrieved and the event was adjudicated by trained clinicians following published guidelines 18,19. Specifically, medical records were examined for the presence of signs or symptoms suggestive of ischemia; a rising and/or falling pattern in cardiac troponin or creatine phosphokinase-MB over 6 or more hours with a peak value greater than or equal to twice the upper limit of normal (diagnostic cardiac enzymes); and ECG changes consistent with ischemia or MI, guided by the Minnesota code and classified as evolving diagnostic, positive, nonspecific or not consistent with ischemia 20,21. Additionally, medical records in the last year of life, death certificates and autopsy reports were collected and reviewed to determine if the death was a CHD death following published guidelines described 18,19. Incident CHD events through December 31, 2009 were available for the current analyses.
All-Cause Mortality
Participant deaths were detected by report of next-of-kin, online sources (e.g., the Social Security Death Index), and the National Death Index. To obtain information surrounding the circumstances of participant death, proxies or next-of-kin were interviewed. Additionally, death certificates and autopsy reports were collected. Deaths occurring through March 31, 2012 were included in the current analysis.
Statistical Analysis
Participant characteristics were calculated for those with aTRH and those without aTRH, overall, and for those with uncontrolled and controlled aTRH, separately. Incidence rates for stroke, CHD, and all-cause mortality were calculated by aTRH status and for participants with uncontrolled and controlled aTRH, separately. Cox proportional hazards regression models were used to calculate the hazard ratio (HR) for stroke, CHD and all-cause mortality associated with aTRH in comparison to no aTRH. HRs were initially adjusted for age, race, sex, and geographic region of residence (model 1) and further adjusted for waist circumference, smoking, physical activity, alcohol consumption, statin use, MMAS score, total cholesterol, HDL-cholesterol, hsCRP and duration of hypertension (model 2). Full covariate adjustment (model 3) included variables in models 1 and 2 plus low eGFR, albuminuria, and diabetes. In model 3, the HR for stroke was also adjusted for history of CHD, the HR for CHD was also adjusted for history of stroke and the HR for all-cause mortality was adjusted for history of CHD and history of stroke. Also, HRs for stroke, CHD and all-cause mortality were calculated comparing controlled aTRH to no aTRH, uncontrolled aTRH to no aTRH, and uncontrolled aTRH to controlled aTRH. The American Heart Association (AHA) definition states that persons with aTRH should ideally be treated with a diuretic1. Therefore, we conducted a sensitivity analysis requiring participants to be taking a diuretic in order to meet the definition of aTRH. For this analysis, we excluded 272 participants who met the definition of aTRH outlined above but who were not taking a diuretic. We were unable to determine if participants taking 1 or 2 antihypertensive medication classes with uncontrolled hypertension would have aTRH if they were more aggressively treated. Therefore, a second sensitivity analysis excluded 2,960 participants in the no aTRH group who were taking 1 or 2 antihypertensive medication classes with uncontrolled hypertension. Analyses were conducted using SAS v. 9.3 (SAS Institute, Cary, NC).
Results
Participant Characteristics
REGARDS participants with aTRH were older than their counterparts without aTRH, more commonly black and male and more likely to have a larger waist circumference, low eGFR, albuminuria, diabetes, and a history of CHD or stroke (Table 1). Persons with aTRH had lower total and HDL-cholesterol and were more likely to be taking statins than persons without aTRH. Duration of hypertension was longer among persons with aTRH, but was not different among subgroups within aTRH. Black race and albuminuria were more common among those with uncontrolled versus controlled aTRH. Diabetes, low eGFR and statin use were more common among those with controlled versus uncontrolled aTRH. The average number of antihypertensive medication classes was higher among those with controlled aTRH versus those with uncontrolled aTRH (4.2 ± 0.4 and 3.4 ± 0.6, respectively). More detailed information about medication classes being taken among those with and without aTRH is provided in Supplemental Table 1.
Table 1.
Baseline characteristics of Reasons for Geographic and Racial Differences in Stroke (REGARDS) study participants with and without apparent treatment resistant hypertension.
| No aTRH | aTRH | |||
|---|---|---|---|---|
|
| ||||
| Characteristic | Overall | Overall | Controlled | Uncontrolled |
|
| ||||
| n = 12,479 | n = 2,043 | n = 723 | n = 1,320 | |
|
| ||||
| Age, years | 66.1 ± 9.0 | 67.6± 8.6 | 67.2 ± 8.5 | 67.8 ± 8.7 |
|
| ||||
| Black, % | 48.6 | 60.5 | 55.3 | 63.3 |
|
| ||||
| Male, % | 41.9 | 49.2 | 51.9 | 47.8 |
|
| ||||
| Geographic Region of Residence, % | ||||
| Belt | 35.3 | 34.8 | 33.9 | 35.3 |
| Buckle | 21.5 | 20.7 | 23.4 | 19.2 |
| Other | 43.3 | 44.5 | 42.7 | 45.5 |
|
| ||||
| Smoking status, % | ||||
| Never | 44.4 | 43.8 | 42.5 | 44.4 |
| Past | 41.8 | 44.2 | 44.7 | 43.9 |
| Current | 13.8 | 12.1 | 12.7 | 11.7 |
|
| ||||
| Physical Activity, % | ||||
| None | 38.0 | 44.2 | 46.2 | 43.1 |
| 1 – 3 times per week | 35.0 | 33.3 | 32.2 | 33.9 |
| ≥4 times per week | 27.0 | 22.5 | 21.6 | 23.0 |
|
| ||||
| Alcohol consumption, % | ||||
| None | 66.6 | 70.1 | 69.2 | 70.5 |
| Moderate | 29.6 | 27.0 | 27.5 | 26.6 |
| Heavy | 3.8 | 3.0 | 3.3 | 2.9 |
|
| ||||
| MMAS score, % | ||||
| 0 | 69.6 | 67.1 | 69.1 | 66.1 |
| 1 | 23.1 | 24.6 | 24.6 | 24.6 |
| ≥ 2 | 7.4 | 8.3 | 6.4 | 9.4 |
|
| ||||
| Waist circumference, cm | ||||
| Men | 102.3 ± 13.4 | 107.2 ± 15.2 | 107.9± 14.1 | 106.8± 15.8 |
| Women | 96.1 ± 16.1 | 101.7 ± 17.3 | 101.5 ± 17.5 | 101.8± 17.3 |
|
| ||||
| HDL-cholesterol, mg/dL | 50.9± 15.9 | 48.2 ± 14.9 | 46.3 ± 15.1 | 49.3 ± 14.8 |
|
| ||||
| Total cholesterol, mg/dL | 188.1 ± 39.9 | 180.1 ± 40.0 | 172.8± 38.5 | 184.1 ± 40.2 |
|
| ||||
| hsCRP, mg/dL | 2.67 (1.14 – 6.04) | 2.86 (1.28 – 6.37) | 2.82 (1.26 – 6.29) | 2.88 (1.28 – 6.46) |
|
| ||||
| eGFR< 60 ml/min/1.73m2, % | 15.3 | 28.0 | 31.0 | 26.4 |
|
| ||||
| ACR ≥ 30 mg/g, % | 18.0 | 33.6 | 27.0 | 37.1 |
|
| ||||
| Statin use, % | 40.2 | 52.1 | 57.0 | 49.4 |
|
| ||||
| Diabetes, % | 28.6 | 46.2 | 49.5 | 44.4 |
|
| ||||
| History of coronary heart disease, % | 21.0 | 35.5 | 40.0 | 33.1 |
|
| ||||
| History of stroke, % | 8.5 | 13.6 | 13.8 | 13.4 |
|
| ||||
| Systolic blood pressure (mm Hg) | 129.6 ± 15.7 | 142.0 ± 18.3 | 123.9 ± 10.4 | 151.8 ± 13.4 |
|
| ||||
| Diastolic blood pressure (mm Hg) | 77.2 ± 9.5 | 79.7 ± 11.7 | 72.7 ± 8.8 | 83.5 ± 11.2 |
|
| ||||
| Hypertension duration (years) | 14.2 ± 12.2 | 20.7 ± 12.9 | 20.6 ± 12.9 | 20.7 ± 12.8 |
|
| ||||
| Number of antihypertensive medications | 1.8 ± 0.7 | 3.6 ± 0.7 | 4.2 ± 0.4 | 3.4 ± 0.6 |
aTRH = apparent treatment resistant hypertension; systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg with ≥ 3 antihypertensive medication classes (uncontrolled aTRH) or systolic blood pressure < 140 mmHg and diastolic blood pressure < 90 mm Hg and treatment with ≥ 4 antihypertensive medication classes (controlled aTRH).
No aTRH = no apparent treatment resistant hypertension; systolic blood pressure < 140 mmHg and diastolic blood pressure <90 mm Hg while treated with < 4 antihypertensive medication classes or systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mm Hg while treated with 1 or 2 antihypertensive medication classes.
eGFR = estimated glomerular filtration rate, ACR = albumin-to-creatinine ratio, hsCRP= high sensitivity creactive protein
Table numbers are % or mean ± standard deviation except hsCRP which is median (25th-75th percentiles).
MMAS=Morisky Medication Adherence Scale
Moderate alcohol consumption defined as between 1 and 7 drinks for women and between 1 and 14 drinks for men.
Heavy alcohol consumption defined as >7 drinks for women and >14 drinks for men.
aTRH and stroke, CHD and all-cause mortality
The median follow-up was 5.9, 4.4, and 6.0 years for the analysis of stroke, CHD, and all-cause mortality, respectively. During follow-up there were 83 strokes, 84 CHD events and 452 deaths among those with aTRH; and 348 strokes, 287 CHD events and 1,616 deaths among those without aTRH (Supplemental Table 2). Overall, 27.0% of deaths were due to cardiovascular causes. The incidence rates for all three outcomes were higher for those with versus without aTRH (Figure 1). Also, the incidence rate for each outcome was higher for participants with uncontrolled versus controlled aTRH in addition to controlled aTRH versus no aTRH.
Figure 1.
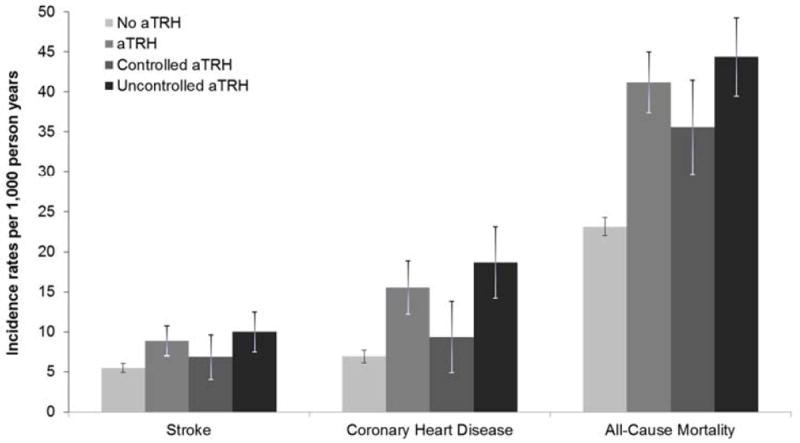
Incidence rates of stroke, coronary heart disease, and all-cause mortality among REGARDS participants with and without apparent treatment resistant hypertension.
aTRH = apparent treatment resistant hypertension; systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg with ≥ 3 antihypertensive medication classes (uncontrolled aTRH) or systolic blood pressure < 140 mmHg and diastolic blood pressure< 90 mm Hg and treatment with ≥ 4 antihypertensive medication classes (controlled aTRH).
No aTRH = no apparent treatment resistant hypertension; systolic blood pressure < 140 mmHg and diastolic blood pressure< 90 mm Hg while treated with < 4 antihypertensive medication classes or systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mm Hg while treated with 1 or 2 antihypertensive medication classes.
Bar represents incidence rate, line represents 95% confidence interval.
See Supplemental Table 2 for the incidence rates and 95% confidence intervals.
After adjustment for age, race, gender, and geographic region of residence (model 1) and further adjustment in model 2, aTRH was associated with increased HRs for stroke, CHD and all-cause mortality (Table 2). In the full multivariable adjusted model (model 3) aTRH in comparison to no aTRH was associated with CHD and all-cause mortality with HRs of 1.69 (1.27 – 2.24) and 1.29 (1.14 – 1.46), respectively. After full multivariable adjustment, the association of aTRH with stroke was not statistically significant (HR =1.25; 95% CI 0.94-1.65).
Table 2.
Hazard ratios for stroke, coronary heart disease, and all-cause mortality associated with apparent treatment resistant hypertension.
| Hazard Ratio (95% confidence interval)
|
Groups being compared
|
||||||
|---|---|---|---|---|---|---|---|
| Stroke | Coronary Heart | All-cause Mortality | Hypertension† | Number of antihypertensive medication | |||
| 1 or 2 | 3 | ≥ 4 | |||||
| Model 1 | 1.49 (1.17 – 1.89) | 2.07 (1.62 – 2.64) | 1.59 (1.43 – 1.77) | Controlled | Reference (no aTRH) | ||
| Model 2 | 1.42 (1.08 – 1.85) | 1.96 (1.49 – 2.58) | 1.56 (1.39 – 1.76) | Uncontrolled | Exposed (aTRH) | ||
| Model 3 | 1.25 (0.94 – 1.65) | 1.69 (1.27 – 2.24) | 1.29 (1.14 – 1.46) | ||||
Hypertension: Controlled: systolic blood pressure < 140 mm Hg and diastolic blood pressure < 90 mm Hg; Uncontrolled: systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg.
aTRH = apparent treatment resistant hypertension; uncontrolled hypertension and treatment with ≥ 3 antihypertensive medication classes or controlled hypertension and treatment with ≥ 4 antihypertensive medication classes.
No aTRH = no apparent treatment resistant hypertension; controlled hypertension and treatment with < 4 antihypertensive medication classes or uncontrolled hypertension and treated with 1 or 2 antihypertensive medication classes.
Model 1 adjusted for age, race, gender, and geographic region of residence.
Model 2 adjusted for variables in Model 1 plus waist circumference, smoking status, physical activity, alcohol consumption, C - reactive protein, statin use, Morisky medication adherence scale score ≥2, total cholesterol, HDL-cholesterol, and hypertension duration.
Model 3 adjusted for variables in Model 1 and 2 plus estimated glomerular filtration rate < 60 ml/min/1.73m2, albuminuria, and diabetes. Hazard ratios for stroke were also adjusted for history of coronary heart disease. Hazard ratios for coronary heart disease were also adjusted for history of stroke. Hazard ratios for all-cause mortality were also adjusted for history of coronary heart disease and stroke.
Participants in the shaded box were not included in the analysis.
When compared to participants with no aTRH, controlled aTRH was associated with an increased risk for all-cause mortality after initial adjustment (Table 3, panel A). This association was attenuated after further multivariable adjustment (model 3 HR=1.14; 95% CI 0.93 – 1.40). The HRs for stroke and CHD were not increased among those with controlled aTRH in comparison to those without aTRH. Compared to participants without aTRH, uncontrolled aTRH was associated with an increased risk for CHD and all-cause mortality; the risk for stroke was increased after initial adjustment, but the HR was attenuated after full multivariable adjustment in model 3 (Table 3, Panel B). Finally, uncontrolled aTRH was associated with a statistically significant increased risk for CHD in comparison to controlled aTRH (Table 4). The HR for stroke and all-cause mortality were not increased for uncontrolled versus controlled aTRH after adjustment for age, race, gender, and geographic region of residence or further multivariable adjustment. Results were markedly similar in sensitivity analyses requiring the use of a diuretic to meet the definition of aTRH (see Supplemental Tables 3-5). Finally, results were also similar after excluding participants with uncontrolled hypertension taking 1 or 2 antihypertensive medication classes from the group without aTRH (see Supplemental Tables 6-7).
Table 3.
Hazard ratios for stroke, coronary heart disease, and all-cause mortality associated with controlled apparent treatment resistant hypertension (Panel A) and uncontrolled apparent treatment resistant hypertension (Panel B) versus no aTRH.
| Panel A | Hazard Ratio (95% confidence interval) | Groups being compared | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Stroke | Coronary Heart | All-cause Mortality | Hypertension† | Number of antihypertensive medication classes | ||||
| 1 or 2 | 3 | ≥ 4 | ||||||
| Model 1 | 1.16 (0.76 – 1.77) | 1.26 (0.77 – 2.05) | 1.39 (1.17 – 1.66) | Controlled | Reference (no aTRH) | Exposed (aTRH) | ||
| Model 2 | 1.12 (0.71 – 1.78) | 0.97 (0.54 – 1.74) | 1.39 (1.15 – 1.68) | Uncontrolled | ||||
| Model 3 | 1.07 (0.67 – 1.71) | 0.83 (0.45 – 1.53) | 1.14 (0.93 – 1.40) | |||||
|
| ||||||||
| Panel B
| ||||||||
| Number of antihypertensive medication classes | ||||||||
| Model 1 | 1.66 (1.26 – 2.18) | 2.48 (1.89 – 3.24) | 1.70 (1.50 – 1.92) | Hypertension† | 1 or 2 | 3 | ≥ 4 | |
| Model 2 | 1.55 (1.14 – 2.11) | 2.44 (1.82 – 3.27) | 1.64 (1.43 – 1.88) | Controlled | Reference (no aTRH) | |||
| Model 3 | 1.31 (0.95 – 1.81) | 2.05 (1.52 – 2.78) | 1.36 (1.18 – 1.57) | Uncontrolled | Exposed (aTRH) | |||
Hypertension: Controlled = systolic blood pressure < 140 mm Hg and diastolic blood pressure < 90 mm Hg; Uncontrolled = systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg.
aTRH = apparent treatment resistant hypertension; uncontrolled hypertension and treatment with ≥ 3 antihypertensive medication classes(i.e., uncontrolled aTRH) or controlled hypertension and treatment with ≥ 4 antihypertensive medication classes (i.e., controlled aTRH).
No aTRH = no apparent treatment resistant hypertension; controlled hypertension and treatment with < 4 antihypertensive medication classes or uncontrolled hypertension and treated with 1 or 2 antihypertensive medication classes.
Model 1 adjusted for age, race, gender, and geographic region of residence.
Model 2 adjusted for variables in Model 1 plus waist circumference, smoking status, physical activity, alcohol consumption, C - reactive protein, statin use, Morisky medication adherence scale score ≥2, total cholesterol, HDL-cholesterol, and hypertension duration.
Model 3 adjusted for variables in Model 1 and 2 plus estimated glomerular filtration rate < 60 ml/min/1.73m2, albuminuria, and diabetes. Hazard ratios for stroke were also adjusted for history of coronary heart disease. Hazard ratios for coronary heart disease were also adjusted for history of stroke. Hazard ratios for all-cause mortality were also adjusted for history of coronary heart disease and stroke.
Participants in the shaded box were not included in the analysis.
Table 4.
Hazard ratios for stroke, coronary heart disease, and all-cause mortality associated with uncontrolled apparent treatment resistant hypertension versus controlled apparent treatment resistant hypertension.
| Hazard Ratio (95% confidence interval)
|
Groups being compared
|
||||||
|---|---|---|---|---|---|---|---|
| Stroke | Coronary Heart | All-cause Mortality | Hypertension† | Number of antihypertensive medication classes | |||
| 1 or 2 | 3 | ≥ 4 | |||||
| Model 1 | 1.41 (0.87 – 2.28) | 1.91 (1.12 – 3.26) | 1.07 (0.84 – 1.36) | Controlled | Reference (aTRH) | ||
| Model 2 | 1.37 (0.80 – 2.33) | 2.52 (1.35 – 4.72) | 1.16 (0.93 – 1.45) | Uncontrolled | Exposed (aTRH) | ||
| Model 3 | 1.05 (0.61 – 1.81) | 2.33 (1.21 – 4.48) | 1.15 (0.91 – 1.45) | ||||
Hypertension: Controlled = systolic blood pressure < 140 mm Hg and diastolic blood pressure < 90 mm Hg; Uncontrolled = systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg.
aTRH = apparent treatment resistant hypertension; uncontrolled hypertension and treatment with ≥ 3 antihypertensive medication classes(i.e. uncontrolled aTRH) or controlled hypertension and treatment with ≥ 4 antihypertensive medication classes (i.e. controlled aTRH).
Model 1 adjusted for age, race, gender, and geographic region of residence.
Model 2 adjusted for variables in Model 1 plus waist circumference, smoking status, physical activity, alcohol consumption, C - reactive protein, statin use, Morisky medication adherence scale score ≥2, total cholesterol, HDL-cholesterol, and hypertension duration.
Model 3 adjusted for variables in Model 1 and 2 plus estimated glomerular filtration rate < 60 ml/min/1.73m2, albuminuria, and diabetes. Hazard ratios for stroke were also adjusted for history of coronary heart disease. Hazard ratios for coronary heart disease were also adjusted for history of stroke. Hazard ratios for all-cause mortality were also adjusted for history of coronary heart disease and stroke
Participants in the shaded box were not included in the analysis.
Discussion
A 2008 scientific statement from the American Heart Association reported on the diagnosis, evaluation and treatment of TRH 1. The authors noted the prognosis for people with TRH was unknown. Since that report, several cross-sectional studies have been published showing aTRH to be associated with an increased prevalence of CVD and CVD risk factors 2-4,22,23. However, there have been few estimates of CVD risk associated with aTRH from prospective cohort studies and no studies have compared CVD risk associated with blood pressure control among people with aTRH 22. The current study, set within a population-based observational cohort, found aTRH to be associated with an increased risk for CHD and all-cause mortality. Additionally, among people with aTRH, having uncontrolled versus controlled hypertension was associated with increased risk for CHD. Finally, participants with aTRH and controlled blood pressure did not have elevated CVD risk in comparison to others without aTRH.
Utilizing Kaiser Permanente healthcare systems data the Cardiovascular Disease Research Network (CVRN) reported the multivariable adjusted HR for the composite outcome of death, stroke, MI, chronic kidney disease (CKD) or heart failure comparing individuals with aTRH versus those without aTRH was 1.47 (95% CI 1.33 – 1.62) 23. aTRH was not associated with risk for any outcome individually, however, few events occurred among the aTRH group (e.g., 54 deaths, 15 strokes and 9 MIs). The association between aTRH and outcomes was also investigated in the Reduction of Atherothrombosis for Continued Health (REACH) registry enriched for patients with atherothrombotic risk factors 24. When defining aTRH as uncontrolled hypertension on ≥3 antihypertensive medication classes (similarly to our definition of uncontrolled aTRH), the HR for the composite endpoint of CVD mortality, MI, or stroke over 4 years of follow-up was 1.11 (95% CI 1.02–1.20). Additionally, aTRH was associated with stroke, but not CVD mortality or all-cause mortality. Redefining aTRH as uncontrolled hypertension on 3 antihypertensive medication classes or ≥4 antihypertensive medication classes regardless of blood pressure control found no association with stroke but increased risk for all-cause mortality, CVD mortality, non-fatal MI and heart failure hospitalizations. Although these results cannot be directly compared to data from the current study, they draw consistent conclusions that persons with aTRH are at higher risk for CVD outcomes.
It has been hypothesized elevated CVD outcome risk among persons with aTRH versus others with less severe hypertension is due to increased accumulated blood pressure burden resulting from more severe hypertension over a longer duration 22. Even after accounting for duration of hypertension, the association of aTRH with CHD and all-cause mortality persisted in the current analyses. However, we cannot rule out that blood pressure differences between the aTRH and no aTRH groups may explain the increased CHD risk associated with aTRH in our study 25. In fact, baseline blood pressure was similar in those with controlled aTRH and no aTRH and our results demonstrate no difference in CVD risk between those two groups.
Other aspects of results presented herein are worthy of comment. Specifically, previous research demonstrates hypertension is the most powerful risk factor for stroke and that hypertension control imparts substantial risk reduction for this outcome 7,26,27. In the current study, aTRH was associated with increased risk for CHD but not stroke after full multivariable adjustment. Additionally, hypertension control in aTRH was protective against CHD but not stroke. Therefore, this pattern of risk is distinct within hypertension and could be related to the increased prevalence of primary aldosteronism, sleep apnea, arterial stiffness, and heightened sympathetic tone in aTRH 1. These conditions are each independently linked to cardiovascular outcomes in the general population 28-33.
Several comorbidities (e.g., diabetes, CKD, history of stroke and history of CHD) may be on the pathway between aTRH and stroke, CHD or all-cause mortality. If so, adjusting for these factors could obscure a true effect between aTRH and outcomes 34,35. Therefore, we considered models with and without adjustment for diabetes, CKD, history of stroke and history of CHD. Overall, adjustment for these comorbidities attenuated the observed associations, but did not remove them. Assessing whether comorbidities considered in the current study are intermediates between aTRH and outcomes or confounders was beyond the scope of the current study. Continued prospective studies with repeated assessments of these factors and aTRH are necessary to determine the etiology of outcomes for people with TRH.
This analysis has several strengths and limitations. A relatively small number of CHD and stroke events were observed among those with aTRH, especially when stratifying by blood pressure control, resulting in wide confidence intervals for some comparisons. We relied on self-report for several covariates. We were unable to confirm optimal dosing of antihypertensive medications as a criterion to define aTRH since dose was not recorded as part of the pill bottle review in REGARDS. Finally, only two blood pressure measurements were available from a single study visit. The association between aTRH and outcomes using blood pressure from more than one visit should be investigated in future studies. Despite these limitations, several strengths are maintained, including the large national population-based sample in the REGARDS study, the use of standardized protocols with stringent quality control procedures for the measurement of BP, and the identification and adjudication of multiple outcomes, including stroke, CHD and all-cause mortality among persons with an extreme form of hypertension.
In this large, population-based sample of persons treated for hypertension, having aTRH was associated with an increased risk for CHD and all-cause mortality but not stroke. Additionally, among people with aTRH, uncontrolled versus controlled hypertension was associated with more than a two-fold higher risk of CHD events. Individuals with aTRH and controlled hypertension did not have elevated CVD risk compared to their counterparts without aTRH. These observations bolster support for continued research to identify unique aspects of pathophysiology related to TRH and strive for continued improvements in treatment paradigms to achieve hypertension control within this patient group.
Supplementary Material
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service and R01 HL080477 from the National Heart Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Footnotes
Conflicts: None to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008 Jun;117(25):e510–526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011 Aug;124(9):1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011 Jun;57(6):1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 4.de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011 May;57(5):898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 5.Irvin MR, Shimbo D, Mann DM, et al. Prevalence and correlates of low medication adherence in apparent treatment-resistant hypertension. J Clin Hypertens (Greenwich) 2012 Oct;14(10):694–700. doi: 10.1111/j.1751-7176.2012.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JG, Staessen JA. Benefits of antihypertensive pharmacologic therapy and blood pressure reduction in outcome trials. J Clin Hypertens (Greenwich) 2003 Jan-Feb;5(1):66–75. doi: 10.1111/j.1524-6175.2003.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999 Sep;138(3 Pt 2):211–219. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008 May;336(7653):1121–1123. doi: 10.1136/bmj.39548.738368.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998 Jun;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 10.Ong HT. Cardiovascular outcomes in the comparative hypertension drug trials: more consensus than controversy. Singapore Med J. 2008 Aug;49(8):599–605. quiz 606. [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 12.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986 Jan;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000 May;31(5):1076–1080. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 16.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989 Oct;20(10):1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 17.Soliman EZ, Howard G, Cushman M, et al. Prolongation of QTc and risk of stroke: The REGARDS (REasons for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2012 Apr;59(16):1460–1467. doi: 10.1016/j.jacc.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007 Nov;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 19.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003 Nov;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 20.Prineas R, Crow R, Blackburn H. The Minnesota code manual of electrocardiographic findings: Standards and procedures for measurement and classification. Boston MA: Wright-OSG; 1982. [Google Scholar]
- 21.Prineas R, Crow R, Zhang Z-M. Minnesota code Manual of Electrocardiographich Findings. 2. London: Springer-Verlag; 2010. [Google Scholar]
- 22.Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation. 2012 Apr;125(13):1594–1596. doi: 10.1161/CIRCULATIONAHA.112.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012 Apr;125(13):1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2012 Nov; doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
- 25.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Collaboration PS. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002 Dec;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Wolf PA, Verter J, McNamara PM. Epidemiologic assessment of the role of blood pressure in stroke. The Framingham study. JAMA. 1970 Oct;214(2):301–310. [PubMed] [Google Scholar]
- 27.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990 Apr;335(8693):827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 28.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008 Jan;168(1):80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 29.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005 Apr;45(8):1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005 Mar 19-25;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010 Feb;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987 Jun;113(6):1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 33.Paul L, Hastie CE, Li WS, et al. Resting heart rate pattern during follow-up and mortality in hypertensive patients. Hypertension. 2010 Feb;55(2):567–574. doi: 10.1161/HYPERTENSIONAHA.109.144808. [DOI] [PubMed] [Google Scholar]
- 34.Rothman KJ, Greenland S. Modern Epidemiology. 2. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 35.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009 Jul;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


