Abstract
Observational studies show a relationship between elevated serum uric acid (UA) and better physical performance and muscle function. The purpose of this paper was to determine whether regular participation in an exercise intervention, known to improve physical functioning, would result in increased serum UA. For this study, 424 older adults at risk for physical disability were randomized to participate in either a 12-mo moderate-intensity physical activity (PA) or a successful aging (SA) health education intervention. UA was measured at baseline, 6, and 12 mo (n = 368, 341, and 332, respectively). Baseline UA levels were 6.03 ± 1.52 mg/dl and 5.94 ± 1.55 mg/dl in the PA and SA groups, respectively. The adjusted mean UA at month 12 was 4.8% (0.24 mg/dl) higher in the PA compared with the SA group (p = .028). Compared with a health education intervention, a 1-yr PA intervention results in a modest increase in systemic concentration of UA in older adults at risk for mobility disability.
Keywords: exercise, aging, health education
Aging is associated with declines in muscle mass and function that often lead to physical disability and loss of independence (Onder et al., 2002; Lindle et al., 1997; Bean et al., 2003; Rantanen, Guralnik, Ferrucci, Leveille, & Fried, 1999). Increased oxidative stress may contribute to several deleterious physical changes that accompany aging (Harman, 1956), including declines in physical function. In older adults, oxidative damage is independently associated with low muscle strength (Howard et al., 2007), as well as with pathophysiologic conditions, diseases, and behavioral factors predictive of disability (Cesari, Kritchevsky, Leeuwenburgh, & Pahor, 2006). Thus, protecting against oxidative stress may be one method of preventing or reducing aging-related disability.
One of the body's most prevalent and potent biologic antioxidants is uric acid (UA), which may account for up to two thirds of all free-radical scavenging activities (Maxwell et al., 1997). While controversy exists as to whether elevations in systemic UA are beneficial or detrimental to cardiovascular health (Feig, Kang, & Johnson, 2008), recent observational data suggest a protective link between modestly elevated UA levels and physical function in older adults (Ruggiero et al., 2007; Macchi et al., 2008). Specifically, in a community-based, cross-sectional study, older adults with intermediate levels of UA (4.8–5.3 mg/dl) had better Short Physical Performance Battery (SPPB) scores and lower instrumental activities of daily living disability than those with lower UA levels (Ruggiero et al., 2007). In addition, in the same cohort, UA levels greater than 5.5 mg/dl were prospectively associated with better muscle function, as measured by handgrip strength and knee extension torque (Macchi et al., 2008). Collectively, these data suggest that slight elevations in UA (i.e., absolute concentration occurring within the normal range of 2.4–7.0 mg/dl) may contribute to maintenance of better physical performance and muscle function with age, possibly due to its antioxidant activity.
Regular performance of physical activity (PA) delays aging-related declines in physical function, and part of this benefit is due to direct increases in muscle strength and size (Evans, 1999). However, such increases do not fully account for the beneficial effects of PA on physical function (Buchner, Larson, Wagner, Koepsell, & de Lateur, 1996; Brown, Sinacore, & Host, 1995). Thus, part of the observed benefit may be mediated by other mechanisms, including enhanced antioxidant potential (Ji et al., 1998), via increased UA. To date, no clinical trials have examined the effect of long-term exercise on UA levels in older adults. The Lifestyle Interventions and Independence for Elders Pilot Trial (LIFE-P) provides an ideal study to investigate this question, as LIFE-P participants were originally recruited based on functional limitations and published results demonstrate improved physical performance (as measured by the SPPB and 400-m walking speed) in the PA group compared with control (Pahor et al., 2006). Therefore, the purpose of this study was to determine the effect of long-term (12 months) PA on circulating levels of UA in older adults at risk for disability. We hypothesized that upon completion of the intervention period, UA levels would be higher in the PA compared with the successful aging (SA) group. Secondarily, we hypothesized that systemic concentrations of UA would be a correlate of muscle mass and function.
Methods
Study Design
This study was conducted as an ancillary to the LIFE-P Trial, a four-site, single-blind, randomized, controlled clinical trial comparing a 12-month PA intervention with an SA education control group in 424 nondisabled, community-dwelling older adults at risk for physical disability. Details of the study design and the main findings on physical function of LIFE-P are published (Rejeski et al., 2005; Pahor et al., 2006). The local institutional review boards at the study sites (Wake Forest University, Cooper Institute, University of Pittsburgh, and Stanford University) approved the study, and all study participants gave written informed consent to participate. The study adhered to the Declaration of Helsinki and was reported following the CONSORT statement.
Study Participants
Detailed randomization, inclusion and exclusion criteria, and a flow diagram of specific numbers of individuals screened and reasons for exclusion are published (Pahor et al., 2006). Briefly, the major inclusion criteria were age of 70–89 years, low functional performance based on a SPPB score of less than 10 (on a scale of 0 [worst] to 12 [best]), sedentary lifestyle, ability to complete a 400-m walk test within 15 min without sitting or using an assistive device, completion of a behavioral run-in that required tracking and logging of health behaviors, and willingness to be randomized to either intervention group. The major exclusion criteria were living in a nursing home; self-reported inability to walk 1 mile; significant cognitive impairment (Mini-Mental Status Examination score <21); severe hearing or visual impairment; and severe cardiac, pulmonary, neurological, orthopedic, renal, or psychiatric disease.
Interventions
The PA intervention consisted of a combination of aerobic, strength, balance, and flexibility exercises and was divided into three phases. For the first 2 months (adoption phase), three center-based exercise sessions (40–60 min) per week were conducted in a supervised setting. During the next 4 months (transition phase), the number of center-based sessions was reduced (2 times/week), and home-based exercises (≥3 times/week) were started. The subsequent maintenance phase (Weeks 25–52) consisted of the home-based intervention, optional 1 or 2 times per week center-based sessions, and monthly telephone contacts.
In addition, the PA intervention included group-based behavioral counseling sessions (1 time/week for the first 10 weeks) that focused on PA participation. The intervention focused on walking as the primary mode of exercise, and the goal was to engage in walking for at least 150 min/week. A brief warm-up proceeded each session, and a brief cooldown period followed. Participants also completed lower extremity strengthening exercises followed by lower extremity stretching exercises. The intensity of training was gradually increased over the first 2–3 weeks. Perceived exertion was quantified using the Borg scale and used to regulate the intensity of exercise. Participants were asked to walk at a target intensity of 12–13 (somewhat hard), and they were discouraged from exercising at levels of 15 or higher (hard) or 11 or less (fairly light). Upper body strengthening exercises using hand weights were performed at a perceived exertion of 15–16.
An SA health education intervention was used as the active control. Participants met in groups weekly for the first 26 weeks and monthly for the remaining weeks. Sessions included health topics relevant to older adults such as nutrition, medications, foot care, and recommended preventive health care. At the end of each session, a short instructor-led intervention (5–10 min) of gentle upper extremity stretching was delivered. Phone calls were made after each missed session to encourage regular participation, and participants received a monthly newsletter.
Measurements
Baseline assessments included an interview, anthropo-metric measures, physical exam, electrocardiogram, and a physician evaluation. Follow-up visits in the clinic occurred at 6 and 12 months. Prevalence of clinical conditions was determined using self-reported physician-diagnosed disease information.
Physical Function Indices
Participants were asked to walk 10 laps at a comfortable, self-directed pace in a corridor between two cones spaced 20 m apart.
A global measure of physical function was performed to characterize functional status of the participants. The SPPB is based on a timed short-distance walk, repeated chair stands, and balance test. The categories computed for walking speed and chair stands are derived from cut-points based on quartiles of time to perform each task assessed in the Established Populations for Epidemiologic Study of the Elderly (Guralnik et al., 1994). A summary score ranging from 0 (worst) to 12 (best) was calculated by summing all scores.
Grip strength in both hands was measured using a hydraulic grip strength dynamometer and reported in kilograms (kg).
Body Composition
Dual-energy X-ray absorptiometry measurements of body composition were conducted in a subsample (n = 222) at the Wake Forest, Cooper, and Pittsburgh sites of the LIFE-P study using a Hologic scanner on each randomized participant at baseline and at the 12-month assessment visit. LIFE-P staff followed manufacturer's recommendations for patient positioning, scan protocols, and scan analysis. Percent lean mass was calculated as lean mass (kg) divided by total mass (kg).
Blood Collection and UA Measurement
Blood samples were collected from LIFE-P study participants in the early morning (between 7 and 9 a.m.) after a 12-hr fast at the baseline and 6- and 12-month assessment visits. The 6- and 12-month blood samples were collected at least 24 hr after the last exercise session and in the case of an acute illness, blood sampling was postponed until 1–2 weeks after full recovery.
All 424 randomized participants consented to the baseline blood draw, and blood was successfully collected from 368 (87%) participants. Blood samples were available from 341 participants (80%) at the 6-month follow-up and from 332 participants (78%) at the 12-month follow-up. Of the 83 participants with missing 6-month data, 2 had died, 22 withdrew consent or dropped from the study, 55 did not have a blood sample drawn due to technical difficulties, and 4 did not have UA measured successfully. Of the 92 participants with missing 12-month data, 4 had died, 25 withdrew consent or dropped from the study, 61 did not have a blood sample drawn due to technical difficulties, and 2 did not have UA measured successfully.
All blood was collected, processed, divided into aliquots, and stored locally at –80 °C until shipment to the Biological Specimen Repository at Wake Forest School of Medicine, where samples were placed for long-term storage at –80 °C until later analysis. UA levels (mg/dl) were analyzed using enzymatic-colorimetric methods and creatinine levels (mg/dl) were analyzed using kinetic colometric methods in a central laboratory. For UA, the lower limit of detection was 0.2 mg/dl, range 0.2–25 mg/ dl, and intra-assay and interassay coefficients of variation were equal to 0.5% and 1.7%, respectively. For creati-nine, the lower limit of detection was 0.2 mg/dl, range 0.2–25 mg/dl, and intra-assay and interassay coefficients of variation were equal to 0.7% and 2.3%, respectively.
Statistical Analysis
All statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC), and a probability level of <.05 was adopted for testing statistical significance. Sample means and standard deviations were computed for the continuous descriptive characteristics, and count and proportions were calculated for the discrete descriptive characteristics according to intervention group.
Unadjusted and adjusted associations among UA and indices of physical function and body composition were determined using Spearman correlation coefficients and partial Spearman correlation coefficients, respectively.
Raw UA values for each intervention group at each time point are reported as means and standard deviations. Differences in mean values of UA between intervention groups were estimated using repeated-measures ANCOVA with results adjusted for gender, baseline UA, study site, BMI, diabetes status, baseline and change in creatinine, intervention arm, visit, intervention arm by gender interaction, and intervention arm by visit interaction reported. Sensitivity analyses were also conducted in which analyses were adjusted for intervention attendance. Hypothesis tests were performed using contrasts of the 6- and 12-month intervention means. Overall comparisons between groups across follow-up visits were obtained using a contrast to compare average effects across both follow-up visits. Estimated least squares means of UA from repeated-measures ANCOVA and their standard errors were reported at the follow-up visits for each randomized group.
Results
Baseline Characteristics and Correlations
The two groups had similar baseline characteristics, except for a slightly higher prevalence of diabetes in the PA group (Table 1). In addition, there were no differences in baseline UA concentrations between groups (Table 2). At baseline, there was a positive correlation between UA and grip strength (r = .19; p < .01; Table 3), but no other relationships between UA and physical function or body composition measures were observed. However, after adjustment for gender, study site, diabetes, creatinine, and BMI, the correlation between UA and grip strength was no longer significant.
Table 1.
Baseline Characteristics of Participants According to Treatment Group
| Successful Aging |
Physical Activity |
|||
|---|---|---|---|---|
| Variable | n | n (%) or M (SD) | n | n (%) or M (SD) |
| Age (years) | 186 | 77.02 (4.36) | 182 | 76.36 (4.12) |
| Female | 186 | 125 (67.2%) | 182 | 126 (69.2%) |
| White | 186 | 141 (75.8%) | 182 | 140 (76.9%) |
| BMI (kg/m2) | 186 | 29.75 (5.51) | 182 | 30.71 (6.01) |
| Former smoking | 186 | 25 (13.4%) | 182 | 28 (15.4%) |
| Current smoking | 186 | 4 (2.2%) | 182 | 7 (3.8%) |
| MMSE (score 0-30) | 186 | 27.45 (2.08) | 182 | 27.04 (2.32) |
| Hypertension | 186 | 129 (69.4%) | 181 | 126 (69.6%) |
| Diabetes mellitus | 186 | 32 (17.2%)a | 182 | 52 (28.6%)a |
| Cancer | 185 | 32 (17.3%) | 182 | 28 (15.4%) |
| Chronic heart disease | 185 | 12 (6.5%) | 181 | 21 (11.6%) |
| Cardiovascular disease | 186 | 12 (6.5%) | 182 | 8 (4.4%) |
| Stroke | 186 | 12 (6.5%) | 182 | 8 (4.4%) |
| Congestive heart failure | 184 | 12 (6.5%) | 180 | 11 (6.1%) |
| Chronic obstructive pulmonary disorder | 185 | 27 (14.6%) | 180 | 26 (14.4%) |
| Walk time for 400 m (s) | 186 | 488.24 (111.99) | 182 | 479.76 (110.57) |
| SPPB | 186 | 7.46 (1.41) | 182 | 7.6 (1.46) |
| Grip strength (kg) | 178 | 25.41 (9.58) | 175 | 24.93 (8.39) |
| Lean mass (%) | 90 | 60.34 (7.16) | 89 | 60.22 (6.37) |
Note. BMI = body-mass index; MMSE = Mini-Mental Status Exam; SPPB = Short Physical Performance Battery.
Only diabetes prevalence shows a significant difference between the two groups (p = .0094).
Table 2.
Unadjusted Uric Acid (UA) Values (mg/dl) by Treatment Group
| Successful Aging |
Physical Activity |
|||
|---|---|---|---|---|
| Time | n | M (SD) | n | M (SD) |
| Baseline | 186 | 5.94 (1.55) | 182 | 6.03 (1.52) |
| 6 months | 170 | 5.91 (1.66) | 171 | 6.08 (1.57) |
| 12 months | 161 | 5.77 (1.46) | 171 | 6.15 (1.62) |
Note. Unadjusted and adjusted associations among UA and indices of physical function and body composition were determined using Spearman correlation coefficients and partial Spearman correlation coefficients, respectively. Raw UA values for each intervention group at each time point are reported as means and standard deviations.
Table 3.
Spearman Correlations Between Baseline UA and Measures of Physical Function and Body Composition at Baseline
| Uric Acid |
||
|---|---|---|
| Variable | Unadjusted | Adjusteda |
| 400-m walk time (s) | ||
| r | .09 | –.02 |
| p | .07 | .74 |
| n | 368 | 368 |
| Total SPPB score | ||
| r | –.02 | .02 |
| p | .77 | .75 |
| n | 368 | 368 |
| Average grip strength (kg) | ||
| r | .19 | –.03 |
| p | <.01 | .56 |
| n | 368 | 368 |
| Lean body mass (%) | ||
| r | .04 | –.07 |
| p | .57 | .37 |
| n | 179 | 179 |
Note: SPPB = Short Physical Performance Battery.
Unadjusted and adjusted associations among UA and indices of physical function and body composition were determined using Spearman correlation coefficients and partial Spearman correlation coefficients, respectively.
Adjusted for gender, study site, diabetes, creatinine, and body mass index.
Effects of the PA Intervention on UA
Compliance to the PA and SA interventions was previously reported (Pahor et al., 2006). Briefly, in the PA group, attendance during the adoption and transition phases averaged 71% and 61%, and during the maintenance phase, participants engaged in an average of 3.7 walking sessions/week and walked an average of 138 ± 149 min/week (median = 119 min/week, interquartile range = 123 min/week). Attendance to the SA group sessions averaged 70% for weeks 1–26 and 73% for weeks 27–52. The number of moderate PA sessions was similar in the two groups at baseline (2.7 vs. 2.8 sessions/week) and significantly higher in the PA group at the 12-month follow-up (3.5 vs. 5.1 sessions/week). No changes in body weight or composition occurred in either group, and there was no effect of PA on total body fat mass or lean mass in the subset of participants with measures of body composition (n = 221, t = –1.19, df = 219, p = .30; and t = 0.41, df = 219, p = .68, respectively).
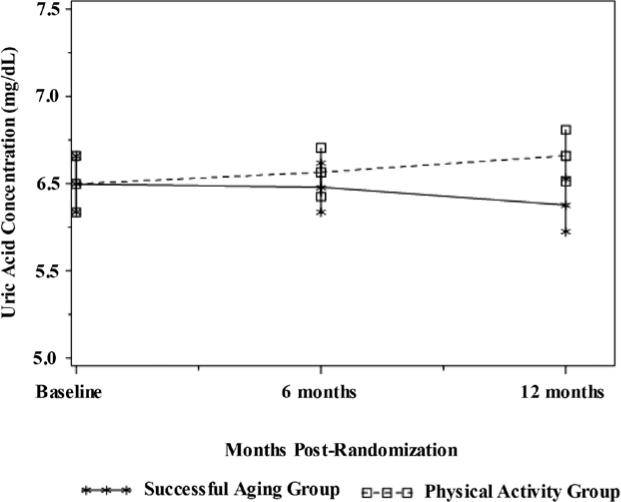
Table 2 shows the unadjusted means for UA in each intervention group at baseline and at each follow-up assessment. On average, the PA intervention resulted in a 2.1% ± 1.4% increase in UA by the end of 12 months, while the SA intervention resulted in a 1.7% ± 1.9% reduction in UA. After adjustment for gender, baseline UA, clinic site, BMI, diabetes status, baseline and change in creatinine, intervention arm, visit, intervention arm by gender interaction, and intervention arm by visit interaction, the PA intervention resulted in a higher (β = 0.46; SE = 0.15; t = 3.11; df = 331; p = .028) UA concentration compared with the SA intervention (Figure 1). Specifically, the adjusted mean UA at month 12 from the mixed-effects model was 4.8% (0.28 mg/dl) higher in the PA compared with the SA group (12-month unadjusted values for the PA and SA groups were 6.15 ± 1.62 mg/dl and 5.77 ± 1.46 mg/dl, respectively). Further adjustment for intervention attendance yielded similar findings. No significant relationships were observed between change in UA and functional status or lean mass over 12 months (Table 4); however, adjusted estimates show a trend for an association between increasing UA levels and decreasing lean mass (r = –.18, p = .07) and increasing 400-m walk time (r = .15, p = .08) .
Figure 1.
Comparison of uric acid concentrations between successful aging and physical activity before and after the 12-month intervention (p = .028). Results adjusted for gender, baseline uric acid, study site, body-mass index, diabetes status, baseline and change in creatinine, intervention arm, visit, intervention arm by gender interaction, and intervention arm by visit interaction.
Table 4.
Spearman Correlations Between Change in UA and Change in Measures of Physical Function and Body Composition From Baseline to 12 Months
| Physical Activity |
Successful Aging |
|||
|---|---|---|---|---|
| Variable | Unadjusted | Adjusteda | Unadjusted | Adjusteda |
| 400-m walk time (s) | ||||
| r | .08 | .15 | .03 | .00 |
| p | .32 | .08 | .68 | .96 |
| n | 149 | 149 | 154 | 154 |
| Total SPPB score | ||||
| r | .07 | .05 | .09 | .07 |
| p | .36 | .55 | .27 | .43 |
| n | 152 | 152 | 153 | 153 |
| Average grip strength (kg) | ||||
| r | .02 | .04 | –.02 | –.04 |
| p | .79 | .58 | .85 | .68 |
| n | 139 | 139 | 143 | 143 |
| Lean body mass (%) | ||||
| r | –.04 | –.18 | .27 | –.09 |
| p | .69 | .07 | .01 | .34 |
| n | 107 | 107 | 102 | 102 |
Note: SPPB = Short Physical Performance Battery.
Adjusted for baseline uric acid, gender, study site, diabetes, body mass index, creatinine, 12-month change in creatinine, treatment group, and gender by treatment interaction.
Discussion
Findings from this randomized controlled trial provide evidence that a 1-year PA intervention results in modest elevation of systemic UA in older adults at risk for disability. No relationships were observed between UA and physical function or body composition at baseline; however, elevation in UA over 12 months was modestly correlated with loss of lean mass and slower 400-m walk time. Although health ramifications of our findings remain to be seen, results should be considered in light of the long-standing controversy surrounding the role of UA as a risk factor for complex disease, as well as relevant study design details—including participant characteristics and exercise modality.
UA (C5H4N4O3) is a heterocyclic, semisolid compound formed during purine metabolism. In the body, UA acts as a reactive-oxygen-species scavenger, and systemic abundance of this molecule is hypothesized to be an adaptive countermeasure to reactive-oxygen-species–mediated damage and disease (Ames, Cathcart, Schwiers, & Hochstein, 1981; Glantzounis, Tsimoyiannis, Kappas, & Galaris, 2005; Mikami, Yoshino, & Ito, 2000). In contrast to this protective role, several epidemiological studies suggest a link between elevated UA and increased prevalence of cardiovascular conditions, including metabolic syndrome (Ford, Li, Cook, & Choi, 2007), renal disease (Siu, Leung, Tong, & Kwan, 2006), coronary artery disease (Tuttle, Short, & Johnson, 2001), and stroke (Lehto, Niskanen, Ronnemaa, & Laakso, 1998). Whether UA serves as a causative agent or a compensatory mechanism of ill health remains to be delineated; however, the nature of this relationship is likely dose dependent and disease specific (Nieto, Iribarren, Gross, Comstock, & Cutler, 2000; Feig et al., 2008).
To gain insight on the relationship between UA, exercise, and physical function, we assessed the effects of a long-term PA intervention—known to improve SPPB score and 400-m walk time (Pahor et al., 2006)—on systemic UA in older adults at risk for mobility disability. Following the 12-month intervention period, we report a 0.28 mg/dl (or 4.8%) higher adjusted UA concentration in the PA group compared with the SA control. Although an effect of this magnitude would not conventionally be considered clinically meaningful (Garg et al., 2005), most studies evaluating the health effects of UA focus on gout or cardiovascular endpoints, not physical function. Recent epidemiologic data assessing the relationship between UA and physical function in a similarly characterized cohort suggest a nonlinear association, where slightly elevated levels of UA are coupled with better physical functioning, compared with much higher or lower levels. Specifically, two recent observational studies from the InCHIANTI cohort demonstrate that older (mean age 75 years) home-dwelling adults with intermediate levels of UA (4.8–5.3 mg/dl) have better SPPB scores and lower instrumental activities of daily living disability than those with lower (or higher) UA levels (Ruggiero et al., 2007). Similarly, UA levels greater than 5.5 mg/ dl are also prospectively associated with better muscle function, as measured by handgrip strength and knee extension torque (Macchi et al., 2008). Hyperuricemia (serum urate concentration >7.5 mg/dl in men and >6.2 mg/dl in women (Ruggiero et al., 2006)) associated with a proinflammatory state, however, has been linked with poorer functional outcomes (Ruggiero et al., 2006; Cesari et al., 2004). Collectively, this data suggests that the modest elevations in UA observed in this study may be associated with clinically significant improvements in physical function.
Despite the significant intervention effect on UA and physical function (Pahor et al., 2006), we did not observe a significant correlation between the two. It is worth noting that the original study was not designed to examine the association between UA and physical function prospectively and may have been underpowered to detect a significant relationship. Alternatively, the unadjusted follow-up UA concentration observed in the PA group was greater than the “optimal range” sited by Ruggiero et al. (2007) and, given the nonlinear trend reported between UA and physical function, may also explain the modest, positive association between UA and 400-m walk time in the current study.
With regard to exercise modality, the majority of research examining the effects of exercise on UA used acute bout designs, with elevations in UA directly proportional to exercise intensity (Green & Fraser, 1988; Francis & Hamrick, 1984). Observational studies in younger adults also show that short-term exercise training results in elevations of systemic UA (Greenleaf, Kaye, & Bosco, 1969; Tekin, 2010)—likely in both acute and training scenarios—in response to exercise-induced free radical production (Mikami et al., 2000). Indeed, increases in plasma 8-iso-prostaglandin F2 (a measurable lipid peroxidation biomarker) following acute aerobic exercise is blunted by administration of intravenous UA (Waring et al., 2003), confirming that elevations in UA may be interpreted as a strategy to counteract damage from reactive oxygen species. Results from the current study affirm and extend current knowledge of the effects of exercise on UA to include long-term PA intervention and an older population at risk for mobility disability.
Findings from this trial should be interpreted in light of certain aspects of the study design and potential limitations. First, the age range of our study population (70–85 years) limits the translatability of our findings to other age groups. Second, the PA intervention was a combination of aerobic (mainly walking) and light lower extremity resistance exercise. Thus, we are unable to distinguish whether one type of training has greater effects on UA over another, or provide information regarding possible dose-response effects of increasing the intensity of PA. Third, our analysis was based on serum UA levels that only partially reflect the total amount of UA stored in peripheral tissues. Last, secondary correlation analyses between UA and measures of muscle function and strength were unadjusted for multiple comparisons. Therefore, despite congruence in the literature regarding the inverse relationship between change in UA and lean mass (Beavers, Beavers, Serra, Bowden, & Wilson, 2009), given the modesty of our findings, it is possible that significant findings are due to chance alone and caution is warranted when interpreting this results. Despite these caveats, however, this study points to the effect of regular PA in modestly, but significantly, increasing systemic UA levels in older adults at risk for mobility disability when compared with control. Future studies should clarify whether UA mediates the known relationship regular PA and improvements in physical function.
Acknowledgments
The Lifestyle Interventions and Independence for Elders (LIFE-P) Pilot Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
Contributor Information
Kristen M. Beavers, Dept. of Internal Medicine, Winston-Salem, NC.
Fang-Chi Hsu, Dept. of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, NC..
Monica C. Serra, Division of Gerontology and Geriatric Medicine, University of Maryland School of Medicine, Baltimore, MD.
Veronica Yank, Dept. of Medicine, Stanford University, Stanford, CA..
Marco Pahor, Dept. of Aging and Geriatric Research, University of Florida, Gainesville, FL..
Barbara J. Nicklas, Dept. of Internal Medicine, Winston-Salem, NC.
References
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proceedings of the National Academy of Science, U.S.A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: Which influences mobility more? Journals of Gerontology, A. Biological Sciences and Medical Sciences. 2003;58(8):728–733. doi: 10.1093/gerona/58.8.m728. PubMed. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Beavers DP, Serra MC, Bowden RG, Wilson RL. Low relative skeletal muscle mass indicative of sarcopenia is associated with elevations in serum uric acid levels: Findings from NHANES III. Journal of Nutrition, Health, and Aging. 2009;13(3):177–182. doi: 10.1007/s12603-009-0054-5. PubMed. [DOI] [PubMed] [Google Scholar]
- Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. Journals of Gerontology, A. Biological Sciences and Medical Sciences. 1995;50:55–59. doi: 10.1093/gerona/50a.special_issue.55. Spec No PubMed. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age and Ageing. 1996;25(5):386–391. doi: 10.1093/ageing/25.5.386. PubMed. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Leeuwenburgh C, Pahor M. Oxidative damage and platelet activation as new predictors of mobility disability and mortality in elders. Antioxidants & Redox Signaling. 2006;8(3-4):609–619. doi: 10.1089/ars.2006.8.609. PubMed doi:10.1089/ars.2006.8.609. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys WG, Ferrucci L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. Journals of Gerontology, A. Biological Sciences and Medical Sciences. 2004;59(3):242–248. doi: 10.1093/gerona/59.3.m242. PubMed. [DOI] [PubMed] [Google Scholar]
- Evans WJ. Exercise training guidelines for the elderly. Medicine and Science in Sports and Exercise. 1999;31(1):12–17. doi: 10.1097/00005768-199901000-00004. PubMed. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. New England Journal of Medicine. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. PubMed doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115(19):2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. PubMed doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- Francis K, Hamrick ME. Exercise and uric acid: Implication in cardiovascular disease. Journal of Orthopaedic Sports and Physical Therapy. 1984;6(1):34–39. doi: 10.2519/jospt.1984.6.1.34. PubMed. [DOI] [PubMed] [Google Scholar]
- Garg JP, Chasan-Taber S, Blair A, Plone M, Bommer J, Raggi P, Chertow GM. Effects of sevelamer and calcium-based phosphate binders on uric acid concentrations in patients undergoing hemodialysis: a randomized clinical trial. Arthritis and Rheumatism. 2005;52(1):290–295. doi: 10.1002/art.20781. PubMed doi:10.1002/art.20781. [DOI] [PubMed] [Google Scholar]
- Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Current Pharmaceutical Design. 2005;11(32):4145–4151. doi: 10.2174/138161205774913255. PubMed. [DOI] [PubMed] [Google Scholar]
- Green HJ, Fraser IG. Differential effects of exercise intensity on serum uric acid concentration. Medicine and Science in Sports and Exercise. 1988;20(1):55–59. doi: 10.1249/00005768-198802000-00008. PubMed. [DOI] [PubMed] [Google Scholar]
- Greenleaf JE, Kaye RL, Bosco JS. Elevated serum uric acid concentration in college athletes: a preliminary study. American Corrective Therapy Journal. 1969;23(3):66–69. PubMed. [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Wallace RB. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journals of Gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: A theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. PubMed. [DOI] [PubMed] [Google Scholar]
- Howard C, Ferrucci L, Sun K, Fried LP, Walston J, Varadhan R, Semba RD. Oxidative protein damage is associated with poor grip strength among older women living in the community. Journal of Applied Physiology. 2007;103(1):17–20. doi: 10.1152/japplphysiol.00133.2007. PubMed doi: 10.1152/japplphysiol.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, Bejma J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Annals of the New York Academy of Sciences. 1998;854:102–117. doi: 10.1111/j.1749-6632.1998.tb09896.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Lehto S, Niskanen L, Ronnemaa T, Laakso M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. 1998;29(3):635–639. doi: 10.1161/01.str.29.3.635. PubMed. [DOI] [PubMed] [Google Scholar]
- Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. Journal of Applied Physiology. 1997;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. PubMed. [DOI] [PubMed] [Google Scholar]
- Macchi C, Molino-Lova R, Polcaro P, Guarducci L, Lauretani F, Cecchi F, Ferrucci L. Higher circulating levels of uric acid are prospectively associated with better muscle function in older persons. Mechanisms of Ageing and Development. 2008;129(9):522–527. doi: 10.1016/j.mad.2008.04.008. PubMed doi: 10.1016/j.mad.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SR, Thomason H, Sandler D, Leguen C, Baxter MA, Thorpe GH, Barnett AH. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. European Journal of Clinical Investigation. 1997;27(6):484–490. doi: 10.1046/j.1365-2362.1997.1390687.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Mikami T, Yoshino Y, Ito A. Does a relationship exist between the urate pool in the body and lipid peroxidation during exercise? Free Radical Research. 2000;32(1):31–39. doi: 10.1080/10715760000300041. PubMed. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000;148(1):131–139. doi: 10.1016/s0021-9150(99)00214-2. PubMed. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, Pahor M. Change in physical performance over time in older women: The Women’s Health and Aging Study. Journals of Gerontology, A. Biological Sciences and Medical Sciences. 2002;57(5):M289–M293. doi: 10.1093/gerona/57.5.m289. PubMed. [DOI] [PubMed] [Google Scholar]
- Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, Studenski SA. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. Journals of Gerontology, A. Biological Sciences and Medical Sciences. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. PubMed. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Ferrucci L, Leveille S, Fried LP. Coimpairments: Strength and balance as predictors of severe walking disability. Journals of Gerontology, A. Biological Sciences and Medical Sciences. 1999;54(4):M172–M176. doi: 10.1093/gerona/54.4.m172. PubMed. [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, Fielding RA, Blair SN, Guralnik JM, Gill TM, Hadley EC, Pahor M. The lifestyle interventions and independence for elders (LIFE) pilot study: Design and methods. Contemporary Clinical Trials. 2005;26(2):141–154. doi: 10.1016/j.cct.2004.12.005. PubMed doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, Ferrucci L. Uric acid and inflammatory markers. European Heart Journal. 2006;27(10):1174–1181. doi: 10.1093/eurheartj/ehi879. PubMed doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero C, Cherubini A, Guralnik J, Semba RD, Maggio M, Ling SM, Ferrucci L. The interplay between uric acid and antioxidants in relation to physical function in older persons. Journal of the American Geriatrics Society. 2007;55(8):1206–1215. doi: 10.1111/j.1532-5415.2007.01260.x. PubMed doi: 10.1111/j.1532-5415.2007.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. American Journal of Kidney Disease. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. PubMed doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Tekin A. Xanthine oxidase and uric acid response to a 6-week pre-season training program in male athletes. African Journal of Pharmacy and Pharmacology. 2010;4(8):511–515. [Google Scholar]
- Tuttle KR, Short RA, Johnson RJ. Sex differences in uric acid and risk factors for coronary artery disease. American Journal of Cardiology. 2001;87(12):1411–1414. doi: 10.1016/s0002-9149(01)01566-1. PubMed. [DOI] [PubMed] [Google Scholar]
- Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clinical Science (London) 2003;105(4):42–430. doi: 10.1042/CS20030149. PubMed doi:10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]