Abstract
Primary ovarian insufficiency (POI) affects 1% of women under the age of 40 and is associated with premature ovarian follicle depletion. TAF4b deficiency in adult female mouse models results in hallmarks of POI including stereotyped gonadotropin alterations indicative of early menopause, poor oocyte quality, and infertility. However, the precise developmental mechanisms underlying these adult deficits remain unknown. Here we show that TAF4b is required for the initial establishment of the primordial follicle reserve at birth. Ovaries derived from TAF4b-deficient mice at birth exhibit delayed germ cell cyst breakdown and a significant increase in Activated Caspase 3 staining compared to control ovaries. Culturing neonatal TAF4b-deficient ovaries with the pan-caspase inhibitor ZVAD-FMK suppresses the excessive loss of these oocytes around the time of birth. These data reveal a novel TAF4b function in orchestrating the correct timing of germ cell cyst breakdown and establishment of the primordial follicle reserve during a critical window of development.
Keywords: TFIID, ovary, aging, oocyte survival, primordial follicle, primary ovarian insufficiency
Introduction
At birth, the mammalian ovary contains a finite number of oocytes, most of which will die during the process of follicle development. In contrast, a small number of high quality oocytes are destined for ovulation during adulthood (Jones and Pepling 2013). Proper regulation of the initial primordial follicle pool is critical for the ovary to maintain sufficient numbers of oocytes for long-term ovulation and fertility. Disruption of the normal balance between oocyte survival and death usually leads to a reduction of the ovarian reserve and impaired fertility (reviewed in (McLaughlin and McIver 2009)). While many effectors of oocyte death in the ovary are known, the upstream transcriptional regulators that ensure for proper primordial follicle number and survival at birth, and their associated mechanisms, remain largely unknown. Towards this goal, we are studying the role the transcriptional co-activator TAF4b during oogenesis and folliculogenesis using a TAF4b-deficient mouse model (Freiman et al. 2001).
TAF4b is a gonadal-enriched subunit of the TFIID complex that is critical for female fertility in the mouse. TFIID is a multi-protein general transcription factor composed of the TATA-box binding protein (TBP) and 14 TBP-associated factors (TAFs) (Freiman et al. 2001). TAF4b-deficient female mice are infertile and suffer from hallmarks of premature ovarian aging including persistent estrous, elevated serum follicle stimulating hormone (FSH) and reduced primordial follicle numbers (Voronina et al. 2007; Lovasco et al. 2010). In addition, the human gene encoding TAF4b has been linked to primary ovarian sufficiency (POI) in women (Knauff et al. 2009) as well as human oocyte quality (Di Pietro et al. 2008). Thus, the study of TAF4b in the mouse is highly relevant to the potential role of TAF4b in the proper regulation of human ovarian follicle assembly and survival.
The fidelity of each step of mammalian oogenesis and folliculogenesis is essential for proper oocyte development, ovulation and the developmental potential of the future embryo. The embryonic timeline of murine primordial follicle assembly is well-documented, beginning with germ cell clusters formed through incomplete cytokinesis during mid-embryogenesis. These clusters constitute “cysts” of oogonia, many of which remain associated by intercellular bridges (Pepling and Spradling 1998; Pepling 2006; Pepling 2012) or cyst aggregation (Mork et al. 2012). At embryonic day (E) 13.5, mitosis ceases, and oogonia enter into meiosis I, becoming “oocytes” which usually arrest in the diplotene stage of Prophase I around the time of birth. Concurrently, cysts of germ cells begin undergoing “breakdown”, in which most of the oocytes are lost through programmed cell death (reviewed in (Pepling 2012)). In contrast, the remaining oocytes become surrounded by a single layer of somatic pre-granulosa cells, forming “primordial follicles”. While seemingly simple, this process of primordial follicle assembly requires the intricate orchestration of somatic cell invasion into oogonial cysts, tightly controlled changes in hormone levels, and a unique Caspase 2-dependent apoptotic cascade (Bergeron et al. 1998; Morita et al. 2001; Takai et al. 2007; Lobascio et al. 2007; Tingen et al. 2009). Cyst breakdown is hypothesized to result from inherent ovarian quality control mechanisms, pre-clearing defective germ cells from the future ovarian reserve. In addition, germ cells that do not receive adequate somatic investment for survival are removed by apoptosis (Tingen, Kim, and Woodruff 2009). However, the molecular and cellular mechanisms required for proper primordial follicle assembly and survival remain largely unknown.
One known regulator of female germ cell cyst breakdown and proper primordial follicle assembly is estrogen (Lei et al. 2010). Estrogen is a steroid hormone that plays important roles in normal ovarian development by promoting granulosa proliferation and differentiation. 17β-estradiol (β-estradiol) is the most bioactive form of endogenous estrogen and is produced by granulosa cells of the ovary. By regulating follicle growth and maturation, β-estradiol modulates the action of FSH and facilitates further β-estradiol production (Drummond and Fuller 2012). Furthermore, β-estradiol has been shown to promote germ cell survival during the late embryonic and early neonatal period, suggesting that separation from maternal estrogen around the time of birth may facilitate cyst breakdown and associated apoptosis (Chen, Breen, and Pepling 2009; Karavan and Pepling 2012). In addition, aberrant activation of estrogen signaling by genistein (Jefferson et al. 2006; Chen et al. 2007) or diethylstilbestrol (Muñoz-de-Toro et al. 2011; Karavan and Pepling 2012) perturbs normal cyst breakdown, leading to multiple oocyte follicles. Additionally, estrogen receptors have been shown to be expressed in the somatic cells of the rodent ovary (Fitzpatrick et al. 1999; Juengel et al. 2006), and disruption of these receptors leads to dramatic reproductive phenotypes (Lubahn et al. 1993; Korach et al, 1996). While the precise role for estrogen in promoting cyst breakdown, primordial follicle assembly, and long-term fertility is unknown, it is clearly an essential regulator of this developmental transition.
In order to elucidate fundamental mechanisms associated with healthy primordial follicle assembly, we have examined ovarian development in the context of TAF4b-deficient ovaries during the late embryonic to early postnatal period. While TAF4b-deficient ovaries appear normal during late embryogenesis, they suffer accelerated germ cell death by postnatal day (PND) 1. Excessive germ cell loss in the TAF4b-deficient ovaries is associated with delayed onset of germ cell cyst breakdown compared to control ovaries. Surprisingly, excessive germ cell loss in the TAF4b-deficient ovaries is associated with a significant increase in Activated Caspase 3, activation not normally observed during normal cyst breakdown which is primarily dependent upon Activated Caspase 2 (Bergeron et al. 1998; Morita et al. 2001; Takai et al. 2007; Lobascio et al. 2007; Tingen et al. 2009). Moreover, this form of germ cell attrition in the TAF4b-deficient ovaries can be prevented by apoptosis inhibition and β-estradiol treatment. Together, these studies highlight the critical role of TAF4b in the establishment of the initial and long-term postnatal primordial follicle pool.
Results
TAF4b is required for postnatal oocyte survival immediately after birth
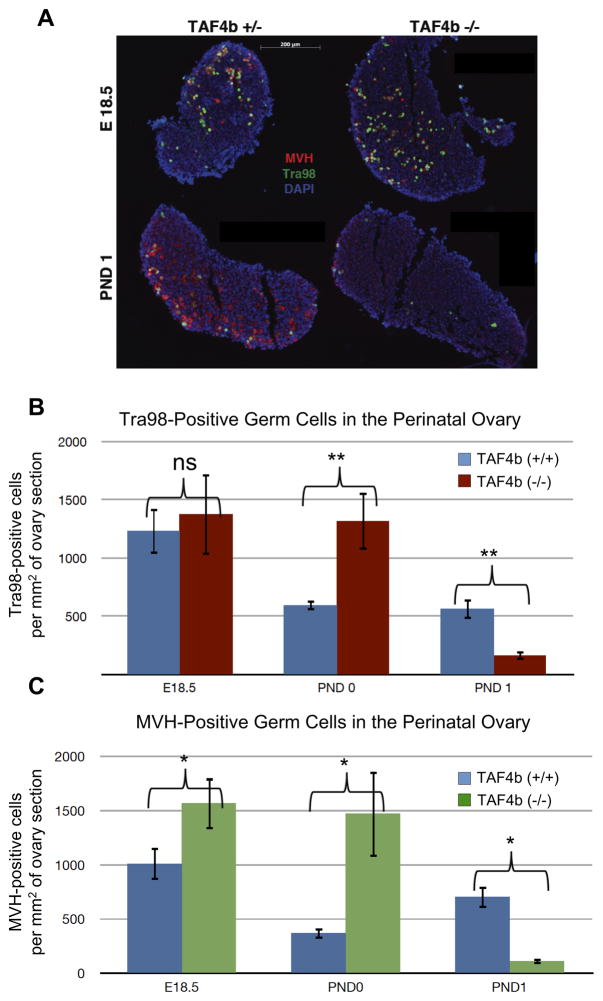
As TAF4b-deficient mice exhibit reduced primordial follicle numbers at PND 21 (Voronina et al. 2007), we aimed to determine the precise timing of their demise. Immuno-staining for germ cell markers Mouse Vasa Homolog (MVH) and Tra98 (Tanaka et al. 1997) within the TAF4b-deficient ovary was performed from late embryogenesis through early postnatal development, and then compared to control ovaries. Ovarian tissue sections obtained from TAF4b-deficient (−/−) or matched heterozygous (+/−) and wildtype (+/+) littermates were stained with antibodies against germ cell markers MVH and Tra98 (Figure 1A). Heterozygous TAF4b mice are completely fertile and were used as control littermates along with their wild-type counterparts. During late embryogenesis at E18.5, ovaries from all genotypes are largely indistinguishable by germ cell density. In contrast, by PND 1, TAF4b (−/−) mice exhibit excessive and significant Tra98-positive germ cell depletion (Figure 1B, p<0.01, two-tailed T-test). When these sections were quantified by MVH-positive cells, this germ cell depletion was similarly observed (Figure 1C, p<0.05, two-tailed T-test). These data are consistent when ovaries are stained via indirect immunohistochemistry for germ cell marker Germ Cell Nuclear Antigen (GCNA, Enders and May 1994) or indirect immunofluorescence of Tra98 (Supplementary Figures 1–3), emphasizing the usefulness of Tra98 as a marker of perinatal oocytes. Additionally, ovary sections stained histologically with hematoxylin and eosin further support these conclusions (Supplementary Figures 4–6). Together, these data indicate that the critical window of germ cell loss in these mice is at or around the time of birth.
Figure 1. Perinatal oocyte depletion in TAF4b (−/−) ovaries.
A) MVH (red) and Tra98 (green) staining were used as cytoplasmic and nuclear germ cell markers, respectively, in 8μm ovary tissue sections. DAPI (blue) denotes nuclei. TAF4b (+/−) and (−/−) ovaries exhibit comparable germ cell densities at E 18.5 (top), while TAF4b (−/−) ovaries suffer excessive depletion of oocyte by PND 1 (bottom). B) Quantification of stained tissue sections. Tra98-positive oocytes were counted per section and total number was divided by total DAPI area. N=4 for PND 0 TAF4b (+/−) and (−/−). N=5 for E18.5 TAF4b (−/−). N=6 for E18.5 TAF4b (+/−), PND 1 TAF4b (+/−), and PND 1 TAF4b (−/−). **: two-tailed T-test, p<0.01. C) Independent quantification of stained tissue sections. MVH-positive oocytes were counted per section and total number was divided by total DAPI area. N=3 for all groups. *: two-tailed T-test, p<0.05.
TAF4b promotes the correct timing of cyst breakdown and primordial follicle development
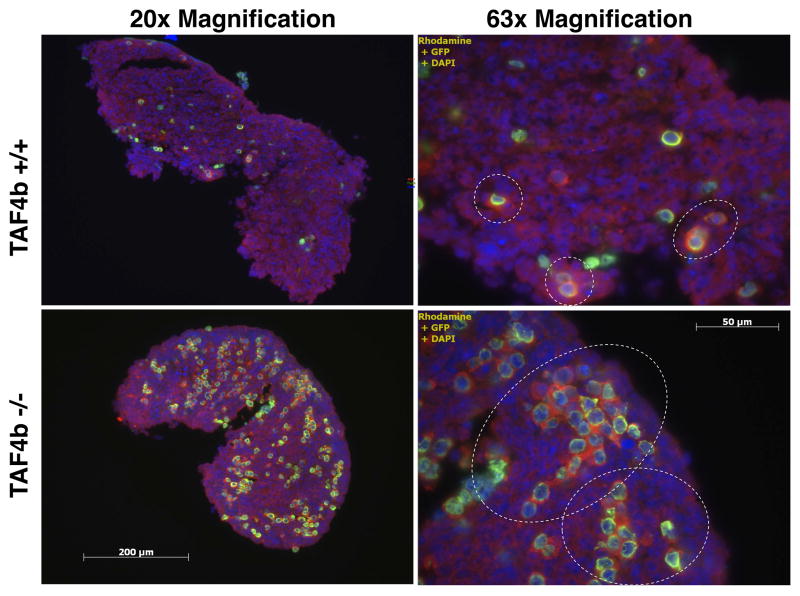
Strikingly, germ cell densities determined at PND 0, or within a few hours of birth, indicate that TAF4b-deficient ovaries have significantly more oocytes than wild-type littermates (Figure 1B,C). As this is counterintuitive to their excessive demise just one day later, cyst breakdown was examined more closely. At PND 0, TAF4b (+/+) and (+/−) mice are undergoing normal cyst breakdown where small cysts becoming primordial follicles are observed in these ovaries (Figure 2, top panel; Supplementary Figures 2 & 5). In contrast, at PND 0 TAF4b (−/−) ovaries retain large cysts of germ cells which have not initiated or completed cyst breakdown commensurate with wild-type littermates (Figure 2, bottom panel; Supplementary Figures 2 & 5). While large germ cell cysts are observed in control mice at earlier time points (data not shown), these data indicate that TAF4b is normally required to initiate or facilitate proper cyst breakdown and complete primordial follicle formation.
Figure 2. Delayed primordial follicle assembly in PND 0 TAF4b (−/−) ovaries.
MVH (red) and Tra98 (green) staining were used as cytoplasmic and nuclear germ cell markers, respectively, in 8μm PND 0 ovary tissue sections. DAPI (blue) denotes nuclei. White dashed circles denote cysts. During the time in which TAF4b (+/+) mice are undergoing normal cyst breakdown and primordial follicle assembly, TAF4b (−/−) oocytes persist in large cysts, indicating a deficit in the process of cyst breakdown.
TAF4b-deficient oocytes and somatic cells exhibit elevated levels of Activated Caspase 3
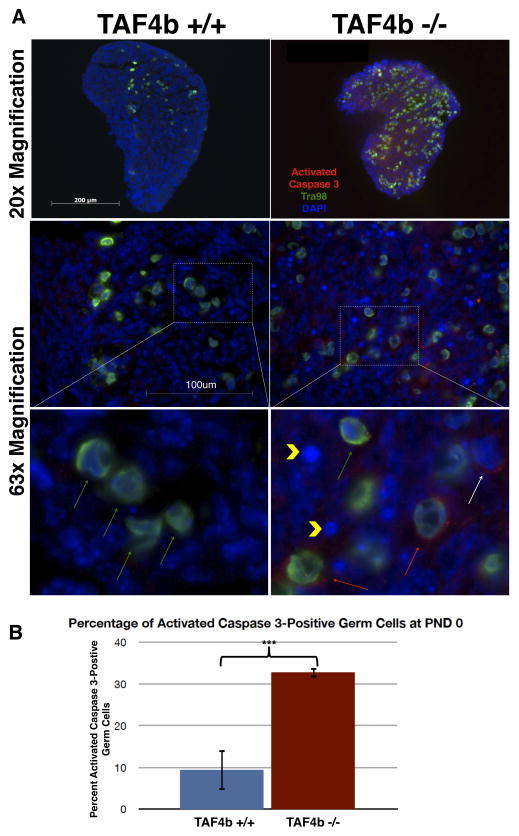
Fetal germ cell death has been described through a variety of mechanisms including apoptosis, autophagy, and shedding from the ovarian cortex (Tingen et al. 2009). As TAF4b (−/−) germ cells are largely absent by PND 1, we wanted to determine the cellular mechanism responsible for their early demise. To this end, PND 0 ovaries were stained with an antibody against Activated Caspase 3 to determine the incidence of apoptosis. While PND 0 TAF4b (+/−) ovaries exhibit little to no Activated Caspase 3 staining (Figure 3A, left panel), TAF4b (−/−) ovaries display many Activated Caspase 3-positive oocytes and somatic cells, as well as pyknotic nuclei (Figure 3A, right panel). Indeed, quantification of percent of Activated Caspase 3 positive oocytes (cells in which both Tra98 and Activated Caspase 3 is apparent by immunofluorescence, divided by total Tra98 positive cells) is significantly greater in TAF4b (−/−) ovaries than in TAF4b (+/+) ovaries (Figure 3B, p<0.001, two-tailed T-test). As this analysis only determined percent of Activated Caspase 3-positive germ cells and excluded other factors including pyknotic nuclei, the percentage of apoptotic germ cells in TAF4b (−/−) ovaries may be even larger. Increased Activated Caspase 3 staining in TAF4b-deficient ovaries at PND 0 is consistent with a role for TAF4b in preventing oocyte apoptosis.
Figure 3. Increased incidence of Activated Caspase 3-positive oocytes and somatic cells in PND 0 TAF4b (−/−) ovaries.
A) Activated Caspase 3 (red) and Tra98 (green) staining was performed in 8μm PND 0 ovary tissue sections. TAF4b (−/−) ovaries (right panel) possess far greater numbers of Activated Caspase 3- positive oocytes relative to TAF4b (+/+) ovaries (left panel). Inset: High magnification insets denote Activated Caspase 3-positive germ cells (red arrows), Activated Caspase 3-positive somatic cells (white arrow), and pyknotic nuclei (yellow arrowheads) in TAF4b (−/−) ovaries, which are absent from TAF4b (+/+) ovaries. Green arrows denote non-apoptotic oocytes. B) Quantification of apoptotic oocytes. Cells positive for both Activated Caspase 3 and Tra98 were counted per section and divided by the total number of Tra98-positive cells per section. ***: n=3, two-tailed T-test, p<0.001.
Ex vivo germ cell loss in TAF4b-deficient ovaries is rescued by Caspase inhibition
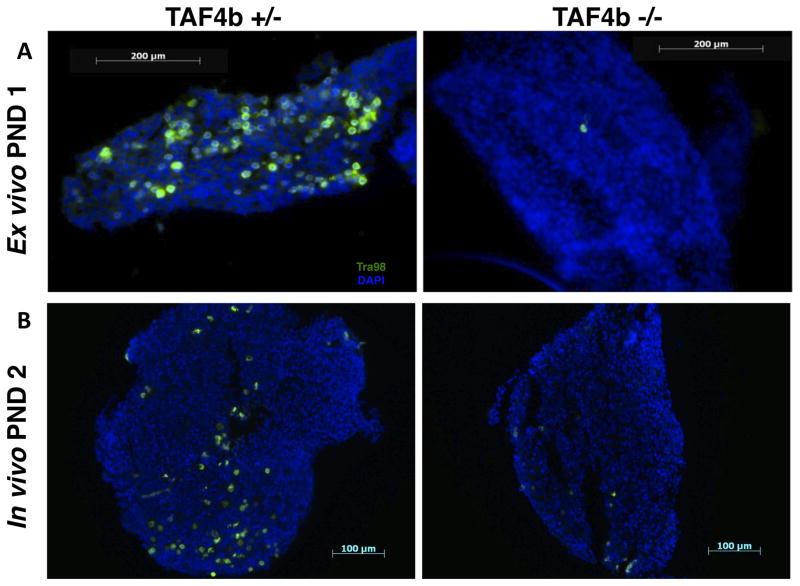
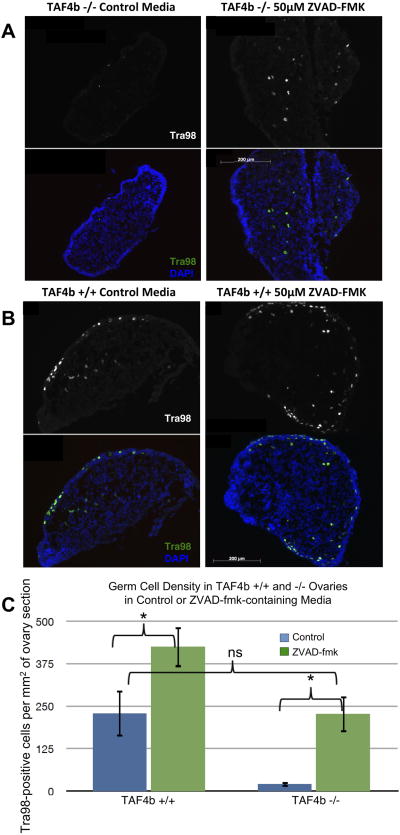
To more definitively test mechanisms of germ cell loss in the context of the TAF4b (−/−) ovaries, ex vivo ovary culture was employed as in (Chen et al. 2007). We first assessed the potential functionality of our ex vivo ovary culture. TAF4b (+/−) and (−/−) ovaries were harvested at E18.5 and cultured for three days until “PND 1”. Ovaries were then sectioned and stained for Tra98-positive germ cells (Figure 4A). Indeed, the TAF4b −/− germ cell loss observed immediately after birth in vivo (Figure 4B) is recapitulated in ex vivo ovary culture, allowing for the use of this method as a complement to our in vivo work. To more directly test the hypothesis that apoptosis is responsible for the germ cell death observed around the time of birth in TAF4b (−/−) ovaries, the pan-caspase inhibitor Z-VAD-FMK was utilized. Ovaries were harvested at E18.5 from TAF4b (+/+) and (−/−) mice and cultured for 4 days. One ovary from each mouse was cultured in media containing 50μM Z-VAD-FMK, while the other ovary was cultured in control media containing an appropriate amount of DMSO vehicle. While TAF4b (−/−) ovaries cultured in control media experience germ cell loss similar to that observed in vivo, TAF4b (−/−) ovaries cultured in Z-VAD-FMK-containing media experience a marked retention of Tra98-positive germ cells (Figure 5A). As expected, TAF4b (+/+) ovaries retain germ cells in both control and treated culture conditions (Figure 5B). Furthermore, while there is some variability in culture results, both TAF4b (+/+) and (−/−) ovaries exhibit a significant increase in germ cell density between control and Z-VAD-FMK-treated culture conditions (p<0.05, two-tailed T-Test) when ovaries are cultured in the presence of Z-VAD-FMK (Figure 5C). This is to be expected of TAF4b (+/+) ovaries as Z-VAD-FMK should inhibit normal cyst breakdown as well as aberrant apoptosis, but this result is particularly notable in TAF4b (−/−) ovaries. Additionally, there is no significant difference between Z-VAD-FMK treated TAF4b (−/−) ovaries and control-treated TAF4b (+/+) ovaries, suggesting that the pan-caspase treatment restores TAF4b-deficient germ cell densities to that which would be expected of wild-type ovaries at the same time point. Furthermore, culture in the presence of Z-VAD-FMK results in the loss of Activated Caspase 3 staining in cultured TAF4b (−/−) ovaries (Supplementary Figure 7). These data further implicate apoptosis as a primary mechanism of germ cell loss in the context of the perinatal TAF4b-deficient ovary.
Figure 4. Ex vivo-cultured PND 1 ovaries recapitulate in vivo oocyte depletion.
A) E18.5 TAF4b (+/−) and (−/−) ovaries were harvested and cultured until PND 1 in ovary culture media. Ovaries were then embedded and stained for Tra98 (green) to assess germ cell numbers. Cultured TAF4b (−/−) experience oocyte depletion similar to in vivo TAF4b (−/−) ovaries (right panel), while cultured TAF4b (+/−) ovaries maintain the oocyte reserve (left panel). B) Representative sections of in vivo PND 2 TAF4b (+/+) and (−/−) ovaries.
Figure 5. TAF4b (−/−) perinatal oocyte depletion is due primarily to apoptosis.
E18.5 TAF4b (+/+) and (−/−) ovaries were harvested and cultured until PND 2 in control media or media containing 50μM ZVAD-FMK. Ovaries were then embedded and stained for Tra98 (green) to assess germ cell numbers. (A) Oocyte depletion in TAF4b (−/−) ovaries is inhibited in the presence of ZVAD-FMK, but not control media. (B) In both culture conditions, TAF4b (+/+) ovaries maintain their oocyte reserve. (C) Quantification of oocyte reserve. Tra98-positive oocytes were counted per section and total number was divided by total DAPI area. N=5 for ZVAD-treated TAF4b (+/+) and (−/−). N=6 for control-treated TAF4b (+/+) and (−/−). **: two-tailed t-test, p<0.01.
Excessive germ cell loss in TAF4b-deficient ovaries can be negated by β-estradiol treatment
As β-estradiol has been shown to inhibit germ cell apoptosis (Lei et al. 2010), ovary cultures in the presence of β-estradiol were also performed. Ovaries from TAF4b (+/+) and (−/−) mice were collected at E18.5 and cultured for 4 days. One ovary per mouse was cultured in media containing 10nM β-estradiol, while the other ovary was cultured in control media containing an appropriate amount of ethanol vehicle. Ovaries were then sectioned and stained with an antibody against Tra98. While TAF4b (−/−) ovaries cultured in control media again experience the expected germ cell loss similar that is observed in vivo, TAF4b (−/−) ovaries cultured in the presence of β-estradiol experience a retention of Tra98-positive germ cells (Supplementary Figure 8A). In contrast, TAF4b (+/+) ovaries experience retain germ cells regardless of culture conditions (Supplementary Figure 8B). However, TAF4b (+/+) ovaries exhibit no significant difference in germ cell density between control and β-estradiol-treated culture conditions while TAF4b (−/−) ovaries exhibit significant retention of germ cells (p<0.05, two-tailed T-Test) when ovaries are cultured in the presence of β-estradiol (Supplementary Figure 8C). Similarly to Z-VAD-FMK treatment, there is no significant difference between β-estradiol-treated TAF4b (−/−) ovaries and control-treated TAF4b (+/+) ovaries, suggesting that the β-estradiol treatment restores TAF4b-deficient germ cell densities to that which would be expected of wild-type ovaries at the same time point. Taken together, these data suggest that a critical aspect of estrogen signaling may be perturbed in the early postnatal TAF4b-deficient ovary leading to aberrant cyst breakdown and reduced oocyte survival.
Discussion
Regulating the precise level of oocyte survival at birth is critical for establishing the proper primordial follicle reserve in the adult ovary that is required for long-term fertility. Premature exhaustion of this primordial follicle reserve is one feature of POI, which affects 1% of women worldwide (Knauff et al. 2009). While known causes of this disorder include accelerated ovarian follicle depletion, the molecular mechanisms and etiology underlying the development of this disorder are largely unknown. Here we demonstrate for the first time that TAF4b is required in the ovary to initiate and/or complete cyst breakdown, ensure germ cell survival, and allow for proper primordial follicle development during this critical window immediately after birth. These data establish further evidence that TAF4b is in part responsible for an early regulatory step that ensures ovarian health and fertility.
Fetal germ cell death is known to occur via a number of mechanisms, including Caspase 2-dependent apoptosis during cyst breakdown, autophagy, and ovarian shedding (Bergeron et al. 1998; Morita et al. 2001; Takai et al. 2007; Lobascio et al. 2007; Tingen et al. 2009). The increased Activated Caspase 3 staining shown here at PND 0 is coincident with the rapid loss of TAF4b-deficient oocytes (Lobascio et al. 2007; Tingen et al. 2009). Previous studies by (Falender et al. 2005) using this TAF4b-deficient mouse model utilized TUNEL staining as an indicator of apoptosis and found no increase in TUNEL-positive cells in TAF4b (−/−) ovaries compared to wild-type (Falender et al. 2005). This is not unexpected, however, as TUNEL staining has been shown to be an unreliable marker of oocyte apoptosis due to unique germ cell chromatin configurations leading to inaccessibility to the terminal deoxynucleotidyl transferase enzyme (Ghafari, Gutierrez, and Hartshorne 2007). Our studies using both immunofluorescent detection of Activated Caspase 3 in vivo, as well as apoptosis inhibition ex vivo, demonstrate that germ cell loss in TAF4b (−/−) ovaries is likely due to classical apoptosis. Germ cell death, and not simply loss of germ cell marker expression, is further supported by the loss of germ cell morphology in TAF4b (−/−) ovaries by PND 1 (Supplementary Figures 3 & 6). While we observe excessive germ cell attrition by PND 1 in the TAF4b-deficient ovary, a small but quantifiable number of TAF4b-deficient germ cells persist at or after this time point (Figures 1B, 1C and Supplementary Figure 3). These surviving oocytes likely represent a small pool of primordial follicles that can mature but usually undergo atresia during early adulthood (Voronina et al. 2007) and are not developmentally competent (Falender et al. 2005). One possible explanation for the cell death observed in TAF4b (−/−) ovaries is that TAF4b may be involved in an inherent ovarian quality control mechanism that ensures the survival of the best quality oocytes. Surprisingly, we have not detected multi-oocyte follicles in the context of the TAF4b-deficient adult ovary, which are well-documented in other mouse models of cyst breakdown disruption (Yan et al. 2001; Xu and Gridley 2013), reviewed in (Silva-Santos and Seneda 2011). Perhaps the rapid loss of oocytes precludes the ability of the TAF4b-deficient ovary to form multi-oocyte follicles. Excessive oocyte apoptosis may result from the aberrant expression of pro-survival and pro-apoptotic genes in the neonatal TAF4b (−/−) ovary. As the expression of these genes is finely balanced to allow for the precise level of oocyte survival (Felici et al. 1999; Poljicanin et al. 2013), fine-tuning of this apoptotic gene expression by TAF4b may allow for appropriate primordial follicle number. Furthermore, TAF4b has been shown to regulate expression of transcriptional regulator c-jun in granulosa cells, and in conjunction with c-jun regulates expression of ovarian genes (Geles et al. 2006). TAF4b may perform a similar function in the oocytes along with transcriptional regulators of oocyte development including factor in the germline alpha (FIGalpha) (Soyal, Amleh, and Dean 2000), Nobox (Suzumori et al. 2002; Rajkovic et al. 2004), and Lhx8 (Choi et al. 2008). Future identification of the direct transcriptional targets of TAF4b in the neonatal ovary is critical for understanding the molecular mechanisms underlying perinatal germ cell survival in the mouse ovary and may reveal potential therapeutic avenues for better managing POI in women.
In addition to ZVAD-fmk treatment, β-estradiol treatment was shown to inhibit germ cell apoptosis in TAF4b (−/−) mice. Previous research has shown β-estradiol treatment to be effective in preventing oocyte apoptosis in wild-type mice as well (Lei et al. 2010). It is hypothesized that the observed survival is due to an estrogen-dependent alteration in pro-survival or oocyte-development genes (Lei et al. 2010). In contrast to these previous studies, retention of single, in contrast to cysts, of Tra98-positive cells in β-estradiol-treated ovaries were observed, though this may be due to differences in culture lengths or the precise time of β-estradiol administration. While there are no significant differences in estrogen receptor beta expression in TAF4b (−/−) mice (Voronina et al. 2007), Aromatase expression was shown to be down-regulated (Freiman et al. 2001), suggesting that they may experience decreased estrogen production as neonates. As β-estradiol treatment helps preserve the oocyte reserve in TAF4b (−/−) mice, it is likely that estrogen level, not estrogen response, is affected in these mice. As estrogen level can be similarly affected in women suffering from POI (Nelson 2009), our understanding of the TAF4b (−/−) phenotype will lend itself to better understanding and treatment of this condition.
The onset of meiotic arrest in prophase I begins around E17.5 and has been proposed to be a steroid hormone-dependent process (reviewed in (Pepling 2012)). Previous research by (Falender et al. 2005) has shown that TAF4b (−/−) oocytes experience spindle defects during meiotic maturation, and fertilized TAF4b (−/−) oocytes do not progress beyond a few embryonic cell divisions, both of which suggest that meiotic defects may be responsible for poor developmental competency. In support of this hypothesis, our previous work in (Lovasco et al. 2010) has shown that a number of critical meiotic genes are down-regulated in 21-day-old TAF4b (−/−) mouse ovaries including SCM1B and Mad2l1. Finally, (Paredes et al. 2005) have shown that loss of SCP1 in rat oocytes facilitates follicle formation, indicating that completion of meiosis I may signal individual oocytes to form primordial follicles. This study emphasizes that primordial follicle assembly may not only follow meiosis I progression arrest, but may be dependent upon the proper completion of these steps. Thus, the potential disruption of steroid hormone production in the TAF4b-deficient embryonic ovary may negatively impact meiotic processes known to affect oocyte survival.
The failure of primordial follicle development in TAF4b (−/−) mice may also result from a variety of ovarian deficiencies, including impaired somatic cell invasion into germ cell cysts (Su et al. 2013), excessive cell-cell adhesion preventing cyst fragmentation (Lechowska et al. 2011), or a decrease or absence of key cyst breakdown regulators (Jones and Pepling 2012). Somatic cell invasion into germ cell cysts is a critical step towards the process of primordial follicle assembly and can be impaired by disruption of cellular pores and channels, altering the motility and invasion capacity of pre-granulosa cells (Su et al. 2013). Improper formation of these cellular connections (Lechowska et al. 2011) may also contribute to the failure of follicle assembly observed in TAF4b-deficient mice. Finally, follistatin expression, a key regulator of germ cell survival, primordial follicle formation (Yao et al. 2004), and developmental competence (Patel et al. 2007) is significantly reduced in juvenile TAF4b (−/−) mice (Lovasco et al. 2010). Follistatin is present in a variety of short and long isoforms which contribute differently to primordial follicle assembly, however mice lacking either long isoform of follistatin (FST305 or FST315) experience increased primordial follicle numbers followed by accelerated oocyte death (Kimura, Bonomi, and Schneyer 2011). While not identical in presentation or timing, this phenotype is similar to the neonatal TAF4b (−/−) phenotype of increased germ cell density followed by accelerated oocyte death soon after birth. As TAF4b (−/−) mice experience reduced expression of many key folliculogenesis genes, the initial preservation of oocytes due to reduction in follistatin may be superseded by deficient cyst breakdown. When oocytes cannot effectively transition from cysts to follicles, an overriding ovarian quality control mechanism may sacrifice the excess oocytes earlier than is observed in mice lacking full-length follistatin. As TAF4b is expressed in both somatic and germ cells in the ovary, it is possible that TAF4b may play multiple roles leading to proper primordial follicle formation. Further studies elucidating these functions will shed light on the precise transcriptional regulatory steps required for proper mammalian ovarian follicle development and fertility, as well as the specific cell death pathways involved in inappropriate oocyte loss and infertility.
Materials and Methods
Mouse Models
Wild-type (TAF4b (+/+)) and TAF4b null (−/−) or heterozygous (+/−) mice were generated by mating heterozygous TAF4b (+/−) male and female mice as described previously (Freiman et al. 2001). Offspring were genotyped by PCR analysis of tail-snip genomic DNA amplifying the region targeted by homologous recombination. All animal protocols were reviewed and approved by Brown University Institutional Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Neonatal Ovary Cultures
Timed matings of 8-week-old mice were set up in the evening; presence of copulation plugs the following morning indicated successful mating and was considered embryonic day 0.5 (E0.5). Pregnant females were euthanized by CO2 inhalation at the appropriate time points and TAF4b (+/+), (+/−) and (−/−) ovaries were harvested from E18.5 embryos for neonatal organ culture until PND 2 as described (Chen et al. 2007) in ovary culture media [DMEM/Ham F-12 supplemented with L-glutamine (Life Technologies), 1mg/ml bovine serum albumin (Sigma), 0.5mg/ml Albumax (Life Technologies), 50 μg/ml ascorbic acid (Sigma), 27.5 μg/ml transferrin (Sigma) and 0.1mg/ml 10000 U/ml penicillin/5 μg/ml streptomycin (Life Technologies)].
One ovary per mouse was cultured in “control media”, while the other was cultured in “treated media”. Treated media contained 50μM Z-VAD-FMK (R&D Systems) in DMSO or 10nm β-estradiol (Sigma-Aldrich) in 100% ethanol, while control media contained an appropriate amount of the respective solvent in the ovary culture media. Media was changed every other day and on PND 2, ovaries were embedded in Optimal Cutting Temperature Compound (Tissue-Tek) for sectioning.
Quantitative Immunofluorescence
Ovaries were removed, cleaned of excess fat, and fixed in 4% formaldehyde solution overnight before embedding in Optimal Cutting Temperature Compound. Ovaries were serially sectioned at 8uM on a Leica Cryostat onto glass slides and washed in 1x Phosphate-Buffered Saline containing 0.01% Triton-X (CalBiochem). The entire tissue was sectioned and the median, adjacent two sections chosen for staining and quantification. Tissue sections were then incubated in blocking buffer (3% Goat Serum (Sigma), 1% Bovine Serum Albumin (Sigma), and 0.5% Tween-20 (Fisher Scientific) in 1X PBS) and stained by incubation with primary antibodies against Tra98 (B-Bridge) and Mouse Vasa Homolog (Abcam) for germ cell counting, or Tra98 and Activated Caspase 3 (Cell Signaling) for apoptosis analysis. A secondary antibody-only control was included to compare background staining. Sections were further stained with DAPI to visualize nuclei and analyzed on an Epifluorescent Zeiss Axioplan microscope.
Germ cell densities were determined by counting total Tra98-positive cells and dividing the total number by the DAPI area to obtain cells/mm2, or density of germ cells in the ovary. For each treatment group, at least two ovary tissues sections each from at least four mice were quantified. Results were averaged and significance determined by two-tailed T-Test. Error bars represent standard error of the mean. Percentages of Activated Caspase 3-positive oocytes were determined by counting cells positive for both Tra98 and Activated Caspase 3 and dividing by the total number of Tra98-positive cells per section. Results were averaged and significance determined by two-tailed T-Test. Error bars represent standard error of the mean.
Ovarian Histology
Ovaries were removed at E18.5, PND 0 and PND 1, cleaned of excess fat and bursal sac, and fixed in 1:10 formalin solution overnight. Standard paraffin embedding, microtome sectioning, and hematoxylin & eosin staining were performed. Images were taken using the Scanscope CS (Aperio).
Ovarian GCNA Immunohistochemistry
Ovaries were removed at E18.5, PND 0 and PND 1, cleaned of excess fat and bursal sac, and fixed in 1:10 formalin solution overnight. Standard paraffin embedding and microtome sectioning were performed. Sections were deparaffinized and antigen unmasking was performed with 1:10 Antigen Unmasking Buffer (Vector Laboratories). Sections were cooled and permeabilized in 0.1% Triton for 10 minutes prior to Avidin-biotin blocking with an Avidin-Biotin Blocking Kit (Vector Laboratories) according to manufacturer’s directions. Sections were blocked in PBS with 1% Bovine Serum Albumin and 6% ready-to-use goat serum before incubating overnight at 4°C with undiluted rat anti- GCNA antibody (gift from laboratory of Dr. David Page). The following day, sections were washed and incubated with biotinylated anti-rat IgG antibodies (Vector Laboratories), for 1 h at room temperature. After PBS wash, sections were incubated in ready-to-use ABC Vectastain reagent (Vector Laboratories) for 45 min and washed. Sections were then developed in Vector NovaRED substrate (Vector Laboratories). Nuclei were counter-stained using Methyl Green (Vector Laboratories). Slides were cleaned with isopropyl alcohol and successive Citrisolv Hybrid (Fisher Scientific) and mounted with Cytoseal 60 mounting media (Richard-Allan Scientific). Stained sections were imaged at 20× magnification with a ScanScope CS imaging system (Aperio).
Supplementary Material
Ovaries were harvested from E18.5 embryos and were embedded for paraffin sectioning. A) Sections were stained using indirect immunohistochemistry for germ cell marker GCNA. GCNA staining (red) was developed using NovaRED substrate, and nuclei (green) were counterstained with Methyl Green. B) Sections were stained using indirect immunofluorescence for germ cell marker Tra98 (green). Nuclei were visualized by DAPI stain (blue).
Ovaries were harvested from PND 0 pups and were embedded for paraffin sectioning. A) Sections were stained using indirect immunohistochemistry for germ cell marker GCNA. GCNA staining (red) was developed using NovaRED substrate, and nuclei (green) were counterstained with Methyl Green. B) Sections were stained using indirect immunofluorescence for germ cell marker Tra98 (green). Nuclei were visualized by DAPI stain (blue). Dashed circles denote large cysts still present at PND 0 in TAF4b (−/−) but not TAF4b (+/−) ovaries.
Ovaries were harvested from PND 1 pups and were embedded for paraffin sectioning. A) Sections were stained using indirect immunohistochemistry for germ cell marker GCNA. GCNA staining (red) was developed using NovaRED substrate, and nuclei (green) were counterstained with Methyl Green. B) Sections were stained using indirect immunofluorescence for germ cell marker Tra98 (green). Nuclei were visualized by DAPI stain (blue). Depletion of germ cells in TAF4b (−/−) ovaries is evident in both staining conditions.
Ovaries were harvested from E18.5 embryos and were embedded for paraffin sectioning. Sections were stained with hematoxylin and eosin by standard procedure. High magnification insets of representative ovarian regions is shown in the top panels.
Ovaries harvested from PND 0 pups and were embedded for paraffin sectioning. Sections were stained with hematoxylin and eosin by standard procedure. High magnification insets of representative ovarian regions is shown in the top panels. Dashed circles denote large cysts still present at PND 0 in TAF4b (−/−) but not TAF4b (+/−) ovaries.
Ovaries harvested from PND 1 pups and were embedded for paraffin sectioning. Sections were stained with hematoxylin and eosin by standard procedure. High magnification insets of representative ovarian regions is shown in the top panels. Red arrows denote primordial follicles present in PND 1 TAF4b (+/+) ovaries but not in TAF4b (−/−).
TAF4b (+/+) and (−/−) ovaries were harvested at E18.5 and cultured until PND 2 in control media or media containing 50μM ZVAD-FMK. Ovaries were then embedded and stained for Tra98 (green) to assess germ cell numbers and Activated Caspase 3 (red) to assess apoptosis. Activated Caspase 3 staining is only seen in TAF4b (−/−) ovaries cultured in control media (upper right panel), and not see when TAF4b (−/−) ovaries are cultured in the presence of Z-VAD-FMK (lower right panel), or in either condition in TAF4b (+/+) ovaries (left panels).
E18.5 TAF4b (+/+) and (−/−) ovaries were harvested and cultured until PND 2 in control media or media containing 10nM β-estradiol. Ovaries were then embedded and stained for Tra98 (green) to assess germ cell numbers. (A) Oocyte depletion in TAF4b (−/−) ovaries is inhibited in the presence of β-estradiol, but not control media. (B) In both culture conditions, TAF4b (+/+) ovaries maintain their oocyte reserve. (C) Quantification of stained tissue sections. Tra98-positive oocytes were counted per section and total number was divided by total DAPI area. N=4 for E2- and control-treated TAF4b (−/−). N=5 for E2- and control-treated TAF4b (+/+). *: two-tailed t-test, p<0.05.
Highlights.
TAF4b-deficient ovaries contain normal germ cell densities late in embryogenesis
TAF4b-deficient mouse ovaries suffer accelerated oocyte depletion at birth
TAF4b is required or the proper timing of normal cyst breakdown in the mouse ovary
Excessive germ cell loss in TAF4b’s absence is coincident with Caspase 3 activation
Apoptosis inhibition promotes germ cell retention in TAF4b-deficient ovaries
Acknowledgments
We thank Drs. Jennifer Wardell, Lindsay Lovasco and Eric Gustafson for insightful suggestions on this manuscript, and members of the Freiman Lab for helpful input throughout these studies. We thank Melinda Golde for her expertise in and assistance with paraffin sectioning and hematoxylin & eosin staining. We thank Drs. Ben Moyer and Mary Hixon for their expertise in ex vivo ovary culture. We thank Dr. Caitlin Brown for her expertise and assistance on the Aperio Scanscope. We thank Drs. David Page and Michelle Carmell for their generous gift of the GCNA antibody. This research is supported by NIH R01 HD065445, NIH COBRE Center for Cancer Signaling Networks RR031153 and ACS Research Scholar Grant DMC-117629 to RNF; and NIH Ruth L. Kirschstein National Research Award F31 AG045016 to KJG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, et al. Defects in Regulation of Apoptosis in Caspase-2-deficient Mice. Genes & Development. 1998;12:1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Breen K, Pepling ME. Estrogen Can Signal through Multiple Pathways to Regulate Oocyte Cyst Breakdown and Primordial Follicle Assembly in the Neonatal Mouse Ovary. J Endocrinol. 2009;202(3):407–417. doi: 10.1677/joe-09-0109. [DOI] [PubMed] [Google Scholar]
- Chen Ying, Jefferson Wendy N, Newbold Retha R, Padilla-Banks Elizabeth, Pepling Melissa E. Estradiol, Progesterone, and Genistein Inhibit Oocyte Nest Breakdown and Primordial Follicle Assembly in the Neonatal Mouse Ovary in Vitro and in Vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Choi Youngsok, Ballow Daniel J, Xin Yun, Rajkovic Aleksandar. Lim Homeobox Gene, Lhx8, Is Essential for Mouse Oocyte Differentiation and Survival. Biology of Reproduction. 2008;79:442–449. doi: 10.1095/biolreprod.108.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro C, Vento M, Ragusa M, Barbagallo D, Guglielmino MR, Maniscalchi T, Duro LR, et al. Expression Analysis of TFIID in Single Human Oocytes: New Potential Molecular Markers of Oocyte Quality. Reproductive Biomedicine Online. 2008;17:338–349. doi: 10.1016/S1472-6483(10)60217-9. [DOI] [PubMed] [Google Scholar]
- Drummond AE, Fuller PJ. Ovarian Actions of Estrogen Receptor-beta: An Update. Semin Reprod Med. 2012;30(1):32–38. doi: 10.1055/s-0031-1299595. [DOI] [PubMed] [Google Scholar]
- Enders GC, May JJ., 2nd Developmentally Regulated Expression of a Mouse Germ Cell Antigen Examined from Embryonic Day 11 to Adult in Male and Female Mice. Developmental Biology. 1994;163(2):331–340. doi: 10.1006/dbio.1994.1152. [DOI] [PubMed] [Google Scholar]
- Falender AE, Shimada M, Lo YK, Richards JS. TAF4b, a TBP Associated Factor, Is Required for Oocyte Development and Function. Dev Biol. 2005;288(2):405–419. doi: 10.1016/j.ydbio.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Felici MD, Carlo AD, Pesce M, Iona S, Farrace MG, Piacentini M. Bcl-2 and Bax Regulation of Apoptosis in Germ Cells During Prenatal Oogenesis in the Mouse Embryo. Cell Death and Differentiation. 1999;6:908–915. doi: 10.1038/sj.cdd.4400561. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Funkhouser JM, Sindoni DM, Stevis PE, Deecher DC, Bapat AR, Merchenthaler I, Frail DE. Expression of Estrogen Receptor-beta Protein in Rodent Ovary. Endocrinology. 1999;140:2581–2591. doi: 10.1210/endo.140.6.6928. [DOI] [PubMed] [Google Scholar]
- Freiman RN, Albright SR, Zheng S, Sha WC, Hammer RE, Tjian R. Requirement of Tissue-selective TBP-associated Factor TAFII105 in Ovarian Development. Science. 2001;293(5537):2084–2087. doi: 10.1126/science.1061935. http://www.ncbi.nlm.nih.gov/pubmed/11557891. [DOI] [PubMed] [Google Scholar]
- Geles KG, Freiman RN, Liu WL, Zheng S, Voronina E, Tjian R. Cell-type-selective Induction of C-jun by TAF4b Directs Ovarian-specific Transcription Networks. Proc Natl Acad Sci U S A. 2006;103(8):2594–2599. doi: 10.1073/pnas.0510764103. http://www.ncbi.nlm.nih.gov/pubmed/16473943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafari Fataneh, Gutierrez Carlos G, Hartshorne Geraldine M. Apoptosis in Mouse Fetal and Neonatal Oocytes During Meiotic Prophase One. BMC Developmental Biology. 2007;7:87. doi: 10.1186/1471-213X-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson Wendy, Newbold Retha, Padilla-Banks Elizabeth, Pepling Melissa. Neonatal Genistein Treatment Alters Ovarian Differentiation in the Mouse: Inhibition of Oocyte Nest Breakdown and Increased Oocyte Survival. Biology of Reproduction. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- Jones Robin L, Pepling Melissa E. Role of the Anti-Apoptotic Proteins BCL2 and MCL1 in the Neonatal Mouse Ovary. Biology of Reproduction. 2012;88:1–8. doi: 10.1095/biolreprod.112.103028. [DOI] [PubMed] [Google Scholar]
- Jones Robin L, Pepling Melissa E. KIT Signaling Regulates Primordial Follicle Formation in the Neonatal Mouse Ovary. Developmental Biology. 2013:1–12. doi: 10.1016/j.ydbio.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Juengel Jennifer L, Heath Derek A, Quirke Laurel D, McNatty Kenneth P. Oestrogen Receptor Alpha and Beta, Androgen Receptor and Progesterone Receptor mRNA and Protein Localisation Within the Developing Ovary and in Small Growing Follicles of Sheep. Reproduction (Cambridge, England) 2006;131:81–92. doi: 10.1530/rep.1.00704. [DOI] [PubMed] [Google Scholar]
- Karavan Jenna R, Pepling Melissa E. Effects of Estrogenic Compounds on Neonatal Oocyte Development. Reproductive Toxicology (Elmsford, NY) 2012;34(1 August):51–6. doi: 10.1016/j.reprotox.2012.02.005. http://www.ncbi.nlm.nih.gov/pubmed/22406039. [DOI] [PubMed] [Google Scholar]
- Kimura Fuminori, Bonomi Lara M, Schneyer Alan L. Follistatin Regulates Germ Cell Nest Breakdown and Primordial Follicle Formation. Endocrinology. 2011;152:697–706. doi: 10.1210/en.2010-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauff EA, Franke L, van Es MA, van den Berg LH, van der Schouw YT, Laven JS, Lambalk CB, et al. Genome-wide Association Study in Premature Ovarian Failure Patients Suggests ADAMTS19 as a Possible Candidate Gene. Hum Reprod. 2009;24(9):2372–2378. doi: 10.1093/humrep/dep197. [DOI] [PubMed] [Google Scholar]
- Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP. Estrogen Receptor Gene Disruption: Molecular Characterization and Experimental and Clinical Phenotypes. Recent Prog Horm Res. 1996;51:159–186. [PubMed] [Google Scholar]
- Lechowska A, Bilinksi S, Choi Y, Shin Y, Kloc M, Rajkovic A. Premature Ovarian Failure in Nobox-deficient Mice Is Caused by Defects in Somatic Cell Invasion and Germ Cell Cyst Breakdown. Journal of Assisted Reproduction and Genetics. 2011;28(7):583–589. doi: 10.1007/s10815-011-9553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Jin S, Mayo KE, Woodruff TK. The Interactions Between the Stimulatory Effect of Follicle-stimulating Hormone and the Inhibitory Effect of Estrogen on Mouse Primordial Folliculogenesis. Biol Reprod. 2010;82(1):13–22. doi: 10.1095/biolreprod.109.077404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobascio AM, Klinger FG, Scaldaferri ML, Farini D, De Felici M. Analysis of Programmed Cell Death in Mouse Fetal Oocytes. Reproduction. 2007;134(2):241–252. doi: 10.1530/rep-07-0141. [DOI] [PubMed] [Google Scholar]
- Lovasco LA, Seymour KA, Zafra K, O’Brien CW, Schorl C, Freiman RN. Accelerated Ovarian Aging in the Absence of the Transcription Regulator TAF4B in Mice. Biol Reprod. 2010;82(1):23–34. doi: 10.1095/biolreprod.109.077495. http://www.ncbi.nlm.nih.gov/pubmed/19684329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of Reproductive Function but Not Prenatal Sexual Development after Insertional Disruption of the Mouse Estrogen Receptor Gene. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin EA, McIver SC. Awakening the Oocyte: Controlling Primordial Follicle Development. Reproduction. 2009;137(1):1–11. doi: 10.1530/rep-08-0118. [DOI] [PubMed] [Google Scholar]
- Morita Y, Maravei DV, Bergeron L, Wang S, Perez GI, Tsutsumi O, Taketani Y, et al. Caspase-2 Deficiency Prevents Programmed Germ Cell Death Resulting from Cytokine Insufficiency but Not Meiotic Defects Caused by Loss of Ataxia Telangiectasia-mutated (Atm) Gene Function. Cell Death and Differentiation. 2001;8:614–620. doi: 10.1038/sj.cdd.4400845. [DOI] [PubMed] [Google Scholar]
- Mork L, Tang H, Batchvarov I, Capel B. Mouse Germ Cell Clusters Form by Aggregation as Well as Clonal Divisions. Mech Dev. 2012;128(11–12):591–596. doi: 10.1016/j.mod.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-de-Toro Mónica, Luque Enrique H, Rivera Oscar E, Varayoud Jorgelina, Rodríguez Horacio A. Neonatal Exposure to Bisphenol A or Diethylstilbestrol Alters the Ovarian Follicular Dynamics in the Lamb. Reproductive Toxicology. 2011 doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed]
- Nelson Lawrence M. Clinical Practice. Primary Ovarian Insufficiency. The New England Journal of Medicine. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes Alfonso, Garcia-Rudaz Cecilia, Kerr Bredford, Tapia Veronica, Dissen Gregory A, Costa Maria E, Cornea Anda, Ojeda Sergio R. Loss of Synaptonemal Complex Protein-1, a Synaptonemal Complex Protein, Contributes to the Initiation of Follicular Assembly in the Developing Rat Ovary. Endocrinology. 2005;146:5267–5277. doi: 10.1210/en.2005-0965. [DOI] [PubMed] [Google Scholar]
- Patel Osman V, Bettegowda Anilkumar, Ireland James J, Coussens Paul M, Lonergan Patrick, Smith George W. Functional Genomics Studies of Oocyte Competence: Evidence That Reduced Transcript Abundance for Follistatin Is Associated with Poor Developmental Competence of Bovine Oocytes. Reproduction (Cambridge, England) 2007;133:95–106. doi: 10.1530/rep.1.01123. [DOI] [PubMed] [Google Scholar]
- Pepling ME. Follicular Assembly: Mechanisms of Action. Reproduction. 2012;143(2):139–149. doi: 10.1530/rep-11-0299. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female Mouse Germ Cells Form Synchronously Dividing Cysts. Development (Cambridge, England) 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Pepling ME. Follicular Assembly: Mechanisms of Action. Reproduction. 2012 doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- Pepling Melissa E. From Primordial Germ Cell to Primordial Follicle: Mammalian Female Germ Cell Development. Genesis New York NY 2000. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Poljicanin Ana, Pusic Tanja Vukusic, Vukojevic Katarina, Caric Ana, Vilovic Katarina, Tomic Snjezana, Soljic Violeta, Saraga-Babic Mirna. The Expression Patterns of Pro-apoptotic and Anti-apoptotic Factors in Human Fetal and Adult Ovary. Acta Histochemica. 2013 doi: 10.1016/j.acthis.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Rajkovic Aleksandar, Pangas Stephanie A, Ballow Daniel, Suzumori Nobuhiro, Matzuk Martin M. NOBOX Deficiency Disrupts Early Folliculogenesis and Oocyte-specific Gene Expression. Science (New York, NY) 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- Silva-Santos KC, Seneda MM. Multioocyte Follicles in Adult Mammalian Ovaries. Animal Reproduction. 2011;8:58–67. [Google Scholar]
- Soyal SM, Amleh A, Dean J. FIGalpha, a Germ Cell-specific Transcription Factor Required for Ovarian Follicle Formation. Development. 2000;127:4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- Su Weiheng, Guan Xingang, Zhang Di, Sun Meiyan, Yang Longfei, Yi Fei, Hao Feng, Feng Xuechao, Ma Tonghui. Occurrence of Multi-oocyte Follicles in Aquaporin 8-deficient Mice. Reproductive Biology and Endocrinology. 2013;11(88) doi: 10.1186/1477-7827-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumori Nobuhiro, Yan Changning, Matzuk Martin M, Rajkovic Aleksandar. Nobox Is a Homeobox-encoding Gene Preferentially Expressed in Primordial and Growing Oocytes. Mechanisms of Development. 2002;111:137–141. doi: 10.1016/S0925-4773(01)00620-7. [DOI] [PubMed] [Google Scholar]
- Takai Y, Matikainen T, Jurisicova A, Kim MR, Trbovich AM, Fujita E, Nakagawa T, et al. Caspase-12 Compensates for Lack of Caspase-2 and Caspase-3 in Female Germ Cells. Apoptosis: an International Journal on Programmed Cell Death. 2007;12:791–800. doi: 10.1007/s10495-006-0022-z. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Pereira LAVD, Nozaki M, Tsuchida J, Sawada K, Mori H, Nishimune Y. A Germ Cell-specific Nuclear Antigen Recognized by a Monoclonal Antibody Raised Against Mouse Testicular Germ Cells. International Journal of Andrology. 1997;20:361–366. doi: 10.1046/j.1365-2605.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Tingen CM, Bristol-Gould SK, Kiesewetter SE, Wellington JT, Shea L, Woodruff TK. Prepubertal Primordial Follicle Loss in Mice Is Not Due to Classical Apoptotic Pathways. Biol Reprod. 2009;81(1):16–25. doi: 10.1095/biolreprod.108.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingen Candace, Kim Alison, Woodruff Teresa K. The Primordial Pool of Follicles and Nest Breakdown in Mammalian Ovaries. Molecular Human Reproduction. 2009;15:795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E, Lovasco LA, Gyuris A, Baumgartner RA, Parlow AF, Freiman RN. Ovarian Granulosa Cell Survival and Proliferation Requires the Gonad-selective TFIID Subunit TAF4b. Dev Biol. 2007;303(2):715–726. doi: 10.1016/j.ydbio.2006.12.011. http://www.ncbi.nlm.nih.gov/pubmed/17207475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Jingxia, Gridley Thomas. Notch2 Is Required in Somatic Cells for Breakdown of Ovarian Germ-cell Nests and Formation of Primordial Follicles. BMC Biology. 2013;11:13. doi: 10.1186/1741-7007-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, et al. Synergistic Roles of Bone Morphogenetic Protein 15 and Growth Differentiation Factor 9 in Ovarian Function. Molecular Endocrinology (Baltimore, Md) 2001;15:854–866. doi: 10.1210/me.15.6.854. [DOI] [PubMed] [Google Scholar]
- Yao Humphrey HC, Matzuk Martin M, Jorgez Carolina J, Menke Douglas B, Page David C, Swain Amanda, Capel Blanche. Follistatin Operates Downstream of Wnt4 in Mammalian Ovary Organogenesis. Developmental Dynamics: an Official Publication of the American Association of Anatomists. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ovaries were harvested from E18.5 embryos and were embedded for paraffin sectioning. A) Sections were stained using indirect immunohistochemistry for germ cell marker GCNA. GCNA staining (red) was developed using NovaRED substrate, and nuclei (green) were counterstained with Methyl Green. B) Sections were stained using indirect immunofluorescence for germ cell marker Tra98 (green). Nuclei were visualized by DAPI stain (blue).
Ovaries were harvested from PND 0 pups and were embedded for paraffin sectioning. A) Sections were stained using indirect immunohistochemistry for germ cell marker GCNA. GCNA staining (red) was developed using NovaRED substrate, and nuclei (green) were counterstained with Methyl Green. B) Sections were stained using indirect immunofluorescence for germ cell marker Tra98 (green). Nuclei were visualized by DAPI stain (blue). Dashed circles denote large cysts still present at PND 0 in TAF4b (−/−) but not TAF4b (+/−) ovaries.
Ovaries were harvested from PND 1 pups and were embedded for paraffin sectioning. A) Sections were stained using indirect immunohistochemistry for germ cell marker GCNA. GCNA staining (red) was developed using NovaRED substrate, and nuclei (green) were counterstained with Methyl Green. B) Sections were stained using indirect immunofluorescence for germ cell marker Tra98 (green). Nuclei were visualized by DAPI stain (blue). Depletion of germ cells in TAF4b (−/−) ovaries is evident in both staining conditions.
Ovaries were harvested from E18.5 embryos and were embedded for paraffin sectioning. Sections were stained with hematoxylin and eosin by standard procedure. High magnification insets of representative ovarian regions is shown in the top panels.
Ovaries harvested from PND 0 pups and were embedded for paraffin sectioning. Sections were stained with hematoxylin and eosin by standard procedure. High magnification insets of representative ovarian regions is shown in the top panels. Dashed circles denote large cysts still present at PND 0 in TAF4b (−/−) but not TAF4b (+/−) ovaries.
Ovaries harvested from PND 1 pups and were embedded for paraffin sectioning. Sections were stained with hematoxylin and eosin by standard procedure. High magnification insets of representative ovarian regions is shown in the top panels. Red arrows denote primordial follicles present in PND 1 TAF4b (+/+) ovaries but not in TAF4b (−/−).
TAF4b (+/+) and (−/−) ovaries were harvested at E18.5 and cultured until PND 2 in control media or media containing 50μM ZVAD-FMK. Ovaries were then embedded and stained for Tra98 (green) to assess germ cell numbers and Activated Caspase 3 (red) to assess apoptosis. Activated Caspase 3 staining is only seen in TAF4b (−/−) ovaries cultured in control media (upper right panel), and not see when TAF4b (−/−) ovaries are cultured in the presence of Z-VAD-FMK (lower right panel), or in either condition in TAF4b (+/+) ovaries (left panels).
E18.5 TAF4b (+/+) and (−/−) ovaries were harvested and cultured until PND 2 in control media or media containing 10nM β-estradiol. Ovaries were then embedded and stained for Tra98 (green) to assess germ cell numbers. (A) Oocyte depletion in TAF4b (−/−) ovaries is inhibited in the presence of β-estradiol, but not control media. (B) In both culture conditions, TAF4b (+/+) ovaries maintain their oocyte reserve. (C) Quantification of stained tissue sections. Tra98-positive oocytes were counted per section and total number was divided by total DAPI area. N=4 for E2- and control-treated TAF4b (−/−). N=5 for E2- and control-treated TAF4b (+/+). *: two-tailed t-test, p<0.05.