Abstract
Sulfur mustard (SM) is a bifunctional alkylating agent causing skin inflammation, edema and blistering. A hallmark of SM-induced toxicity is follicular and interfollicular epithelial damage. In the present studies we determined if SM-induced structural alterations in hair follicles and sebaceous glands were correlated with cell damage, inflammation and wound healing. The dorsal skin of hairless mice was treated with saturated SM vapor. One to seven days later, epithelial cell karyolysis within the hair root sheath, infundibulum and isthmus were apparent, along with reduced numbers of sebocytes. Increased numbers of utriculi, some with connections to the skin surface, and engorged dermal cysts were also evident. This was associated with marked changes in expression of markers of DNA damage (phospho-H2A.X), apoptosis (cleaved caspase-3), and wound healing (FGFR2 and galectin-3) throughout pilosebaceous units. Conversely, fatty acid synthase and galectin-3 were down-regulated in sebocytes after SM. Decreased numbers of hair follicles and increased numbers of inflammatory cells surrounding the utriculi and follicular cysts were noted within the wound 3–7 days post SM exposure. Expression of phospho-H2A.X, cleaved caspase-3, FGFR2 and galectin-3 was decreased in dysplastic follicular epidermis. Fourteen days after SM, engorged follicular cysts which expressed galectin-3 were noted within hyperplastic epidermis. Galectin-3 was also expressed in basal keratinocytes and in the first few layers of suprabasal keratinocytes in neoepidermis formed during wound healing indicating that this lectin is important in the early stages of keratinocyte differentiation. These data indicate that hair follicles and sebaceous glands are targets for SM in the skin.
Keywords: sulfur mustard, hair follicles, sebaceous glands, phosphorylated histone H2A.X
Introduction
Sulfur mustard (SM) is a highly reactive bifunctional alkylating agent known to target the skin (Ghabili et al., 2011; Ghanei et al., 2010; Laskin et al., 2010). In humans, SM initially induces skin injury associated with an early inflammatory response (Vogt et al., 1984). Depending on the dose and time following exposure, tissue damage and blistering can ensue (Arck and Paus, 2006; Ghabili et al., 2011). SM also induces injury in rodent skin models where at low doses, it has been reported to induce inflammation, epidermal hyperplasia and microblisters (Snider et al., 1999). The precise mechanisms by which SM induces tissue injury and blistering are poorly understood. SM has been reported to modify many cellular components including nucleic acids, lipids and proteins (Kehe et al., 2009; Naghii, 2002). It also stimulates tissue production of growth factors and proinflammatory mediators. In the skin, this can disrupt cell and tissue functioning, processes that lead to structural alterations in the epidermis, and cytotoxicity (Laskin et al., 2010; Levitt et al., 2003; Naghii, 2002).
It is well recognized that dermal appendages including hair follicles and sebaceous glands, which comprise the pilosebaceous unit, are targets for dermal toxicants (Haslam et al., 2013; Paus et al., 2013). Pilosebaceous units are self-renewing structures important in thermoregulation, sensation and protection against the external environment (Schneider et al., 2009). Using the hairless mouse skin model, we previously reported that SM-induced structural changes in the skin including epidermal thinning, stratum corneum shedding and basal cell karyolysis were associated with expression of markers of apoptosis and DNA damage (Joseph et al., 2011). This was followed by epithelial cell hypertrophy, edema, parakeratosis and loss of epidermal structure. In the present studies, we extended these studies to characterize the effects of SM on hair follicles and sebaceous glands. We found that SM damage to the follicular and interfollicular epidermis of hairless mouse skin is distinct; thus, toxicity in hair follicles and sebaceous glands was delayed, when compared to interfollicular epidermis. Additionally, whereas epidermal regeneration and stratification were evident in interfollicular epidermis during wound healing, hair follicles and sebaceous glands degenerated and were not replaced. Identification of molecular and biochemical changes in the skin induced by SM in different skin compartments will aid in the development of novel therapeutic approaches to mitigating tissue damage and improving wound healing.
Materials and Methods
Animals and Treatments
All experiments with SM were performed at Battelle Memorial Laboratories (Columbus, OH). Male CRL: SKH1-Hr mice, 5 weeks of age (Charles River Laboratories, Wilmington, MA), were housed in microisolation cages and maintained on food and water ad libitum. Animals received humane care in compliance with the institution's guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Mice were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg), and randomly assigned to treatment groups. Animals were exposed to SM using a vapor cup model, modified from Ricketts et al. (2000), was used to expose animals to SM. Thirteen mm diameter vapor cups were mounted on the dorsal skin on both sides of the spine producing a SM exposed site and a contralateral control. SM vapor was produced by absorbing 10 μl of neat SM onto a filter disk within the vapor cup assembly. Animals were exposed to air or SM vapor, estimated from earlier studies as 1.4 g/m3 for 6 min (Ricketts et al., 2000). Mice were sacrificed 1, 3, 7, and 14 days post exposure; full thickness skin punch biopsies were immediately collected, trimmed, and stored in ice cold phosphate buffered saline (PBS) containing 2% paraformaldehyde/3% sucrose. After 24 hr at 4°C, skin samples were rinsed in ice cold PBS containing 3% sucrose, transferred to ethanol (50%), and paraffin embedded. Skin sections (5 μm) were prepared and stained with hematoxylin and eosin (H & E) for analysis of tissue injury or Gomori’s trichrome, which contains methyl (aniline) blue, for analysis of collagen I/III (Goode Histolabs, New Brunswick, NJ).
Immunostaining
For immunohistochemistry, tissue sections were deparaffinized, and blocked with 10% or 100% goat serum at room temperature for 2 hr. Tissue sections were incubated at overnight at 4°C with rabbit affinity purified polyclonal antibodies against cleaved caspase-3 (1:200, Cell Signaling, Danvers, MA), fatty acid synthase (1:100, Cell Signaling), fibroblast growth factor receptor 2 (FGFR2) (1:2000, Abcam, Cambridge, MA), phospho-histone 2A.X (Ser139) (phospho-H2A.X) (1:100, Cell Signaling), proliferating cell nuclear antigen (PCNA) (1:250, Abcam), goat affinity purified polyclonal antibody galectin-3 (1:2000, R&D Systems, Minneapolis, MN) or appropriate controls. Slides were then incubated for 30 min with biotinylated goat anti-rabbit or rabbit anti-goat secondary antibody (Vector Labs, Burlingame, CA). Antibody binding was visualized using a DAB Peroxidase Substrate Kit (Vector Labs).
Results
SM induces alterations in hair follicles
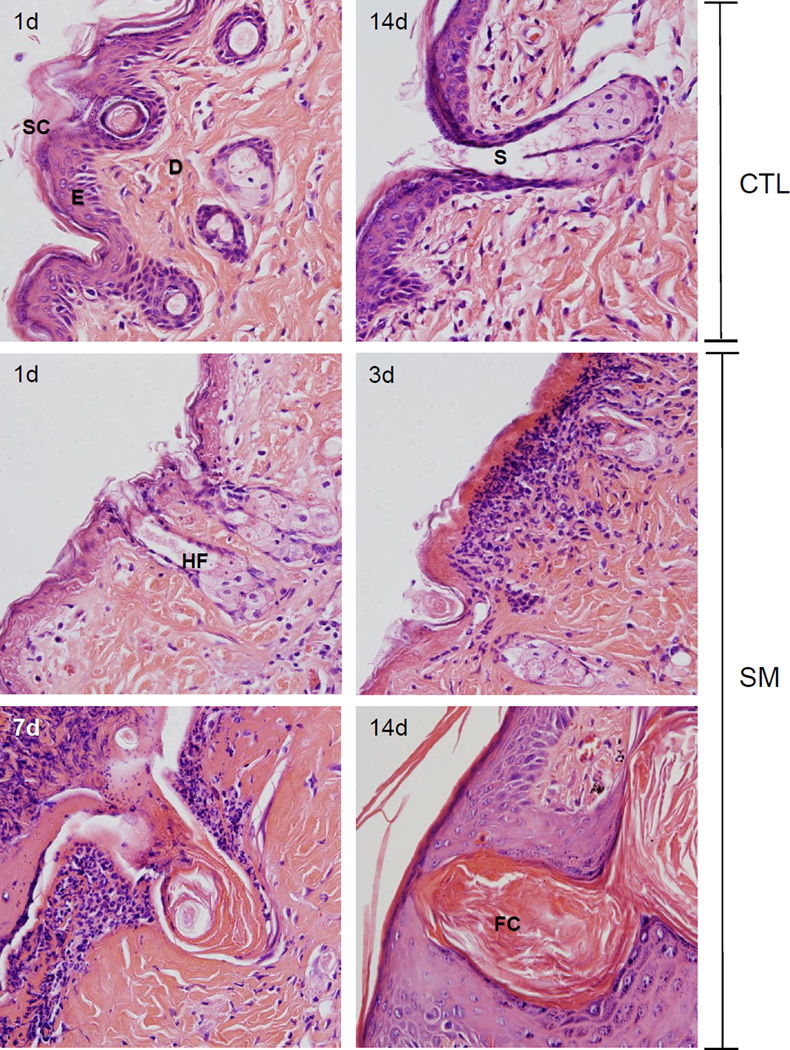
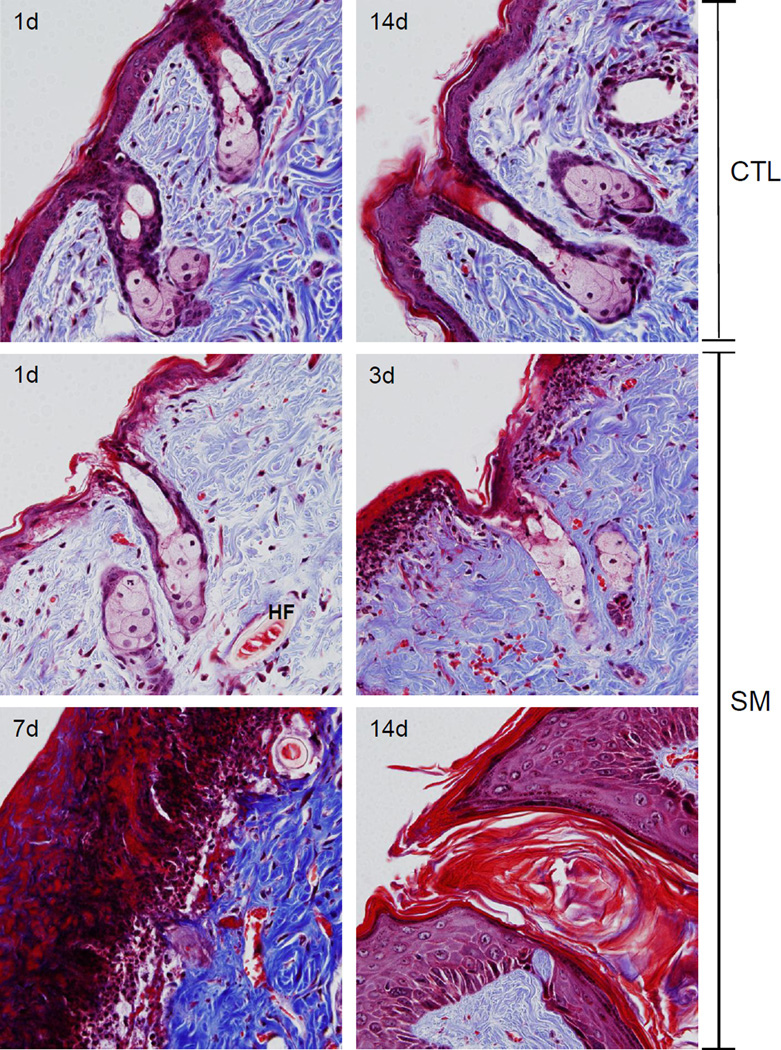
In initial studies we analyzed morphological changes in the hair follicles and sebaceous glands following SM exposure. In control skin, prominent sebaceous glands, atypical hair shafts, isolated utriculi, and small dermal cysts were evident (Figures 1 and 2, upper panels). One day post SM, distortions in the hair follicles and sebaceous glands were noted including karyolysis of epidermal cells in the hair root sheath, the infundibular epidermis and isthmus, karyolysis of sebocytes, dystrophic utriculi, and engorged dermal cysts (Figures 1 and 3). At 3 days post SM, decreased numbers of hair follicles, inflammatory cells surrounding utriculi, and small follicular cysts were also evident. Sebocytes within both unilobate and multilobate sebaceous glands were swollen, with disintegrated nuclei and poorly defined cell margins. Trichrome staining revealed hemorrhage in the compacted dermal collagen (Figures 2 and 4). By day 7 post SM, a thick eschar had formed over the wound site, which was characterized by necrosis, hemorrhage, and an inflammatory infiltrate (Figures 1 and 2). Increased collagen deposition was observed in the dermis which was not easily demarcated following H&E staining (compare Figures 1 and 2). Areas of hemorrhage were still observed in trichrome stained dermis. In the dermis underlying the eschar, pilosebaceous gland vestiges were noted, while within the eschar, only sebaceous gland remnants were present (Figure 2). Fourteen days post SM exposure, hyperplastic interfollicular epidermis and engorged follicular cysts were identified in the dermis underlying the newly formed epidermis (Figure 2). Of note, at this time hair follicles had degenerated to form lamellated keratin structures; these were surrounded by stratum granulosum, stratum spinosum and basal stratum which resemble the infundibulum (Figures 1 and 2). In contrast, the stratum corneum, epidermis, dermal appendages, dermal/epidermal junction, and dermis from the control skin appeared normal (Figures 1 and 2) (Massironi et al., 2005).
Figure 1. Structural changes in hairless mouse skin following SM.
Histological sections prepared 1 and 14 days after air exposure (CTL), and 1, 3, 7 and 14 days after SM exposure were stained with H&E. One representative section from 3 mice/treatment group is shown (original magnification, × 400). Stratum corneum (SC), epidermis (E), hair follicle (HF), dermis (D), sebaceous gland (S), follicular cyst (FC).
Figure 2. Trichrome staining of hairless mouse skin following SM.
Histological sections prepared 1 and 14 days after air exposure (CTL), and 1, 3, 7 and 14 days after SM exposure were stained with Gomori's trichrome containing hematoxylin which stains nuclei blue/black, eosin which stains keratin and cytoplasm red, and aniline blue which stains collagen I/III royal blue. One representative section from 3 mice/treatment group is shown (original magnification, × 400). Hair follicle (HF).
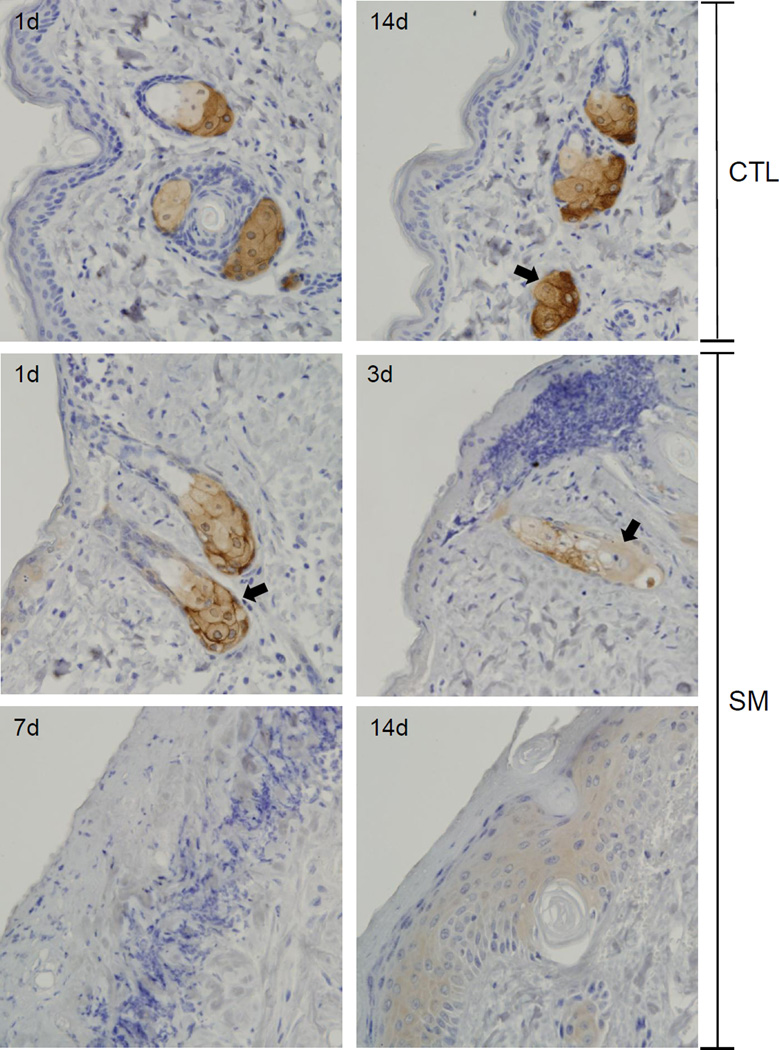
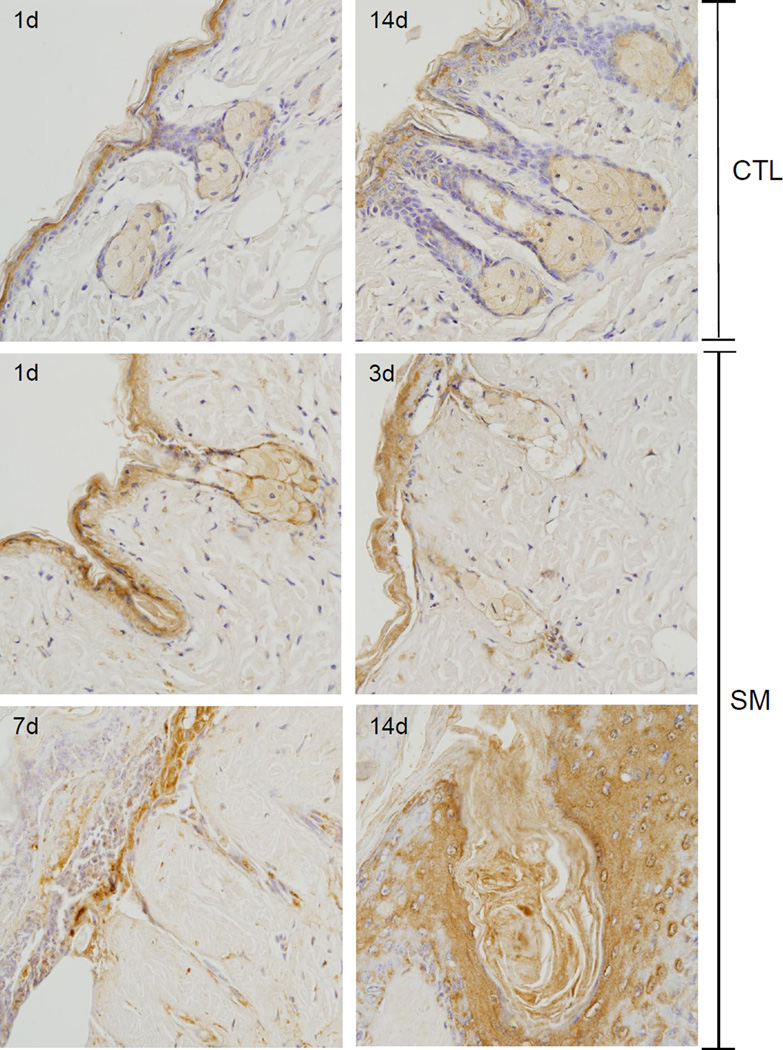
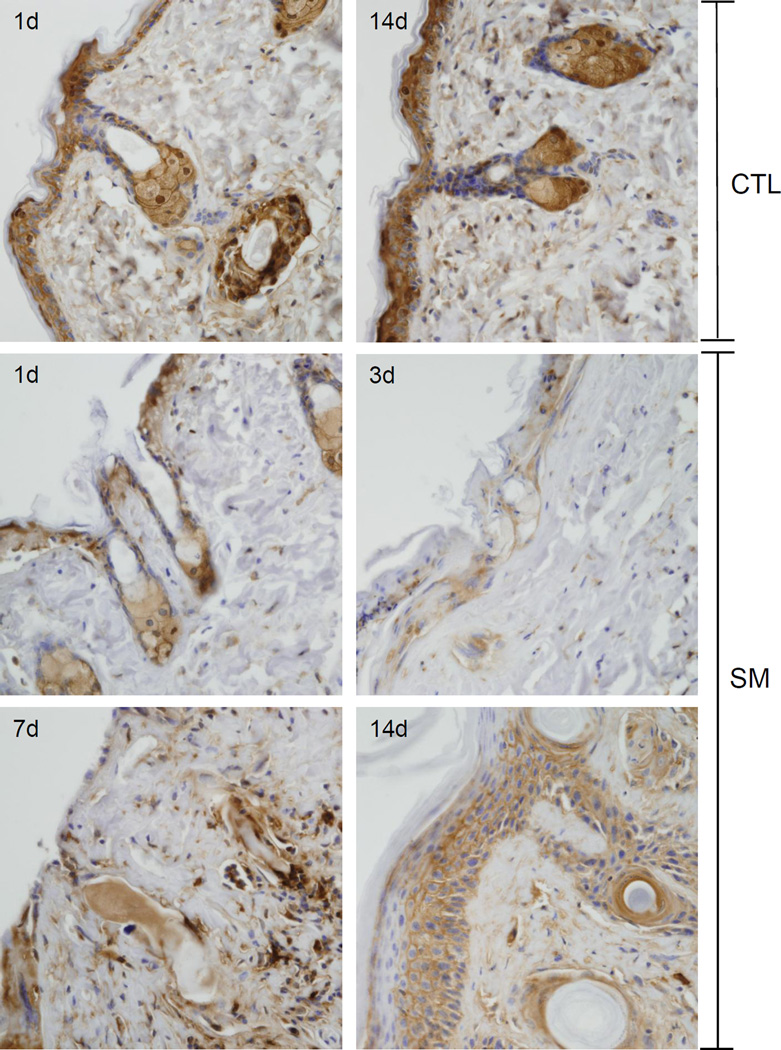
Figure 3. Effects of SM on expression of fatty acid synthase.
Histological sections prepared 1 and 14 days after air exposure (CTL), and 1, 3, 7 and 14 days after SM exposure were stained with an antibody to fatty acid synthase. Antibody binding was visualized using a Vectastain Elite ABC kit (original magnification, × 400). One representative section from 3 mice/treatment group is shown. Arrows indicate sebaceous glands.
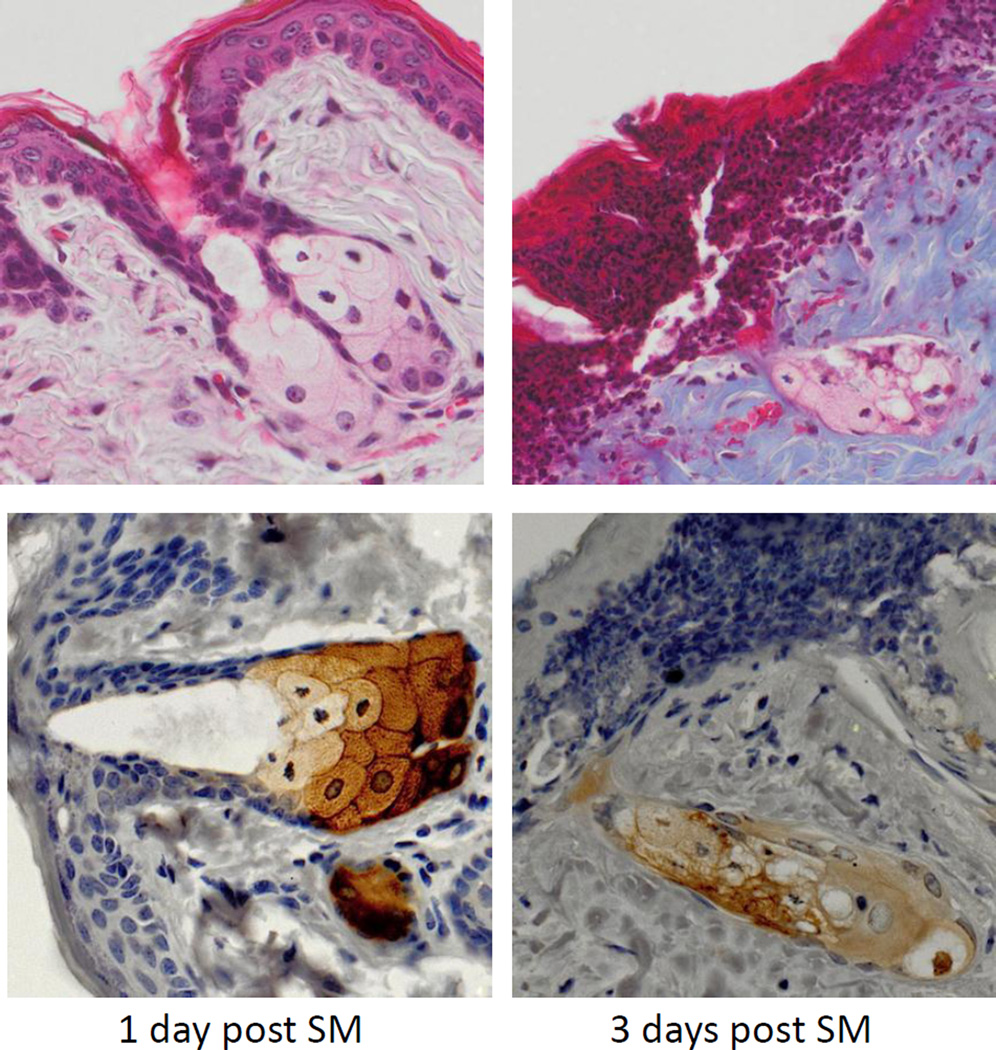
Figure 4. Effects of SM on sebaceous glands.
Histological sections prepared 1 day and 3 days after SM exposure were stained with Gomori’s trichrome followed by aniline blue counterstaining (upper panels) or with anti-fatty acid synthase antibody (lower panels). One representative section from 3 mice/treatment group is shown (original magnification, × 400).
In control skin, fatty acid synthase, an enzyme important in de novo fatty acid synthesis, was expressed in sebocytes in both the hair germ region and the dermal papilla (Figures 3 and 4) (Jensen-Urstad and Semenkovich, 2012). Expression of fatty acid synthase was greatest in cells near the distal end of the sebaceous gland and in nucleated sebocytes in both control and SM-exposed skin. Changes in expression of fatty acid synthase were evident 1 and 3 days post SM. After these times, degradation of sebocytes was apparent. Thus, by 3 days, only ghostly remnants of sebocytes where observed which expressed low levels of fatty acid synthase (Figure 3 and 4). Seven and fourteen days post SM, sebaceous glands were not evident within the wound.
SM induces DNA damage and apoptosis in the hair follicle
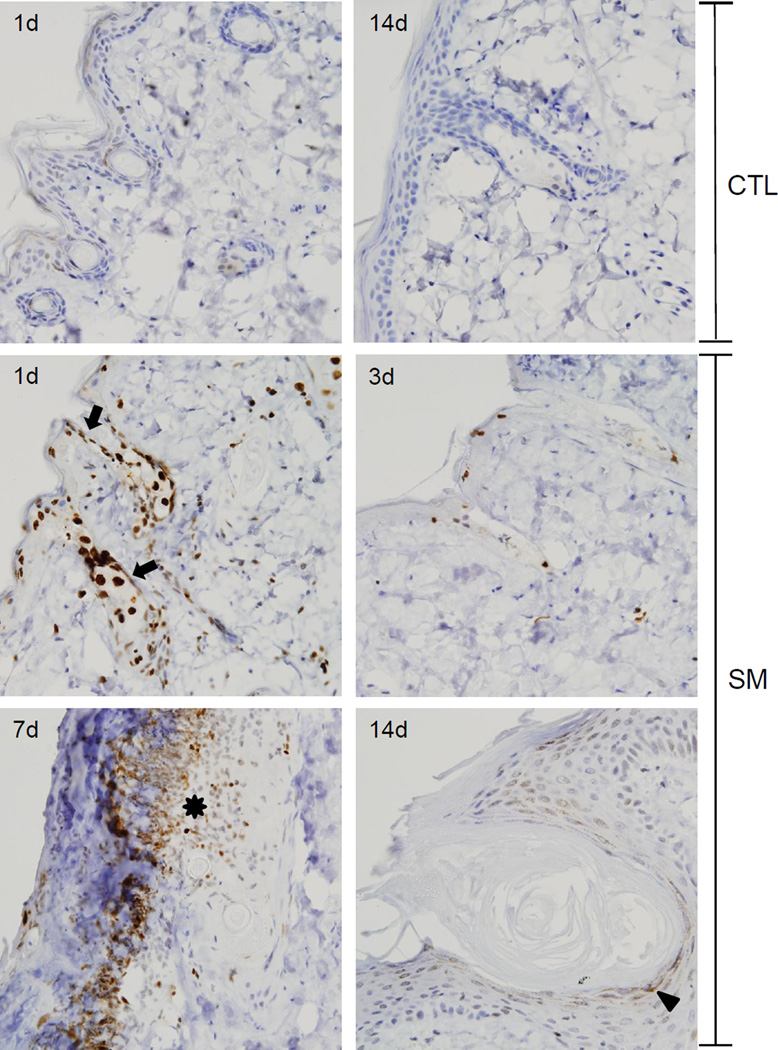
Previous studies have shown that SM induces DNA double strand breaks, a process that activates DNA repair (Black et al., 2010; Jowsey et al., 2010). Histone phospho-H2A.X binds to double stranded DNA breaks creating a repair recognition domain that is important in controlling DNA damage (Clingen et al., 2008; Mah et al., 2010). Phospho-H2A.X was not evident in control skin (Figure 5). Within 1 day of SM exposure, phospho-H2A.X was detected in the interfollicular epidermis, the outer root sheath surrounding the hair shaft, dermal papilla, bulge area and sebocytes (Figure 5). Inflammatory cells and fibroblasts in the dermis also expressed phospho-H2A.X. Expression of phospho-H2A.X declined in the epidermis 3 days post SM exposure and only sporadic expression was evident in the interfollicular epidermis and dermal papilla of remnant hair follicles. By 7 days post SM, expression of phospho-H2A.X was increased in infiltrating inflammatory cells and flattened keratinocytes at the dermal/epidermal junction. At 14 days post SM, phospho-H2A.X expression was observed sporadically in the hyperplastic regenerating epidermis and within the granular squamous epithelium surrounding follicular cysts (Figure 5).
Figure 5. Effects of SM on phospho-H2A.X expression.
Histological sections were prepared 1 and 14 days after air exposure (CTL), and 1, 3, 7 and 14 days after SM exposure were stained with an antibody to phospho-H2A.X. Antibody binding was visualized using a Vectastain Elite ABC kit (original magnification, × 400). One representative section from 3 mice/ treatment group is shown. Arrows indicates sebaceous glands, asterisk indicates inflammatory cells at the dermal/epidermal junction, and arrowhead indicates interfollicular and outer root sheath basal keratinocytes.
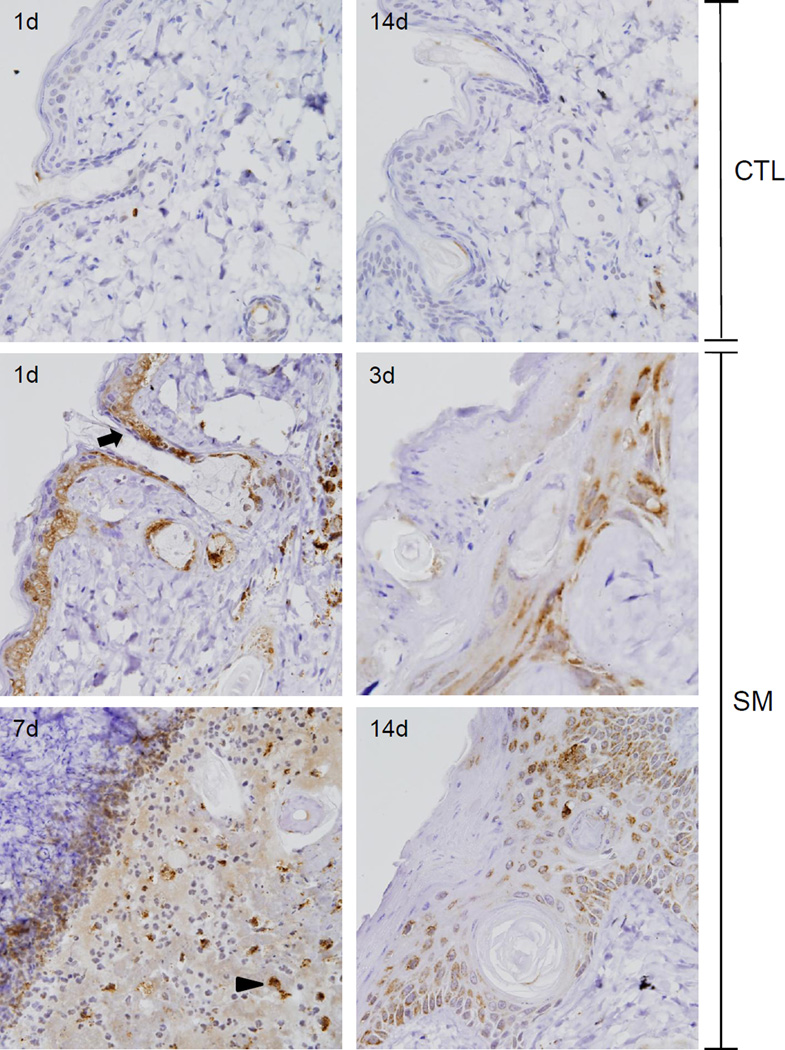
Cleaved caspase-3 is a cysteine-dependent aspartate-directed protease important in the execution phase of apoptosis (Lamkanfi and Kanneganti, 2010; Yoshida, 2007). Low constitutive levels of cleaved caspase-3 were noted throughout the basal layer of control skin (Figure 6). At 1 day post SM exposure, there was a marked increase in expression of cleaved caspase-3 in basal keratinocytes, including those in the outer root sheath and in sebaceous glands, with scattered staining of inflammatory cells in the dermis (Figure 6). Three days post SM; cleaved caspase-3 was noted in the flattened interfollicular epidermis and within the epithelium of the degenerating follicular outer root sheath. By 7 days post SM, expression of cleaved caspase-3 was restricted to basophilic cells situated within the dermis below the eschar. Fourteen days post SM exposure; cleaved caspase-3 was detected in cells in the granular layer of the hyperplastic epidermis and in cells surrounding follicular cysts.
Figure 6. Effects of SM on cleaved caspase-3 expression.
Histological sections prepared 1 and 14 days after air exposure (CTL), and 1, 3, 7 and 14 days after SM exposure were stained with an antibody to cleaved caspase-3. Antibody binding was visualized using a Vectastain Elite ABC kit (original magnification, × 400). One representative section from 3 mice/treatment group is shown. Arrow indicates interfollicular and outer root sheath basal keratinocytes and arrowhead indicates basophilic cells in the dermis.
The effects of SM on markers of wound repair
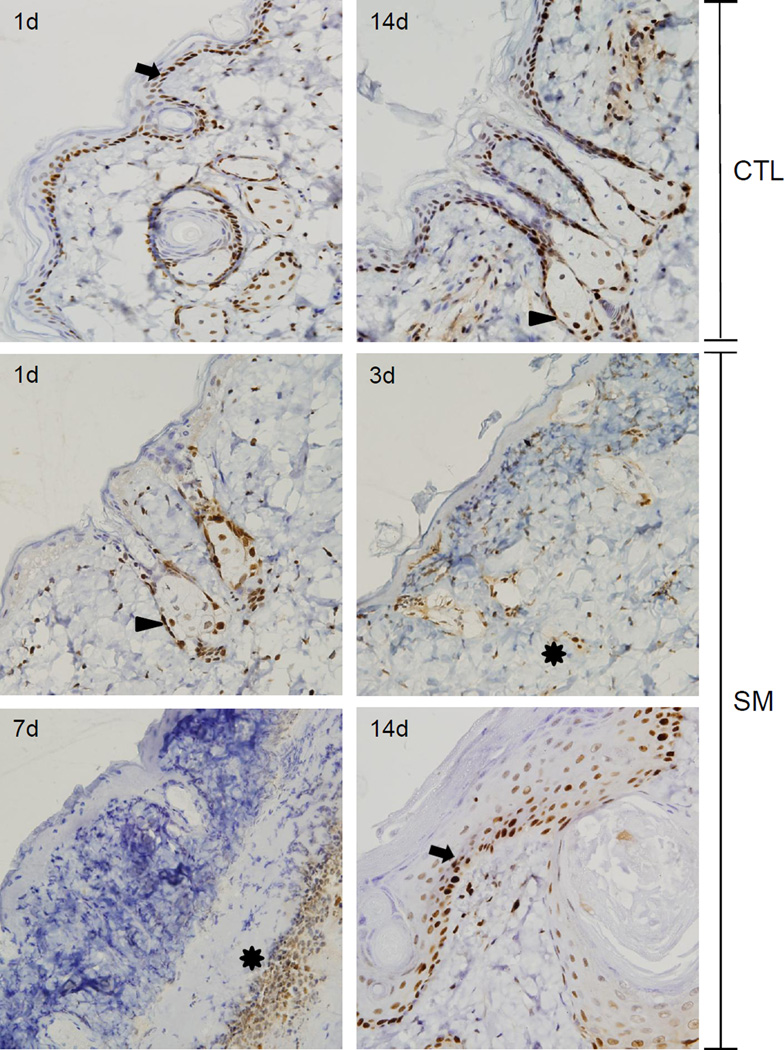
In control mice PCNA, a marker of cellular proliferation, was constitutively expressed in nuclei of interfollicular basal keratinocytes, keratinocytes within the outer root sheath of hair follicles, and sebaceous glands (Figure 7). Within sebaceous glands, PCNA was predominantly localized in the nucleus of sebocytes adjacent to the dermal papilla. Following SM exposure, decreased PCNA expression was evident in cells in the interfollicular epidermis, infundibulum, and the upper portion of the isthmus of the hair follicle; this was observed within 1 day of SM treatment. PCNA was expressed in the outer root sheath, within proliferating sebocytes, and at the presumptive hair bulge. Three days post SM exposure, cells expressing PCNA were scattered throughout the dermis in inflammatory cells and remnants of pilosebaceous units. By 7 days, PCNA was confined to inflammatory cells beneath the eschar. At 14 days post SM exposure, PCNA was expressed within hyperplastic basal cells in the epidermis undergoing wound repair, as well as with the stratum spinosum surrounding follicular cysts (Figure 7).
Figure 7. Effects of SM on PCNA expression.
Histological sections prepared 1 and 14 days after air exposure (CTL), and 1, 3, 7 and 14 days after SM exposure were stained with an antibody to PCNA. Antibody binding was visualized using a Vectastain Elite ABC kit (original magnification, × 400). One representative section from 3 mice/treatment group is shown. Arrows indicate interfollicular and outer root sheath basal keratinocytes, arrowheads indicate sebocytes and asterisks indicate inflammatory cells at the dermal/epidermal junction.
FGFR2, a member of a family of fibroblast growth factor receptors, is important in regulating tissue repair (Steiling and Werner, 2003; Yang et al., 2010). Suprabasal keratinocytes, keratinocytes in the infundibulum, and sebocytes expressed FGFR2 in control skin; low levels of the protein were also expressed in keratinocytes of the outer hair root sheaths (Figure 8). One day post SM exposure, FGFR2 was evident in the interfollicular epidermis, infundibulum, sebocytes, and in the outer root sheath surrounding the whole pilosebaceous unit (Figure 8). By 3 days post SM exposure, FGFR2 expression was evident in the outer root sheath surrounding both the hair shaft and the sebaceous gland. At 7 days post SM, FGFR2 was evident at the base of the eschar, in the remnant hair follicles and within the dermis. Fourteen days post SM, FGFR2 expression was apparent in the suprabasal layer hyperplastic stratum granulosum in both the follicular and interfollicular epidermis. FGFR2 was also observed in the infundibulum and the lamellated keratin of the remnant hair follicles.
Figure 8. Effects of SM on FGFR2 expression.
Histological sections prepared 1 and 14 days after air exposure (CTL), and 1, 3, 7 and 14 days after SM exposure were stained with an antibody to FGFR2. Antibody binding was visualized using a Vectastain Elite ABC kit (original magnification, × 400). One representative section from 3 mice/treatment group is shown.
Galectin-3 is a lectin binding protein essential for wound re-epithelialization; it also regulates the cell cycle and the hair growth cycle in mice (Wollina et al., 2000). In control mice, galectin-3 was expressed in interfollicular suprabasal keratinocytes and in keratinocytes along both the inner and outer root sheath of the hair follicle (Figure 9). There was also prominent expression of galectin-3 in the cytoplasm and nucleus of sebocytes and in fibroblasts in the dermal matrix. At 1 day and 3 days post SM, galectin-3 was decreased in basal epidermal cells, sebocytes and dermis. Seven days post SM, galectin-3 expression increased in the dermis directly below the eschar, in fibroblasts, inflammatory cells, and degenerative hair follicles. Increased galectin-3 was also evident in epithelial cells within the infundibulum. Of note was our observation that galectin-3 was expressed in non-cellular portions of the dermal matrix and in the lamellated keratin in the degenerative hair follicles. By 14 days post SM, galectin-3 was distributed within the hyperplastic interfollicular epidermis, specifically, the stratum granulosum and the stratum basale, in the outer root sheath surrounding the remnant hair follicles, and in dermal fibroblasts.
Figure 9. Effects of SM on galectin-3 expression.
Histological sections prepared 1 and 14 days after air exposure (CTL), and 1, 3, 7 and 14 days after SM exposure were stained with an antibody to galectin-3. Antibody binding was visualized using a Vectastain Elite ABC kit (original magnification, × 400). One representative section from 3 mice/treatment group is shown.
Discussion
The present studies characterized the responses of hair follicles and sebaceous glands to SM in a hairless mouse model. Within one day, of SM vapor exposure, aberrant hair follicles developed in SM treated skin; notable changes were evident in epithelial cell layers including a progressive loss of outer root sheath cells from the infundibulum to the root bulb. This was associated with epidermal karyolysis in both the outer root sheath of the hair follicle and interfollicular epidermis. With time, hair follicles degenerated into ghostly remnants and by 14 days, only engorged follicular cysts remained within the wound. Degenerative changes in sebaceous glands were only evident 3–7 days post SM exposure. At these times, decreased numbers of intact sebocytes were noted in sebaceous glands and by 14 days, sebaceous glands were not evident in the wound. These degenerative changes were associated with a marked increase in flaky xerotic skin in the wound (unpublished studies). These data are consistent with earlier studies in hairless guinea pig skin showing that SM vapor induces rapid (within 24 hr) follicular necrosis (Snider et al., 1999; Yourick et al., 1993). It has also been reported that hairless guinea pigs, like hairless mice, experience a loss of hair follicles and sebaceous glands 14 days post SM exposure (Benson et al., 2011). In the guinea pig studies, loss of hair follicles and sebaceous glands was accompanied by increased collagen deposition and dermal compaction and xerosis. As observed in hairless mice, humans also develop xerosis following SM exposure (Firooz et al., 2011; Tewari-Singh et al., 2013). This may be due to decreased sebum production and decreased skin barrier lipids as a consequence of dermal scarring and loss of the sebaceous glands (Poursaleh et al., 2012; Rowell et al., 2009).
Fatty acid synthase is an enzyme responsible for the production of long-chain fatty acids in sebaceous glands (Hinde et al., 2013). These fatty acids are important in maintaining the protective barrier of the skin and in keeping moisture in the skin and hair. Sebaceous glands are composed of proliferating sebocytes which mature as they migrate from the gland periphery into the central region of the lobule at which point they secrete sebum (Niemann, 2009). Of note is our finding that in normal hairless mouse skin, fatty acid synthase expression was most pronounced in peripheral sebocytes, with decreased expression in maturing sebocytes as they migrate to the center of the sebaceous glands. SM exposure was found to alter expression of fatty acid synthase in the sebocytes. Thus, by 3 days post exposure, enzyme expression was decreased throughout the sebaceous glands. These data suggest that SM either decreases synthesis of fatty acid synthase in sebocytes or induces maturation of these cells. The absence of sebaceous glands in the wounded skin at 14 days post exposure indicates that SM is cytotoxic not only for differentiating sebocytes, but also for stem cells that have the potential to regenerate sebaceous glands.
As a DNA polymerase cofactor important in DNA replication and repair, PCNA is constitutively expressed in the nuclei of proliferating basal cells in the interfollicular epidermis, as well as in basal cells surrounding hair follicles, in sebocytes, and in the dermal papilla. These results are in accord with earlier studies assessing PCNA expression in hairless mouse skin (Bacchi and Gown, 1993; Fuchs and Horsley, 2008; Guo et al., 2013). PCNA expression was found to be decreased in the interfollicular epidermis and the upper portion of the infundibulum of the hair follicle one day following SM exposure, with no effect on the outer root sheath, hair bulb and sebaceous glands. By day 3 post SM exposure, PCNA expression was only observed in inflammatory cells within the dermis. This is similar to observed changes in PCNA expression in rodent skin following thermal burns (Kulac et al., 2013). Decreases in PCNA expression in hair follicles and sebaceous glands are likely due to inhibition of epithelial cell proliferation associated with SM-induced injury. The longer times required for inhibition of PCNA expression in central and lower portions of the hair follicles may be due to differences in localized concentrations of SM. In this regard, lipids are known to sequester SM and lipid containing sebum in the hair follicle may delay the actions of SM (Hattersley et al., 2008). By seven days post SM exposure, only inflammatory cells within the dermis expressed PCNA. This is consistent with findings that PCNA prevents neutrophil activation and acts as an antiapoptotic protein (De Chiara et al., 2013; Witko-Sarsat et al., 2011). Fourteen days post SM exposure, PCNA was evident in the hyperplastic epidermis, a finding in accord with enhanced cell proliferation during wound healing. The epidermis surrounding follicular cysts also expressed PCNA indicating aberrant growth of remnant hair follicles. Similar PCNA expression has been observed in hyperplastic epidermis of hairless guinea pig skin treated with SM (Benson et al., 2011).
Earlier studies have shown that alkylating agents can induce double strand DNA breaks in keratinocytes (Joseph et al., 2011; Kehe et al., 2009; Moser and Meier, 2000). A key component of repair of DNA double strand breaks is the phosphorylation of histone H2A variant, H2A.X (Clingen et al., 2008; Inturi et al., 2011; Jowsey et al., 2010). We have previously demonstrated that SM treatment of mouse epidermis causes rapid (within 1 day) increases in expression of phospho-H2A.X in the nucleus of basal keratinocytes (Joseph et al., 2011). The half mustard, 2-chloroethylethylamine, also induces phospho-H2A.X in basal keratinocytes of human full-skin constructs (Black et al., 2010). The present studies demonstrate that SM also induces double strand DNA breaks in hair follicles and sebaceous glands within 1 day of treatment. However, this decreased 3–7 days post SM treatment and by 7 days, phospho-H2A.X was primarily localized in infiltrating leukocytes at the base of the eschar, presumably due to apoptosis of these cells. Of interest were our findings of low levels of phospho-H2A.X in the interfollicular hyperplastic regenerating epidermis and in follicular cysts in areas adjacent to the stratum corneum 14 days post SM exposure. This likely represents apoptosis of differentiating keratinocytes as they regenerate the epidermis during wound repair. Taken together these data indicate that SM-induced damage to hair follicles and sebaceous glands is due, at least in part, to DNA damage.
Caspase-3 is a key protease in the execution phase of apoptosis. It is expressed throughout the hair follicle and is thought to be important in regulating the hair growth cycle, as well as keratinocyte proliferation and wound recovery in the skin (Fang et al., 2010; Schneider et al., 2009; Zarach et al., 2004). We found that SM exposure resulted in increased cleaved caspase-3 expression in both interfollicular and follicular basal keratinocytes, but not in sebocytes, after 1 day and 3 days. These data indicate that interfollicular and follicular basal cells are susceptible to apoptosis and are in accord with earlier reports showing that cleaved caspase-3 is associated with SM-induced cell death in mouse and human keratinocytes (Joseph et al., 2011; Rosenthal et al., 2003). The fact that sebocytes do not express cleaved caspase-3 following SM exposure indicates that either cell death is not mediated by apoptosis, or that apoptosis in these cells is independent of cleaved caspase-3. Increased expression of cleaved caspase-3 was also evident at the dermal-epidermal junction within the eschar 7 days post SM exposure. This is consistent with rapid turnover and apoptosis of leukocytes that have infiltrated into the wound site. By 14 days post SM exposure, cleaved caspase-3 was expressed in cells surrounding follicular cysts and in the suprabasal epithelium, which is likely related to the rapid turnover of keratinocytes during wound repair.
SM was also found to alter keratinocyte expression of FGFR2, a transmembrane tyrosine kinase that is critical for normal epidermal growth and development, as well as hair follicle morphogenesis (Li et al., 2001; Petiot et al., 2003) In control skin, expression of FGFR2 was restricted to the stratum granulosum and stratum corneum in the interfollicular epidermis, the infundibulum of the hair follicles, and to sebocytes. This suggests that fibroblast growth factor or related growth factors that bind to FGFR2 are important in suprabasal keratinocyte differentiation, as well as hair follicle and sebocyte maturation. In this regard, earlier studies have shown that mutant mice lacking FGFR2 display epidermal thickening and dysplasia, along with abnormal hair and sebaceous gland development (Grose et al., 2007). One day following SM exposure, increased FGFR2 expression was noted in the outer hair root sheath, possibly related to damage of these epithelial cells. This is consistent with decreased proliferation of these cells. After 3–7 days, SM-induced toxicity was associated with decreased FGFR2 throughout the hair follicle including sebocytes. Interestingly, by 14 days post SM, FGFR2 was upregulated in hyperplastic follicular and interfollicular suprabasal keratinocytes, suggesting that signaling via this receptor is important in epidermal growth during wound repair (Jang, 2005; Price et al., 2009; Steiling and Werner, 2003).
Galectin-3 belongs to a family of highly conserved lectins and is widely distributed in many tissues including the skin (Larsen et al., 2011; Sundblad et al., 2011). It is a galactoside-binding protein whose expression and secretion are important in regulating inflammation, cell adhesion, migration and proliferation (Dragomir et al., 2012; Ochieng et al., 2004). By binding to extracellular matrix components, galectin-3 and related molecules also regulate cell-matrix interactions important in wound repair (Cao et al., 2002; Elola et al., 2007). In control skin, we found that galectin-3 was primarily expressed in non-dividing differentiating keratinocytes, in the inner root hair sheath, and in immature sebocytes, suggesting that it plays a role in keratinocyte differentiation as well as sebocyte proliferation and maturation. Similar expression patterns of galectin-3 have been described in human skin (Konstantinov et al., 1994). SM exposure resulted in a progressive loss of galectin-3, beginning within 1 day and by 7 days; it was expressed only in inflammatory cells adjacent to dermal matrix proteins. Increases in galectin-3 expression after 7 days may be important in preparing the dermis for keratinocyte adherence and migration during would repair (Liu et al., 2012). It is also consistent with a role for galectin-3 in mediating inflammatory reactions associated with SM-induced tissue injury. We also noted increased galectin-3 expression in basal keratinocytes, in the first few layers of suprabasal keratinocytes in neoepidermis formed during wound healing, and in keratinocytes surrounding epidermal cysts 14 days post SM exposure. These data indicate that galectin-3 is important in the early stages of keratinocyte differentiation (Plzak et al., 2002). Galectin-3 also continued to be expressed in the dermis, a finding in accord with the idea of prolonged inflammation in the skin following SM exposure.
In summary, the present studies demonstrate that hair follicles and sebaceous glands are targets for SM-induced toxicity in the skin. Within the first 7 days following SM exposure, rapid degradation of these dermal appendages is apparent. This is associated with reduced epidermal proliferation and increased expression of markers of inflammation, DNA damage and apoptosis. After 14 days, markers of proliferation and wound repair were evident in the neoepidermis, although there was no evidence for the regeneration of hair follicles and sebaceous glands. This is likely due to SM-induced damage to the hair follicle and sebocyte stem cell compartments. Overall, these findings are consistent with the pathophysiology of SM-induced skin injury in humans confirming that the hairless mouse can be used to investigate the dermatotoxicity of vesicants and the potential efficacy of countermeasures.
Highlights.
Hairless mice have dystrophic hair follicles and sebaceous glands
DNA damage in hair follicles and sebocytes are induced by sulfur mustard
Sulfur mustard alters fatty acid synthase and causes sebocyte maturation
Sulfur mustard causes follicular degeneration
Acknowledgements
This work was supported in part by National Institutes of Health grants CA093798, CA132624, ES004738, ES005022, NS079249 and AR055073. This work was also supported in part by the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award U54AR055073). Its contents are the responsibility of the authors and do not necessarily represent the official views of the federal government.
List of Abbreviations
- FGFR2
fibroblast growth factor receptor 2
- H&E
hematoxylin and eosin
- phospho-H2A.X
phosphorylated histone H2A.X
- PCNA
proliferating cell nuclear antigen
- SM
sulfur mustard
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arck P, Paus R. From the brain-skin connection: the neuroendocrine-immune misalliance of stress and itch. Neuroimmunomodulation. 2006;13:347–356. doi: 10.1159/000104863. [DOI] [PubMed] [Google Scholar]
- Bacchi CE, Gown AM. Detection of cell proliferation in tissue sections. Braz J Med Biol Res. 1993;26:677–687. [PubMed] [Google Scholar]
- Benson JM, Seagrave J, Weber WM, Santistevan CD, Grotendorst GR, Schultz GS, March TH. Time course of lesion development in the hairless guinea-pig model of sulfur mustard-induced dermal injury. Wound Repair Regen. 2011;19:348–357. doi: 10.1111/j.1524-475X.2011.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AT, Hayden PJ, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD. Expression of proliferative and inflammatory markers in a fullthickness human skin equivalent following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2010;249:178–187. doi: 10.1016/j.taap.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and-7, but not galectin-1, play a role in reepithelialization of wounds. J Biol Chem. 2002;277:42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- Clingen PH, Wu JY, Miller J, Mistry N, Chin F, Wynne P, Prise KM, Hartley JA. Histone H2AX phosphorylation as a molecular pharmacological marker for DNA interstrand crosslink cancer chemotherapy. Biochem Pharmacol. 2008;76:19–27. doi: 10.1016/j.bcp.2008.03.025. [DOI] [PubMed] [Google Scholar]
- De Chiara A, Pederzoli-Ribeil M, Mocek J, Candalh C, Mayeux P, Millet A, Witko-Sarsat V. Characterization of cytosolic proliferating cell nuclear antigen (PCNA) in neutrophils: antiapoptotic role of the monomer. J Leukoc Biol. 2013;94:723–731. doi: 10.1189/jlb.1212637. [DOI] [PubMed] [Google Scholar]
- Dragomir AC, Sun R, Choi H, Laskin JD, Laskin DL. Role of galectin-3 in classical and alternative macrophage activation in the liver following acetaminophen intoxication. J Immunol. 2012;189:5934–5941. doi: 10.4049/jimmunol.1201851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci. 2007;64:1679–1700. doi: 10.1007/s00018-007-7044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Hunag Q, Chen J, OPeng Y, Roop DR, Bedford JS, Li C-Y. Apoptotic cells activate the"phoenix rising" pathway to promote wound healing and tissue regeneration. Science Signaling. 2010;3:1–10. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firooz A, Sadr B, Davoudi SM, Nassiri-Kashani M, Panahi Y, Dowlati Y. Long-term skin damage due to chemical weapon exposure. Cutan Ocul Toxicol. 2011;30:64–68. doi: 10.3109/15569527.2010.529547. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Horsley V. More than one way to skin. Genes Dev. 2008;22:976–985. doi: 10.1101/gad.1645908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Panahi Y, Shoja MM. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Crit Rev Toxicol. 2011;41:384–403. doi: 10.3109/10408444.2010.541224. [DOI] [PubMed] [Google Scholar]
- Ghanei M, Poursaleh Z, Harandi AA, Emadi SE, Emadi SN. Acute and chronic effects of sulfur mustard on the skin: a comprehensive review. Cutan Ocul Toxicol. 2010;29:269–277. doi: 10.3109/15569527.2010.511367. [DOI] [PubMed] [Google Scholar]
- Grose R, Fantl V, Werner S, Chioni AM, Jarosz M, Rudling R, Cross B, Hart IR, Dickson C. The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. EMBO J. 2007;26:1268–1278. doi: 10.1038/sj.emboj.7601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Hutchenreuther J, Carter DE, Leask A. TAK1 is required for dermal wound healing and homeostasis. J Invest Dermatol. 2013;133:1646–1654. doi: 10.1038/jid.2013.28. [DOI] [PubMed] [Google Scholar]
- Haslam IS, Pitre A, Schuetz JD, Paus R. Protection against chemotherapy-induced alopecia: targeting ATP-binding cassette transporters in the hair follicle? Trends Pharmacol Sci. 2013;34:599–604. doi: 10.1016/j.tips.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Hattersley IJ, Jenner J, Dalton C, Chilcott RP, Graham JS. The skin reservoir of sulphur mustard. Toxicol In Vitro. 2008;22:1539–1546. doi: 10.1016/j.tiv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Hinde E, Haslam IS, Schneider MR, Langan EA, Kloepper JE, Schramm C, Zouboulis CC, Paus R. A practical guide for the study of human and murine sebaceous glands in situ. Exp Dermatol. 2013;22:631–637. doi: 10.1111/exd.12207. [DOI] [PubMed] [Google Scholar]
- Inturi S, Tewari-Singh N, Gu M, Shrotriya S, Gomez J, Agarwal C, White CW, Agarwal R. Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free Radic Biol Med. 2011;51:2272–2280. doi: 10.1016/j.freeradbiomed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH. Stimulation of human hair growth by the recombinant human keratinocyte growth factor-2 (KGF-2) Biotechnol Lett. 2005;27:749–752. doi: 10.1007/s10529-005-5624-y. [DOI] [PubMed] [Google Scholar]
- Jensen-Urstad AP, Semenkovich CF. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta. 2012;1821:747–753. doi: 10.1016/j.bbalip.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph LB, Gerecke DR, Heck DE, Black AT, Sinko PJ, Cervelli JA, Casillas RP, Babin MC, Laskin DL, Laskin JD. Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage. Exp Mol Pathol. 2011;91:515–527. doi: 10.1016/j.yexmp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowsey PA, Williams FM, Blain PG. The role of homologous recombination in the cellular response to sulphur mustard. Toxicol Lett. 2010;197:12–18. doi: 10.1016/j.toxlet.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Konstantinov KN, Shames B, Izuno G, Liu FT. Expression of epsilon BP, a beta-galactoside- binding soluble lectin, in normal and neoplastic epidermis. Exp Dermatol. 1994;3:9–16. doi: 10.1111/j.1600-0625.1994.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Kulac M, Aktas C, Tulubas F, Uygur R, Kanter M, Erboga M, Ceber M, Topcu B, Ozen OA. The effects of topical treatment with curcumin on burn wound healing in rats. J Mol Histol. 2013;44:83–90. doi: 10.1007/s10735-012-9452-9. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD. Caspase-7: a protease involved in apoptosis and inflammation. Int J Biochem Cell Biol. 2010;42:21–24. doi: 10.1016/j.biocel.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen L, Chen HY, Saegusa J, Liu FT. Galectin-3 and the skin. J Dermatol Sci. 2011;64:85–91. doi: 10.1016/j.jdermsci.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin JD, Black AT, Jan YH, Sinko PJ, Heindel ND, Sunil V, Heck DE, Laskin DL. Oxidants and antioxidants in sulfur mustard-induced injury. Ann N Y Acad Sci. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JM, Lodhi IJ, Nguyen PK, Ngo V, Clift R, Hinshaw DB, Sweeney JF. Low-dose sulfur mustard primes oxidative function and induces apoptosis in human polymorphonuclear leukocytes. Int Immunopharmacol. 2003;3:747–756. doi: 10.1016/S1567-5769(03)00075-4. [DOI] [PubMed] [Google Scholar]
- Lu C, Guo H, Xu X, Weinberg W, Deng CX. Fibroblast growth factor receptor 2 (Fgfr2) plays an important role in eyelid and skin formation and patterning. Dev Dyn. 2001;222:471–483. doi: 10.1002/dvdy.1205. [DOI] [PubMed] [Google Scholar]
- Liu W, Hsu DK, Chen HY, Yang RY, Carraway KL, 3rd, Isseroff RR, Liu FT. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J Invest Dermatol. 2012;132:2828–2837. doi: 10.1038/jid.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- Massironi SM, Giacoia MR, Maiorka PC, Kipnis TL, Dagli ML. Skin morphology of the mutant hairless USP mouse. Braz J Med Biol Res. 2005;38:33–39. doi: 10.1590/s0100-879x2005000100006. [DOI] [PubMed] [Google Scholar]
- Moser J, Meier HL. Comparison of cell size in sulfur mustard-induced death of keratinocytes and lymphocytes. J Appl Toxicol. 2000;20(Suppl 1):S23–S30. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat693>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Naghii MR. Sulfur mustard intoxication, oxidative stress, and antioxidants. Mil Med. 2002;167:573–575. [PubMed] [Google Scholar]
- Niemann C. Differentiation of the Sebaceous Gland. Dermato-Endocrinology. 2009;1:64–76. doi: 10.4161/derm.1.2.8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:e50–e59. doi: 10.1016/S1470-2045(12)70553-3. [DOI] [PubMed] [Google Scholar]
- Petiot A, Conti FJ, Grose R, Revest JM, Hodivala-Dilke KM, Dickson C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development. 2003;130:5493–5501. doi: 10.1242/dev.00788. [DOI] [PubMed] [Google Scholar]
- Plzak J, Holikova Z, Smetana K, Jr, Dvorankova B, Hercogova J, Kaltner H, Motlik J, Gabius HJ. Differentiation-dependent glycosylation of cells in squamous cell epithelia detected by a mammalian lectin. Cells Tissues Organs. 2002;171:135–144. doi: 10.1159/000063707. [DOI] [PubMed] [Google Scholar]
- Poursaleh Z, Ghanei M, Babamahmoodi F, Izadi M, Harandi AA, Emadi SE, Taghavi NO, Sayad-Nouri SS, Emadi SN. Pathogenesis and treatment of skin lesions caused by sulfur mustard. Cutan Ocul Toxicol. 2012;31:241–249. doi: 10.3109/15569527.2011.636119. [DOI] [PubMed] [Google Scholar]
- Price JA, Rogers JV, McDougal JN, Shaw MQ, Reid FM, Graham JS. Transcriptional changes in porcine skin at 7 days following sulfur mustard and thermal burn injury. Cutan Ocul Toxicol. 2009;28:129–140. doi: 10.1080/15569520903097754. [DOI] [PubMed] [Google Scholar]
- Ricketts KM, Santai CT, France JA, Graziosi AM, Doyel TD, Gazaway MY, Casillas RP. Inflammatory cytokine response in sulfur mustard-exposed mouse skin. J Appl Toxicol. 2000;20(Suppl 1):S73–S76. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat685>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Rosenthal DS, Velena A, Chou FP, Schlegel R, Ray R, Benton B, Anderson D, Smith WJ, Simbulan-Rosenthal CM. Expression of dominant-negative Fas-associated death domain blocks human keratinocyte apoptosis and vesication induced by sulfur mustard. J Biol Chem. 2003;278:8531–8540. doi: 10.1074/jbc.M209549200. [DOI] [PubMed] [Google Scholar]
- Rowell M, Kehe K, Balszuweit F, Thiermann H. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263:9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Snider TH, Matthews MC, Braue EH., Jr Model for assessing efficacy of topical skin protectants against sulfur mustard vapor using hairless guinea pigs. J Appl Toxicol. 1999;19(Suppl 1):S55–S58. doi: 10.1002/(sici)1099-1263(199912)19:1+<s55::aid-jat616>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Steiling H, Werner S. Fibroblast growth factors: key players in epithelial morphogenesis, repair and cytoprotection. Curr Opin Biotechnol. 2003;14:533–537. doi: 10.1016/j.copbio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Sundblad V, Croci DO, Rabinovich GA. Regulated expression of galectin-3, a multifunctional glycan-binding protein, in haematopoietic and non-haematopoietic tissues. Histol Histopathol. 2011;26:247–265. doi: 10.14670/HH-26.247. [DOI] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, White CW, Agarwal R. Clinically-relevant cutaneous lesions by nitrogen mustard: useful biomarkers of vesicants skin injury in SKH-1 hairless and C57BL/6 mice. PLoS One. 2013;8:e67557. doi: 10.1371/journal.pone.0067557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt RF, Jr, Dannenberg AM, Jr, Schofield BH, Hynes NA, Papirmeister B. Pathogenesis of skin lesions caused by sulfur mustard. Fundam Appl Toxicol. 1984;4:S71–S83. doi: 10.1016/0272-0590(84)90139-8. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Pederzoli-Ribeil M, Hirsch E, Sozzani S, Cassatella MA. Regulating neutrophil apoptosis: new players enter the game. Trends Immunol. 2011;32:117–124. doi: 10.1016/j.it.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Wollina U, Lange D, Paus R, Burchert M, Gabius HJ. Expression of galectin-1 and-3 and of accessible binding sites during murine hair cycle. Histol Histopathol. 2000;15:85–94. doi: 10.14670/HH-15.85. [DOI] [PubMed] [Google Scholar]
- Yang J, Meyer M, Muller AK, Bohm F, Grose R, Dauwalder T, Verrey F, Kopf M, Partanen J, Bloch W, Ornitz DM, Werner S. Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J Cell Biol. 2010;188:935–952. doi: 10.1083/jcb.200910126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K. PKCdelta signaling: mechanisms of DNA damage response and apoptosis. Cell Signal. 2007;19:892–901. doi: 10.1016/j.cellsig.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Yourick JJ, Dawson JS, Benton CD, Craig ME, Mitcheltree LW. Pathogenesis of 2,2'-dichlorodiethyl sulfide in hairless guinea pigs. Toxicology. 1993;84:185–197. doi: 10.1016/0300-483x(93)90116-a. [DOI] [PubMed] [Google Scholar]
- Zarach JM, Beaudoin GM, 3rd, Coulombe PA, Thompson CC. The co-repressor hairless has a role in epithelial cell differentiation in the skin. Development. 2004;131:4189–4200. doi: 10.1242/dev.01303. [DOI] [PubMed] [Google Scholar]