Abstract
Objective
To indirectly compare aflibercept, bevacizumab, dexamethasone, ranibizumab and triamcinolone for treatment of macular oedema secondary to central retinal vein occlusion using a network meta-analysis (NMA).
Design
NMA.
Data sources
The following databases were searched from January 2005 to March 2013: MEDLINE, MEDLINE In-process, EMBASE; CDSR, DARE, HTA, NHSEED, CENTRAL; Science Citation Index and Conference Proceedings Citation Index-Science.
Eligibility criteria for selecting studies
Only randomised controlled trials assessing patients with macular oedema secondary to central retinal vein occlusion were included. Studies had to report either proportions of patients gaining ≥3 lines, losing ≥3 lines, or the mean change in best corrected visual acuity. Two authors screened titles and abstracts, extracted data and undertook risk of bias assessment. Bayesian NMA was used to compare the different interventions.
Results
Seven studies, assessing five drugs, were judged to be sufficiently comparable for inclusion in the NMA. For the proportions of patients gaining ≥3 lines, triamcinolone 4 mg, ranibizumab 0.5 mg, bevacizumab 1.25 mg and aflibercept 2 mg had a higher probability of being more effective than sham and dexamethasone. A smaller proportion of patients treated with triamcinolone 4 mg, ranibizumab 0.5 mg or aflibercept 2 mg lost ≥3 lines of vision compared to those treated with sham. Patients treated with triamcinolone 4 mg, ranibizumab 0.5 mg, bevacizumab 1.25 mg and aflibercept 2 mg had a higher probability of improvement in the mean best corrected visual acuity compared to those treated with sham injections.
Conclusions
We found no evidence of differences between ranibizumab, aflibercept, bevacizumab and triamcinolone for improving vision. The antivascular endothelial growth factors (VEGFs) are likely to be favoured because they are not associated with steroid-induced cataract formation. Aflibercept may be preferred by clinicians because it might require fewer injections.
Systematic review registration
Not registered.
Strengths and limitations of this study.
Important topic area, with significant policy implications.
Robust method used to identify studies.
Network meta-analyses are based on a number of assumptions.
Network meta-analysis is the best method to compare interventions in the absence of head-to-head trials.
Introduction
Central retinal vein occlusion (CRVO) dramatically reduces an individual's functioning and quality of life.1 It is estimated that the 15-year cumulative incidence of CRVO is 0.5%.2 Visual loss is caused by thrombosis of the central retinal vein which leads to a rise in venous pressure and an increase in vascular endothelial growth factor (VEGF), consequently causing an increase in vascular permeability. Macular oedema subsequently ensues with varying degrees of ischaemia and neovascularisation. Although CRVO is generally classified as ischaemic or non-ischaemic, ischaemia should be regarded as a spectrum.3 Cases with ischaemia carry a considerably worse prognosis as, in around one-third of them, neovascular glaucoma, the most devastating complication of CRVO, may develop.4
CRVO is more common in older people with risk factors such as diabetes, hypertension or hyperlipidaemia, but can occur in young people with inflammatory disorders. Hayreh et al, in a 27-year cohort study, found that only 13% of people with CRVO were under 45 years of age.3 In 95% of cases, CRVO affects only one eye.3 However, visual loss in this already comorbid patient group significantly compounds their already impaired functioning and quality of life. Patients can lose confidence, struggle with daily activities and become increasingly dependent on friends and family.1
For many years, laser photocoagulation was the only effective therapeutic strategy that could be used in the management of patients with CRVO. It was only useful for reducing the risk of neovascular glaucoma, but not effective for the treatment of macular oedema in CRVO.5 Over the past decade, a number of drugs to treat macular oedema have been introduced, including the steroids, triamcinolone and dexamethasone, and the anti-VEGFs, ranibizumab, bevacizumab, pegaptanib and aflibercept. Dexamethasone, ranibizumab and aflibercept have been assessed in large commercially funded trials.6–13 Bevacizumab was originally developed as an anticancer drug and has been found to be effective in treating macular oedema secondary to age-related macular degeneration,14 diabetic macular oedema,15 branch retinal vein occlusion16 and CRVO.17 Like triamcinolone, bevacizumab is used off license in the eye. Ranibizumab is derived from the same parent molecule of the bevacizumab monoclonal antibody and was developed and commercially marketed specifically for use in the eye.
In the UK, the National Institute of Health and Care Excellence (NICE) has recommended the use of dexamethasone, ranibizumab and aflibercept for the treatment of macular oedema secondary to CRVO in separate appraisals.18–20 Therefore clinicians have three NICE-recommended treatments for CRVO without head-to-head trials or clear guidance in which one may be best for their patients. On this basis, the aim of this study was to indirectly compare, in a network meta-analysis (NMA), the clinical effectiveness of aflibercept, ranibizumab, bevacizumab, dexamethasone and triamcinolone for the treatment of macular oedema secondary to CRVO.
Methods
Information sources and search strategy
To identify suitable studies, initially for a systematic review of treatment of macular oedema after CRVO (submitted for publication), the following databases were searched from January 2005 to March 2013: MEDLINE, MEDLINE In-process, EMBASE (all via OVID); CDSR, DARE, HTA, NHSEED, CENTRAL (all via The Cochrane Library); Science Citation Index and Conference Proceedings Citation Index-Science (via Web of Knowledge). The MEDLINE search strategy is shown in the online supplementary appendix 1. This search strategy was modified for other databases. In addition to the bibliographic database searching, supplementary searches were undertaken to look for recent and unpublished studies in the WHO International Clinical Trials Registry Platform and ophthalmology conference websites (American Academy of Ophthalmology, Association for Research in Vision and Ophthalmology from 2010 to 2012).
Study selection
Only randomised controlled trials which included patients with macular oedema secondary to CRVO were included. It was acceptable for a study to include branch retinal vein occlusion and CRVO provided that the CRVO group was reported separately. The following drugs were included: dexamethasone, triamcinolone, ranibizumab, bevacizumab and aflibercept. Pegaptanib was not included because it is not used routinely in clinical practice. Only doses that are used in clinical practice were included. Studies had to report at least one of the following outcomes: proportions of patients gaining ≥3 lines from baseline to 6 months, proportions of patients losing ≥3 lines from baseline to 6 months and the mean change in best corrected visual acuity (BCVA) from baseline to 6 months.
Risk of bias assessment
The Cochrane Collaboration's tool was used for assessing risk of bias.21 The trials were graded (unclear, high or low risk of bias) based on: (1) sequence generation, (2) allocation concealment, (3) blinding of outcome assessor, (4) incomplete outcome data and (5) selective outcome reporting.
Study selection and data abstraction
Two authors independently assessed the eligibility and methodological quality of the studies identified during the literature search. Two authors extracted and compared the data. For each study identified that met the selection criteria, details on study design, study population characteristics, intervention, outcome measures and study quality were extracted. Discrepancies were resolved by consensus through discussion. Studies were assessed for comparability based on the populations included, trial arms, outcome measures and duration of follow-up. Common comparators were identified from the trials and a network diagram was created.
Summary measures
The primary measures of treatment effects were relative risk (RR) for the proportions of patients gaining ≥3 lines of vision, proportions of patients losing ≥3 lines of vision and the weighted mean difference (WMD) for mean change BCVA. We used the following methods to calculate SDs when incompletely reported: (1) contact with the corresponding author or (2) estimation of the SD on the basis of the sample size, median and range as suggested by Hozo et al22 or on the basis of the sample size and p value.
In one trial (SCORE),23–36 6-month data were not available because patients were followed up every 4 months. For the dichotomous outcomes, that is, proportions of patients gaining and losing ≥3 lines, we averaged 4 and 8-month data to get the 6 months follow up data. For the third outcome, that is, mean change BCVA, again data from two time-points were used. The weighted mean and SDs for each treatment arm were calculated using the mean and SDs of two time-points.
Data synthesis and model implementation
Bayesian NMA37 38 was used to compare the different interventions. NMA is a generalisation of meta-analysis methods because it allows comparisons of agents not addressed within individual primary trials. Bayesian statistical inference provides probability distributions for treatment effect parameters (RR and WMD), with 95% credible intervals (95% CrI), rather than 95% CIs (95% CI). A 95% CrI can be interpreted as there being a 95% probability that the parameter takes a value in the specified range.37 38
All analyses were conducted using a Bayesian Markov Chain Monte Carlo (MCMC) method and fitted in the freely available Bayesian software, WinBUGS V.1.4.3.39 Two Markov chains were run simultaneously using different initial values. Convergence to a stable solution was checked by viewing plots of the sampled simulations and using the Brooks-Gelman-Rubin diagnostic tool.40 Convergence was found to be adequate after running 20 000 samples for both chains. These samples were then discarded and a further 70 000 sampled simulation was then run, on which the results were based. We also calculated the probability of treatment being the most effective (first best), the second best, the third best and so on, and presented the results graphically with rankograms.41
Like standard meta-analysis comparison, an NMA can be either a fixed-effect or a random-effect model. We used the Bayesian Deviation Information Criterion (DIC) to compare fixed-effect and random-effect models. The most appropriate NMA model can be identified as the one with the lowest DIC. The DIC measures the fit of the model while penalising it for the number of effective parameters. The fixed-effect model was chosen because of the small number of trials available for each comparison, and difficulty in estimating between studies variance, if random-effect model, was implemented, and the difference in DIC was less than 5.
Results
Study selection and characteristics
The literature search identified 945 articles, as shown in figure 1. Seven studies were judged to be sufficiently comparable to be included in the NMA. Tables 1 and 2 present the characteristics and results of the included trials. Two studies11–13 compared aflibercept 2 mg against sham; two identical studies6–8 compared dexamethasone 0.7 mg (Ozurdex) against sham; one study9 10 compared ranibizumab 0.5 mg against sham; one study42–44 compared bevacizumab 1.25 mg against sham and, finally, one study23–36 compared triamcinolone 4 mg against observation. Sham or observation was used as the common comparator. The number of included participants varied from 6042–44 to 437.6–8 Most studies required patients to be treatment naïve and have macular oedema with retinal thickness measuring at least 250 or 300 μm on optical coherence tomography. Sham injection was undertaken by placing a needleless syringe on the eye. All studies, except for Epstein et al,42–44 were multi-centre, international studies. Most studies had an extension phase after the primary outcome, but this was not included in the NMA.
Figure 1.
Study selection flow diagram.
Table 1.
Baseline characteristics and results of all included studies
| Study | Participants | Intervention/outcomes |
|---|---|---|
| DEXAMETHASONE | ||
|
GENEVA 20106–8 International Setting: multicentre (167 centres in 24 countries, so a mean of 2.6 patients per centre) Design: 2 identical double-blind, sham-controlled RCTs, phase 3 Follow-up: primary endpoint for the masked trial: 6 months; primary endpoint for the open-label extension: 12 months |
N: CRVO—437 eyes of 437 patients randomised; 94% follow-up at 6 months Participants: adults with visual acuity reduced because of macular oedema due to CRVO or BRVO |
1. Dexamethasone 0.7 mg (n=136) Single dose 2. Dexamethasone 0.35 mg (n=154) Single dose 3. Sham (n=147) Single dose—a needleless applicator was placed against the conjunctiva to simulate the placement of study medication. Primary end point: gain of ≥15 ETDRS letters; for the open-label extension: safety |
| TRIAMCINOLONE | ||
|
SCORE 200923–36 USA Setting: multicentre Design: RCT Follow-up: primary end point 12 months, FU planned up to 36 months |
N: 271 eyes of 271 patients randomised; 83% (observation) and 90% (triamcinolone) completed 12 months Participants: centre-involved macular oedema secondary to CRVO |
1. Triamcinolone 1 mg (n=92) Every 4 months depending on retreatment regimen (average 2.2 injections at 12 months) 2. Triamcinolone 4 mg (n=91) Every 4 months depending on retreatment regimen (average 2.0 injections at 12 months) (The form of triamcinolone used was Trivaris, no longer available. It was made by the manufacturer of Ozurdex (Allergan)) 3. Observation (n=88) Primary end point: gain of ≥15 ETDRS letters |
| AFLIBERCEPT | ||
|
COPERNICUS 201212
13 International Setting: multicentre, 70 sites in North and South America, India and Israel. Mean 2.7 patients per centre Design: double-blind, sham-controlled RCT, phase 3 Follow-up: primary end point 24 weeks, FU 2 years |
N: 189 eyes of 189 patients randomised; 95.7% (aflibercept) and 81.1% (sham) completed 24 weeks; 93% (aflibercept) and 77% (sham) completed 52 weeks Participants: adult patients with centre-involved CRVO for a maximum of 9 months |
1. Aflibercept 2 mg (n=114) Every 4 weeks for 6 months (average number not available) 2. Sham (n=73) Every 4 weeks for 6 months (average number not available) (empty syringe without needle pressed to conjunctival surface) Primary end point: gain of ≥15 ETDRS letters |
|
GALILEO 201211 International Setting: multicentre, 10 countries in Europe and Asia; 63 centres in total Design: double-blind, sham-controlled RCT, phase 3 Follow-up: primary end point 24 weeks, FU up to 12 months, planned up to 76 weeks |
N: 177 eyes of 177 patients randomised; 90.6% (aflibercept) and 78.9% (sham) completed 24 weeks Participants: treatment-naïve patients with centre-involved CRVO for a maximum of 9 months |
1. Aflibercept 2 mg (n=103) Every 4 weeks for 6 months (average number not available) 2. Sham (n=71) Every 4 weeks for 6 months (average number not available) (empty syringe without needle pressed to conjunctival surface) Primary end point: gain of ≥15 ETDRS letters |
| RANIBIZUMAB | ||
|
CRUISE 20109
10 USA Setting: multicentre Design: double-blind, sham-controlled RCT, phase 3 Follow-up: primary end point 6 months, FU up to 12 months |
N: 392 eyes of 392 patients randomised; 97.7% (ranibizumab 0.3 mg), 91.5% (ranibizumab 0.5 mg) and 88.5% (sham) completed 6 months Participants: patients with foveal centre-involved macular oedema secondary to CRVO diagnosed within 12 months |
1. Ranibizumab 0.3 mg (n=132) Every 4 weeks for 6 months (average number not available) 2. Ranibizumab 0.5 mg (n=130) Every 4 weeks for 6 months (average number not available) 3. Sham (n=130) Every 4 weeks for 6 months (average number not available) (empty syringe without needle pressed to the injection site) Primary end point: mean change from baseline BCVA |
| BEVACIZUMAB | ||
|
EPSTEIN 201242–44 Sweden Setting: Single centre; St. Eriks Eye Hospital Stockholm Design: sham-injection controlled, double maskedRCT Follow-up: primary endpoint 6 months; open label extension up to 12 months |
N: 60 eyes of 60 patients randomised; 93% completed open label extension Participants: patients with CRVO of ≤6 months |
1. Bevacizumab 1.25 mg (n=30) Every 6 weeks for 6 months (average number not available) 2. Sham (n=30) Every 6 weeks for 6 months (averege number not available) (syringe without needle pressed to the globe) Primary end point: gain of ≥15 ETDRS letters |
BCVA, best corrected visual acuity; BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; ETDRS, Early Treatment Diabetic Retinopathy Study; FU, follow-up; N, number; RCT, randomised controlled trial.
Table 2.
Baseline characteristics and results of included trials
| Copernicus12 13 | Galileo11 | Cruise9 10 | Geneva6–8 | Epstein et al42–44 | Score23–36 |
|---|---|---|---|---|---|
| Baseline similarities | |||||
| Number (%) of patients | |||||
| Aflib 2 mg: 114 | Aflib 2 mg: 103 | Rani 0.5 mg: 130 | Dexa 0.7 mg: 136 | Beva 1.25 mg: 30 | Triam 4 mg: 91 |
| Sham: 73 | Sham: 68 | Sham: 130 | Sham: 147 | Sham: 30 | Obser: 88 |
| Age (years) | |||||
| Aflib 2 mg: 65.5 SD 13.6 | Aflib 2 mg: 59.9 SD 12.4 | Rani 0.5 mg: 67.6 SD 12.4 | Dexa 0.7 mg: NR | Beva 1.25 mg: 70.6 SD 12.6 | Triam 4 mg: 67.5 SD 12.0 |
| Sham: 67.5 SD 14.3 | Sham: 63.8 SD 13.3 | Sham: 65.4 SD 13.1 | Sham: NR | Sham: 70.4 SD 10.4 | Obser: 69.2 SD 12.8 |
| BCVA at baseline (SD) | |||||
| Aflib 2 mg: 50.7 SD 13.90 | Aflib 2 mg: 53.6 SD 15.8 | Rani 0.5 mg: 48.1 SD 14.6 | Dexa 0.7 mg: NR | Beva 1.25 mg: 44.4 SD 15.3 | Triam 4 mg: 51.0 SD 14.4 |
| Sham: 48.9 SD 14.42 | Sham: 50.9 SD 15.4 | Sham: 49.2 SD 14.7 | Sham: NR | Sham: 43.6 SD 16.0 | Obser: 52.1 SD 13.1 |
| Duration of MO from diagnosis to screening | |||||
| Aflib 2 mg: 2.73 SD 3.09 (in months) | Aflib 2 mg: 50.9 SD 15.4) (in days) | Rani 0.5 mg: – | Dexa 0.7 mg: NR | Beva 1.25 mg: NR | Triam 4 mg: 4.2 SD 3.6 (in months) |
| Sham: 1.88 SD 2.19 (in months) | Sham: 87.6 SD 79.1 (in days) | Sham: – | Sham: NR | Sham: NR | Obser: 4.2 SD 3.1 (in months) |
| Results | |||||
| Number (%) of patients gaining ≥15 letters improvement from baseline to 6 months | |||||
| Aflib 2 mg: 64 (56.1) | Aflib 2 mg: 62 (60.2) | Rani 0.5 mg: 62 (47.7) | Dexa 0.7 mg: 25 (18) | Beva 1.25 mg: 18 (60%) | Triam 4 mg: 18 (19.5%) (average of 4 and 8 months) |
| Sham: 9 (12.3) | Sham: 15 (22.1) | Sham: 22 (16.9) | Sham: 18 (12) | Sham: 6 (20%) | Obser: 3 (4%) (average of 4 and 8 months) |
| Number (%) of patients losing ≥15 letters of BCVA from baseline to 6 months | |||||
| Aflib 2 mg: 2 (1.8) | Aflib 2 mg: 8 (7.8) | Rani 0.5 mg: 2 (1.5) | Dexa 0.7 mg: NR | Beva 1.25 mg: 2 (6.7%) | Triam 4 mg: 19 (20.5%) (average of 4 and 8 months) |
| Sham: 20 (27.4) | Sham: 15 (22.1) | Sham: 20 (15.4) | Sham: NR | Sham: 7 (23.3%) | Obser: 31 (35.5%) (average of 4 and 8 months) |
| Mean change (SD) from baseline in BCVA | |||||
| Aflib 2 mg: 17.3 (12.8) | Aflib 2 mg: 18.0 (12.2) | Rani 0.5 mg: 14.9 (13.2) | Dexa 0.7 mg: 0.1 (NR) | Beva 1.25 mg: 14.1 SD 18.7 | Triam 4 mg: −0.15 SD 20.67 (n=85) (weight mean and SD of 4 and 8 months) |
| Sham: −4 (18) | Sham: 3.3 (14.1) | Sham: 0.8 (16.2) | Sham: −1.8 (NR) | Sham: −2.0 SD 20.5 | Obser: −9.66 SD 18.04 (n=75) (weighted mean and SD of 4 and 8 months) |
Aflib, aflibercept; BCVA, best corrected visual acuity; Dexa, dexamethasone; NR, not reported; Obser, observation; Rani, ranibizumab; Triam, triamcinolone.
The sufficiently comparable studies were combined into a network analysis based on a common comparator. The network for the proportions of patients gaining ≥3 lines is shown in figure 2. This network is the same for the other two outcomes, but without dexamethasone, because the trial did not report these outcomes.
Figure 2.

Network of randomised controlled trials comparing different treatments for proportions of gaining three or more lines of vision.
Risk of bias of included trials
Risk of bias is shown in table 3. Included studies were generally of high quality, with all studies being judged to be of low or unclear bias for all criteria. The non-commercially funded bevacizumab trial had fewer patients and, inevitably, results had wider CIs.42–44 In no study does it appear that patients were asked at the end of the trial which arm they thought they had been assigned. It is unclear how many could distinguish injections (intervention arm) from punctureless pressure (sham arm).
Table 3.
Risk of bias
| Study (author and year) | Adequate sequence generation | Allocation concealment | Masking | Incomplete outcome data addressed | Free of selective reporting | Free of other bias (eg, similarity at baseline, power assessment) | Funder |
|---|---|---|---|---|---|---|---|
| Geneva 20106–8 | Low | Low | Partial: patients and assessors of efficacy variables | Low: ITT analysis, 94% FU at 6 months | Low |
Power: 81% power to detect difference in primary outcome with n=495 for each trial Similarity at baseline: yes |
Allergan Inc |
| Score 200923–36 | Low | Unclear | Partial (physicians and patients masked to dose but not triamcinolone vs observation) | Low: ITT analysis, 83–90% FU at 12 months | Low |
Power: 80% power to detect difference in primary outcome with n=486 (but only 271 randomised) Similarity at baseline: yes |
National Eye Institute grants, Allergan |
| Copernicus 201212 13 | Low | Unclear | Low: double-blind | Low: ITT analysis, 89.9% assessed at primary end point | Low |
Power: 90% power to detect difference in primary outcome with n=165 Similarity at baseline: yes |
Bayer HealthCare, Regeneron Pharmaceuticals |
| Galileo 201211 | Unclear | Unclear | Low: double-blind | Low: ITT analysis, 86% assessed at primary end point | Low |
Power: 90% power to detect difference in primary outcome with n=150 Similarity at baseline: yes |
Bayer HealthCare, Regeneron Pharmaceuticals |
| Cruise 20109 10 | Low | Unclear | Low: patients and evaluating examiners, injecting physicians masked to dose | Low: ITT analysis, 88.5–97.7% completed 6 months | Low |
Power: not reported Similarity at baseline: yes |
Genentech Inc. |
| Epstein 201242–44 | Unclear | Low | Low: patients, outcome assessors | Low: ITT analysis; missing data for 2 patients (primary endpoint) | Low |
Power: 80% power to detect difference in primary outcome with n=24 per group Similarity at baseline: yes |
Unclear; authors are consultants for Allergan, Novartis, Alcon, Bayer |
FU, follow-; ITT, intention to treat.
Effects of interventions on proportions of patients gaining ≥3 lines
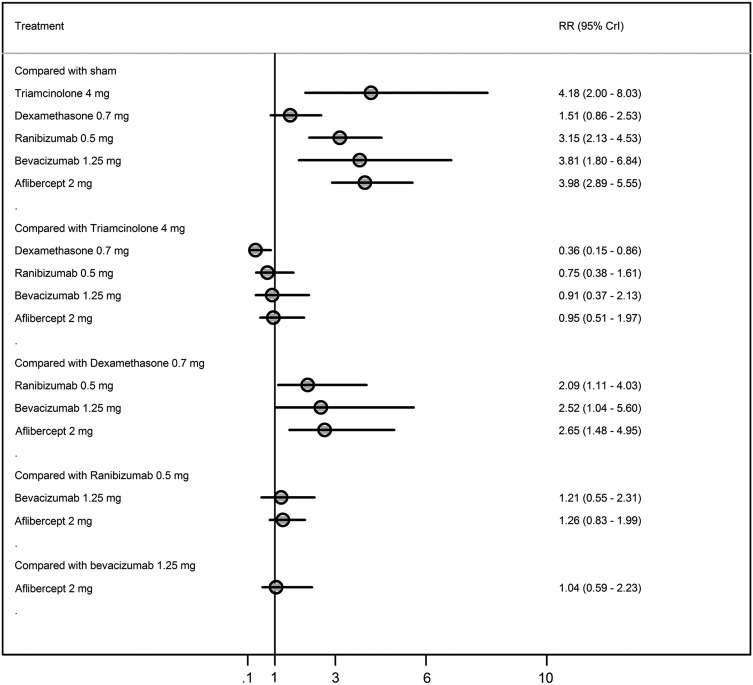
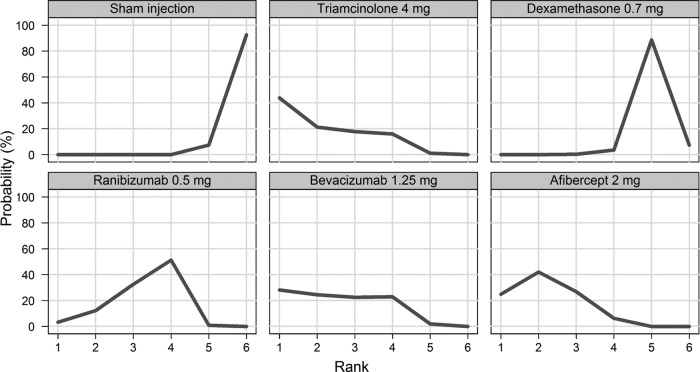
Figure 3 displays a forest plot of the risk ratio and 95% CrI in proportions of patients gaining ≥3 lines for all the possible pairwise comparisons. In terms of proportions of patients gaining ≥3 lines, triamcinolone 4 mg, ranibizumab 0.5 mg, bevacizumab 1.25 mg and aflibercept 2 mg had a higher probability of being more effective than a sham and dexamethasone (figure 4). There was no difference in the proportions of patients gaining ≥3 lines between triamcinolone 4 mg, ranibizumab 0.5 mg, bevacizumab 1.25 mg and aflibercept 2mg.
Figure 3.
Proportions of patients gaining three lines or more from baseline to 6 months.
Figure 4.
Rankogram for gaining ≥3 lines—distribution of the probabilities of every treatment being ranked at each of the possible six positions.
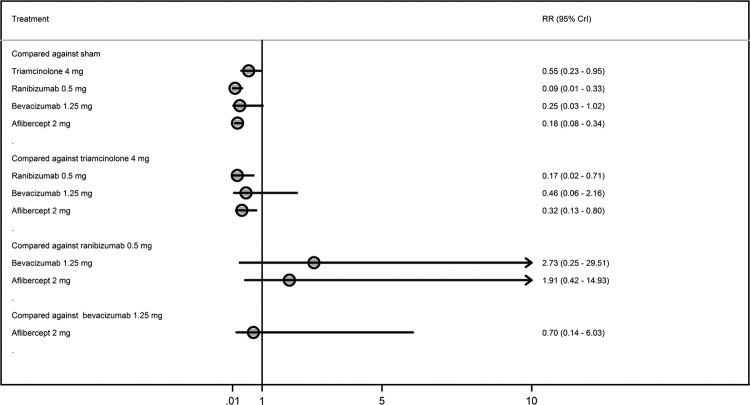
Effects of interventions on proportions of patients losing ≥3 lines
Figure 5 displays forest plot of the risk ratio and 95% CrI of proportions of patients losing ≥3 lines for all the possible pairwise comparisons. A smaller proportion of patients treated with triamcinolone 4 mg, ranibizumab 0.5 mg or aflibercept 2 mg lost ≥3 lines of vision than those treated with sham. There was no difference in the proportions of patients losing ≥3 lines between triamcinolone 4 mg, ranibizumab 0.5 mg, bevacizumab 1.25 mg and aflibercept 2 mg. Figure 6 shows ranking for efficacy in terms of proportions of patients losing ≥3 lines.
Figure 5.
Proportions of patients losing three lines or more from baseline to 6 months.
Figure 6.
Rankogram for losing ≥3 lines—distribution of the probabilities of every treatment being ranked at each of the possible six positions.
Effects of interventions on mean change in BCVA
Figure 7 displays a forest plot of the mean changes and 95% CrIs of improvement in BCVA for all the possible pairwise comparisons. Patients treated with triamcinolone 4 mg, ranibizumab 0.5 mg, bevacizumab 1.25 mg or aflibercept 2 mg had a higher probability of improvement in BCVA compared to those treated with sham injections. Patients treated with aflibercept 2 mg had a higher probability of improvement in BCVA compared with those treated with triamcinolone 4 mg (figure 8). There was no difference in the mean change in BCVA from baseline between patients treated with ranibizumab 0.5 mg, bevacizumab 1.25 mg and aflibercept 2 mg.
Figure 7.
Mean best corrected visual acuity change from baseline to 6 months.
Figure 8.
Rankogram for mean change in best corrected visual acuity—distribution of the probabilities of every treatment being ranked at each of the possible six positions.
Discussion
Statement of principal findings
Our results show no evidence of a difference in effectiveness between aflibercept, ranibizumab and triamcinolone. Bevacizumab was similar to these drugs in terms of letters gained and the mean change in BCVA. Dexamethasone was less effective compared with these drugs.
Strengths and limitations
This is the first study providing an indirect comparison of drugs to treat macular oedema secondary to CRVO. A robust search strategy, screening process and data extraction were used, and this analysis drew on a systematic review. The studies included had, in general, a low risk of bias. Safety was not considered in this study but is described in detail elsewhere.45 Five different drugs were suitable for NMA. Unpublished data were obtained from one author.42–44 Bayesian methods were used for the NMA. There was good model fit and convergence within the analysis.
However, pre-specified outcomes were not reported in all studies and the sample size varied considerably. For example, Epstein et al, 42–44 assessing bevacizumab, only included 30 participants in each arm. This resulted in wide CrIs from the NMA, which could have led to a type 1 error, especially with regard to the proportions of patients losing ≥3 lines. The SCORE study compared triamcinolone to observation.23–36 The NMA assumes a11 similar effect of sham and observation and this may result in a small degree of bias. Only 6 months of data were included, and the long-term effects are not known. Using a 6-month follow-up period may disadvantage dexamethasone because peak effect in the GENEVA trials was seen at 90 days, and by 6 months, benefits had been largely lost.6–8
As with most network meta-analyses, methodological heterogeneity was present. There were some differences among the trials. For example, CRUISE,9 10 assessing ranibizumab, did not include as many patients with ischaemic CRVO as the aflibercept trials.12 13 There were also some small differences in the chronicity of macular oedema and the mean BCVA at baseline.
Meaning of the study: possible explanations and implications for clinicians and policymakers
No head-to-head trials comparing aflibercept, bevacizumab, ranibizumab, triamcinolone and dexamethasone have been published in CRVO. Part of the reason for this is that the Food and Drug Administration requires proof of the safety and effectiveness of a drug.46 The easiest and quickest method for pharmaceutical companies to produce this proof is through placebo controlled trials. Trials comparing new medications to current best treatment would be considerably more useful to clinicians and patients.
Head-to-head trials comparing some of these drugs are available in other conditions. For example, a comparison of ranibizumab and bevacizumab was undertaken in age-related macular degeneration in the Comparison of Age-related macular degeneration Treatment Trials (CATT)47 and alternative treatments to Inhibit VEGF in patients with Age-related choroidal Neovascularisation (IVAN)48 trials. Both of these trials found no difference in effectiveness between ranibizumab and bevacizumab. Furthermore, an indirect comparison of ranibizumab and bevacizumab found no evidence of a difference between these drugs.49 Thus, it is highly probable that this may also apply in CRVO. The difference seen in our results regarding bevacizumab may be due to the low number of patients included in Epstein et al.42–44 In the CATT trial, more patients were hospitalised in the bevacizumab arm, but the authors did not believe that this was explained by a direct effect of bevacizumab.47 The 2-year results from the IVAN showed little difference in cardiovascular events, with the number being insignificantly lower with bevacizumab.50 Ranibizumab and aflibercept were directly compared in two similarly designed trials, VEGF Trap-eye: investigation of Efficacy and safety in Wet age-related macular degeneration (VIEW 1 and 2).51 Similar efficacy and safety was found in both drugs.
From the included trials it is clear that intraocular steroids are associated with complications, including increased intraocular pressure and cataract formation.6–8 23–36 These are substantial drawbacks for using steroids to treat macular oedema in CRVO. However, many affected patients may already be pseudophakic and, on these, the use of intraocular steroids may be reasonable. Steroids may have a place in the treatment pathway of patients who have failed on anti-VEGF therapy, but this is yet to be tested. The anti-VEFG drugs have a good safety profile and do not cause cataract formation.9–13 42–44 For this reason they are more likely to be favoured by clinicians than steroids.
Aflibercept, compared with ranibizumab and bevacizumab, targets a wider range of cytokines and may have a stronger binding affinity.52 Initial results suggested that aflibercept would require fewer injections than ranibizumab.51 Heier and colleagues compared aflibercept and ranibizumab in two similarly designed randomised controlled trials in age-related macular degeneration. They found that 2 mg aflibercept administered every 8 weeks produced similar effects at 96 weeks to 0.5 mg ranibizumab administered every 4 weeks.51 This was reflected in the FDA Dermatologic and Ophthalmic Drugs Advisory Committee recommendation that aflibercept should be given every 2 months following three initial monthly doses in age-related macular oedema.53 This may be because aflibercept also appears to last longer in the eye than ranibizumab.54 Age-related macular degeneration is a more aggressive condition than CRVO and so it is unlikely that more frequent dosing would be needed. Therefore, aflibercept may be preferred because it would reduce pressure on outpatient clinics. Furthermore, there is some evidence from patients with age-related macular degeneration that aflibercept may be effective in patients who have not responded to ranibizumab.55 56 This may be due to the higher affinity and wider number of cytokines that are targeted. There is no reason to suspect that these effects are any different for the macular oedema caused by CRVO. However, we have as yet no evidence as to whether ranibizumab would be effective after aflibercept has failed.
The National Institute of Health and Care Excellence has recommended dexamethasone, ranibizumab and aflibercept as options in the treatment of macular oedema secondary to CRVO.18–20 Until these technologies are reviewed together and compared with each other, clinicians are left with three recommended drugs. It should be noted that during the appraisal of ranibizumab the evidence review group found that in the cost-effectiveness analysis dexamethasone was extendedly dominated by ranibizumab (an intervention is judged not be cost-effective because it has an ICER that is greater than that of a more effective intervention). The committee appraising ranibizumab did not re-consider the previous appraisal decision on dexamethasone.
Our results show that dexamethasone was not as effective as ranibizumab or aflibercept, at 6 months follow-up and with the dosing regimens in the trials. However, these results do not assess quality of life or cost effectiveness. Bevacizumab is likely to prove more cost effective than both aflibercept and ranibizumab because it is substantially less expensive.57 However, the National Institute for Health and Care Excellence has not issued guidance on bevacizumab because it does not have a license for use in the eye.
Unanswered questions and future research
Not all patients benefit from the use of anti-VEGF drugs; only about 60% gain 15 or more letters. It is not clear why some patients benefit more than others. Future research should focus on identifying subgroups of patients who are likely to benefit. Only a few of the trials included ischaemic patients, and in those trials only a few patients with ischaemia were included.11–13 More research assessing the effectiveness of these drugs in severely ischaemic patients is needed.
Head-to-head trials comparing ranibizumab, aflibercept, bevacizumab and triamcinolone are needed. These should include assessment of cost effectiveness. To assist this, a better measure of quality of life is needed for patients with eye conditions. The widely used EQ5D may not be sensitive enough to measure changes that are important to patients, such as the ability to drive.
In conclusion, we have found no evidence of differences between ranibizumab, bevacizumab, aflibercept and triamcinolone for improving vision. The anti-VEGFs are likely to be favoured because they are not associated with steroid-induced cataract formation. Clinicians may prefer Aflibercept because it might require fewer injections.
Supplementary Material
Acknowledgments
The authors thank Christine Clar, Sian Thomas and Rachel Court for assisting with searches, screening and data extraction for the systematic review that precedes this study. They also thank the authors of the Epstein 2012 trial for providing addition data.
Footnotes
Contributors: NW conceived the idea. All authors contributed to the design of the study. DS and OAU undertook the statistical analysis. JAF, DS and OAU wrote the first draft of the manuscript. All authors redrafted and agreed on the final article. JAF is the guarantor.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Deramo VA, Cox TA, Syed AB, et al. Vision-related quality of life in people with central retinal vein occlusion using the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2003;121:1297–302 [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Moss SE, Meuer SM, et al. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol 2008;126:513–18 [DOI] [PubMed] [Google Scholar]
- 3.Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology 2011;118:119–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 2010;117:1113–23 [DOI] [PubMed] [Google Scholar]
- 5.The Royal College of Ophthalmologists. Interim Guidelines for Management of Retinal Vein Occlusion. Secondary Interim Guidelines for Management of Retinal Vein Occlusion. 2010. http://www.rcophth.ac.uk/core/core_picker/download.asp?id=728& filetitle=Interim+Guidelines+for+Management+of+Retinal+Vein+Occlusion+2010
- 6.Haller JA, Bandello F, Belfort R, Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117:1134–46 [DOI] [PubMed] [Google Scholar]
- 7.Haller JA, Bandello F, Belfort R. Jret al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 2011;118:2453–60 [DOI] [PubMed] [Google Scholar]
- 8.Yeh WS, Haller JA, Lanzetta P, et al. Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology 2012;119:1190–8 [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1124–33 [DOI] [PubMed] [Google Scholar]
- 10.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 2011;118:2041–9 [DOI] [PubMed] [Google Scholar]
- 11.Holz FG, Roider J, Ogura Y, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol 2013;97:278–84 [DOI] [PubMed] [Google Scholar]
- 12.Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology 2012;119:1024–32 [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol 2013;155:429–37 [DOI] [PubMed] [Google Scholar]
- 14.Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 2005;112:1035–47 [DOI] [PubMed] [Google Scholar]
- 15.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology 2007;114:743–50 [DOI] [PubMed] [Google Scholar]
- 16.Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina 2007;27:419–25 [DOI] [PubMed] [Google Scholar]
- 17.Algvere PV, Epstein D, von Wendt G, et al. Intravitreal bevacizumab in central retinal vein occlusion: 18-month results of a prospective clinical trial. Eur J Ophthalmol 2011;21:789–95 [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence. Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein occlusion: NICE technology appraisal guidance 283. 2013. http://guidance.nice.org.uk/TA283/Guidance/pdf/English
- 19.National Institute for Health and Clinical Excellence. Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion: NICE technology appraisal guidance 229. 2011. http://guidance.nice.org.uk/TA229/Guidance/pdf/English
- 20.National Institute for Health and Care Excellence. Aflibercept for treating visual impairment caused by macular oedema secondary to central retinal vein occlusion. NICE technology appraisal guidance 305. 2014. http://guidance.nice.org.uk/TA305
- 21.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhavsar AR, Ip MS, Glassman AR, et al. The risk of endophthalmitis following intravitreal triamcinolone injection in the DRCRnet and SCORE clinical trials. Am J Ophthalmol 2007;144:454–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blodi BA, Domalpally A, Scott IU, et al. Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study system for evaluation of stereoscopic color fundus photographs and fluorescein angiograms: SCORE Study Report 9. Arch Ophthalmol 2010;128:1140–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan CK, Ip MS, VanVeldhuisen PC, et al. SCORE Study report #11: incidences of neovascular events in eyes with retinal vein occlusion. Ophthalmology 2011;118:1364–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ip M, Oden N, VanVeldhuisen P, et al. The standard care vs. corticosteroid for retinal vein occlusion study: design and baseline characteristics. Am Acad Ophthalmol 2008;260 [Google Scholar]
- 27.Ip MS, Oden NL, Scott IU, et al. SCORE Study report 3: study design and baseline characteristics. Ophthalmology 2009;116:1770–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol 2009;127:1101–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers D, Blodi B, Ip M, et al. Reading center evaluation of OCT images from patients enrolled in the standard care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) Study. IOVS 2006;47:ARVO E-abstract 5194 [Google Scholar]
- 30.Oden NL, Veldhuisen PC, Scott IU, et al. Temporal variability of OCT in retinal vein occlusion participants in the SCORE study. IOVS 2007;48:ARVO E-abstract 107 [Google Scholar]
- 31.Scott IU, Blodi BA, Ip MS, et al. SCORE study report 2: Interobserver agreement between investigator and reading center classification of retinal vein occlusion type. Ophthalmology 2009;116:756–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott IU, Oden NL, VanVeldhuisen PC, et al. SCORE Study Report 7: incidence of intravitreal silicone oil droplets associated with staked-on vs luer cone syringe design. Am J Ophthalmol 2009;148:725–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott IU, VanVeldhuisen PC, Oden NL, et al. Baseline predictors of visual acuity and retinal thickness outcomes in patients with retinal vein occlusion: Standard Care Versus COrticosteroid for REtinal Vein Occlusion Study report 10. Ophthalmology 2011;118:345–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott IU, VanVeldhuisen PC, Oden NL, et al. SCORE Study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology 2009;116:504–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott IU, VanVeldhuisen PC, Oden NL, et al. Baseline characteristics and response to treatment of participants with hemiretinal compared with branch retinal or central retinal vein occlusion in the standard care vs COrticosteroid for REtinal vein occlusion (SCORE) study: SCORE study report 14. Arch Ophthalmol 2012;130:1517–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren K, Blodi BA, Oden N, et al. Reading center evaluation of baseline retinal images in the standard care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) Study. IOVS 2008;ARVO E-abstract 2136 [Google Scholar]
- 37.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24 [DOI] [PubMed] [Google Scholar]
- 39.Spiegelhalter D, Thomas A, Best N, et al. WinBUGS User Manual: Version 1.4. Cambridge, MA: MRC Biostatistics Unit, 2003 [Google Scholar]
- 40.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434–55 [Google Scholar]
- 41.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71 [DOI] [PubMed] [Google Scholar]
- 42.Epstein D, Algvere P, Von WG, et al. Long-term benefit from bevacizumab for macular edema in central retinal vein occlusion: 12-month results of a prospective study. Acta Ophthalmol 2012;90:48. [DOI] [PubMed] [Google Scholar]
- 43.Epstein DL, Algvere PV, Von WG, et al. Benefit from bevacizumab for macular edema in central retinal vein occlusion: twelve-month results of a prospective, randomized study. Ophthalmology 2012;119:2587–91 [DOI] [PubMed] [Google Scholar]
- 44.Epstein DL, Algvere PV, Von WG, et al. Bevacizumab for macular edema in central retinal vein occlusion: a prospective, randomized, double-masked clinical study. Ophthalmology 2012;119:1184–9 [DOI] [PubMed] [Google Scholar]
- 45.Ford JA, Clar C, Lois N, et al. Treatments for macular oedema following central retinal vein occlusion: systematic review. BMJ Open 2014;4:e004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FDA. Drug Study Designs—Information Sheet. Guidance for Institutional Review Boards and Clinical Investigators. Secondary Drug Study Designs—Information Sheet. Guidance for Institutional Review Boards and Clinical Investigators. 2011. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126501.htm
- 47.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119:1399–411 [DOI] [PubMed] [Google Scholar]
- 49.Ford JA, Elders A, Shyangdan D, et al. The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: an indirect comparison in a systematic review. BMJ 2012;345:e5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258–67 [DOI] [PubMed] [Google Scholar]
- 51.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537–48 [DOI] [PubMed] [Google Scholar]
- 52.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012;15:171–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.FDA. VEGF TRAP-EYE (aflibercept ophthalmic solution). Ophthalmologic Drugs Advisory Committee. Secondary VEGF TRAP-EYE (aflibercept ophthalmic solution). Ophthalmologic Drugs Advisory Committee. 2011. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOphthalmicDrugsAdvisoryCommittee/UCM259143.pdf
- 54.Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol 2008;92:667–8 [DOI] [PubMed] [Google Scholar]
- 55.Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 2013;156:15–22.e1 [DOI] [PubMed] [Google Scholar]
- 56.Cho H, Shah CP, Weber M, et al. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol 2013;97:1032–5 [DOI] [PubMed] [Google Scholar]
- 57.Raftery J, Clegg A, Jones J, et al. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol 2007;91:1244–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.