Abstract
T cell exhaustion is thought to be a natural mechanism for limiting immune pathology, although it may be desirable to circumvent this mechanism, to help eliminate viral reservoirs or tumors. Although there are no definitive markers, a fingerprint for exhausted T cells has been described, which includes the transmembrane proteins PD-1, LAG3 and Tim-3. However, apart from the recruitment of tyrosine phosphatases to PD-1, little is known about the biochemical mechanisms by which these proteins contribute to development or maintenance of exhaustion. Tim-3 contains no known motifs for the recruitment of inhibitory phosphatases, but may actually increase signaling downstream of TCR/CD3, at least under acute conditions. Other studies have shown that T cell exhaustion results from chronic stimulation that extends the effector phase of T cell activation, at the expense of T cell memory. We suggest that Tim-3 may contribute to T cell exhaustion in part by enhancing TCR-signaling pathways.
Introduction to T cell exhaustion
T cell activation, including development of a robust memory response, is critical for the development of an efficient immune response to viral infection, and can also be instrumental in mounting an immune response to solid tumors. However, overly vigorous or sustained immune responses can cause immune mediated pathology, which is detrimental to the host. Such a problem is particularly evident with viruses that cause chronic infections (1). In these cases, the sustained presence of viral antigens appears to drive the formation of a state of antigen-specific T cell “exhaustion.” While this has the beneficial effect of limiting immune pathology, it can result in the establishment of a viral reservoir, which may become re-activated under conditions of physiological stress. T cell exhaustion can also be detrimental when it impairs the ability of an adaptive immune response to eliminate a tumor.
Functionally, the development of T cell exhaustion is characterized by the gradual loss of expression of various cytokines and effector molecules, with IL-2, cytotoxicity and proliferation among the earliest, and IFN-γ among the latest (1, 2). Exhausted T cells may also become “addicted” to antigen receptor signals, and lose responsiveness to the homeostatic cytokine IL-7, the latter due at least in part to loss of CD127 (IL-7r alpha chain) expression (2). Importantly for possible therapeutic reversal, exhausted T cells also gain high-level and persistent, as opposed to transient, expression of several proteins, including the transcription factor BLIMP-1 and the transmembrane proteins PD-1, Tim-3, LAG-3 (1, 2). The latter proteins, so-called “check point” receptors, have attracted attention as possible dominant mediators of T cell exhaustion, since antibodies to these proteins or their ligands can, under some circumstances, “rescue” the function of exhausted T cells (2–4). Since this topic has been covered extensively in other relatively recent reviews (1, 2), we will focus here mainly on recent studies of Tim-3, which has attracted substantial pre-clinical attention of late as a novel therapeutic target for reversal of T cell exhaustion. We will also review what is known regarding signal transduction pathways implicated in Tim-3 function. Finally, we will discuss the role of TCR signaling in driving the development of exhaustion, and how this might be influenced by Tim-3.
Lessons from tumors
The tumor microenvironment is known to be immunosuppressive, due to inhibitory signals from cell surface and soluble mediators (5), although the precise strategies employed by different tumors can vary by tissue, and even from patient-to-patient. Thus, while T cells specific to tumor antigens can be readily isolated from solid tumors of patients and in mouse models, these cells often respond poorly to ex vivo stimulation. This T cell dysfunction is thought to result at least in part from exhaustion of effector tumor-infiltrating lymphocytes (TILs), due to chronic antigenic stimulation, inhibitory co-receptor and cytokine expression, among other factors (6). Based on the recent success of CTLA-4 antibody therapy (7), and accumulating data from pre-clinical models, there is now considerable excitement surrounding molecules whose targeting may allow for broad enhancement of T cell responses against tumors. Solid tumor-infiltrating T cells often express high levels of one or more inhibitory or exhaustion-associated receptors, including PD-1, LAG3 and/or Tim-3. Indeed, and consistent with antigen acting as a driver of exhaustion, a recent study on melanoma patients demonstrated that PD-1 can be used to prospectively distinguish tumor-specific T cells at the tumor site (8). Tim-3 expression on T cells is also seen in the context of non-solid tumors. For example, upregulation of Tim-3 (possibly driven by IL-12) on effector T cells of patients with follicular B cell non-Hodgkin lymphoma was associated with poor outcomes (9).
PD-1 has been extensively studied as a potential therapeutic target, and recent clinical trial data suggest that mAb’s to PD-1 or one of its ligands, PD-L1, are clinically effective against certain solid tumors, including melanoma, as well as non-small cell lung cancer (NSCLC), generally regarded as a non-immunogenic tumor (10–12). Monoclonal antibodies specific for Tim-3 have also been shown to promote rejection of solid tumors in murine models (13, 14), and mAb’s to human Tim-3 can rescue the ex vivo function of apparently exhausted T cells from tumor-bearing patients (15). Interestingly, in the former case, the efficacy of Tim-3 mAb therapy appeared to result at least in part from effects on regulatory T cells (Treg), which can also express Tim-3 (16). Strikingly, these Tim-3+ Treg appear to be among the most potent at inhibiting effector T cell function and express greater levels of IL-10. At least in this study, monotherapy with Tim-3 antibody alone was not sufficient to augment anti-tumor immunity in vivo, but rather could cooperate with primary anti-PDL1 treatment (13). High levels of Tim-3 have also been observed on tumor-infiltrating Treg from human patients with NSCLC, a finding that correlated with poor clinical outcomes (17). Thus, at least in the case of tumors, it will clearly be important to parse out the effects of Tim-3 manipulation on effector/exhausted T cells from the effects on regulatory T cells. In addition, the precise role played by Tim-3 may vary, depending on tumor type.
Tim-3 in infectious disease: recent studies
T cell exhaustion has been observed not only in mouse models (discussed above), but also in human disease, mainly in the context of various chronic viral infections (18–25). For instance, a population of Tim-3+CD8+ T cells is readily identified in HIV patients (26), and the expression of Tim-3 correlates positively with progression to AIDS, and inversely with viral control (27). In addition, ex vivo stimulation of these HIV-associated, exhausted, CD8+ T cells in the presence of Tim-3 mAb can at least partially rescue their function (26), while anti-retroviral therapy may reduce Tim-3 expression (28). Similarly, study of hepatitis C virus (HCV)-infected patients has revealed the presence of dysfunctional CD4+ and CD8+ T cells with high level Tim-3 expression (29, 30). In both HIV and HCV, up-regulation of Tim-3 was associated with the accumulation of central memory (CD45RA-CCR7+) T cells (26, 29). Thus, a large body of evidence now supports a net in vivo negative impact of Tim-3 expression on T cell-dependent antiviral immune responses.
The case of Tim-3 in tuberculosis (Tb) has proven to be quite complex, and points out that effects of Tim-3 expression (or its therapeutic targeting) on infectious disease outcomes may be disease- and/or context-specific. An elegant study from Behar and colleagues revealed a novel and unexpected function for Tim-3 in a murine model of Tb (31). Thus, the authors found that interaction of Tim-3 with one of its ligands – galectin-9 (gal9), expressed on macrophages – stimulated the production of the cytokine IL-1b, enhancing bacterial killing. This effect was mimicked by administration of Tim3-Ig fusion protein, through its interaction with gal9. A similar phenomenon has also been observed in human macrophages (32). At this point, the nature of the signal transmitted by gal9 after Tim-3 binding is unknown. Still, this finding is of possible relevance for the interpretation of multiple other studies, since a soluble Tim3-Ig fusion protein is often used as a “blocking” reagent in other settings. Returning to T cells and human patients, active Tb, as opposed to latent infection, is associated with the up-regulation of Tim-3 on both CD4+ and CD8+ T cells (33). Surprisingly, however, these Tim-3+ T cells display more potent anti-Tb responses, contrary to what has been observed in chronic viral infection. While the reasons for these differences are not clear, the authors speculated that they may be due at least in part to the somewhat divergent phenotypes of Tim-3+ Tb-specific T cells, compared with Tim-3+ T cells found in HIV and HCV patients. For example, Tim-3+ T cells in Tb patients were found to still express CD127 (IL-7r alpha chain) (33), while this marker is well known to be lost in exhausted T cells (including those that are Tim-3+) during chronic viral infections of humans and mice (1, 2).
A recently published study using a newly generated Tim-3 knockout mouse model provides further support for a context-dependent positive role for Tim-3 in vivo, in this case in an acute bacterial infection model (34). Thus, Colgan and colleagues first infected wild type mice with L. monocytogenes (LM) and followed the expression of Tim-3. They noted that Tim-3 is robustly expressed on CD8+ T cells during this infection, with particularly high expression on effector T cells. They then infected mice with a germline deletion of Tim-3 expression and found that both primary and secondary T cell responses to LM were significantly impaired. This was also true, although the effect of Tim-3 deletion was less severe, when Tim-3 deficient T cells were transferred to WT hosts, along with WT T cells. Finally, the authors provided evidence that the decreased T cell responses in the absence of Tim-3 were due at least in part to compromised survival of the knockout T cells. It should be noted that the Tim-3 deficient mice used in this study were derived from 129 strain ES cells and then backcrossed to C57BL/6 for ten generations. As the Tim locus is polymorphic between 129 and C57BL/6, the carry-over of polymorphisms in Havcr1 (encoding Tim-1) and in other neighboring genes should be taken into account as potential confounding variables in this study. Nonetheless, this report provides additional compelling evidence that the effects of Tim-3 expression during infection are complex, and may lead to enhanced, rather than diminished, T cell responses.
Tim-3 Signaling and T cell exhaustion
Surprisingly, at this point there is relatively sparse evidence to support the model that Tim-3 directly mediates suppression of T cell activation or cytokine secretion in a manner similar to PD-1. Rather, despite evidence that Tim-3 manipulation can ameliorate T cell exhaustion, there are a number of observations that do not fit with a simple narrative of direct inhibition of T cell activation by Tim-3. First is the fact that Tim-3+ T cells from HIV-infected patients, while displaying defective stimulation-induced phosphorylation of Stat5, ERK1/2 and p38, actually possess higher basal phosphorylation of all of the same pathways (26). This is consistent with our own data, obtained with ectopic expression of Tim-3 in T cell lines and primary T cells. Thus, ectopic expression of Tim-3 actually enhanced T cell activation, leading to increased activation of NFAT/AP-1 and NF-κB transcriptional reporters, and even enhanced cytokine production, from both T cell lines and primary T cells (35).
When considering how Tim-3 might signal to regulate the development or activation of exhausted T cells, it may be instructive to consider the reported functions of Tim-3 in myeloid lineage cells. Thus, ligation of Tim-3, with specific antibodies or one of the reported Tim-3 ligands, can (at least under some circumstances) enhance the activation and function of various myeloid lineage cell types (36). This is consistent with induction of the transcription factor NF-κB after Tim-3 mAb treatment of dendritic cells (37). In addition, antibody ligation of Tim-3 on murine bone marrow-derived mast cells has been shown to augment IgE/Ag-induced cytokine production (38), a finding that we have reproduced with multiple antibodies to Tim-3 (B. Phong and L.P. Kane, unpublished). Another recently described function for Tim-3 in DC’s is to inhibit an innate response to nucleic acids, an effect that involves interaction of Tim-3 with the endogenous “danger” signal HMGB1 (39). This unusual pathway seems to prevent the trafficking of exogenously acquired DNA into endosomes. The precise mechanism underlying this effect is still unclear (e.g. whether it is mediated by active Tim-3 signaling or by passive blockade of HMGB1 function), but may have relevance for the effects of Tim-3 in T cell responses against viral and/or tumor antigens. It should be noted here that this is one of the few published studies to directly demonstrate that a particular Tim-3 antibody could block interaction with the ligand under study (in this case HMGB1). Several studies have also suggested a negative regulatory role for Tim-3 in myeloid cells (40–42). Thus, as in the case of T cells, it would appear the precise effects of Tim-3 on myeloid cells depend upon the context in which Tim-3 ligation occurs.
Administration of certain Tim-3 antibodies can rescue the function of exhausted T cells, in both in vivo mouse models (13, 23) and in vitro experiments with cells from patients with chronic viral infections or tumors (15, 26). However, particularly in the case of the aforementioned in vivo experiments, it is not clear whether the effects of the Tim-3 antibodies are the direct result of blocking the interaction of Tim-3 on exhausted T cells with one more of the known ligands for Tim-3 (e.g. galectin-9 and/or HMGB1, or other yet-to-be discovered ligands). Indeed the presence of Tim-3 on other cells, including APC’s and Treg, might also explain the function of at least some Tim-3 antibodies in certain disease settings. In such cases, one might imagine that either “agonistic” or “antagonistic” activities of such antibodies could eventually lead to the observed downstream functional effects. However, at this point, specific agonistic or antagonistic activity has not been directly ascribed to the most commonly used Tim-3 antibodies. One approach to this problem would be to compare the activities of Tim-3 mAb’s in their native forms, vs. the effects of Fab’ fragments of the same antibodies. This will be an important point to clarify in future studies.
Tim-3 proximal signaling – tyrosine kinases and beyond
When we began studying signaling pathways linked to Tim-3, we were intrigued by the presence of multiple tyrosine residues in the cytoplasmic tails of both murine and human Tim-3 (Fig. 1A). However, the sequences around these tyrosines do not conform to any known inhibitory signaling motifs (e.g. ITIM, ITSM), such as those found in PD-1. Analysis of the cytoplasmic tail of murine Tim-3 with the Scansite algorithm (http://scansite3.mit.edu) revealed that several of these tyrosines conformed well to putative sites of phosphorylation by multiple tyrosine kinases, particularly those of the Src family (Fig. 1B). Importantly, the analogous tyrosines in human Tim-3 also scored as possible sites of phosphorylation by the same classes of kinases (not shown). We and others have demonstrated that the cytoplasmic tail of Tim-3 can indeed be phosphorylated on multiple tyrosine residues (35, 43, 44). While the identity of the kinases that phosphorylate the Tim-3 cytoplasmic tail have not been definitively shown in vivo, our data are consistent with such phosphorylation being carried out by the Src family kinases Fyn and/or Lck, at least in T cells (35). However, it should be pointed out that the Tec family kinase Itk has also been implicated in the phosphorylation of Tim-3 (43). Tyrosine phosphorylation often functions to recruit downstream signaling proteins, particularly those containing Src homology 2 (SH2) domains. Thus, we found that the SH2 domain of Fyn and one of the SH2 domains of the PI3K adaptor protein p85 could bind to a phosphorylated peptide corresponding to the region around Y256 and Y263 of murine Tim-3 (35). Consistent with these findings, we also found that ectopic expression of Tim-3 enhanced the phosphorylation of both PLC-γ1 (which is dependent on Src and Syk family kinases) and ribosomal protein S6, which lies downstream of the PI3K/Akt/mTOR pathway (35). Some of these experiments were performed with the work-horse Jurkat T cell model (45), which does have some limitations, including lack of PTEN expression. Importantly, however, key findings of these studies were also reproduced in the murine T cell clone D10, as well as in primary murine T cells (35). Thus, at the levels of both signal transduction and function (cytokine production), acute upregulation of Tim-3 expression can enhance T cell activation. A current challenge is deciphering how these observations fit into the current paradigm of Tim-3 function in vivo.
Figure 1.
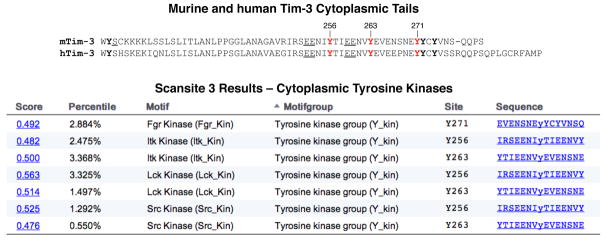
Amino acid sequence of the Tim-3 cytoplasmic tail and possible sites of phosphorylation. (Top) Alignment of the murine and human Tim-3 cytoplasmic tail sequences. Predicted sites of tyrosine phosphorylation are numbered, while other tyrosines are in bold type. Conserved charged residues (suggesting favorable sites for phosphorylation) upstream of Y256 and Y263 are underlined. (Bottom) Results of a Scansite search using the murine Tim-3 cytoplasmic domain, and restricting results to cytoplasmic tyrosine kinases. Analogous tyrosines in human Tim-3 were also predicted to be phosphorylated by the same kinases (not shown).
A recent report suggested that interaction of Tim-3 with a chaperone protein known variously as Bat3, Bag6 or Scythe, regulates suppression of T cell responses by Tim-3 (46). One of the more intriguing findings reported in this paper was that knock-down of Bat3 led to a dramatic up-regulation of Tim-3 and other (although not all) phenotypic and functional “markers” of T cell exhaustion. The authors also identified an interaction between Bat3 and a pool of the Lck tyrosine kinase that was phosphorylated on its activation loop tyrosine (Y394), but not the inhibitory tyrosine near the C-terminus (Y505), suggesting a preferential interaction of Bat3 with active Lck. Furthermore, this interaction appeared to be inhibited by antibody ligation of Tim-3, which itself was also seen to interact with Lck (46). The latter finding is consistent with our own observation that Tim-3 could interact with Lck, as well as with the related Src family tyrosine kinase Fyn (35). Importantly, we have confirmed that the T cell lines used in our signaling experiments do express Bat3 (L.P.K., unpublished data). These results suggest a possible mechanism for inhibition of proximal TCR signaling by Tim-3 and Bat3. It should also be noted that this study did not address the effects of TCR signaling on the Tim-3/Bat3 interaction. Thus, further investigation will be necessary to determine the precise relationship between these different pools of Tim-3 and Src family kinases, as well as the consequences on downstream signaling pathways, in exhausted T cells.
Another recent study examined the interaction of Tim-3 with transmembrane proteins expressed on T cells, and reported that Tim-3 can associate with the transmembrane phosphatases CD45 and CD148, which the authors proposed might promote de-phosphorylation of downstream mediators of T cell activation (44). Furthermore, Tim-3, CD45 and CD148 were all found to be recruited into the immunological synapse. Such a mechanism could help explain the observed negative effects of Tim-3 on T cell activation during exhaustion. It remains to be seen to what extent these various interactions affect Tim-3 function, including whether the interaction of these phosphatases with Tim-3 modulates its interaction with Bat3 or Lck, as discussed above.
TCR signaling and the development of T cell exhaustion
T cell exhaustion appears to be maintained by transcriptional re-programming (e.g. through BATF) and/or active negative signaling through receptors like PD-1 (2). Indeed, tyrosine phosphatases like SHP1/2 have been shown to mediate dominant suppression of TCR signaling, after ligation of PD-1 (47). However, it is quite clear that development of T cell exhaustion is driven, at least in part, by high levels - and/or the sustained presence - of cognate antigen (48, 49). Evidence (much of it still indirect) has begun to emerge regarding how this process may be controlled by TCR signaling pathways. Several studies have now implicated Akt- and/or mTOR-dependent signaling in this checkpoint. Thus, a study from Kaech and colleagues demonstrated that ectopic expression of Akt led to reduced memory T cell formation (50). In addition, Cantrell and colleagues showed that while inhibition of Akt impairs the function of CTL effector cells, it actually promotes development of memory cells (51). Similar conclusions were also reached in a study by Suresh and colleagues (52). Among the many downstream effects of Akt signaling is the activation of an mTOR-containing complex known as mTOR complex 1 or mTORC1 (53). Consistent with the above data, several groups have demonstrated that limiting the activation of mTOR enhances development of T cell memory. This has been shown through the use of the relatively specific mTORC1 inhibitor rapamycin (54, 55) or by reducing expression of the Raptor gene, which encodes a critical subunit of the mTORC1 complex (54).
These previous studies on Akt and mTOR in T cell memory are also intriguing in light of a recent report describing a human immunodeficiency associated with activating mutations of the PI3K catalytic protein p110δ, with consequent hyper-activation of downstream Akt and mTOR signaling (56). Of relevance for the discussion of T cell exhaustion, patients with these mutations present with an accumulation of terminally differentiated T cells. These T cells are refractory to stimulation with mitogen, and display a deficient recall response to tetanus toxoid, consistent with a defect in generation of stable memory T cell responses. It should be noted that patients with the activating p110δ mutations have a combined immunodeficiency, since B cells are also affected. Strikingly, treatment of one of the affected patients with the mTORC1 inhibitor rapamycin partially restored the T cell compartment and improved clinical outcomes (56).
The studies discussed above provide compelling evidence that excessive and/or sustained activation of one or more signaling pathways is critical for driving the development of T cell exhaustion. However, it is still not known whether such signals are initiated solely from the TCR itself, or whether other molecules might also contribute. Given what is known about the multi-factorial regulation of T cell activation by co-stimulatory receptors, the latter seems more likely. Based on our previous findings demonstrating the ability of Tim-3 to enhance TCR signaling under acute conditions (35), we propose that Tim-3 might function at least in part to help drive T cell exhaustion, by enhancing TCR/CD28-dependent signaling (Fig. 2). In such a scenario, upregulation of Tim-3 during an extended effector phase of T cell activation would act in a feed-forward loop to further enhance T cell activation signals, and drive T cells even more towards an exhausted phenotype, at the expense of T cell memory (Fig. 2A). An additional non-exclusive possibility is that positive signals from Tim-3 might positively signal to augment the (suppressive) function of Treg, contributing to the overall inhibitory in vivo effects of Tim-3 antibodies. With respect to the specific signaling pathways downstream of Tim-3, work described above suggests that at least one such pathway is the PI3K/Akt/mTOR pathway, with upstream involvement of Src or Tec kinases, and downstream involvement of transcription factors like NFAT or NF-κB (Fig. 2B). Coming back to the role of signaling in driving T cell exhaustion (discussed above), we propose that an accessory receptor like Tim-3 could help drive this process in part through enhanced PI3K/Akt/mTOR signaling.
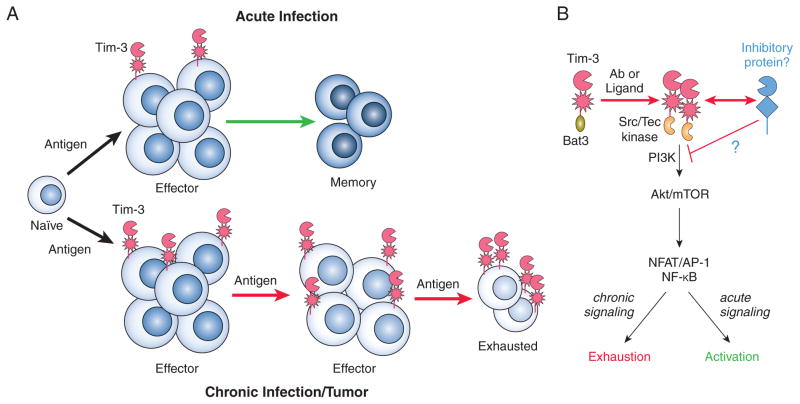
Figure 2.
Speculative model for development of T cell exhaustion. (A – Top) Acute infection results in elimination of a pathogen (and its antigens) and allows for the formation of a pool of memory T cells. This response may be accompanied by transient and/or low level expression of Tim-3. (A – Bottom) Establishment of a chronic infection (or significant tumor burden) promotes the development of T cell exhaustion, marked in part by upregulation of Tim-3. This process may be accelerated by Tim3-derived signals that enhance TCR/CD3 signaling in the short-term. In addition, these positive signals might contribute to the “reversal” of exhaustion by some Tim-3 mAb’s. (B) Tim3-derived “positive” signals that may contribute to the induction of T cell exhaustion or activation. These positive signals could contribute to the reversal of exhaustion by some Tim-3 antibodies, although the latter could be the result of qualitatively different signals or blocking of an as-yet-undefined inhibitory co-receptor.
As discussed above, it will be critical to more thoroughly define the biophysical and biochemical properties of the various Tim-3 antibodies being used in both mouse and human studies. Thus, it is still a distinct possibility that some antibodies might be acting to enhance signaling in T cells (or other cell types). In this regard, it is interesting to note that the efficacy of mAb’s targeting CTLA-4 for immunotherapy of melanoma, at least in mouse models, was just recently attributed to FcR-dependent depletion of Treg (57, 58), despite two decades of study of CTLA-4 biology. Finally, the unintended consequences of antibody manipulation of CD28 offer a cautionary tale with regard to the clinical translation of a target that regulates T cell activation (59–62).
Conclusions
Recent investigation into Tim-3 function has clearly elucidated its importance, in part due to its frequent up-regulation during antiviral T cell responses or in the tumor microenvironment. Pre-clinical studies in mouse models are also encouraging. However, these studies are generally restricted by a lack of suitable reagents to clarify the significance and function of a putatively stimulatory signal on a population of “exhausted” T cells. These gaps in the field are slowly being rectified, and must be accelerated as therapeutic manipulation in patients is being seriously contemplated.
Acknowledgments
Funding: Work in the investigators’ laboratories is supported by grants DE019727 and CA097190 (to R.L.F.), CA167229 and CA097190 (to B.L.) and AI109605 and AI073748 (to L.P.K.).
References
- 1.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 3.Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. Journal of immunology. 2012;188:2957–2965. doi: 10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends in immunology. 2011;32:345–349. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Advances in immunology. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 6.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends in immunology. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. The Journal of clinical investigation. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. The Journal of clinical investigation. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer research. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 15.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. The Journal of experimental medicine. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC. TIM3FOXP3 regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2:e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PloS one. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. Journal of virology. 2011;85:4184–4197. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelosanto JM, Wherry EJ. Transcription factor regulation of CD8+ T-cell memory and exhaustion. Immunological reviews. 2010;236:167–175. doi: 10.1111/j.1600-065X.2010.00927.x. [DOI] [PubMed] [Google Scholar]
- 20.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 23.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS pathogens. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Current opinion in immunology. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. The Journal of experimental medicine. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, Palmer BE. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. Journal of immunology. 2010;185:3007–3018. doi: 10.4049/jimmunol.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Palmer BE. Suppression of HIV replication by antiretroviral therapy reduces TIM-3 expression on HIV-specific CD8(+) T cells. AIDS research and human retroviruses. 2011;27:1–3. doi: 10.1089/aid.2010.0156. [DOI] [PubMed] [Google Scholar]
- 29.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. Journal of virology. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. The Journal of clinical investigation. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, Behar SM. Tim3 binding to galectin-9 stimulates antimicrobial immunity. The Journal of experimental medicine. 2010;207:2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sada-Ovalle I, Chavez-Galan L, Torre-Bouscoulet L, Nava-Gamino L, Barrera L, Jayaraman P, Torres-Rojas M, Salazar-Lezama MA, Behar SM. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. Journal of immunology. 2012;189:5896–5902. doi: 10.4049/jimmunol.1200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu Y, Chen J, Liao H, Zhang Y, Wang H, Li S, Luo Y, Fang D, Li G, Zhou B, Shen L, Chen CY, Huang D, Cai J, Cao K, Jiang L, Zeng G, Chen ZW. Tim-3-Expressing CD4(+) and CD8(+) T Cells in Human Tuberculosis (TB) Exhibit Polarized Effector Memory Phenotypes and Stronger Anti-TB Effector Functions. PLoS pathogens. 2012;8:e1002984. doi: 10.1371/journal.ppat.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorman JV, Starbeck-Miller G, Pham NL, Traver GL, Rothman PB, Harty JT, Colgan JD. Tim-3 directly enhances CD8 T cell responses to acute Listeria monocytogenes infection. Journal of immunology. 2014;192:3133–3142. doi: 10.4049/jimmunol.1302290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, Smithgall TE, Kuchroo VK, Kane LP. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Molecular and cellular biology. 2011;31:3963–3974. doi: 10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han G, Chen G, Shen B, Li Y. Tim-3: An Activation Marker and Activation Limiter of Innate Immune Cells. Frontiers in immunology. 2013;4:449. doi: 10.3389/fimmu.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 38.Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nature immunology. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Jia ZS, Moorman JP, Yao ZQ. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PloS one. 2011;6:e19664. doi: 10.1371/journal.pone.0019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. Journal of leukocyte biology. 2012;91:189–196. doi: 10.1189/jlb.1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, Zhou T, Li Y, Guo X, Xiao H, Hou C, Wang R, Lin Z, Li X, Feng J, Ma Y, Shen B, Li Y, Han G. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. Journal of immunology. 2013;190:2068–2079. doi: 10.4049/jimmunol.1202661. [DOI] [PubMed] [Google Scholar]
- 43.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochemical and biophysical research communications. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 44.Clayton KL, Haaland MS, Douglas-Vail MB, Mujib S, Chew GM, Ndhlovu LC, Ostrowski MA. T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases. Journal of immunology. 2014;192:782–791. doi: 10.4049/jimmunol.1302663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nature reviews Immunology. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 46.Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, Okada H, McKinnon PJ, Mak TW, Addo MM, Anderson AC, Kuchroo VK. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nature medicine. 2012;18:1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. Journal of immunology. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 48.Richter K, Brocker T, Oxenius A. Antigen amount dictates CD8+ T-cell exhaustion during chronic viral infection irrespective of the type of antigen presenting cell. European journal of immunology. 2012;42:2290–2304. doi: 10.1002/eji.201142275. [DOI] [PubMed] [Google Scholar]
- 49.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, Feijoo C, Okkenhaug K, Cantrell DA. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. Journal of immunology. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, Stoddard J, Ouyang W, Frucht DM, Rao VK, Atkinson TP, Agharahimi A, Hussey AA, Folio LR, Olivier KN, Fleisher TA, Pittaluga S, Holland SM, Cohen JI, Oliveira JB, Tangye SG, Schwartzberg PL, Lenardo MJ, Uzel G. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nature immunology. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. The Journal of experimental medicine. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, Burns C, Thorpe R, Stebbings R. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. British journal of pharmacology. 2010;161:512–526. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horvath C, Andrews L, Baumann A, Black L, Blanset D, Cavagnaro J, Hastings KL, Hutto DL, MacLachlan TK, Milton M, Reynolds T, Roberts S, Rogge M, Sims J, Treacy G, Warner G, Green JD. Storm forecasting: additional lessons from the CD28 superagonist TGN1412 trial. Nature reviews Immunology. 2012;12:740. doi: 10.1038/nri3192-c1. author reply 740. [DOI] [PubMed] [Google Scholar]
- 61.Hunig T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nature reviews Immunology. 2012;12:317–318. doi: 10.1038/nri3192. [DOI] [PubMed] [Google Scholar]
- 62.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. The New England journal of medicine. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]