Abstract
Background
Research has demonstrated a consistent relationship between early sexual experience and subsequent sexual risk-taking behaviors. We hypothesized that this relationship is due to a general predisposition towards behavioral disinhibition (BD), and that relationships among BD, early sex, and subsequent risky sexual behavior may be influenced by common genetic influences for males and common environmental influences for females.
Methods
A prospective sample of 1,512 same-sex adolescent twins (50.2% female) was used. Adolescent BD was measured by clinical symptom counts of conduct disorder, oppositional defiant disorder, and self-reported delinquent behavior (age 14). Age of sexual initiation was defined as first age of consensual oral or penetrative sex (mean age ~17). Adult risky sexual behavior was defined by sexual behaviors under the influence of drugs and alcohol and number of casual sexual partners in the past year (age 24).
Results
Multivariate analyses showed evidence for substantial common genetic variance among age 14 BD, age at sexual initiation, and adult risky sexual behavior for males, but not females. There was no significant difference in the degree of common environmental influence on these variables for females compared to males. Notably, age of sexual initiation was not significantly correlated with age 24 risky sexual behavior for females.
Conclusion
The relationship between early sex and later risky sex can be better understood through a general liability towards BD, which is influenced primarily by genetic factors for males. The association between age 14 BD and age of sexual initiation was influenced through a combination of genetic and environmental factors for females; however, age of sexual initiation does not appear to be a salient predictor of adult women’s sexual risk-taking behavior. Findings suggest that prevention programs aimed at reducing sexual risk behavior might target youth exhibiting BD by age 14, particularly males. More research is needed on what predicts adult sexual risk-taking behavior for females.
Keywords: Behavior genetics, Behavioral disinhibition, Externalizing disorder, Gender differences, Sexual behavior
Introduction
Previous research has demonstrated a consistent association between age of sexual initiation and subsequent risky sexual behavior (Sandfort, Orr, Hirsch, & Santelli, 2008; Wellings, Nachahal, Macdowall, McManus, Erens, Mercer, et al., 2001). There is growing evidence that these two variables may be related because of a general liability to externalizing behaviors and impulsivity (Boislard & Poulin, 2011; Cooper, Wood, Orcutt, & Albino, 2003; Donohew, Zimmerman, Cupp, Novak, Colon, & Abell, 2000; Kahn, Kaplowitz, Goodman, & Emans, 2002). In fact, previous studies (Boislard & Poulin, 2011; Epstein, Bailey, Manhart, Hill, & Hawkins, 2013; Ramrakha, Bell, Paul, Dickson, Moffitt, & Caspi, 2007) have reported that the strongest predictors of both early sexual initiation and subsequent risky sexual behavior were antisocial behavior and behavioral disinhibition (BD, defined as an inability to constrain impulses or social undesirable behavior; Iacono, Malone, & McGue, 2008). These findings follow Iacono et al.’s (2008) model, which proposes that a genetic liability to BD accounts for the developmental progression of a variety of specific externalizing attributes, such as early initiation of substance use and later co-occurring substance use disorders. In this study, we evaluate whether this hypothesis applies to early sexual initiation and subsequent risky sexual behavior.
There is some genetically-informed research that has addressed whether the experience of having sex at an early age has a causal influence on the likelihood of sexual behavior and related psychopathology in young adulthood (Donahue, Lichtenstein, Långström, & D’Onofrio, 2012; Huibregste, Bornovalova, Hicks, McGue, & Iacono, 2011; Kiselica, Cummings, Bornovalova, Samek, McGue, & Iacono, 2013). For example, Huibregste et al. (2011) utilized a longitudinal discordant twin design and found that a twin who had sex early (before age 16) did not report more sexual risk behavior at age 24 than the co-twin who did not have early sex. Other research has shown that twins discordant on early sex were not more likely to be discordant on other externalizing attributes, such as substance use disorders and criminal history (Donahue, et al., 2012). Together, these findings suggest the link between early sex and later externalizing behaviors (including sexual risk behavior) is not causal, but is instead due to genetic and environmental factors common to both.
What is not known is how genetic and environmental influences affect the developmental progression of sexual risk-taking behavior, such as whether the predisposition for BD gives rise to early sexual initiation and subsequent sexual risk-taking through primarily common genetic or environmental influences. Following Iacono et al.’s (2008) model, we hypothesized that the genetic and environmental influences evident for BD in adolescence (age ~14) would influence both early age of sexual initiation and subsequent risky sexual behavior in young adulthood (age ~24). An additional gap we aimed to fill was to evaluate potential gender differences in the relationship among BD, early sex, and later risky sex. The etiology behind these relationships may be different for men and women; for example, there is some evidence (Hicks, et al., 2007) that the genetic influences on BD become increasingly more important for men as they transition from adolescence into adulthood, whereas environmental influences become increasingly important for women. Following this, we hypothesized that common genetic influences would be important in the developmental progression from early adolescent BD, early age of sexual initiation, and subsequent adult risky sexual behavior for men, but that for women, common environmental influences would predominate.
Methods
Participants
An epidemiologic sample of same-sex twins from the Minnesota Twin Family Study, initially assessed at age 11, was used for this investigation (Iacono, Carlson, Taylor, Elkins, & McGue, 1999). Twins born between the years of 1977 and 1984 were recruited to participate. Families were identified from birth certificates made public in the state of Minnesota and located through a variety of public databases (e.g., internet searches). At intake, ~90% of twin families were successfully located and contacted to determine study eligibility. To participate, families had to live within driving distance of the university, neither twin could have a mental or physical handicap precluding participation, and twins could not have been adopted by nonrelatives (determined by a pre-screen telephone interview with one parent, usually the mother). About 17% of the eligible families declined to participate. The majority of participating parents were married (79%) and of European ancestry (97%), consistent with Minnesota demographics in the relevant birth years. Mothers were on average 39.3 years (SD = 4.6) and fathers were on average 42.0 years (SD = 5.3). The majority of parents had a high school degree/GED or higher (97% mothers, 96% fathers). A moderate proportion of parents had a bachelor’s degree (23% mothers, 21% fathers) and a small number had a professional degree (3% mothers, 6% fathers).
A total of 1,512 children completed the first intake assessment (756 twin pairs, 50.2% female). On average, participants were 11.7 years old at the first assessment (SD = .43). Following intake, twins were invited to participate in follow-up assessments approximately every 3 years. A total of 93% of eligible twins from the first assessment participated in the age 14 assessment (M age = 14.8, SD = .53), and 87% or more participated in the age 17 (M age = 18.2, SD = .70) and subsequent assessments (age 20 assessment M age = 21.5, SD = .82; age 24 assessment M age = 25.29, SD = .74). BD levels were assessed at age 14, adult sexual risk behavior was measured at age 24, and age of sexual initiation was measured throughout all assessments. Twins included 486 monozygotic (MZ) and 270 dizygotic (DZ) pairs. All sets of twins were same-sex (253 MZ males, 233 MZ females, 123 DZ males, 147 DZ females). Zygosity was determined at intake by a questionnaire administered to parents concerning the resemblance of twin pairs, anthropometric measurements, and an algorithm comparing twins on fingerprint ridge counts; if results were not in agreement for these three measures, DNA was analyzed to resolve zygosity.
Notably, we are using the same sample that was used by Huibregste et al. (2012). Huibregste et al. analyzed the relationship between early sex and adult risky sex using a discordant twin analysis, and found that having sex early was not an environmental experience that had a causal influence on subsequent sexual behavior in adulthood. Our manuscript extends Huibregste’s work (and others) by using a multivariate, longitudinal analysis of twin data to evaluate whether the genetic liability for BD present at age 14 can account for the association between age of sexual initiation and subsequent risky sexual behavior in adulthood. Of additional significance, we evaluated for gender differences in the nature of the genetic and environmental influences governing the linkage between BD and subsequent sexual behavior.
Procedure
All adult participants provided written informed consent. Minor children provided written assent, and a parent provided written consent on their child’s behalf. This project was approved by the University of Minnesota’s Institutional Review Board. If twins were not able to participate in an in-person follow-up assessment, they were interviewed by phone and completed all materials except the computerized assessments. Approximately 8% of participants were interviewed by phone at the age 14 assessment and 16% at the age 24 assessment.
Measures
Age 14 BD
DSM-IIIR symptoms of Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD), as well as self-reported delinquency (the Delinquent Behavior Inventory [DBI]; Gibson, 1967) were used to measure BD at age 14. ODD and CD were assessed using the Diagnostic Interview for Children and Adolescents-Revised (DICA-R; Welner, Reich, Herjanic, Jung, & Amado, 1987). Both child and parent (usually the mother) were interviewed about the presence of ODD and CD symptoms in the child. All interviews were reviewed by at least two individuals with advanced clinical training, blind to the diagnoses of other family members, who coded by consensus, every DSM-IIIR symptom and diagnostic criterion. We utilized a best-estimate approach so that if either the child or parent reported a symptom, the symptom was considered present (kappas > .74 for all clinical disorder diagnoses). The DBI is a self-reported, 36-item inventory of delinquent behaviors, e.g., “cutting classes at school,” “smashing, slashing, or damaging things” (α = .91 for males, α = .86 for females).
ODD, CD, and DBI scores were log transformed to better approximate normality assumptions, and then used to estimate a latent factor of age 14 BD using Mplus (Muthén & Muthén, 1998–2013); the maximum likelihood estimator with robust standard errors (MLR) was used and individuals were clustered by family id. As shown in parentheses, standardized loadings suggested adequate reliability of the factor: age 14 BD = CD (.89), ODD (.71), DBI (.70). Also demonstrating good reliability of the factor, the factor score determinacy value was .92 (range 0–1, 1 being the best value). The estimated factor score was exported for the multivariate biometric decomposition analysis.
Age of sexual initiation
Age at first sex was assessed using reports from the Life Events Interviews (LEI) and the Sexual Behavior Inventory (SBI) at the age 24 assessment (Huibregste, et al., 2011; Kiselica, et al., 2013). Both assessments captured whether the individual had ever had sex (SBI included oral or penetrative; LEI included penetrative only) and age at first occurrence of consensual sex. To deal with outliers and better approximate normality assumptions, initiation at age 13 or younger was coded as 5; age 14–15 as 4; age 16–18 as 3; age 19–22 as 2; and age 23 or older as 1.
Sexual Risk Behavior at age 24
Sexual risk behavior was measured using self-report items from the SBI at the age 24 assessment. Three indicators were used to measure risky sex (Kiselica, et al., 2013), including frequency of 1) oral and 2) penetrative sex with casual partners in the past 12 months (1 = None, 2 = 1–2, 3 = 3–5, 4 = 6–9, 5 = 10–19, 6 = 20+), and the 3) frequency of engaging in sexual behavior under the influence of alcohol and drugs in the past 12 months (α = .78; e.g., “In the last 12 months, I have had unprotected sex (not used a condom) because I was drinking or using drugs”) answered on a scale of 1 = Never, 2 = 1–2 times, 3 = 3–10 times, 4 = More than 10 times. These three indicators were log-transformed to better approximate normality assumptions and were then standardized and averaged to create the age 24 sexual risk behavior composite (α = .84 for males, .76 for females).
Attrition
We found that those missing age 24 risky sex data had a significantly greater age 14 BD score (M = .09, SD = .32) compared to those with risky sex data (M = −.02, SD = .24; p < .001). Follow-up analyses confirmed this was only true for males (missing data: M = .18, SD = .33; not missing: M = .07, SD = .26; p < .001), and not females (missing data: M = −.08, SD = .20; not missing M = −.10, SD = .18; p = .44). This suggests some bias in attrition for males (although minimal as the difference was less than ½ of one standard deviation).
Data Analysis
Our hypothesis was that age 14 BD would influence the age at sexual initiation and the degree of adult sexual risk-taking primarily through common genetic mechanisms for males and common environmental mechanisms for females. To test this, we conducted multivariate biometric decomposition using Mx software (Neale, 2006), which uses full information maximum likelihood (FIML) estimation to estimate missing data (shown to be superior over listwise, pairwise, or other maximum likelihood techniques; Enders & Bandalos, 2001).
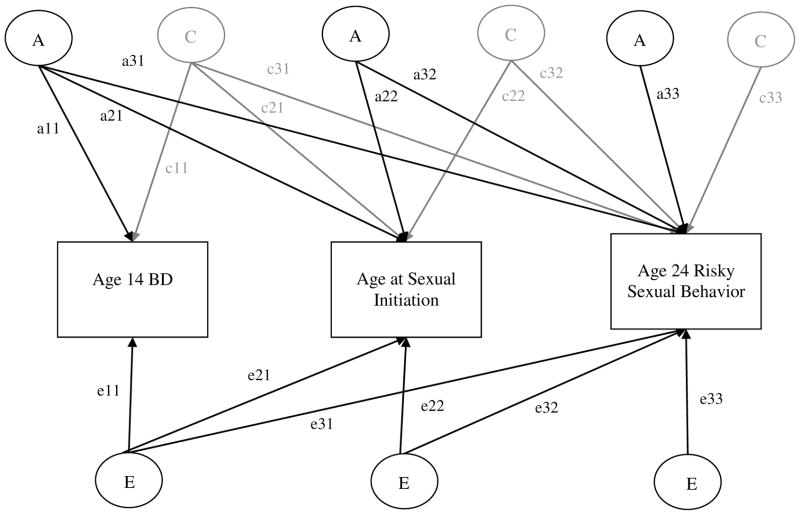
The conceptual model is diagrammed in Figure 1. Variables were entered to reflect the hypothesized developmental chronology (age 14 BD entered first, then age at sexual initiation, then age 24 risky sexual behavior); however, there were few cases (<6% of the sample) that reported sex before age 14. Model fitting makes use of the facts that MZ pairs share 100% of their DNA, DZ pairs share 50% of their DNA, and the assumption that environmental similarity does not vary by zygosity. Additive genetic effects (A) refer to the effect of all alleles added up over the course of the entire genome on twin similarity; A effects are present if rMZ > rDZ. Shared environmental effects (C) refer to the environmental causes of twin similarity; they are present if rDZ > 1/2rMZ. Nonshared environmental effects (E) cause members of a twin pair to be different from each other and also capture measurement error; E effects are present if rMZ < 1.0.
Figure 1. Standard Multivariate Biometric Decomposition.
BD = Behavior Disinhibition. This model represents the path diagram of the Cholesky decomposition. Variance of each variable (BD at 14, age at sexual initiation, adult risky sexual behavior) is decomposed into additive genetic effects (A1, A2, A3), shared environmental effects (C1, C2, C3, shown in gray for clarity of presentation), and non-shared environmental effects (E1, E2, E3). Path coefficients, represented by lowercase letters follow by two numbers (e.g., c11, c21) must be squared to estimate the proportion of variance accounted for. For example, a11, c11, and e11 are paths representing effects for BD only; a21, c21, and e21 are effects on BD that also influence age at sexual initiation; a31, c31, and e31 are effects of BD that also influence risky sexual behavior in adulthood.
As described in Figure 1, the total variance in, and covariance between variables are decomposed into components attributed to genetic (all “a” paths, e.g., a11, a21), shared environmental (all “c” paths, e.g., c11, c21), and nonshared environmental effects (all “e” paths, e.g., e11, e21). The proportions of A, C, or E variance accounted for in age 24 risky sexual behavior by age 14 BD and age at sexual initiation are calculated by standardizing the path coefficients, squaring them, and dividing by the total ACE variance. For example, to determine the proportion of additive genetic variance in risky sex explained by age 14 BD and age at sexual initiation, the standardized a31 and a32 coefficients should be squared, summed, and divided by the total additive genetic variance in risky sex [(a312 + a322)/(a312 + a322 + a332)]. Paths are statistically significant if the 95% confidence interval (CI) does not include zero.
Results
Preliminary Analyses
There were 117 (8.2%) participants who had not yet had sex (penetrative or oral) by the age 24 assessment (57 males, 60 females); for all analyses, we analyzed the data by excluding those who had not had sex by the FU4 assessment and also by using FIML to estimate data for those missing age at first sex (they were coded to the lowest categories for risky sex at age 24). We found the same pattern of results whether they were excluded or included in the analysis but were coded as missing on age at first sex using FIML. Therefore we reported the FIML results (which utilized the whole sample) to optimize statistical power.
Table 1 provides the means, standard deviations, and correlations for study variables. In general, age 14 BD, age at sexual initiation, and age 24 risky sex were significantly correlated for both males and females, with one exception: the correlation between age of sexual initiation and age 24 risky sex for females was not significant (r = .08, p = .06). Moreover, the correlations for all pairwise combinations were significantly greater for males than females (age 14 BD and age at first sex χ2 (1) = 11.90, p = .001; age of sexual initiation and risky sex χ2 (1) = 15.38, p < .001; age 14 BD and age 24 risky sex χ2 (1) = 6.29, p = .01). Additionally, males had higher mean scores on age 14 BD (χ2 (1) = 155.96, p < .001) and age 24 risky sex (χ2 (1) = 46.20, p < .001), but not age at first sex (χ2 (1) = .85, p = .85). Note, the average age at first sex for males was 17.66 years (SD = 2.59) and was 17.79 years (SD = 2.46) for females, which follows national statistics for this age cohort (Chandra, Martinez, Mosher, Jabma, & Jones, 2005).
Table 1.
Correlations, Means (M), and Standard Deviations (SD) among Study Variables across Gender
| 1 | 2 | 3 | M | SD | |
|---|---|---|---|---|---|
|
|
|||||
| 1. Age 14 BD | -- | .34 | .18 | −.09 | .18 |
| 2. Age at sexual initiation | .44 | -- | .08a | 17.79 | 2.46 |
| 3. Age 24 risky sex | .24 | .27 | -- | −.15 | .69 |
| M | .09 | 17.66 | .17 | -- | -- |
| SD | .29 | 2.59 | .99 | -- | -- |
NOTE: BD = behavioral disinhibition. Values for females are shown above the diagonal (n = 760), values for males are shown below the diagonal (n = 752). The raw mean M and SD of age of sexual initiation are given in the table for purposes of sample description; correlations used the scaled score (higher score indicates lower ages; females M = 2.80, SD = .82; males M = 2.85, SD = .86). All correlations were significant at p < .001, with the exception of a, p = .06.
Hypothesis Testing: Multivariate Biometric Results
Our main goal was to evaluate the genetic and environmental architecture of the developmental progression of adolescent BD leading to early sexual initiation and subsequent adult sexual risk-taking. We also evaluated gender differences with the expectation that common genetic influences would be important for this developmental progression for men, and that common environmental influences would predominate for women. We ran multivariate biometric decomposition analyses separately for males and females then tested for significant differences across gender utilizing multigroup modeling with the chi-square difference test.
The multivariate analysis also provides the ACE results for each of the three variables; these results are presented in Table 2. Three important findings emerge from this analysis. First, for both males and females, age 14 BD, age of sexual initiation, and risky sex at age 24 were all moderately heritable, with heritabilities ranging from .29 to .50. Second, all the effects for nonshared environmental influence were also significant (ranging from .23 to .70). Third, for the two variables measured in adolescence (age 14 BD and age of sexual initiation), significant shared environmental effects were found, with coefficients ranging from .23 to .42. For age 24 risky sexual behavior, the effect of shared environment is negligible, producing values of .01 and .05 for males and females. These results are in line with expectation given other studies that show the effect of the shared environment for many behavioral measures is stronger in adolescence than in adulthood.
Table 2.
Proportions of Genetic and Environmental Variance across Gender
| Total Proportion of Variance
|
|||
|---|---|---|---|
| Age 14 BD | Age at Sexual Initiation | Age 24 Risky Sexual Behavior | |
|
|
|||
| Males | |||
| A | .35 * (.21, .53) | .36* (.14, .55) | .29* (.11, .39) |
| C | .42* (.24, .56) | .23* (.06, .42) | .01 (.00, .15) |
| E | .23* (.20, .27) | .42* (.36, .49) | .70* (.61, .80) |
| Females | |||
| A | .50* (.36, .65) | .31* (.09, .54) | .38* (.23, .50) |
| C | .25* (.11, .38) | .28* (.06, .47) | .05 (.00, .14) |
| E | .25* (.21, .29) | .42* (.36, .48) | .57* (.48, .67) |
NOTE: BD = Behavioral Disinhibition, A = Additive Genetic Influence, C = Shared Environmental Influence, E = Nonshared Environmental Influence. Standardized coefficients are presented, as well as 95% confidence intervals (in parentheses). Significance denoted by the 95% confidence interval not bracketing zero and by * p < .05. No significant differences were found in ACE estimates across gender, all χ2 < 3.85 on 1 degree of freedom.
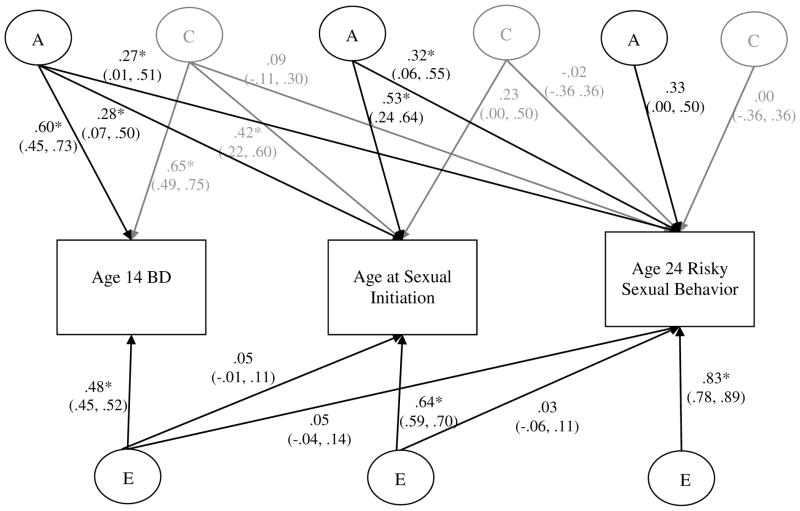
For males (Figure 2), a key question was whether the additive genetic influence on BD was shared with age at sexual initiation and age 24 risky sex. Inspection of paths a11, a21, and a31 reveals they were. Squaring the standardized path coefficient and dividing by the total A, C, or E influence (given in Table 2) gives the proportion of ACE variance explained. The genetic influence of age 14 BD accounted for 22% of the variance in age at sexual initiation, and 25% of the variance in age 24 risky sex. Age at sexual initiation accounted for an additional 37% of the genetic variance in age 24 risky sex. Notably, the residual genetic influence on age 24 risky sex was not significantly different from zero (path a33), suggesting substantial common genetic influences among age 14 BD, age at sexual initiation, and age 24 risky sex. Shared environmental influences were also evident in the relationship between age 14 BD and age at sexual initiation (path c21, 77% of shared environmental influences explained), but not in the relationship between age 14 BD and age 24 risky sexual behavior (path c31 was not significant). Notably, although 70% of the variance in age 24 risky sex was attributable to the nonshared environment (see Table 2), almost all of this nonshared effect (98%) was due to novel environmental influences present only at age 24.
Figure 2. Standardized Coefficients from the Multivariate Biometric Decomposition: Males.
BD = Behavior Disinhibition. The variance within and the covariance amongst study variables are decomposed into genetic (A), shared environmental (C, shown in gray for clarity of presentation) and nonshared environmental components (E). Ninety-five percent confidence intervals (CIs) are also presented. Significant paths are indicated by CIs not crossing zero and also by * p < .05. Paths are squared and summed to determine the proportion of variance explained.
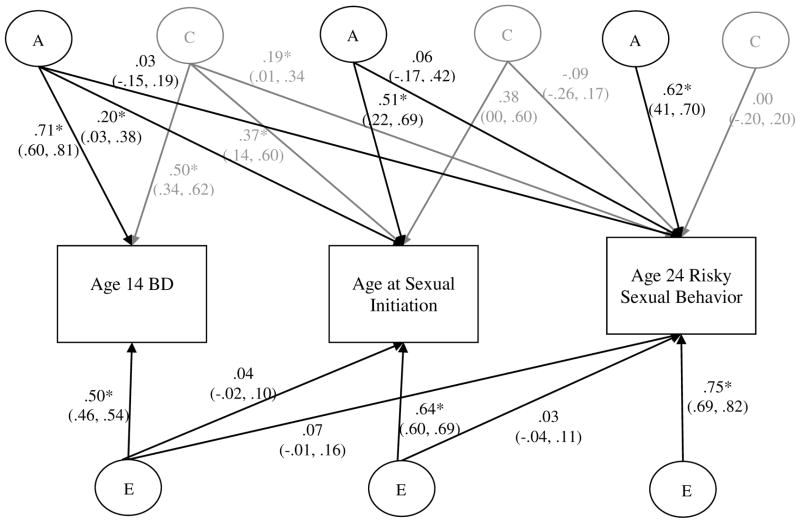
A different pattern of results was observed for females (see Figure 3). While genetic influences on age 14 BD were common to age at sexual initiation, they were not common to age 24 risky sex (see a11, a21, and a31 paths). Path a33 shows there was a large residual genetic influence on age 24 risky sex; thus, the majority (99%) of the genetic influence on age 24 risky sex was due to novel genetic influences present at only age 24. The same large residual effect was found for nonshared environmental influences on age 24 risky sex (path e33, a similar effect as was found in males). Unlike males, and as expected, there were common shared environmental influences on age 14 BD, age at sexual initiation, and age 24 risky sex (see paths c11, c21, and c31). The shared environmental influence of age 14 BD accounted for 48% of the variance in age at sexual debut and 82% of the variance in age 24 risky sex. Age at sexual initiation did not account for significant unique sources of genetic influence on age 24 risky sexual behavior (as evident by the non-significant a32 path). However, only 5% of the total variance in age 24 risky sex was explained by shared environmental influences (see Table 2), suggesting a trivial influence of the shared environment in the overall etiology of age 24 risky sex.
Figure 3. Standardized Coefficients from the Multivariate Biometric Decomposition: Females.
BD = Behavior Disinhibition. The variance within and the covariance amongst study variables are decomposed into genetic (A), shared environmental (C, shown in gray for clarity of presentation) and nonshared environmental components (E). Ninety-five percent confidence intervals (CIs) are also presented. Significant paths are indicated by CIs not crossing zero and also by * p < .05. Paths are squared and summed to determine the proportion of variance explained.
We next tested for significant differences in these coefficients and proportions of ACE variance accounted for across gender. As expected, age 14 BD and age at sexual debut combined explained a substantial proportion of genetic variance in age 24 risky sex for males (.61, 95% CI: .17, 1.0), but not females (.01; 95% CI: .00, .50), and this difference was significant (χ2 (1) = 3.89, p = .049). On the other hand, there was no difference in the proportion of shared environmental influence accounted for by age 14 BD and age at sexual debut in age 24 risky sex across gender (χ2 (1) = .00, p = 1.0). For both males and females, 100% of the shared environmental influence on age 24 risky sex was explained by the combination of age 14 BD and age at sexual debut; however, only 1–5% of the total variance in age 24 risky sex was attributed to the shared environment (as shown in Table 2, column 3). Therefore, explaining 100% of this influence is somewhat trivial. There was also no significant difference in the proportion of nonshared environmental influence explained in age 24 risky sex by age 14 BD and age at sexual initiation (about 1% explained for both males and females, χ2 (1) = .23, p = .63).
To summarize, the hypotheses were partially supported; findings suggest a greater degree of common genetic influence on the relationships between age 14 BD, age at sexual debut, and adult risky sexual behavior for males compared to females. Results do not suggest environmental mechanisms are more important in this developmental progression for females compared to males.
Discussion
Following previous research (Cooper, et al., 2003; Donohew, et al., 2000; Harden, Mendle, Hill, Turkheimer, & Emery, 2008; Huibregste, et al., 2011), and Iacono et al.’s (2008) developmental model of BD, study findings suggest that general liability to BD present at age 14 predisposes an individual to both an earlier age of sexual initiation and subsequent adult sexual risk-taking, at least for males. The present report also indicates that the relationships among BD, age at sexual initiation, and adult risky sexual behavior are predominantly due to common genetic influences for males, but not females. While we found significant shared environmental influence in these relationships for females, they were not significantly different from males, and the magnitude of the effect was quite small. Thus, hypotheses were only partially supported. In total, results suggest that early sex is not the beginning of a causal chain that ends with subsequent sexual risk-taking behavior, but rather is a part of a predisposition towards BD that is manifested as early as age 14, particularly for males.
It seems likely that gender differences in the proportion of genetic variance explained is a result of differences in the zero-order correlations among age 14 BD, age of sexual initiation, and age 24 risky sexual-behavior for males versus females. Notably, the correlation between age 14 BD and age of sexual initiation was significant and moderately correlated for both males and females; however, the correlation between age of sexual initiation and adult risky sexual behavior was not significantly different from zero for females. We were surprised by this finding, as it suggests a developmental progression between age 14 BD and age of sexual initiation (as hypothesized), but not one that continues into adult sexual risk-taking behavior for females. It is possible that dispositional and behavioral traits other than BD are relevant to the developmental progression of sexual risk-taking behaviors for females (e.g., negative emotionality, age of first committed romantic relationship). Additionally, it may be that the hypothesized developmental progression of age 14 BD leading to earlier age of sexual initiation and subsequent sexual risk-taking behavior is more relevant for women earlier or later in their development rather than at age 24. Additional research is needed to investigate these hypotheses.
Study findings indicate that prevention and intervention efforts aimed at reducing risky sexual behaviors may be more successful if they target individuals that are at most at risk (i.e., those high in BD at age 14), rather than targeting early sex alone (e.g., abstinence only programs). For example, Multisystemic therapy (MST; Henggeler, Schoenwald, Borduin, Rowland, & Cunningham, 2009) is based on a social ecology framework (Brofenbrenner, 1979) and suggests interventions across multiple “systems,” including the individual, family, and community levels are particularly important for adolescents that are most at risk for externalizing problems. MST aims to foster a greater degree of parent-child and family bonding, mitigate effects of affiliation with deviant peers, and increase engagement in school and extra-curricular activities. In general, MST has been shown to be quite effective in reducing juvenile recidivism and substance use (Curtis, Ronan, & Borduin, 2004; Henggeler, 2011), even continuing into adulthood (e.g., up to 21 years later; Sawyer & Borduin, 2011; Schaeffer & Borduin, 2005). Other systemic based interventions (e.g., Communities That Care; Hawkins, Oesterle, Brown, Monahan, Abbott, Arthur, et al., 2012) have been shown to reduce BD in adolescence, as well, and may likely impact long term sexual-risking taking and related externalizing behaviors.
It should be noted that while early age of sexual initiation has been linked to problem behaviors such as early adolescent substance use (McGue & Iacono, 2005) and alcohol use disorders (Bailey, Pollock, Martin, & Lynch, 1999; Kahn, Berger, Wells, & Cleland, 2012), sexual initiation is indeed a normative part of human development (Tolman & McClelland, 2011). Benefits of sexual initiation include affirmation of love and commitment and achieving a greater degree of intimacy with a committed romantic partner, which are indicative of healthy development. For example, Harden & Mendle (2011) showed that for older adolescents, while sexual activity in a non-committed relationship was associated with a greater degree of delinquency, sexual activity that occurred in committed romantic relationships was associated with a lower degree of delinquency.
There are limitations to this study. While our community sample was representative of the region from which it was sampled (Holdcraft & Iacono, 2004; Iacono, et al., 1999), participants were predominately of European ancestry. Studies are needed to determine if the results will generalize to other populations (e.g., age at first sexual initiation varies substantially across ethnicity; Cavazos-Rehg, et al., 2009). As expected in a community sample, BD was less pronounced among females than males. Also, males who scored high in BD at age 14 were less likely to participate at the age 24 assessment, perhaps reducing the likelihood of our finding effects in males. However, we obtained strong results for the male sample, suggesting that attrition effects were unlikely to play an important role in our findings. Additionally, while not evaluated here, it may be that alternative mechanisms of gene-environment interplay are important in the developmental progression of BD, age at first sex, and risky sex (e.g., gene-environment interaction). Finally, while our measure of sexual risk behavior included multiple aspects (oral and penetrative sexual partners, sex under the influence of drugs and alcohol), it may have been beneficial to have measured more detailed sexual history (e.g., frequency of condom use, number of sexually transmitted infections). Strengths of the study include the use of a large, genetically-informed twin design, the use of prospective data through age 24, the inclusion of both genders, and the evaluation of gender differences.
Conclusion
This study builds on previous research (Donahue et al., 2012; Huibregste, et al., 2011) and theory (Iacono, et al., 2008) to show that genetic and environmental influences on BD help explain the developmental progression of early age of sexual initiation and subsequent adult sexual risk-taking behaviors, particularly for males. Considering the present study’s findings along with research showing that abstinence only programs have generally failed to work (Kirby, 2008; Manlove, Papillio, & Ikramullah, 2004; Santelli, Ott, Lyon, Rogers, Summers, & Schleifer, 2006), it may be that allocating abstinence only funds to cover in addition MST or other systemic interventions could be effective in reducing both risky sex and co-occurring externalizing behaviors. Our results suggest that prevention efforts would be optimized if sex education was coupled with systemic interventions aimed at reducing a wide variety of externalizing behaviors, including early age of sexual initiation and risky sexual behaviors.
Key Points.
Growing evidence suggests that early age of sexual initiation and subsequent sexual risk-taking behaviors are linked as a result of a general liability to behavioral disinhibition. Less research has evaluated for the developmental etiology of these relationships, as well as potential gender differences in this etiology.
The present study found evidence for substantial shared genetic variance among age 14 BD, age of sexual initiation, and age 24 sexual risk-taking behaviors for males, but not females.
Results suggest that early age of sexual initiation is not the beginning of a causal chain that ends with subsequent sexual risk-taking behavior, but rather is a part of a predisposition towards BD that is manifested as early as age 14, particularly for males.
Taken together with previous research, study findings suggest that prevention programs aimed at reducing sexual risk behavior might target youth exhibiting BD by age 14, rather than focusing on abstinence alone. This strategy may especially beneficial for males.
Acknowledgments
This research was supported by Grants R37DA05147 from the National Institute on Drug Abuse and Grant R01AA09367 from the National Institute of Alcohol Abuse and Alcoholism. Author, DRS was also supported by Grant MH017069 from the National Institute of Mental Health postdoctoral training grant and ME was supported by R01DA009679 and R01DA024411 from the National Institute of Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare they have no competing or potential conflicts of interest.
Footnotes
Conflicts of interest statement: No conflicts declared.
References
- Bailey SL, Pollock NK, Martin CS, Lynch KG. Risky sexual behaviors among adolescents with alcohol use disorders. Journal of Adolescent Health. 1999;25:179–181. doi: 10.1016/s1054-139x(99)00023-3. [DOI] [PubMed] [Google Scholar]
- Boislard M, Poulin F. Individual, familial, friends-related and contextual predictors of early sexual intercourse. Journal of Adolescence. 2011;34:289–300. doi: 10.1016/j.adolescence.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Brofenbrenner U. The ecology of human development: Experiments by design and nature. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Schootman M, Bucholz KK, Peipert JF, et al. Age of sexual debut among US adolescents. Contraception. 2009;80:158–162. doi: 10.1016/j.contraception.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Martinez GM, Mosher WD, Jabma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: Data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23 Retrieved from http://www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. [PubMed] [Google Scholar]
- Cooper ML, Wood PK, Orcutt HK, Albino A. Personality and the predisposition to engage in risky or problem behaviors during adolescence. Journal of Personality and Social Psychology. 2003;84:390–410. doi: 10.1037//0022-3514.84.2.390. [DOI] [PubMed] [Google Scholar]
- Curtis N, Ronan KR, Borduin CM. Multisystemic treatment: A meta-analysis of outcome studies. Journal of Family Psychology. 2004;18:411–419. doi: 10.1037/0893-3200.18.3.411. [DOI] [PubMed] [Google Scholar]
- Donahue KL, Lichtenstein P, Långström N, D’Onofrio BM. Why Does Early Sexual Intercourse Predict Subsequent Maladjustment? Exploring Potential Familial Confounds. Health Psychology. 2012;32:180–189. doi: 10.1037/a0028922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohew L, Zimmerman R, Cupp PS, Novak S, Colon S, Abell R. Sensation seeking, impulsive decision-making, and risky sex: Implications for risk-taking and design of interventions. Personaltiy and Indivual Differences. 2000;28:1079–1091. [Google Scholar]
- Enders CK, Bandalos DL. The Relative Performance of Full Information Maximum Likelihood Estimation for Missing Data in Structural Equation Models. Structural Equation Modeling-a Multidisciplinary Journal. 2001;8:430–457. [Google Scholar]
- Epstein M, Bailey JA, Manhart LE, Hill KG, Hawkins JD. Preventing sexual risk behavior in young adulthood: Broadening the scope beyond early sexual initiation. Manuscript under review. 2013 doi: 10.1080/00224499.2013.849652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson HB. Self-reported delinquency among schoolboys, and their attitudes to the police. British Journal of Clinincal Psychology. 1967;6:168–173. doi: 10.1111/j.2044-8260.1967.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Harden KP, Mendle J, Hill JE, Turkheimer E, Emery RE. Rethinking timing of first sex and delinquency. Journal of Youth Adolescence. 2008;37:373–385. doi: 10.1007/s10964-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Mendle J. Adolescent sexual activity and the development of delinquent behavior: The role of relationship context. Journal of Youth and Adolescence. 2011;40:825–838. doi: 10.1007/s10964-010-9601-y. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Oesterle S, Brown EC, Monahan KC, Abbott RD, Arthur MW, et al. Sustained decreases in risk exposure and youth problem behaviors after installation of the Communities that Care prevention system in a randomized trial. Arcives of Pediatics & Adolescent Medicine. 2012;166:141–148. doi: 10.1001/archpediatrics.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler SW. Efficacy studies to large-scale transport: The development and validation of multisystemic therapy programs. Annual Review of Clinical Psychology. 2011;7:351–381. doi: 10.1146/annurev-clinpsy-032210-104615. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, Schoenwald SK, Borduin CM, Rowland MD, Cunningham PB. Multisystemic therapy for antisocial behavior in children and adolescents. New York, NY: The Guilford Press; 2009. [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, et al. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal study. Journal of Abnormal Psychology. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cross-generational effects on gender differences in psychoactive drug abuse and dependence. Drug and Alcohol Dependence. 2004;74:147–158. doi: 10.1016/j.drugalcdep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Huibregste BM, Bornovalova MA, Hicks BM, McGue M, Iacono WG. Testing the role of adolescent sexual initation in late-life sexual risk behavior: A longitudinal design. Psychological Science. 2011;22:924–933. doi: 10.1177/0956797611410982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavior disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Developmental Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Kahn JA, Kaplowitz RA, Goodman E, Emans SJ. The association between impulsiveness and sexual risk behaviors in adolescent and young adult women. Journal of Adolescent Health. 2002;30:229–232. doi: 10.1016/s1054-139x(01)00391-3. [DOI] [PubMed] [Google Scholar]
- Khan MR, Berger AT, Wells BE, Cleland CM. Longitudinal associations between adolescent alcohol use and adulthod sexual risk behavior and sexually transmitted infection in the United States: Assessment of differences by race. American Journal of Public Health. 2012;102:867–876. doi: 10.2105/AJPH.2011.300373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby DB. The impact of abstinence and comprehensive sex and STD/HIV education programs on adolescent sexual behavior. Sexuality Research & Soialc Policy. 2008;5:18–27. [Google Scholar]
- Kiselica AM, Cummings JR, Bornovalova MA, Samek DR, McGue M, Iacono WG. Genetic factors account for the association between age of sexual initiation and adult sexual risk behavior. Manuscript under review 2013 [Google Scholar]
- Manlove J, Papillio AR, Ikramullah E. Not yet: Programs to delay first sex among teens. Washington, DC: National Campaign to Prevent Teen Pregnancy; 2004. [Google Scholar]
- McGue M, Iacono WG. The assocation of early adolescent problem behavior with adult psychopathology. American Journal of Psychiatry. 2005;162:1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén L. Mplus, Version 6.12. Los Angeles, CA: Muthén & Muthén; 1998–2013. [Google Scholar]
- Neale MC. Mx: Statistical modeling. 7. Richmond, VA: Department of Psychiatry; 2006. [Google Scholar]
- Ramrakha S, Bell ML, Paul C, Dickson N, Moffitt TE, Caspi A. Childhood behavior problems linked to sexual risk taking in young adulthood: A birth cohort study. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:1272–1279. doi: 10.1097/chi.0b013e3180f6340e. [DOI] [PubMed] [Google Scholar]
- Sandfort TGM, Orr M, Hirsch JS, Santelli J. Long-term health correlates of timing of sexual debut: Results from a national US study. American Journal of Public Health. 2008;98:155–161. doi: 10.2105/AJPH.2006.097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelli J, Ott MA, Lyon M, Rogers J, Summers D, Schleifer R. Abstinence and abstinence-only education: A review of U.S. policies and programs. Journal of Adolescent Health. 2006;38:72–81. doi: 10.1016/j.jadohealth.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Sawyer AM, Borduin CM. Effects of multisystemic therapy through midlife: A 21.9 year follow-up to a randomized clinical trial with serious and violent juvenile offenders. Journal of Consulting & Clinical Psychology. 2011;79:643–652. doi: 10.1037/a0024862. [DOI] [PubMed] [Google Scholar]
- Schaeffer CM, Borduin CM. Long-term follow-up to a randomized clinical trial of multisystemic therapy with serious and violent juvenile offenders. Journal of Consulting & Clinical Psychology. 2005;73:445–453. doi: 10.1037/0022-006X.73.3.445. [DOI] [PubMed] [Google Scholar]
- Tollman DL, McClelland SI. Normative sexuality development in adolescence: A decade in review 2000–2009. Journal of Research of Adoelscence. 2011;21:242–255. [Google Scholar]
- Wellings K, Nachahal K, Macdowall W, McManus S, Erens B, Mercer CH, et al. Sexual behavior in Britain: Early heterosexual experience. Lancet. 2001;358(9296):1843–1850. doi: 10.1016/S0140-6736(01)06885-4. [DOI] [PubMed] [Google Scholar]
- Welner Z, Reich W, Herjanic B, Jung K, Amado H. Reliability, validity, and parent-child agreement studies of the Diagnostic Interview for Children and Adolescents (DICA) Journal of the American Academy of Child & Adolescent Psychiatry. 1987;26(5):649–653. doi: 10.1097/00004583-198709000-00007. [DOI] [PubMed] [Google Scholar]