Abstract
Objective
To provide a broader evidence summary to inform dietary guidelines of the effect of tree nuts on criteria of the metabolic syndrome (MetS).
Design
We conducted a systematic review and meta-analysis of the effect of tree nuts on criteria of the MetS.
Data sources
We searched MEDLINE, EMBASE, CINAHL and the Cochrane Library (through 4 April 2014).
Eligibility criteria for selecting studies
We included relevant randomised controlled trials (RCTs) of ≥3 weeks reporting at least one criterion of the MetS.
Data extraction
Two or more independent reviewers extracted all relevant data. Data were pooled using the generic inverse variance method using random effects models and expressed as mean differences (MD) with 95% CIs. Heterogeneity was assessed by the Cochran Q statistic and quantified by the I2 statistic. Study quality and risk of bias were assessed.
Results
Eligibility criteria were met by 49 RCTs including 2226 participants who were otherwise healthy or had dyslipidaemia, MetS or type 2 diabetes mellitus. Tree nut interventions lowered triglycerides (MD=−0.06 mmol/L (95% CI −0.09 to −0.03 mmol/L)) and fasting blood glucose (MD=−0.08 mmol/L (95% CI −0.16 to −0.01 mmol/L)) compared with control diet interventions. There was no effect on waist circumference, high-density lipoprotein cholesterol or blood pressure with the direction of effect favouring tree nuts for waist circumference. There was evidence of significant unexplained heterogeneity in all analyses (p<0.05).
Conclusions
Pooled analyses show a MetS benefit of tree nuts through modest decreases in triglycerides and fasting blood glucose with no adverse effects on other criteria across nut types. As our conclusions are limited by the short duration and poor quality of the majority of trials, as well as significant unexplained between-study heterogeneity, there remains a need for larger, longer, high-quality trials.
Trial registration number
Keywords: Nutrition & Dietetics
Strengths and limitations of this study.
This is the first systematic review and meta-analysis to look at the effect of tree nuts on metabolic syndrome criteria.
This systematic review and meta-analysis involved a large number of trials (49 randomised controlled trials) in participants with a range of metabolic phenotypes.
Most of the trials (74.4%) were of low quality (Methodological Quality Score (MQS) <8).
Most of the trials (68.8%) were of short duration (<12 weeks).
Substantial interstudy heterogeneity remained unexplained.
Introduction
Dietary patterns including tree nuts have received particular attention for their cardiovascular benefits, and the Food and Drug Administration (FDA) has granted a qualified health claim to tree nuts for cardiovascular risk reduction.1 General dietary guidelines2 and heart health guidelines3 4 also continue to recommend tree nuts alone or as part of the Mediterranean, Portfolio and Dietary Approaches to Stop Hypertension (DASH) dietary patterns for cardiovascular disease prevention and management.
Although these recommendations are based primarily on the low-density lipoprotein cholesterol (LDL-C)-lowering benefits of tree nuts,4 the cardiovascular risk reduction seen with tree nuts is beyond that which would be predicted by this effect alone. The Prevención con Dieta Mediterránea (PREDIMED) trial showed that despite a non-significant effect on LDL-C early on in the trial,5 a Mediterranean diet supplemented with mixed nuts (30 g/day) compared with a low-fat control diet reduced major cardiovascular events by 30% in high cardiovascular risk participants.6 Nut consumption of >3 servings/week was also associated with other metabolic advantages such as a decreased risk of obesity, metabolic syndrome (MetS) and type 2 diabetes mellitus.7 Individual large trials of tree nuts have also shown that nuts improve criteria of the MetS: waist circumference,8 9 triglycerides,5 10–12 high-density lipoprotein cholesterol (HDL-C),13–18 blood pressure (BP)5 8 and glycaemic control.19–22
The overall evidence for these additional metabolic benefits, however, remains uncertain. Guidelines have not recommended tree nuts directly for managing these risk factors. Although the Canadian Diabetes Association (CDA) 2013 clinical practice guidelines for nutrition therapy23 did acknowledge some of these metabolic benefits, the evidence was deemed insufficient for making a recommendation. Tree nuts consumption was recommended only insofar as it was part of Mediterranean or DASH dietary patterns.23 To synthesise the evidence on which recommendations are based for the metabolic benefits of tree nuts beyond LDL-C lowering, we conducted a systematic review and meta-analysis of randomised controlled dietary trials of the effect of tree nuts on criteria of the MetS.
Methods
Protocol and registration
We followed the guidelines of the Cochrane Handbook for Systematic Reviews of Intervention for the planning and conduct of this meta-analysis.24 Reporting of results followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.25 The review protocol is available at ClinicalTrials.gov (registration number: NCT01630980).
Study selection
We searched MEDLINE, EMBASE, CINAHL and the Cochrane Library (through 4 April 2014) to identify randomised controlled dietary trials of tree nuts. Details of the search strategy are presented in online supplementary appendix table 1. The electronic database searches were supplemented by manual searches of the reference list of included trials and reviews. No language restriction was used.
We included randomised dietary trials that reported the effect of diets rich in tree nuts (almonds, Brazil nuts, cashews, hazelnuts, macadamia nuts, pecans, pine nuts, pistachios, walnuts and mixed nuts)1 as a whole compared with diets without tree nuts, but matched for energy on at least one of the five criteria of the MetS: waist circumference, triglycerides, HDL-C, BP and fasting blood glucose. Included trials were ≥3 weeks’ duration, a duration that satisfies the minimum follow-up requirement for lipid-lowering health claims by the FDA used in the scientific evaluation of lipid-lowering health claims.26 We excluded trials that incorporated tree nuts as paste, oil or skin nuts into the treatment diets and also those trials that added tree nuts as part of a dietary pattern and did not have a matched control group. The former exclusion was intended to eliminate contamination from the other nutritional aspects, and to isolate the effect of tree nuts. Where multiple intervention or control groups were presented, we only included those groups which allowed us to isolate the effect of tree nuts. When multiple publications existed for the same trial, data from the most recent report were included. Publications including additional relevant data were used as companion reports. The MetS end points were selected according to the 2009 harmonised definition for MetS.27
Data extraction
Studies that met the inclusion criteria were extracted in full by two independent reviewers (SBM and one of EV, LSA, VH or AM) for study characteristics and data for end points. Study characteristics included: study design (cross-over or parallel), participant characteristics, comparator, nut dose, nut type, duration of follow-up, dietary adherence measures, macronutrient profile, statistical analysis and funding sources. All disagreements among reviewers were resolved by consensus.
The Heyland Methodological Quality Score (MQS) was used for assessment of study quality.28 Scores from 0 to 2 points were given for each of the following evaluated criteria: methods (randomisation, blinding and analysis), sample (selection, compatibility and follow-up) and intervention (protocol, cointervention and cross-overs). This scale gave a maximum MQS of 13 points. Studies with a score of ≥8 were considered of high quality.
The Cochrane Collaboration Risk of Bias Tool was used to assess the study risk of bias.24 Trials were classified as ‘unclear risk of bias’ when insufficient information was provided to permit judgement, ‘high risk of bias’ when the methodological flaw was likely to have affected the true outcome and ‘low risk of bias’ when a methodological flaw was deemed inconsequential to determine the true effect within a study. As blinding of participants in dietary trials is difficult to achieve, we scored the trials based on the intensity of the dietary advice given to the randomised groups. If treatment intensity was judged to be more intensive in one intervention over another, then trials were classified as ‘high risk’. If both interventions were emphasised equally, then trials were classified as ‘low risk of bias’. Trials reported in abstract format only were not included in assessments of MQS or of bias owing to a lack of information.
Means (SD) for baseline values, end values, change from baseline differences, end differences and mean differences (MD) were recorded for primary end points (waist circumference, triglycerides, HDL-C, BP and fasting blood glucose). Reported t values or F statistics and p values for differences were also recorded. Missing information for any end point data or study details was requested directly from authors. Where SDs were not reported or given directly by authors, we attempted to calculate these missing SDs from the available statistics using methods recommended by the Cochrane Collaboration.24 If this was not possible, then we imputed these missing SDs using a pooled correlation coefficient derived from a meta-analysis of correlation coefficients from those trials reporting sufficient data.24 These correlation coefficients were then transformed into z-scores and meta-analysed using inverse-variance weighing. The pooled effect estimate from the z-scores was then back transformed to impute the missing SDs. We used a derived pooled correlation coefficient of 0.635 for triglycerides, 0.856 for HDL-C, 0.327 for systolic BP, 0.508 for diastolic BP and 0.446 for fasting blood glucose.
Statistical analyses
Data were analysed using Review Manager (RevMan) 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) for primary analyses and Stata (V.12, College Station, USA) for subgroup analyses. Pooled analyses were conducted using the generic inverse variance method with random effects models. Data were expressed as MD with 95% CI and considered significant at p<0.05. Paired analyses were applied to all cross-over trials.29 In cases where there were multiple intervention or control groups, we combined either intervention or control groups to create single pairwise comparisons with the aim of diminishing the unit-of-analysis error.24
The presence of between-studies heterogeneity was assessed by the Cochran Q statistic (significance set at p<0.10) and quantified by the I² statistic. We interpreted the I2 statistic as follows: <50% indicates ‘moderate’ heterogeneity; ≥50%, ‘substantial’ heterogeneity; and ≥75%, ‘considerable’ heterogeneity.24 Analyses were stratified by participant health status: otherwise healthy, dyslipidaemia, MetS criteria and type 2 diabetes mellitus based on trial entry criteria. Sources of heterogeneity were explored using sensitivity and subgroup analyses. To determine if any single trial exerted an undue influence on the overall results, sensitivity analyses were preformed, in which each individual trial was removed from the meta-analysis, and the effect size recalculated with the remaining trials. Sensitivity analyses were also undertaken using correlation coefficients of 0.25, 0.5 and 0.75 to determine whether the overall results were robust to the use of different derived correlation coefficients in paired analyses of cross-over trials. A priori subgroup analyses were performed for baseline values (according to MetS diagnostic criteria),27 absolute fibre intake on the tree nut diet (<25 vs ≥25 g/day23), change in fibre intake within the tree nut diet (<5.3 vs ≥5.3 g/day) and between the tree nut and control diets (<3.8 vs ≥3.8 g/day), absolute saturated fatty acid (SFA) intake on the tree nut diet (<7% vs ≥7% of total energy23), change in SFA intake within the tree nut diet (<−2% vs ≥−2% of total calories) and between the tree nut and control diets (<−2% vs ≥−2% of total calories), tree nut dose (<50 vs ≥50 g/day), tree nut type (almonds, Brazil nuts, cashews, hazelnuts, macadamia nuts, pecans, pine nuts, pistachios, walnuts and mixed nuts), duration of follow-up (<3 vs ≥3 months), study design (cross-over vs parallel) and study quality (MQS <8 vs ≥8). Post hoc subgroup analyses were conducted for the difference in per cent carbohydrate intake between the control and tree nut diets (carbohydrate displacement). The significance of between-subgroup differences was assessed using metaregression (p<0.05). Publication bias was assessed by visual inspection of funnel plots and formally complemented by Begg's and Egger's tests.
Results
Trial selection
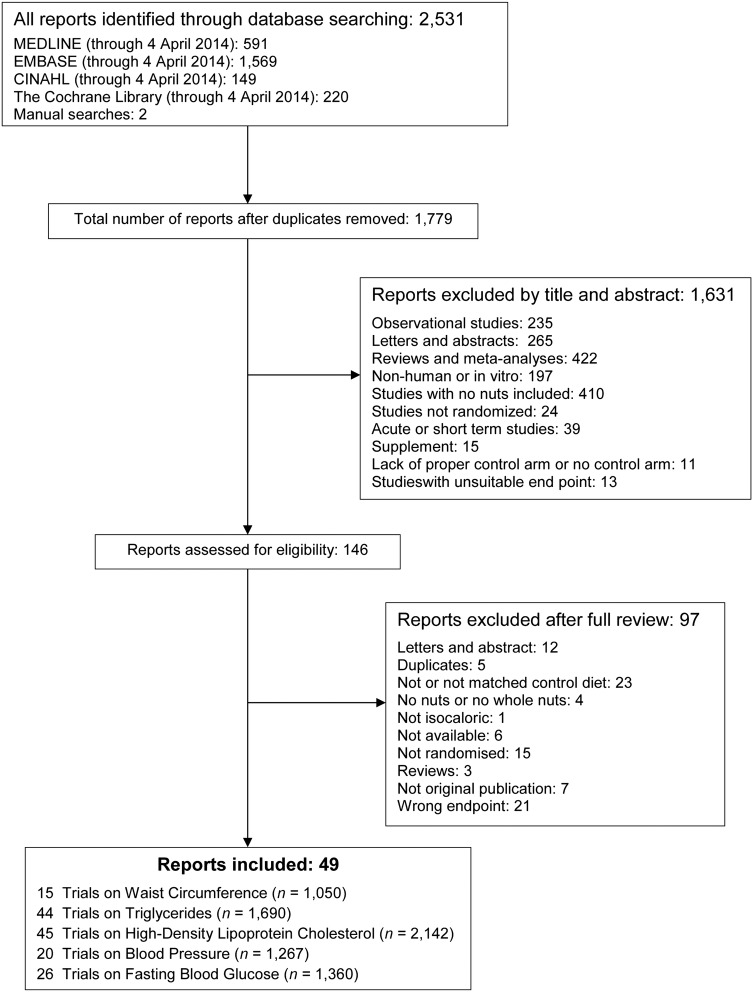
Figure 1 shows flow of studies through the search and selection process. We identified a total of 2531 reports, from which 752 reports were duplicates and 1631 reports were deemed irrelevant (determined by review of title and abstract). The remaining 146 reports were reviewed in full, of which 97 reports were excluded for not meeting inclusion criteria. A total of 49 reports on 47 trials8–23 30–59 as well as four companion reports60–63 that addressed at least one criterion of the MetS (waist circumference (15 trials, n=1050), triglycerides (44 trials, n=1690), HDL-C (45 trials, n=2142), BP (20 trials, n=1267) and fasting blood glucose (26 trials, n=1360)) were included.
Figure 1.
Summary of evidence search and selection.
Trial characteristics
Table 1 presents characteristics of the included trials. There were 47 trials involving 49 comparisons in 2211 participants. Twelve trials (26.7%)10 12 14 16 30 32 34 36 39 43 49 59 were conducted in otherwise healthy participants. Two of these trials contained a minority of participants with dyslipidaemia who had been classified as otherwise healthy.36 43 Eleven trials (24.4%)8 18–21 35 37 44 45 54 55 were conducted in participants with type 2 diabetes mellitus or a mix of patients with overweight and type 2 diabetes mellitus in one case.8 The remaining trials were conducted in people with dyslipidaemia (9 trials (20%)13 15 17 31 33 38 41 42 53), MetS (5 trials22 40 47 48 58), some MetS criteria (overweight (7 trials(15.6%)9 11 50–52 56 57 and prediabetes (1 trial (2.2%)46). Median age for participants was 50.2 years (IQR 42.5–55.8 years). Median body weight for participants was 81.4 kg (IQR 72.1–91.7 kg).
Table 1.
Characteristics of RCTs investigating the effect of tree nuts on criteria of the MetS
| Study (year) (reference) | Participants | Mean age (SD or range), years | Mean body weight or BMI (SD or range)* | Setting | Design | Feeding control | Nut type | Nuts dose (g/day)† | Comparator | Diet‡ | Energy balance | Follow-up | MQS§ | Funding sources¶ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sabate et al (1993)30 | ||||||||||||||
| Walnut | 18 (18 M) | 30 | 73 | OP, USA | Cross-over | Met | Walnut | 84 | 55:31:14 | Isocaloric | 4 weeks | 6 | Agency | |
| Control | NCEP step 1 diet | 56:30:14 | ||||||||||||
| Chisholm et al (1998)13 | ||||||||||||||
| Walnut | 16 HLP | 45 (6.8) | 28.4 (4.3) | OP, New Zealand | Cross-over | DA | Walnut | 78 | 40:38:17 | Isocaloric | 4 weeks | 4 | Agency | |
| Control | Low-fat diet | 46:30:19 | ||||||||||||
| Spiller et al (1998)31 | ||||||||||||||
| Almond | 30 HLP | 53 (10) | 66 (13) | OP, Italy | Parallel | Supp | Almond | 100 | 45:39:16 | Isocaloric | 4 weeks | 6 | Agency | |
| Control | Matched macronutrient diet | 47:36:17 | ||||||||||||
| Curb et al (2000)10 | ||||||||||||||
| Macadamia | 30 (15 M, 15 W) | 35.25 (18–53) | 23 (19.1–28.3) | OP, USA | Cross-over | Met | Macadamia | 46 | 48:35:17 | Isocaloric | 4 weeks | 4 | Agency–industry | |
| Control | AHA | 54:30:16 | ||||||||||||
| Control | AAD | 48:35:17 | ||||||||||||
| Morgan and Clayshulte (2000)32 | ||||||||||||||
| Pecan | 19 (4 M, 15 W) | 37 (12) | 24 (5) | OP, USA | Parallel | Supp | Pecan | 68 | 45:43:12 | Isocaloric | 8 weeks | 6 | Agency | |
| Control | 45 (10) | 24 (4) | Self-selected diet | 46:36:18 | ||||||||||
| Zambon et al (2000)33 | ||||||||||||||
| Walnut | 49 HC (26 M, 23 W) | 56 (11) | 70.6 (12.1) | OP, Spain | Cross-over | Supp | Walnut | 48.5 | 48:34:18 | Isocaloric | 6 weeks | 6 | Agency | |
| Control | Mediterranean diet | 50:31:19 | ||||||||||||
| Rajaram et al (2001)14 | ||||||||||||||
| Pecan | 23 (14 M, 9 W) | 25–55 | 74.4 (16.7) | OP, USA | Cross-over | Met | Pecan | 72 | 47:40:13 | Isocaloric | 4 weeks | 8 | Agency | |
| Control | NCEP step 1 diet | 57:28:15 | ||||||||||||
| Iwamoto et al (2002)34 | ||||||||||||||
| Walnut | 40 (20 M, 20 W) | 23.8 (3.1)** | 22.2 (0.5) | OP, Japan | Cross-over | Met | Walnut | 52†† | 60:26:14 | Isocaloric | 4 weeks | 8 | Agency | |
| Control | 23.6 (4.6)** | 20.7 (0.5) | Average Japanese diet | 62:24:14 | ||||||||||
| Jenkins et al (2002)15 | ||||||||||||||
| Almond | 27 HLP (15 M, 12 W) | 64 (9) | 71.2 (2.5) | OP, Canada | Cross-over | Supp | Almond | 73 | 47:36:17 | Isocaloric | 4 weeks | 6 | Agency | |
| Control | 71.0 (2.4) | NCEP step 2 diet + muffin | 57:26:18 | |||||||||||
| Lovejoy et al (2002)35 | ||||||||||||||
| High-fat almond | 30 DM2 (13 M, 17 W) | 53.8 (10.4) | 33.0 (5.5) | OP, USA | Cross-over | Met | Almond | 85†† | 48:37:15 | Isocaloric | 4 weeks | 5 | Agency | |
| Low-fat almond | Almond | 60:25:15 | ||||||||||||
| High-fat control | High-fat diet | 48:37:15 | ||||||||||||
| Low-fat control | Low-fat diet | 60:25:15 | ||||||||||||
| Sabate et al (2003)36 | ||||||||||||||
| High almond | 25 NL-HC (14 M, 11 W) | 41 (13) | NA | OP, USA | Cross-over | Met | Almond | 83 | 46:39:14 | Isocaloric | 4 weeks | 5 | Agency–industry | |
| Low almond | Almond | 42 | 35:51:14 | |||||||||||
| Control | NCEP step 1 diet | 56:30:14 | ||||||||||||
| Wien et al (2003)8 | ||||||||||||||
| Almond | 65 OW/DM2 (28 M, 37 W) | 53 (2) | 113 (5) | OP, USA | Parallel | Supp | Almond | 84 | 53:18:29 | Isocaloric | 24 weeks | 8 | Agency | |
| Control | 57 (2) | 114 (5) | CHO-LCD | 32:39:29 | ||||||||||
| Tapsell et al (2004)37 | ||||||||||||||
| Walnut | 37 DM2 | 57.7 (9) | 87.6 (12.8) | OP, Australia | Parallel | Supp | Walnut | 30 | 44:32:22 | Isocaloric | 6 months | 6 | Agency | |
| Control | 59.3 (7.1) | 81.9 (11.2) | Modified fat | 41:33:23 | ||||||||||
| Tamizifar et al (2005)38 | ||||||||||||||
| Almond | 30 HC (17 M, 13 W) | 56 (6.1) | 63 (8.9) | OP, Iran | Cross-over | Supp | Almond | 25 | 47:37:17 | Isocaloric | 4 weeks | 5 | NA | |
| Control | NCEP step 1 diet | 45:29:15 | ||||||||||||
| Kocyigit et al (2006)16 | ||||||||||||||
| Pistachio | 44 (24 M, 20 W) | 32.8 (6.7) | 24.2 (6.1) | OP, Turkey | Parallel | DA | Pistachio | 69 | NA | Isocaloric | 3 weeks | 8 | Agency | |
| Control | 24.6 (5.6) | Regular diet | ||||||||||||
| Kurlandsky and Stoke (2006)39 | ||||||||||||||
| Almond | 47 (47 W) | 41.8 (11.7) | 25.3 (3.5) | OP, USA | Parallel | Supp | Almond | 60 | 51:34:15 | Isocaloric | 6 weeks | 5 | Agency–industry | |
| Almond + dark chocolate | 46.2 (7.8) | 27.2 (4.2) | Almond | 46:39:15 | ||||||||||
| Dark chocolate | 36.5 (11.9) | 23.9 (3.3) | NCEP ATP III diet + chocolate | 55:30:15 | ||||||||||
| Control | 51.3 (6.3) | 26.1 (4.1) | NCEP ATP III diet | 57:27:16 | ||||||||||
| Schutte et al (2006)60‡‡ | ||||||||||||||
| Walnut | 62 MetS | 45.5 | 35.9 | OP, South Africa | Parallel | Met | Walnut | 85.5 | 47:36:17 | Isocaloric | 8 weeks | 7 | Agency–industry | |
| Cashew | 45.7 | 34.7 | Cashew | 47:36:17 | ||||||||||
| Control | 44.4 | 35.5 | Control diet | 50:33:18 | ||||||||||
| Mukuddem-Petersen et al (2007)40 | ||||||||||||||
| Walnut | 64 MetS | 45 (10) | 107 | OP, South Africa | Parallel | Met | Walnut | 85.5†† | 49:35:16 | Isocaloric | 8 weeks | 7 | Agency–industry | |
| Cashew | 99 | Cashew | 44:37:19 | |||||||||||
| Control | 106 | Habitual diet | 47:33:20 | |||||||||||
| Sheridan et al (2007)17 | ||||||||||||||
| Pistachio | 15 HC | 60 (11.2) | 175 (26) | OP, USA | Cross-over | Supp | Pistachio | 35 | 52:31:17 | Isocaloric | 4 weeks | 6 | Agency | |
| Control | Regular diet | 53:31:16 | ||||||||||||
| Gebauer et al (2008)41 | ||||||||||||||
| 1 Pistachio | 28 HLP (10 M, 18 W) | 48 (7.9) | 76.6 (13.2) | OP, USA | Cross-over | Met | Pistachio | 37 | 53:34:16 | Isocaloric | 4 weeks | 5 | Agency | |
| 2 Pistachio | Pistachio | 74 | 57:29:16 | |||||||||||
| Control | NCEP step 1 diet | 62:25:15 | ||||||||||||
| Griel et al (2008)42 | ||||||||||||||
| Macadamia | 25 HC | 50.2 (8.4) | 26.3 (3.3) | OP, USA | Cross-over | Met | Macadamia | 42.5§§ | 50:33:19 | Isocaloric | 5 weeks | 8 | Agency–industry | |
| Control | AAD | 52:33:17 | ||||||||||||
| Jenkins et al (2008)61‡‡ | ||||||||||||||
| Almond | 27 HLP (15 M, 12 W) | 64 (9) | 71.2 (2.5) | OP, Canada | Cross-over | Supp | Almond | 73 | 47:36:17 | Isocaloric | 4 weeks | 6 | Agency | |
| Control | 71.0 (2.4) | NCEP step 2 diet + muffin | 57:26:18 | |||||||||||
| Rajaram et al (2009)43 | ||||||||||||||
| Walnut | 25 NL-HLP (14 M, 11 W) | 23–65 | 71.9 (15.5) | OP, USA | Cross-over | Met | Walnut | 42.5 | 60:31:15 | Isocaloric | 4 weeks | 5 | Agency | |
| Control | 71.7 (15.5) | AAD | 57:30:14 | |||||||||||
| Tapsell et al (2009)44 | ||||||||||||||
| Walnut | 35 DM2¶¶ | 54 (8.7) | 92.3 (15.7) | OP, Australia | Parallel | Supp | Walnut | 30 | 42:29:24 | Isocaloric | 12 months | 7 | Agency | |
| Control | 93.4 (3) | Low-fat diet | 41:34:20 | |||||||||||
| Li et al (2010)11 | ||||||||||||||
| Almond | 52 OW¶¶ | 45.4 (2.0) | 86 (26.8) | OP, USA | Parallel | Supp | Pistachio | 53 | 55:30:15 | Hypocaloric | 12 weeks | 7 | Agency | |
| Control | 47.3 (2.3) | 85.5 (40.2) | Pretzel | 65:20:15 | Hypocaloric | |||||||||
| Ma et al (2010)45 | ||||||||||||||
| Walnut | 22 DM2¶¶ | 58.1 (9.2) | 89 (15.5) | OP, USA | Cross-over | Supp | Walnut | 56 | 39:44:17 | Isocaloric | 8 weeks | 5 | NA | |
| Control | Ad libitum diet | 43:38:19 | ||||||||||||
| Torabian et al (2010)12 | ||||||||||||||
| Walnut | 87 (38 M, 49 W) | 54 (10.2) | 75.6 (13.2) | OP, USA | Cross-over | Supp | Walnut | 46 | NA | Isocaloric | 6 months | 6 | Agency | |
| Control | Habitual diet | |||||||||||||
| Wien et al (2010)46 | ||||||||||||||
| Almond | 65 PD (17 M, 48 W) | 53 (9) | 82.9 (14.4) | OP, USA | Parallel | Supp | Almond | 58 | 42:39:19 | Isocaloric | 16 weeks | 9 | Agency | |
| Control | 54 (11) | 80.5 (14.4) | AAD | 48:30:21 | ||||||||||
| Wu et al (2010)47 | ||||||||||||||
| Walnut | 189 MetS | 48.2 (8.4) | 72.2 (11.4) | OP, USA | Parallel | Supp | Walnut | 30 | 48:37:15 | Isocaloric | 12 weeks | 9 | Agency | |
| Control | 48.6 (8) | 70.6 (10.9) | AHA | 51:34:15 | ||||||||||
| Casas-Agustench et al (2011)48 | 50 MetS (28 M, 22 W) | OP, Spain | Parallel | Supp | ||||||||||
| Mixed nuts | 52.9 (8.4) | 31.6 (2.8) | Mixed nuts | 30 | 41:36:19 | Isocaloric | 12 weeks | 6 | Agency | |||||
| Control | 50.6 (8.4) | 30.0 (3.3) | Prudent diet | 42:36:19 | ||||||||||
| Cohen and Johnston (2011)19 | ||||||||||||||
| Almond | 13 DM2 (7 M, 6 W) | 66 (11.9) | 96.1 (40.4) | OP, USA | Parallel | Supp | Almond | 28 | NA | Isocaloric | 12 weeks | 7 | Agency | |
| Control | 105.1 (32.1) | Cheese sticks | ||||||||||||
| Jenkins et al (2011)20 | ||||||||||||||
| Mixed nuts | 79 DM2 (52 M, 27 W) | 63 (9) | 80 (15) | OP, Canada | Parallel | Supp | Mixed nuts | 75†† | 41:41:18 | Isocaloric | 12 weeks | 8 | Agency | |
| Control | 61 (10) | 83 (15) | NCEP step 2 diet + muffin | 46:35:19 | ||||||||||
| Li et al (2011)21 | ||||||||||||||
| Almond | 20 DM2 (9 M, 11 W) | 58 (8.9) | 26 (3.1) | OP, Taiwan | Cross-over | Met | Almond | 56 | 47:37:17 | Isocaloric | 4 weeks | 5 | Agency | |
| Control | NCEP step 2 diet | 57:27:17 | ||||||||||||
| Tey et al (2011)49 | ||||||||||||||
| Hazelnut | 61 | 38.9 (14.3) | 72 (11.1) | OP, New Zealand | Parallel | Supp | Hazelnut | 42 | 45:39:16*** | Isocaloric | 12 weeks | 9 | Agency | |
| Control | 36.1 (15.2) | 67.3 (9.5) | Regular diet | 50:33:17 | ||||||||||
| Damavandi (2012)18 | ||||||||||||||
| Cashew | 43 DM2 (9 M, 34 W) | 51 (7.9) | 72.1 (13.1) | OP, Iran | Parallel | Supp | Cashew | 30 | 53:32:16 | Isocaloric | 8 weeks | 3 | NA | |
| Control | 56 (5.7) | 71.9 (9.7) | Regular diet | 57:27:16 | ||||||||||
| Foster et al (2012)50 | ||||||||||||||
| Almond | 123 OW (11 M, 112 W) | 47 (12) | 94 (13.1) | OP, USA | Parallel | Supp | Almond | 56 | NA | Hypocaloric | 18 months | 9 | Agency | |
| Control | 46.7 (13) | 91.5 (11.9) | Nut-free diet | Hypocaloric | ||||||||||
| Katz et al (2012)51 | ||||||||||||||
| Walnut | 40 OW¶¶ | 57.4 (11.9) | 33.2 (4.4) | OP, USA | Cross-over | Supp | Walnut | 56 | 41:41:17 | Isocaloric | 8 weeks | 7 | Industry | |
| Control | Ad libitum diet | 45:34:20 | ||||||||||||
| Wang et al (2012)22 | ||||||||||||||
| Pistachios | 86 MetS | 51.9 (8.8) | 28.1 (3.2) | OP, China | Supp | Pistachio | 42 | NA | Isocaloric | 12 weeks | 5 | Industry | ||
| High pistachios | 51.8 (9.4) | 28 (4.5) | Pistachio | 70 | ||||||||||
| Control | 50.7 (9.9) | 28 (4.4) | AHA step 1 diet | |||||||||||
| West et al (2012)62‡‡ | ||||||||||||||
| 1 Pistachio | 28 HLP (10 M, 18 W) | 48 (7.9) | 76.6 (13.2) | OP, USA | Cross-over | Met | Pistachio | 37 | 53:34:16 | Isocaloric | 4 weeks | 5 | Agency | |
| 2 Pistachio | Pistachio | 74 | 57:29:16 | |||||||||||
| Control | NCEP step 1 diet | 62:25:15 | ||||||||||||
| Anderson et al (2013)52 | ||||||||||||||
| Pistachio | 22 OW | 55 (2) | 90 (3.6) | OP, USA | Parallel | NA | Pistachio | 35.4 | NA | NA | 6 weeks | 5 | NA | |
| Control | NA | |||||||||||||
| Berryman et al (2013)53 | ||||||||||||||
| Almond | 53 HC | NA | NA | OP, USA | Cross-over | NA | Almond | 42.5 | 51:33:16 | Isocaloric | 6 weeks | NA | NA | |
| Control | Muffin | 59:26:15 | ||||||||||||
| Damavandi et al (2013)54 | ||||||||||||||
| Hazelnut | 48 DM2¶¶ | 55.7 (7.7) | 72.1 (10.3) | OP, Iran | Parallel | Supp | Hazelnut | 29 | 55:31:16 | Isocaloric | 8 weeks | 6 | None | |
| Control | 72 (9.6) | Self-selected diet | 60:25:17 | |||||||||||
| Holligan et al (2013)63‡‡ | ||||||||||||||
| 1 Pistachio | 28 HLP (10 M, 18 W) | 48 (7.9) | 76.6 (13.2) | OP, USA | Cross-over | Met | Pistachio | 37 | 53:34:16 | Isocaloric | 4 weeks | NA | Agency | |
| 2 Pistachio | Pistachio | 74 | 57:29:16 | |||||||||||
| Control | NCEP step 1 diet | 62:25:15 | ||||||||||||
| Sauder et al (2013)55 | ||||||||||||||
| Pistachio | 30 DM2 (15 M, 15 W)¶¶ | 56.1 (1.4) | 31.2 (1.1) | OP, USA | Cross-over | Met | Pistachio | 73.4 | 51:33:17 | Isocaloric | 4 weeks | NA | Industry | |
| Control | Low-fat diet | 55:27:18 | ||||||||||||
| Somerset et al (2013)9 | ||||||||||||||
| Macadamia | 64 OW (10 M, 54 W) | 43.7 (8.4) | 95 (14.7) | OP, Australia | Parallel | DA | Macadamia | 46 | 36:38:21 | Isocaloric | 10 weeks | 9 | Agency | |
| Control | 43.2 (10.9) | 99.6 (15.2) | Regular diet | 41:38:17 | ||||||||||
| Tan and Mattes (2013)56 | ||||||||||||||
| Almond (breakfast) | 137 OW (48 M, 89 W) | 32.9 (11.5) | 80.5 (15) | OP, USA | Parallel | Supp | Almond | 43 | 50:16:15 | Isocaloric | 4 weeks | 5 | Industry | |
| Almond (morning snack) | 27.8 (10.7) | 83.2 (21.1) | Almond | 43 | 51:15:14 | |||||||||
| Almond (lunch) | 29.3 (13.5) | 84.8 (13.7) | Almond | 43 | 48:16:17 | |||||||||
| Almond (afternoon snack) | 29 (11.9) | 81.8 (14.6) | Almond | 43 | 49:15:16 | |||||||||
| Control | 28.7 (9.6) | 77.2 (16.8) | Regular diet | 48:15:16 | ||||||||||
| Tey et al (2013)57 | ||||||||||||||
| Hazelnut 30 g | 107 OW (46 M, 61W) | 43.8 (13.5) | 86.2 (11.8) | OP, New Zealand | Parallel | Supp | Hazelnut | 30 | 42:39:17 | Isocaloric | 12 weeks | 6 | Agency | |
| Hazelnut 60 g | 42.8 (10.6) | 92 (19.6) | Hazelnut | 60 | 38:42:16 | |||||||||
| Control | 41.1 (13.1) | 88.7 (16.7) | Usual diet | 47:33:17 | ||||||||||
| Gulati et al (2014)58 | ||||||||||||||
| Pistachio | 68 MetS (37 M, 31 W) | 41.6 (8.4) | 81.6 (12.9) | OP, India | Parallel | DA | Pistachio | 50§§ | 51:29:20 | Isocaloric | 24 weeks | 4 | Industry | |
| Control | 43.3 (8.1) | 80.3 (10.3) | Standard diabetic diet | 60:25:15 | ||||||||||
| Wu et al (2014)59 | ||||||||||||||
| Walnut | 40 (10 M, 30 W) | 60 (1) | 24.9 (0.6) | OP, Germany | Cross-over | Supp | Walnut | 43 | 50:35:15 | Isocaloric | 8 weeks | 7 | Industry | |
| Control | Western-type diet | |||||||||||||
*Body weight is reported in kg and BMI is reported in kg/m2. BMI is reported only when no data on weight were available.
†Nut dose is given based on g/day, 1 oz=28 g.
‡Energy from carbohydrate:fat:protein.
§Trials with scores ≥8 were considered to be of high quality.
¶Agency funding is that from government, university or not-for-profit health agency sources.
**Mean age was given separately for men and women.
††Medians were calculated from the ranges reported: Iwamoto et al34 range 50–54 g/day; Jenkins et al20 range 50–75 g/day; Lovejoy et al35 range 57–113 g/day; Mukuddem-Petersen et al40 range 63–108 g/day; Torabian et al12 range 28–64 g/day; Zambon et al33 range 41–56 g/day.
‡‡Companion reports: Jenkins et al61 for Jenkins et al15; Schutte et al60 for Mukuddem-Petersen et al40; West et al62 and Holligan et al63 for Gebauer et al41.
¶¶Baseline characteristics were based on the number of randomised participants for Li et al11 n=70; Ma et al45 n=24; Zambon et al33 n=55; Katz et al51 n=46; Sauder et al55 n=30; Gulati et al58 n=68 for recruited participants for Tapsell et al44 (n=50), and for age for Damavandi et al54 (n=50).
***Values for carbohydrates are reported as geometric means.
AAD, Average American Diet; AHA, American Heart Association; BMI, body mass index; CHO-LCD, self-selected complex carbohydrate diet; DA, dietary advice; DM2, type 2 diabetes mellitus; HC, hypercholesterolaemic; HLP, hyperlipidaemic; M, men; Met, metabolic; MetS, metabolic syndrome; MQS, Heyland Methodological Quality Score; NA, not available; NCEP, National Cholesterol Education Program; NL-HC, normal to hypercholesterolaemic; NL-HLP, normal to mildly hyperlipidaemic; PD, prediabetes; OP, out-patient; OW, overweight; RCT, randomised controlled trial; SUPP, supplement; W, women.
Trials tended to be of considerable size, with a median number of 40 participants (IQR 25–61 participants). The majority were conducted in the USA (24 trials (53.3%)) with the rest conducted in various other countries: 3 trials (6.7%) each in Australia, New Zealand and Iran; 2 trials (4.4%) each in Canada and Spain and 1 trial (2.2%) each in Japan, Turkey, Italy, China, Taiwan, Germany, India and South Africa. A similar number of trials used parallel (24 trials (53.3%)) and cross-over (21 trials (46.7%)) designs. All trials were conducted in an outpatient setting.
Control diets included usual diets (nine trials, 20%), a National Cholesterol Education Program step 1 diet (five trials, 11.1%), an average American diet (three trials, 6.7%), a low-fat diet (three trials, 6.7%), among others. Twenty-seven trials (60%) provided test food supplements, 12 trials (26.7%) provided all study foods under metabolic feeding control conditions and 4 trials provided dietary advice (8.9%). Five trials (11.1%) used a control diet in which a muffin or pretzel11 15 20 53 or cheese sticks19 were exchanged for nuts. The test and control diets were matched for energy in all cases; however, two of the trials11 50 featured a negative energy balance tree nut diet compared with a matched negative energy balance control diet. Tree nut types included almonds (13 trials, 28.3%), cashews (2 trials, 4.3%), hazelnuts (3 trials, 6.5%), macadamia nuts (3 trials, 6.5%), pecans (2 trials, 4.3%), pistachios (8 trials, 17.4%), walnuts (13 trials, 28.3%) and mixed nuts (2 trials, 4.3%). We were unable to find studies on Brazil nuts or pine nuts. Median nut dose intake was 49.3 g/day (IQR 42–70.5 g/day). Median follow-up was 8 weeks (IQR 4–12 weeks).
Macronutrient profiles varied across studies and between treatment and control groups; median values reported for carbohydrate intake were 48% (IQR 44–51%) for the treatment group and 50.5% (IQR 46–57%) for the control group. Median values for fat intake were 35% (IQR 31–39%) and 30% (IQR 27.3–34%) for tree nut and control groups, respectively. Median values for protein intake were 16% (IQR 15–17%) and 17% (IQR 15–18.8%) for tree nut and control groups, correspondingly.
Online supplementary appendix table 2 and appendix figure 1 present the assessment and summary of the risk of bias by using The Heyland MQS and The Cochrane Risk of Bias Tool. The Heyland MQS ranged from 3 to 9. Thirty-two trials (74.4%) were considered to be low quality (MQS<8) and 11 trials (25.6%) high quality (MQS≥8). The main contributors of low scores were absence of double blinding, loss of participants to follow-up and poor description of cross-overs in the control group. The Cochrane Risk of Bias Tool showed that 34 trials (70.8%) were unclear risk and 14 trials (29.2%) were low risk for random sequence generation; 29 trials (60.4%) were unclear risk and 19 trials (39.6%) were low risk for allocation concealment; 26 trials (54.2%) were unclear risk and 22 trials (45.8%) were low risk for blinding of participants and personnel; 5 trials (10.4%) were unclear risk, 35 trials (72.9%) were low risk and 8 trials (16.7%) were high risk for incomplete outcome data and 28 trials (58.3%) were unclear risk, 19 trials (39.6%) were low risk and 1 trial (2.1%) was high risk for selective reporting.
Most of the trials reported research funding from an agency (28 trials (62.2%)), while others were funded from a combination of agency and industry (5 trials (11.1%)) or industry alone (6 trials (13.3%)). One trial (2.2%) reported no funding. Five trials18 38 45 52 53 did not report their funding source (11.1%).
Waist circumference
Online supplementary appendix figure 2 presents data on the effect of tree nuts on waist circumference. Tree nuts did not significantly decrease waist circumference (MD=−0.62 cm (95% CI −1.54 to 0.30 cm)) in the overall analyses with evidence of substantial heterogeneity (I2=67%, p<0.001). Stratification by health status failed to demonstrate a significant effect for any of the subsamples. Sensitivity analyses did not alter the results (data not shown).
Online supplementary appendix table 3A and appendix figure 3 present the a priori continuous and categorical subgroup analyses, respectively, for waist circumference. There was evidence of statistically significant effect modification by the difference in carbohydrate intake in the continuous subgroup analyses (p<0.05) between tree nut and control interventions. Trials with lower carbohydrate intakes in the tree nut intervention arms showed larger reductions in waist circumference. No other subgroup analyses were statistically significant.
Triglycerides
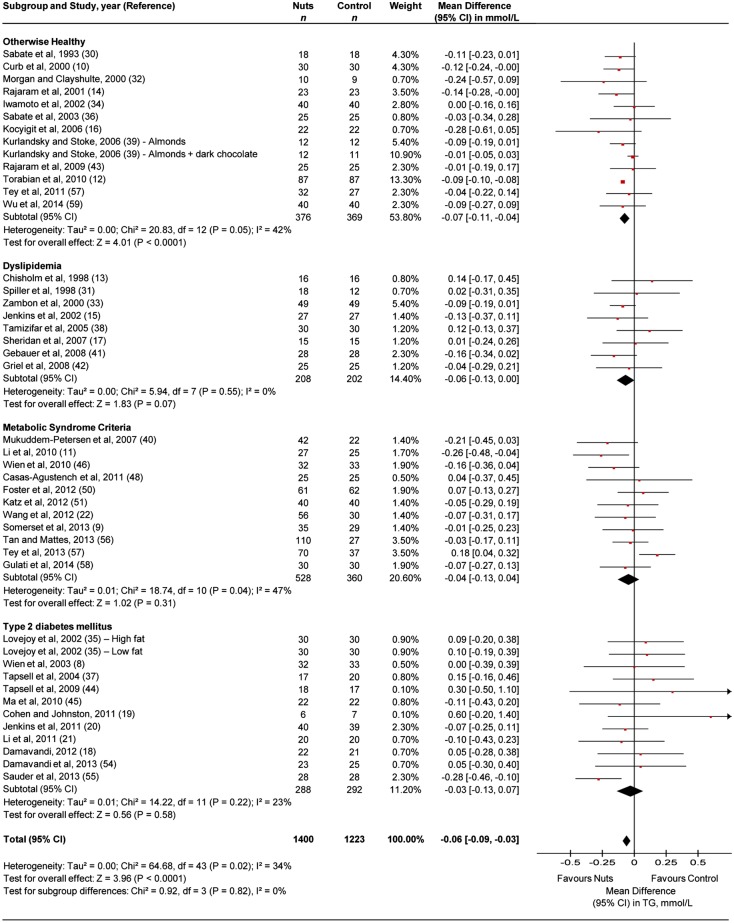
Figure 2 presents data on the effect of tree nuts on triglycerides. Tree nuts showed a significant triglyceride-lowering effect (MD=−0.06 mmol/L (95% CI −0.09 to −0.03 mmol/L)) in the overall analysis with evidence of moderate heterogeneity (I2=34%, p=0.02). The same effect was seen with evidence of moderate heterogeneity (I2=42%, p=0.05) in the subsample of participants who were otherwise healthy (MD=−0.07 mmol/L (95% CI −0.11 to −0.04 mmol/L)). Although the reductions were not statistically significant in people with dyslipidaemia, MetS criteria or type 2 diabetes mellitus, they did not significantly differ from the reductions in participants who were otherwise healthy. Sensitivity analyses did not alter the results (data not shown).
Figure 2.
Forest plot of the randomised controlled trials (RCTs) investigating the effect of tree nuts on triglycerides (TG). Pooled effect estimates are shown as diamonds, one each for trials conducted in otherwise healthy, dyslipidaemia, metabolic syndrome criteria, type 2 diabetes mellitus and their combination (total). Paired analyses were applied to all cross-over trials (20) and one substudy. Data are expressed as mean differences with 95% CI, using generic inverse-variance random effects models. Interstudy heterogeneity was tested by using the Cochran Q statistic (Chi2) at a significance level of p<0.10 and quantified by the I2 statistic.
Online supplementary appendix table 3B and appendix figure 4 present data from the a priori continuous and categorical subgroup analyses, respectively, for triglycerides. There was significant effect modification by nut type in categorical analyses (p<0.05). Pairwise comparisons showed that pecan, walnut and pistachio interventions all significantly decreased triglycerides more than almond interventions (p<0.05) and almond, macadamia, pecan, pistachio and walnut more than hazelnut (p<0.05). No other subgroup analyses were statistically significant.
High-density lipoprotein cholesterol
Online supplementary appendix figure 5 presents the effect of tree nuts on HDL-C. Tree nuts did not significantly affect HDL-C (MD=0.00 mmol/L (95% CI −0.01 to 0.01 mmol/L)) in the overall analysis with evidence of considerable heterogeneity (I2=86%, p<0.001). Stratification by health status failed to demonstrate a significant effect for any of the subsamples. Sensitivity analyses did not alter the results (data not shown).
Online supplementary appendix table 3C and appendix figure 6 present the a priori continuous and categorical subgroup analyses, respectively, for HDL-C. None of the subgroup analyses were significant.
Blood pressure
Online supplementary appendix figures 7A and 7B present the effect of tree nuts on systolic and diastolic BP, respectively. Tree nuts did not significantly increase either systolic (MD=0.07 mm Hg (95% CI −1.54 to 1.69 mm Hg)) or diastolic BP (MD=0.23 mm Hg (95% CI −0.38 to 0.83 mm Hg)) in the overall analysis with evidence of substantial heterogeneity in the systolic BP analysis (I2=64%, p<0.001) and evidence of moderate heterogeneity in the diastolic BP analysis (I2=34%, p=0.07). Stratification by health status failed to demonstrate an effect for any of the subsamples. Sensitivity analyses did not alter the results (data not shown).
Online supplementary appendix tables 3D and 3E present the a priori continuous subgroup analyses and online supplementary appendix figures 8A and 8B present the a priori categorical subgroup analyses for systolic and diastolic BP, respectively. There was evidence of statistically significant effect modification by difference in fibre intake and by the difference in carbohydrate intake in the continuous subgroup analyses, for systolic BP (p<0.05 and p<0.01, respectively) between tree nut and control interventions. Trials with higher fibre intakes in the tree nut intervention arms showed larger reductions in systolic BP. Trials in which tree nuts displaced more carbohydrates or contained lower levels of SFA intake leading to larger differences between the tree nut and control interventions were more likely to favour the tree nut diet in systolic BP. Tree nut intervention arms with higher fibre intake showed reductions in diastolic BP and also explained the heterogeneity in the overall analyses reducing the residual I2 to 1.6%. No other subgroup analyses were statistically significant for either systolic or diastolic BP.
Fasting blood glucose
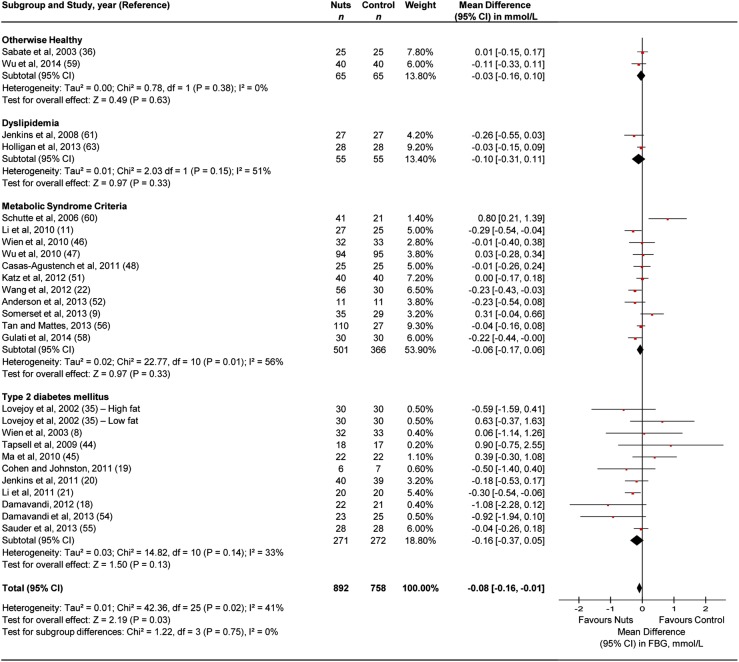
Figure 3 presents the effect of tree nuts on fasting blood glucose. Tree nuts showed a significant fasting blood glucose-lowering effect (MD=−0.08 mmol/L (95% CI −0.16 to −0.01 mmol/L)) in the overall analysis, with evidence of moderate heterogeneity (I2=41%, p<0.05). Stratification by health status failed to demonstrate an effect for any of the subsamples. Sensitivity analyses did not alter the results (data not shown).
Figure 3.
Pooled effect estimates are shown as diamonds, one each for trials conducted in otherwise healthy, dyslipidaemia, metabolic syndrome criteria, type 2 diabetes mellitus and their combination (total). Paired analyses were applied to all cross-over trials (10) and one substudy. Data are expressed as mean differences with 95% CI, using generic inverse-variance random effects models. Interstudy heterogeneity was tested by using the Cochran Q statistic (Chi2) at a significance level of p<0.10 and quantified by the I2 statistic. FBG, fasting blood glucose; RCT, randomised controlled trial.
Online supplementary appendix table 3F and appendix figure 9 present the a priori continuous and categorical subgroup analyses, respectively, for fasting blood glucose. None of the subgroup analyses were significant.
Publication bias
Online supplementary appendix figure 10 presents the funnel plots for publication bias for each end point. Visual inspection of the funnel plots revealed some evidence of asymmetry in several of the end points. There was a small trial with larger effect estimate favouring tree nuts than control for waist circumference, which argues that the ‘small-study’ effect was actually not a source of potential bias (ie, smaller studies that favoured control were published). On the other hand, there were more small trials with larger effect estimates favouring control than tree nuts for triglycerides. Egger's test confirmed these small study effects for triglycerides (p<0.05). No other evidence of small study effects was detected by Egger's and Begg's tests.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to look at the effect of tree nuts on MetS criteria. Our systematic review and meta-analysis included 47 randomised trials in 2211 participants who were otherwise healthy or had MetS criteria, dyslipidaemia or type 2 diabetes mellitus. Tree nut consumption at a median dose of ∼50 g/day was found to decrease triglycerides significantly by ∼0.06 mmol/L, and decrease fasting blood glucose significantly by ∼0.08 mmol/L over a median follow-up of 8 weeks. No adverse effects were seen on waist circumference, HDL-C or BP, suggesting an overall net metabolic benefit of tree nuts.
Results in relation to other studies
Our findings of a reduction in triglycerides without the expected reciprocal increase in HDL-C are in accordance with previous evidence. Although Sabate et al64 did not show a triglyceride-lowering effect of nut interventions (non-specific to tree nuts) in overall pooled analyses in a patient-level meta-analysis of controlled feeding trials, they did show that nut interventions lowered triglycerides when analyses were restricted to a subsample of participants with baseline triglycerides ≥1.7 mmol/L, without an increase in HDL-C. A triglyceride benefit has also been seen in individual trials and meta-analyses of trials investigating the effect of a Mediterranean dietary pattern containing tree nuts in people with type 2 diabetes mellitus.65 66 This triglyceride-lowering effect, however, was accompanied by an HDL-C increasing effect.65 66 Our findings add to these data by showing a similar triglyceride-lowering effect, especially for walnuts, pistachios, macadamia and pecans, in the absence of an HDL-C increasing effect, across all subsamples of participants, without differences in triglycerides by baseline levels. The lipid benefits of tree nuts can be attributed to numerous cardioprotective nutrients such as unsaturated fatty acids, plant protein, fibre and phytochemicals.67 The fibre content and high unsaturated fat content, with its ability to displace high-glycaemic index carbohydrate from the diet and so effect a lower glycaemic load diet, are likely the main factors in lowering triglycerides.20
Our results of a reduction in fasting blood glucose are in accordance with an evidence-based review for the 2013 CDA guidelines that found evidence to support small improvements in overall glycaemic control in people with type 2 diabetes mellitus.23 Individual trials have shown evidence of improvements in other aspects of glycaemic control.19–22 A fasting blood glucose-decreasing effect has also been seen in long-term glycaemic control as assessed by glycated haemoglobin for tree nuts as part of Mediterranean65 66 68 and DASH69 dietary patterns in people with type 2 diabetes mellitus.70 The ability of tree nuts to decrease fasting blood glucose in our analyses may relate to the proposed displacement mechanism by which tree nuts reduce the glycaemic load of the diet, as this mechanism would be expected to improve long-term glycaemic control through a reduction in postprandial glycaemia,71 and possibly decrease insulin resistance,48 neither of which was assessed in our review.
The lack of effect we observed on waist circumference reinforces the view that tree nuts do not have an adverse effect on body weight. Dietary guidelines have raised concerns about the potential of tree nuts to contribute to weight gain,2 owing to their high energy density; however, prospective cohort studies and randomised trials have shown the opposite. A pooled analysis of Harvard cohorts showed that an increase in one serving per day of nuts was associated with significant weight loss.72 Controlled trials of tree nuts alone or as part of Mediterranean,65 66 68 Portfolio73 or DASH69 dietary patterns have shown neutral or weight loss effects, and no influence on body fat mass or body fat percentage.74 Dietary patterns that incorporated nuts have reported weight loss under isocaloric conditions or no weight gain under hypercaloric feeding conditions,75 perhaps because the metabolically available energy from nuts is less than the calculated value, as incomplete digestion of nuts leads to energy excretion in the faeces.76 Our findings further suggest that tree nuts do not have a significant effect on the most metabolically adverse weight gain involving an increase in waist circumference. We observed a tendency for a reduction in waist circumference, especially where nuts displaced high-glycaemic index carbohydrate to effect a lower glycaemic load diet (as opposed to where tree nuts were used to displace saturated fat). These data suggest that the inclusion of a greater number of long-term trials in which tree nuts are used to displace high-glycaemic index carbohydrate to effect a low-glycaemic load diet may yet demonstrate a waist circumference benefit in future meta-analyses.
We were surprised not to see an improvement in BP. Individual trials have shown evidence of improvements in BP.5 8 A BP-decreasing effect of tree nuts has also been seen in the context of Portfolio73 and DASH69 77 78 dietary patterns across a range of participant types. As elevated BP in the MetS often relates to the underlying insulin resistance, the lack of effect on BP may also be explained by a lack of trials using tree nuts to displace high-glycaemic index carbohydrate to decrease the low-glycaemic load of the diet (trials taking advantage of this mechanism were more likely to show reductions than trials that did not in subgroup analyses). Alternatively, it may be explained by the need for tree nuts to be combined with the other aspects of a DASH dietary pattern, which collectively results in larger amounts of potassium, calcium, magnesium, dietary fibre and protein.
Limitations
There are some limitations to our work. First, the majority of trials (74.4%) were of low quality (MQS<8). Factors that contributed the most to low-quality scores were incomplete outcome data and poor reporting. However, in our a priori subgroup analyses, there was no effect modification by study quality. Second, the risk of bias remains uncertain for most of the available trials owing to poor reporting. This point is particularly concerning given that the majority of the trials were conducted after the Consolidated Standards of Reporting Trials (CONSORT) guidelines were first reported in 1993 and published in 1996.79 Third, the majority of available trials were <3 months, which is perhaps, too short a time to observe an effect for some outcomes (waist circumference, BP). This also made it difficult to assess the sustainability of the observed effects over the long term. We did not, however, observe significant effect modification by follow-up in categorical or continuous subgroup analyses for any of the end points. Finally, our analyses were complicated by significant unexplained heterogeneity for waist circumference, and HDL-C, which we attempted to accommodate using random effects models, but it remains a source of uncertainty in the summary effect estimates for these end points.
Practical implications
Tree nuts are a high-energy food that contain cardioprotective nutrients.67 Although the median fat intake of the tree nut containing diets (33.6%) was above that of the control diets (30.5%), but within the recommended limits of dietary guidelines (20–35%),23 a beneficial effect was seen only in the tree nut containing diets. The median dose of ∼50 g/day of tree nuts can be easily integrated as a snack into the dietary pattern or as a substitution for animal fats or carbohydrates. No increase in side effects compared with control diets was reported in any of the trials, suggesting diets which emphasise tree nuts are as safe as conventional diets (except in individuals with tree nut allergies).
Conclusion
In conclusion, our pooled analyses indicate that daily tree nut consumption has an overall metabolic benefit, through modest decreases in triglycerides and fasting blood glucose while preserving waist circumference, HDL-C and BP in people who are otherwise healthy or have dyslipidaemia, MetS criteria or type 2 diabetes mellitus. These data support recommendations to consume tree nuts alone or as part of heart healthy dietary patterns such as the Mediterranean, Portfolio, Vegetarian and DASH dietary patterns as a mean for improving metabolic control.69 80–83 Careful interpretation of the results is advised, as our conclusions are limited by the short duration and poor quality of the majority of trials, as well as the presence of significant unexplained heterogeneity in our analyses. These limitations highlight the need for larger, longer, high-quality trials. Trials in which tree nuts are used to displace high-glycaemic index carbohydrate to decrease the glycaemic load of the diet will be especially relevant to understand the role of tree nuts in reducing cardiometabolic risk associated with the MetS.
Supplementary Material
Acknowledgments
The authors wish to thank Teruko Kishibe for her help in the development of search terms used.
Footnotes
Contributors: SBM, CWCK, LSA and JLS were involved in conception and design. SBM, CWCK, EV, LSA, VH, AIC, AM, AM, LC, LAL, RJdS, DJAJ and JLS were involved in analysis or interpretation of the data. SBM and JLS were involved in drafting of the article. SBM, CWCK, EV, LSA, VH, AIC, AM, AM, LC, LAL, RJdS, DJAJ and JLS were involved in critical revision of the article for important intellectual content. RJdS was involved in statistical expertise. CWCK, LSA, DJAJ and JLS were involved in obtaining funding. CWCK, EV, LSA, VH, AIC, AM, AM and LC were involved in administrative, technical or logistic support. SBM, EV, LSA, VH, AIC and AM were involved in collection and assembly of data. CWCK and JLS are the guarantors. All authors approved the final version of the article.
Funding: This work was supported by the International Tree Nut Council Nutrition Research & Education Foundation (Davis, CA), and the Canadian Institutes of Health Research (funding reference number, 129920) through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3-D) Centre, funded through the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation's Ontario Research Fund (ORF), provided the infrastructure for the conduct of this project. VH and AIC were funded by Province of Ontario Graduate Scholarships. AIC was also funded by a Canadian Institutes of Health Research (CIHR)-Fredrick Banting and Charles Best Canada Graduate Scholarship and Banting and Best Diabetes Centre (BBDC)-Novo Nordisk Studentship. RJdS was funded by a CIHR Postdoctoral Fellowship Award. DJAJ was funded by the Government of Canada through the Canada Research Chair Endowment.
Competing interests: CWCK has received research support from the Advanced Foods and Material Network, Agrifoods and Agriculture Canada, the Almond Board of California, the American Pistachio Growers, Barilla, the California Strawberry Commission, the Calorie Control Council, CIHR, the Canola Council of Canada, the Coca-Cola Company (investigator initiated, unrestricted grant), Hain Celestial, the International Tree Nut Council Nutrition Research and Education Foundation, Kellogg, Kraft, Loblaw Companies Ltd, Orafti, Pulse Canada, Saskatchewan Pulse Growers, Solae and Unilever. He has received travel funding, consultant fees and/or honoraria from Abbott Laboratories, the Almond Board of California, the American Peanut Council, the American Pistachio Growers, Barilla, Bayer, the Canola Council of Canada, the Coca-Cola Company, Danone, General Mills, the International Tree Nut Council Nutrition Research and Education Foundation, Kellogg, Loblaw Companies Ltd, the Nutrition Foundation of Italy (NFI), Oldways Preservation Trust, Orafti, Paramount Farms, the Peanut Institute, PepsiCo, Pulse Canada, Sabra Dipping Co, Saskatchewan Pulse Growers, Solae, Sun-Maid, Tate and Lyle, and Unilever. He is on the Dietary Guidelines Committee for the Diabetes Nutrition Study Group of the European Association for the Study of Diabetes and has served on the scientific advisory board for the Almond Board of California, the International Tree Nut Council, Oldways Preservation Trust, Paramount Farms and Pulse Canada. VH has received research support from the CIHR and the World Health Organization (WHO) for work on a systematic review and meta-analysis commissioned by WHO of the relation of saturated fatty acids with health outcomes. She received a travel award to attend a science day hosted by PepsiCo Inc and the New York Academy of Sciences. LC has received research support from the CIHR and the Agricultural Bioproducts Innovation Program through the Pulse Research Network (PURENet) and Saskatchewan Pulse Growers. She is also a casual clinical research coordinator at Glycemic Index Laboratories. RJdS is funded by a CIHR Postdoctoral Fellowship Award and has received research support from the CIHR, the Calorie Control Council, the Canadian Foundation for Dietetic Research and the Coca-Cola Company (investigator initiated, unrestricted grant). He has served as an external resource person to WHO's Nutrition Guidelines Advisory Group and received travel support from WHO to attend group meetings. He is the lead author of two systematic reviews and meta-analyses commissioned by WHO of the relation of saturated fatty acids and trans fatty acids with health outcomes. DJAJ has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd, Unilever, Barilla, the Almond Board of California, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, the Canola and Flax Councils of Canada, the Calorie Control Council, the CIHR, the Canada Foundation for Innovation and the Ontario Research Fund. He has been on the speaker's panel, served on the scientific advisory board, and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, Saskatchewan Pulse Growers, Sanitarium Company, Orafti, the Almond Board of California, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamental for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, the Coca-Cola Company, the Griffin Hospital, Abbott Laboratories, the Canola Council of Canada, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, the Nutritional Fundamentals for Health, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Saskatchewan Pulse Growers, the Soy Foods Association of North America, the NFI, Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the US Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). His wife is a director and partner of Glycemic Index Laboratories, and his sister received funding through a grant from the St Michael's Hospital Foundation to develop a cookbook for one of his studies. JLS has received research support from the CIHR, Calorie Control Council, the Coca-Cola Company (investigator initiated, unrestricted grant), Pulse Canada and the International Tree Nut Council Nutrition Research and Education Foundation. He has received travel funding, speaker fees and/or honoraria from the American Heart Association, ASN, the National Institute of Diabetes and Digestive and Kidney Diseases, CDA, the CNS, the Calorie Control Council, the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD), the International Life Sciences Institute (ILSI) North America, ILSI Brazil, the University of South Carolina, the University of Alabama at Birmingham, the Canadian Sugar Institute, Oldways Preservation Trust, NFI, Abbott Laboratories, Pulse Canada, Dr Pepper Snapple Group, Corn Refiners Association, the Coca-Cola Company and World Sugar Research Association. He has consulting arrangements with Winston & Strawn LLP and Tate and Lyle. He is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the CDA and the EASD, and he is on the ASN writing panel for a scientific statement on the metabolic and nutritional effects of fructose, sucrose and high-fructose corn syrup. He is a member of the Carbohydrate Quality Consortium and an unpaid scientific advisor for the Food, Nutrition and Safety Program of ILSI North America. His wife is an employee of Unilever Canada.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.US Food and Drug Administration. Qualified health claims: letter of enforcement discretion—nuts and coronary heart disease (Docket No 02P-0505), 2003
- 2.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans. 7th edn Washington, DC: US Government Printing Ofice, 2010 [Google Scholar]
- 3.Lichtenstein AH, Appel LJ, Brands M, et al. American Heart Association Nutrition Committee. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82–96 [DOI] [PubMed] [Google Scholar]
- 4.McKelvie RS, Moe GW, Ezekowitz JA, et al. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol 2013;29:168–81 [DOI] [PubMed] [Google Scholar]
- 5.Estruch R, Martinez-Gonzalez MA, Corella D, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006;145:1–11 [DOI] [PubMed] [Google Scholar]
- 6.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90 [DOI] [PubMed] [Google Scholar]
- 7.Ibarrola-Jurado N, Bullo M, Guasch-Ferre M, et al. Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: the PREDIMED study. PLoS ONE 2013;8:e57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wien MA, Sabate JM, Ikle DN, et al. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord 2003;27:1365–72 [DOI] [PubMed] [Google Scholar]
- 9.Somerset SM, Graham L, Markwell K. Isoenergetic replacement of dietary saturated with monounsaturated fat via macadamia nuts enhances endothelial function in overweight subjects. e-SPEN J 2013;8(3):e113–e119 [Google Scholar]
- 10.Curb JD, Wergowske G, Dobbs JC, et al. Serum lipid effects of a high-monounsaturated fat diet based on macadamia nuts. Arch Intern Med 2000;160:1154–8 [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Song R, Nguyen C, et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J Am Coll Nutr 2010;29:198–203 [DOI] [PubMed] [Google Scholar]
- 12.Torabian S, Haddad E, Cordero-MacIntyre Z, et al. Long-term walnut supplementation without dietary advice induces favorable serum lipid changes in free-living individuals. Eur J Clin Nutr 2010;64:274–9 [DOI] [PubMed] [Google Scholar]
- 13.Chisholm A, Mann J, Skeaff M, et al. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur J Clin Nutr 1998;52:12–16 [DOI] [PubMed] [Google Scholar]
- 14.Rajaram S, Burke K, Connell B, et al. A monounsaturated fatty acid-rich pecan-enriched diet favorably alters the serum lipid profile of healthy men and women. J Nutr 2001;131:2275–9 [DOI] [PubMed] [Google Scholar]
- 15.Jenkins DJ, Kendall CW, Marchie A, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation 2002;106:1327–32 [DOI] [PubMed] [Google Scholar]
- 16.Kocyigit A, Koylu AA, Keles H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr Metab Cardiovasc Dis 2006;16:202–9 [DOI] [PubMed] [Google Scholar]
- 17.Sheridan MJ, Cooper JN, Erario M, et al. Pistachio nut consumption and serum lipid levels. J Am Coll Nutr 2007;26:141–8 [DOI] [PubMed] [Google Scholar]
- 18.Damavandi RD. The effects of cashew consumption on serum glucose, insulin and lipoprotein in type 2 diabetic patients. Iran J Endocrinol Metab 2012;14:325–34 [Google Scholar]
- 19.Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism 2011;60:1312–17 [DOI] [PubMed] [Google Scholar]
- 20.Jenkins DJ, Kendall CW, Banach MS, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011;34:1706–11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Li SC, Liu YH, Liu JF, et al. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011;60:474–9 [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Li Z, Liu Y, et al. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr J 2012;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dworatzek PD, Arcudi K, Gougeon R, et al. Clinical practice guidelines nutrition therapy. Can J Diabetes 2013;37(Suppl 1):S45–55 [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
- 25.Graf C, Battisti WP, Bridges D, et al. Research methods & reporting. Good publication practice for communicating company sponsored medical research: the GPP2 guidelines. BMJ 2009;339:b4330. [DOI] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. Guidance for industry: evidence-based review system for the scientific evaluation of health claims—final, 2009 [Google Scholar]
- 27.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5 [DOI] [PubMed] [Google Scholar]
- 28.Heyland DK, Novak F, Drover JW, J, et al. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001;286:944–53 [DOI] [PubMed] [Google Scholar]
- 29.Elbourne DR, Altman DG, Higgins JP, et al. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140–9 [DOI] [PubMed] [Google Scholar]
- 30.Sabate J, Fraser GE, Burke K, et al. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med 1993;328:603–7 [DOI] [PubMed] [Google Scholar]
- 31.Spiller GA, Jenkins DA, Bosello O, et al. Nuts and plasma lipids: an almond-based diet lowers LDL-C while preserving HDL-C. J Am Coll Nutr 1998;17:285–90 [DOI] [PubMed] [Google Scholar]
- 32.Morgan WA, Clayshulte BJ. Pecans lower low-density lipoprotein cholesterol in people with normal lipid levels. J Am Diet Assoc 2000;100:312–18 [DOI] [PubMed] [Google Scholar]
- 33.Zambon D, Sabate J, Munoz S, et al. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med 2000;132:538–46 [DOI] [PubMed] [Google Scholar]
- 34.Iwamoto M, Imaizumi K, Sato M, et al. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur J Clin Nutr 2002;56:629–37 [DOI] [PubMed] [Google Scholar]
- 35.Lovejoy JC, Most MM, Lefevre M, et al. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr 2002;76:1000–6 [DOI] [PubMed] [Google Scholar]
- 36.Sabate J, Haddad E, Tanzman JS, et al. Serum lipid response to the graduated enrichment of a step I diet with almonds: a randomized feeding trial. Am J Clin Nutr 2003;77:1379–84 [DOI] [PubMed] [Google Scholar]
- 37.Tapsell LC, Gillen LJ, Patch CS, et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004;27:2777–83 [DOI] [PubMed] [Google Scholar]
- 38.Tamizifar B, Rismankarzadeh M, Vosoughi A, et al. A low-dose almond-based diet decreases LDL-C while preserving HDL-C. Arch Iran Med 2005;8:45–51 [Google Scholar]
- 39.Kurlandsky S, Stoke K. Cardioprotective effects of chocolate and almond consumption in healthy women. Nutr Res 2006;26:509–16 [Google Scholar]
- 40.Mukuddem-Petersen J, Stonehouse Oosthuizen W, et al. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br J Nutr 2007;97:1144–53 [DOI] [PubMed] [Google Scholar]
- 41.Gebauer SK, West SG, Kay CD, et al. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose-response study. Am J Clin Nutr 2008;88:651–9 [DOI] [PubMed] [Google Scholar]
- 42.Griel AE, Cao Y, Bagshaw DD, et al. A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. J Nutr 2008;138:761–7 [DOI] [PubMed] [Google Scholar]
- 43.Rajaram S, Haddad EH, Mejia A, et al. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr 2009;89:S1657–63 [DOI] [PubMed] [Google Scholar]
- 44.Tapsell LC, Batterham MJ, Teuss G, et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr 2009;63: 1008–15 [DOI] [PubMed] [Google Scholar]
- 45.Ma Y, Njike VY, Millet J, et al. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care 2010;33:227–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wien M, Bleich D, Raghuwanshi M, et al. Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr 2010;29:189–97 [DOI] [PubMed] [Google Scholar]
- 47.Wu H, Pan A, Yu Z, et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr 2010;140:1937–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casas-Agustench P, Lopez-Uriarte P, Bullo M, et al. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis 2011;21:126–35 [DOI] [PubMed] [Google Scholar]
- 49.Tey SL, Brown R, Gray A, et al. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. J Nutr Metab 2011;2011:357350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster GD, Shantz KL, Vander Veur SS, et al. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am J Clin Nutr 2012;96:249–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz DL, Davidhi A, Ma Y, et al. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. J Am Coll Nutr 2012;31:415–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anderson AD, Anderson MM, Jacobson JL, et al. Metabolic effects of bedtime pistachio consumption for 6 weeks in overweight persons. FASEB J 2013;27:1072.20. [Google Scholar]
- 53.Berryman CE, West SG, Bordi PL, et al. Daily inclusion of almonds (1.5 ounces) in a cholesterol-lowering diet maintains HDL-cholesterol and HDL subclasses in mildly hypercholesterolemic adults. Ann Nutr Metab 2013;63:1338 [Google Scholar]
- 54.Damavandi RD, Eghtesadi S, Shidfar F, et al. Effects of hazelnuts consumption on fasting blood sugar and lipoproteins in patients with type 2 diabetes. J Res Med Sci 2013;18:314–21 [PMC free article] [PubMed] [Google Scholar]
- 55. Sauder KA, McCrea CE, Kris-Etherton PM, et al. Effect of pistachios on lipids, lipoproteins, glucose metabolism, and insulin sensitivity in type 2 diabetes. FASEB J 2013;27:368.4. [Google Scholar]
- 56.Tan SY, Mattes RD. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur J Clin Nutr 2013;67:1205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tey SL, Gray AR, Chisholm AW, et al. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. J Nutr 2013;143:1254–62 [DOI] [PubMed] [Google Scholar]
- 58.Gulati S, Misra A, Pandey RM, et al. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition 2014;30:192–7 [DOI] [PubMed] [Google Scholar]
- 59.Wu L, Piotrowski K, Rau T, et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism 2014;63:382–91 [DOI] [PubMed] [Google Scholar]
- 60.Schutte AE, Van Rooyen JM, Huisman HW, et al. Modulation of baroreflex sensitivity by walnuts versus cashew nuts in subjects with metabolic syndrome. Am J Hypertens 2006;19:629–36 [DOI] [PubMed] [Google Scholar]
- 61.Jenkins DJ, Kendall CW, Marchie A, et al. Almonds reduce biomarkers of lipid peroxidation in older hyperlipidemic subjects. J Nutr 2008;138:908–13 [DOI] [PubMed] [Google Scholar]
- 62.West SG, Gebauer SK, Kay CD, et al. Diets containing pistachios reduce systolic blood pressure and peripheral vascular responses to stress in adults with dyslipidemia. Hypertension 2012;60:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Holligan S, West SG, Gebauer SK, et al. A moderate-fat diet with pistachios lowers small-dense LDL and improves markers of insulin sensitivity in subjects with moderately-elevated cholesterol Levels. FASEB J 2013;27:1057.13. [Google Scholar]
- 64.Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med 2010;170:821–7 [DOI] [PubMed] [Google Scholar]
- 65.Esposito K, Maiorino MI, Ciotola M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med 2009;151:306–14 [DOI] [PubMed] [Google Scholar]
- 66.Esposito K, Maiorino MI, Ceriello A, et al. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 2010;89:97–102 [DOI] [PubMed] [Google Scholar]
- 67.Kris-Etherton PM, Hu FB, Ros E, et al. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr 2008;138:S1746–51 [DOI] [PubMed] [Google Scholar]
- 68.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–41 [DOI] [PubMed] [Google Scholar]
- 69.Azadbakht L, Fard NR, Karimi M, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care 2011;34:55–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sievenpiper JL, Dworatzek PD. Food and dietary pattern-based recommendations: an emerging approach to clinical practice guidelines for nutrition therapy in diabetes. Can J Diabetes 2013;37:51–7 [DOI] [PubMed] [Google Scholar]
- 71.Josse AR, Kendall CW, Augustin LS, et al. Almonds and postprandial glycemia—a dose-response study. Metabolism 2007;56:400–4 [DOI] [PubMed] [Google Scholar]
- 72.Mozaffarian D, Capewell S. United Nations’ dietary policies to prevent cardiovascular disease. BMJ 2011;343:d5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenkins DJ, Jones PJ, Lamarche B, et al. Effect of a dietary portfolio of cholesterol-lowering foods given at 2 levels of intensity of dietary advice on serum lipids in hyperlipidemia: a randomized controlled trial. JAMA 2011;306:831–9 [DOI] [PubMed] [Google Scholar]
- 74.Sabate J, Cordero-Macintyre Z, Siapco G, et al. Does regular walnut consumption lead to weight gain? Br J Nutr 2005;94:859–64 [DOI] [PubMed] [Google Scholar]
- 75.Almario RU, Vonghavaravat V, Wong R, et al. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am J Clin Nutr 2001;74:72–9 [DOI] [PubMed] [Google Scholar]
- 76.Jenkins DJ, Kendall CW, Popovich DG, et al. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metabolism 2001;50:494–503 [DOI] [PubMed] [Google Scholar]
- 77.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24 [DOI] [PubMed] [Google Scholar]
- 78.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 79.Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637–9 [DOI] [PubMed] [Google Scholar]
- 80.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:S1588–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rizzo NS, Sabate J, Jaceldo-Siegl K, et al. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: the adventist health study 2. Diabetes Care 2011;34:1225–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jenkins DJ, Kendall CW, Marchie A, et al. The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism 2003;52:1478–83 [DOI] [PubMed] [Google Scholar]
- 83.Jenkins DJ, Kendall CW, Marchie A, et al. Direct comparison of a dietary portfolio of cholesterol-lowering foods with a statin in hypercholesterolemic participants. Am J Clin Nutr 2005;81:380–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.