Summary
Heterozygous germline inactivating mutations in the aryl hydrocarbon receptor-interacting protein (AIP) gene lead to pituitary adenomas that most frequently present in the setting of familial isolated pituitary adenoma syndrome, usually as somatotropinomas and prolactinomas. More recently, they have been found in a significant percentage of young patients presenting with pituitary macroadenoma without any apparent family history. We describe the case of a 19-year-old man who presented with a gigantic somatotropinoma. His family history was negative. His peripheral DNA showed a heterozygous AIP mutation (p.I13N), while tumor tissue only had the mutated allele, showing loss of heterozygosity (LOH) and suggesting that the mutation caused the disease.
Learning points
AIP mutations may be observed in sporadic somatotrope adenomas occurring in young patients.
LOH is a strong indicator that an AIP variant is disease causing.
Somatotrope adenomas in carriers of AIP mutations are generally larger and more difficult to cure.
Background
Germline inactivating mutations in the aryl hydrocarbon receptor-interacting protein (AIP) gene lead to pituitary adenomas that most frequently present in the setting of familial isolated pituitary adenoma (FIPA) syndrome, usually as somatotropinomas and prolactinomas (1) (2). AIP mutation-associated cases of acromegaly differ from AIP WT cases, in that they are statistically significantly larger, more frequently invasive, occur at a younger age, and are relatively resistant to the hormonal and tumoral effects of somatostatin analogs (2) (3) (4) (5). In AIP-mutated tumors, the heterozygous inactivating germline mutations are frequently associated with the loss of heterozygosity (LOH) due to a second genetic hit (mutation and deletion) of the other allele at the same locus at the level of tumor DNA. Recently, it has been reported that germline AIP mutations are present in a significant percentage of patients who present with a pituitary macroadenoma in pediatric age despite the absence of a family history of pituitary adenomas (6). Knowledge of the presence of such an AIP mutation helps the physician in predicting biological behavior and may assist in the choice of therapy, as multimodal treatment is usual in these cases where somatostatin analog responses are poor (2) (7). Herein, we report the case of a young man who presented with a very large (>4 cm) somatotropinoma, whose DNA analysis revealed an AIP mutation that was previously unreported in the clinical setting.
Case presentation
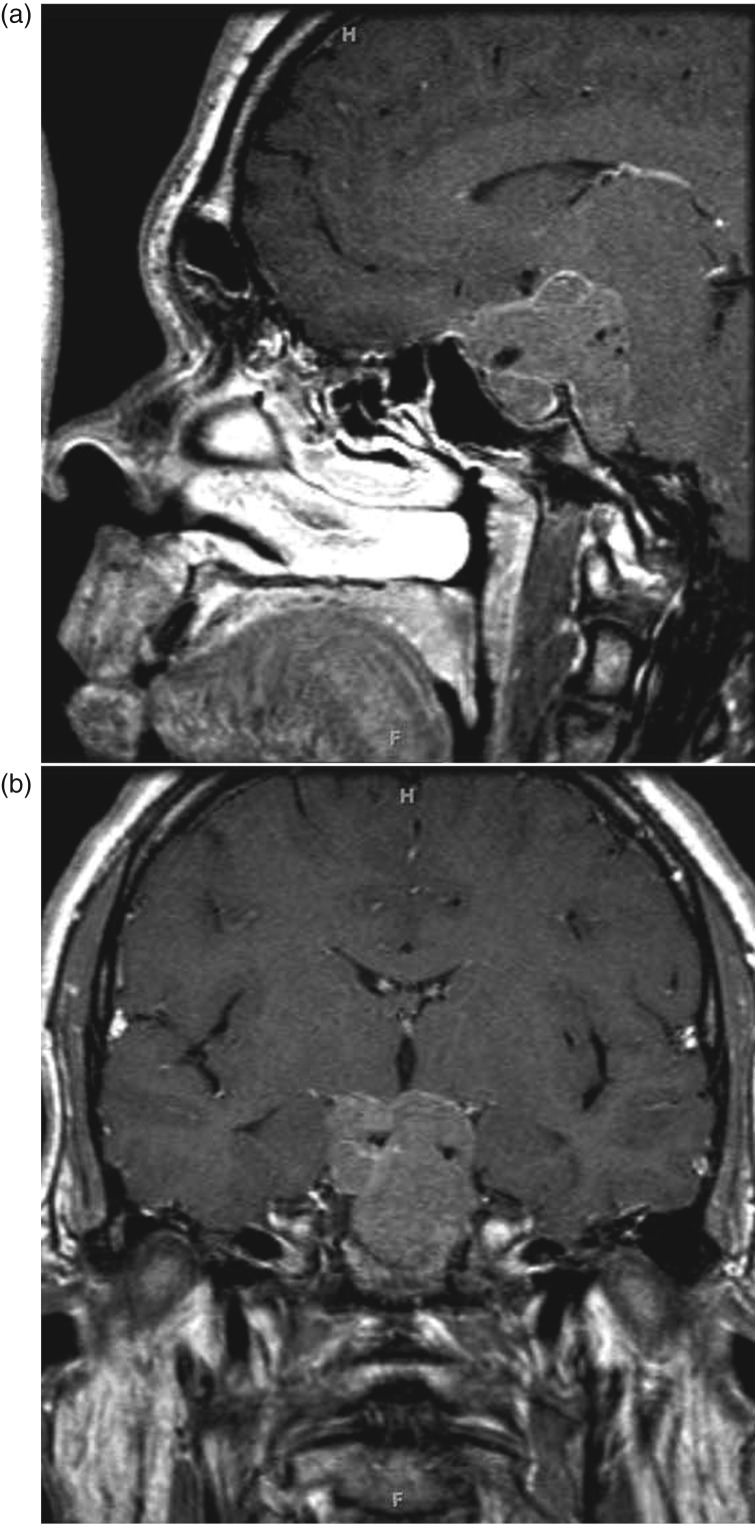
The patient, an adolescent Caucasian male, first noticed enlargement of his hands and worsening headaches beginning at the age of 16. His mother reported that he grew ∼22 cm between 15 and 17 years of age. When aged 17, he noticed some visual disturbances, which were ascribed to astigmatism and were treated with corrective lenses. A temporary improvement occurred but he reported that his vision again began to worsen. At the time, he also noted widening of his feet, pain in his knees and lower back, excessive sweating, and the expansion of his interdental spaces, associated with malocclusion due to a newly developed underbite. He denied symptoms of carpal tunnel syndrome or sleep apnea. Owing to the continuing headaches at the age of 19, he underwent brain magnetic resonance imaging (MRI) that revealed an extensive skull base mass, centered in the sellar and suprasellar region with compression of the optic chiasm and prechiasmatic nerves along with invasion of bilateral cavernous sinuses, encasement of the basilar artery, and significant extension into the prepontine cistern (Fig. 1). He was referred to an endocrinologist.
Figure 1.
Sagittal (a) and coronal (b) post-gadolinium MRI showing a large sellar mass with extensive skull base invasion.
He is an only child. His family history was negative for pituitary, parathyroid, pancreatic, and gastrointestinal tumor, and for recurrent kidney stones and hypercalcemia. His mother was 157.2 cm in height and his father was 177.8 cm (midparental height, 174.0 cm). On physical examination, he looked frankly acromegalic, with frontal bossing, prognathism, widely spaced teeth, and thick doughy hands. His height was 190.5 cm (+16.5 cm above midparental height) and he weighed 135.2 kg (calculated BMI 37.25 kg/m2). His blood pressure was 130/70 mmHg. He was normally androgenized, with a full beard and Tanner V genital development. Testicular volume was ∼8 ml.
A neuro-ophthalmological examination showed mild bitemporal changes and more prominent inferonasal field loss in both eyes, consistent with prechiasmal optic nerve compression. Visual acuity was diminished bilaterally (L 20/40 and R 20/50), color vision was impaired with changes consistent with moderate bilateral optic neuropathy, and compression of optic nerves. There were no oculomotor abnormalities and no optic nerve evidence of pallor.
Laboratory evaluation showed a frankly elevated serum insulin-like growth factor 1 level of 1056 ng/ml (normal range for age/sex, 108–548 ng/ml; Z score, +3.8) and an elevated random growth hormone (GH) level of 138 ng/ml (0–2.9). There was biochemical evidence of hypogonadism with a low serum testosterone of 16 ng/dl (241–827), accompanied by low luteinizing hormone (LH) (<0.2 mIU/ml) and follicle-stimulating hormone (FSH) (0.3 mIU/ml) concentrations. His prolactin level on repeated sampling (after dilution) was low at 2.3 ng/ml (normal range, 3.0–14.7), his thyroid function tests were normal, and his serum cortisol after adrenocorticotropic hormone (ACTH) simulation was 28.3 μg/dl.
Investigation
Genetic analysis was performed on patient's leukocytes on fresh neoplastic tissue. DNA from blood leukocytes was extracted by routine alkaline lysis. DNA was extracted from two separate fragments of fresh tumor tissue using the DNeasy Kit (Qiagen) following the manufacturer's recommendations. The six exons and the flanking intronic sequences of the AIP gene were amplified by PCR from peripheral blood DNA with previously reported primers (8) using standard techniques. The PCR products were purified and sequenced in two separate reactions (one with a forward primer and the other with a reverse primer) using capillary electrophoresis (model 730 XL; Applied Biosystems), by the Johns Hopkins Genetic Core. GH, prolactin, and ACTH immunostainings were carried out with commercially available antibodies. AIP immunostaining was performed using a monoclonal IgG antibody (Novus Biological, Cambridge, UK) at a dilution of 1:1000 on preserved paraffin-embedded tissue from the neurosurgical specimens. The study was approved by the Johns Hopkins Institutional Review Board, and written informed consent for the genetic studies was obtained from the patient.
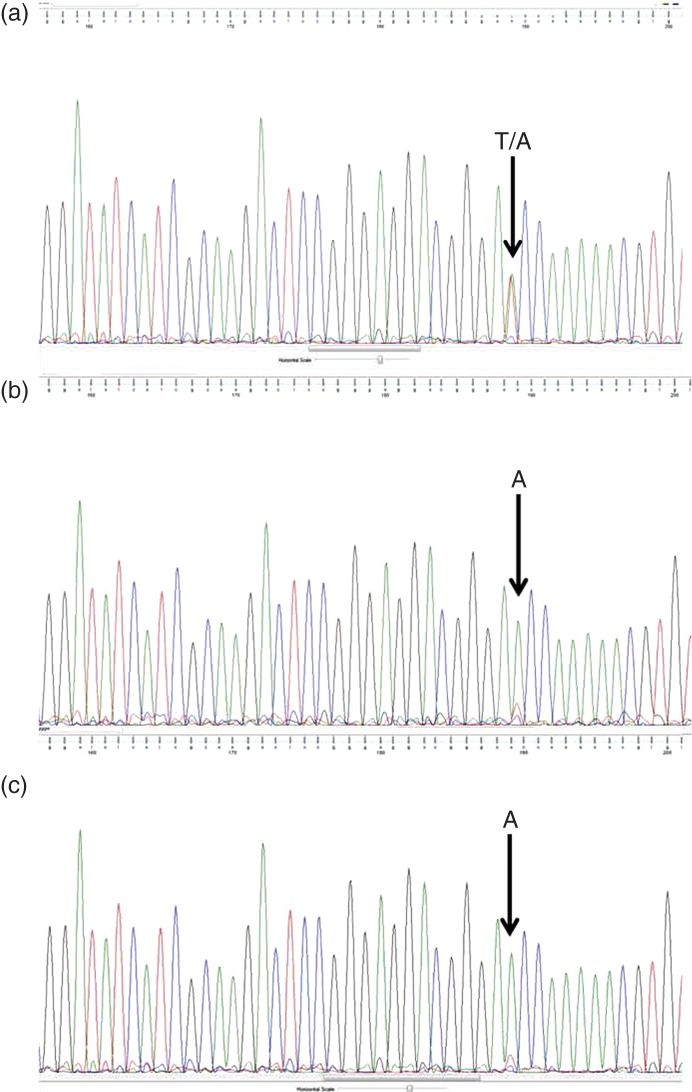
Sequence analysis from peripheral blood leukocyte DNA demonstrated a novel heterozygous mutation in exon 1 of AIP (c.38T>A), corresponding to a change of amino acid 13 from isoleucine to asparagine (p.I13N) (ATC>AAC) (Fig. 2a). The two tumor DNA samples demonstrated the loss of the normal sequence (Fig. 2b). in silico prediction of the impact of amino acid changes on functional stability of proteins was performed with PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/). This interpreted the I13N change as ‘probably damaging’ with a score of 0.999 (sensitivity, 0.14 and specificity, 0.99). The histopathology was consistent with a pituitary adenoma. Staining for GH was diffusely positive, prolactin staining was focally positive, while ACTH and other hormones (thyroid-stimulating hormone, FSH, and LH) were negative. Ki67 labeling index was estimated to be 10–15%. Cytokeratin staining demonstrated a sparsely granulated pattern.
Figure 2.
Sequence analysis of part of AIP gene exon 1 performed in blood DNA showing the heterozygous mutation 38T/38A (corresponding to p.I13N) (a) and in two different areas of the somatotropinoma (b and c) showing only the mutated allele sequence.
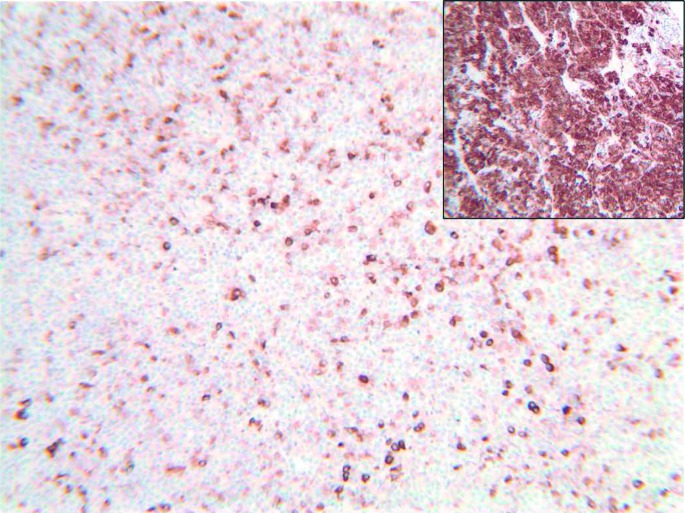
AIP immunostaining is shown in Fig. 3. It showed a low intensity of staining within the pituitary adenoma with some heterogeneity due to scattered cells having a closer to normal intensity of staining (for comparison, strong cytoplasmic staining for AIP is shown in the normal pituitary (Fig. 3, inset)).
Figure 3.
AIP immunostaining (×100) showing heterogeneous staining, with predominantly low-intensity or negative-staining cells with occasional cells displaying normal intensity. Staining intensity in the normal pituitary is shown in the inset image.
Treatment
The patient underwent an initial transsphenoidal surgery, which resulted only in partial tumor removal. A second surgery via supraorbital approach was performed.
Outcome and follow-up
The second debulking surgery resulted in complete decompression of the optic chiasm. GH level remained elevated at 4 weeks after surgery (59.4 ng/ml). Prolactin was further reduced to 0.3 ng/ml. Residual disease remains particularly in the prepontine area, with future plans for adjuvant medical (somatostatin analogs) and radiological therapy.
Discussion
Germline AIP mutations are being reported with an increasing frequency in FIPA families and in patients with apparently sporadic early-onset pituitary macroadenomas (2). Patients with these mutations usually present as children/adolescents or as young adults with large aggressive macroadenomas that are usually somatotropinomas (although all pituitary subtypes have been described in association with AIP germline mutations). Acromegaly patients with AIP mutations have statistically significantly higher GH levels at diagnosis when compared with sporadic AIP WT tumors (3). As our patient's clinical, hormonal, and radiological presentation fit this profile and was in line with recent genetic screening recommendations (2), we decided to sequence the AIP gene despite the lack of evidence of familial disease.
Although he did not meet the criteria for gigantism based on not being >+2 s.d. above the current mean height for United States (US) males, he was markedly taller than his midparental stature. As he appeared fully androgenized, we suspect that hypogonadism had appeared recently, and that his epiphyses closed before his stature could increase further. It is also interesting that prolactin level was low. This may reflect the destruction of normal pituitary tissue, as reported in 25% of pituitary macroadenomas (9).
The I13N change has not been previously reported in the clinical setting. This amino acid change substitutes a hydrophobic residue with a hydrophilic residue. This isoleucine residue is highly conserved in baboon, bovine, horse, rat, mouse, and zebrafish AIP, suggesting functional importance (www.ensemble.org), and the PolyPhen-2 prediction suggests that this change is probably damaging to the protein structure. Data from the Exome Variant Server reported one instance of this change (rs376913545) in an individual without clinical details; predicted effects of this change were also classified as probably damaging. Although more knowledge about the function of the AIP protein is being gained (2) (10) (11), no readily available functional assays exist to determine whether an AIP amino acid change is a mutation or a rare polymorphism. Much of the functional data regarding AIP protein have focused on the C-terminal domain, but recent information about the N-terminal structure and its binding of the important chaperone hsp90 has provided a possible pathophysiological pathway for the current and previous N-terminal AIP mutations (12) (13). It is generally believed that the AIP protein acts as a tumor suppressor gene and that the product of a single normal allele is sufficient to prevent tumorigenesis. A second somatic mutation (‘second hit’) is necessary to lose the expression of the normal allele and to cause disease. When AIP activity is lost, it has been shown recently that the mechanism of tumorigenesis is related to the loss of inhibition of cAMP synthesis due to alteration in the function of inhibitory G-protein Gαi2 (11). Accordingly, DNA sequence from two different areas of the macroadenoma showed only the mutated allele, with this LOH strongly suggesting that this amino acid change causes protein function abnormalities. Regrettably, DNA from the parents was not available, and therefore we cannot determine whether the mutation was inherited or if it occurred de novo.
The AIP immunostaining was consistent with the patterns observed in other pathological mutations, with decreased intensity when compared with the normal pituitary (14). This pattern indicates that, in this case, the AIP p.I13N change is associated with decreased expression of the AIP protein. Low AIP staining intensity is now well established to be a marker for more aggressive pituitary adenomas, including poor responses to somatostatin analogs (14) (15).
AIP belongs to the family of tetratricopeptide repeat (TPR) domain-containing proteins. It contains an inactive peptidyl-prolyl cis–trans isomerase (PPIase)-like domain, and three TPR motifs and a final α7 helix at the C-terminus (16). The TPR domains are highly degenerate consensus sequences of 34 amino acids, often arranged in tandem repeats, formed by two α-helices forming an antiparallel amphipathic structure that mediates intra- and intermolecular interactions in many proteins (10).
Owing to the lack of family history of pituitary tumors and of DNA from family members, we have no data on the segregation of the I13N change. Although the finding of LOH is strong evidence, it is not necessarily a proof that this variant is itself pathogenic. Other possibilities could explain LOH, such loss of AIP locus (common in pituitary tumors), or the existence of another AIP variant (in a non-coding or non-sequenced regions) that could be in fact the pathogenic one. Indeed, Toledo et al. (17) reported a patient with an AIP mutation diagnosed with both acromegaly and adrenocortical carcinoma (ACC). Although both tumors presented AIP LOH, it does not necessarily mean that the AIP mutation also caused the ACC.
In summary, we describe a young man with a very large somatotropinoma, who carries a germline AIP missense mutation described for the first time in the clinical setting, with the LOH in tumor samples. The p.I13N change should be considered as a likely disease-associated AIP mutation.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and associated images.
Author contribution statement
Dr R Salvatori identified the patient, performed genetic studies, and drafted the manuscript. Drs A Daly and A Beckers performed in silico analysis and contributed to the manuscript writing and editing. Dr A Quinones-Hinojosa operated the patient and collected tumor samples. Dr A Thiry performed AIP and other immunohistochemistry analyses.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The part of research performed at the Centre Hospitalier Universitaire de Liège was supported by the Fonds d'Investissement de Recherche Scientifique (FIRS) 2013.
References
- 1.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke Ret al. 2014Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 31220061228–1230 10.1126/science.1126100 [DOI] [PubMed] [Google Scholar]
- 2.Beckers A, Aaltonen LA, Daly AF & Karhu A. 2013Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocrine Reviews. 34: 239–277 10.1210/er.2012-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly AF, Tichomirowa MA, Petrossians P, Heliovaara E, Jaffrain-Rea ML, Barlier A, Naves LA, Ebeling T, Karhu A, Raappana Aet al. 2010Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. Journal of Clinical Endocrinology and Metabolism. 95: E373–E383 10.1210/jc.2009-2556 [DOI] [PubMed] [Google Scholar]
- 4.Daly AF, Vanbellinghen JF, Khoo SK, Jaffrain-Rea ML, Naves LA, Guitelman MA, Murat A, Emy P, Gimenez-Roqueplo AP, Tamburrano Get al. 2007Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. Journal of Clinical Endocrinology and Metabolism. 92: 1891–1896 10.1210/jc.2006-2513 [DOI] [PubMed] [Google Scholar]
- 5.Korbonits M, Storr H & Kumar AV. 2012Familial pituitary adenomas – who should be tested for AIP mutations?. Clinical Endocrinology. 77: 351–335 10.1111/j.1365-2265.2012.04445.x [DOI] [PubMed] [Google Scholar]
- 6.Tichomirowa MA, Barlier A, Daly AF, Jaffrain-Rea ML, Ronchi C, Yaneva M, Urban JD, Petrossians P, Elenkova A, Tabarin Aet al. 2011High prevalence of AIP gene mutations following focused screening in young patients with sporadic pituitary macroadenomas. European Journal of Endocrinology. 165: 509–515 10.1530/EJE-11-0304 [DOI] [PubMed] [Google Scholar]
- 7.Oriola J, Lucas T, Halperin I, Mora M, Perales MJ, Alvarez-Escola C, Paz de MN, Diaz SG, Salinas I, Julian MTet al. 2013Germline mutations of AIP gene in somatotropinomas resistant to somatostatin analogues. European Journal of Endocrinology. 168: 9–13 10.1530/EJE-12-0457 [DOI] [PubMed] [Google Scholar]
- 8.Cazabat L, Libe R, Perlemoine K, Rene-Corail F, Burnichon N, Gimenez-Roqueplo AP, Dupasquier-Fediaevsky L, Bertagna X, Clauser E, Chanson Pet al. 2007Germline inactivating mutations of the aryl hydrocarbon receptor-interacting protein gene in a large cohort of sporadic acromegaly: mutations are found in a subset of young patients with macroadenomas. European Journal of Endocrinology. 157: 1–8 10.1530/EJE-07-0181 [DOI] [PubMed] [Google Scholar]
- 9.Wichers-Rother M, Hoven S, Kristof RA, Bliesener N & Stoffel-Wagner B. 2004Non-functioning pituitary adenomas: endocrinological and clinical outcome after transsphenoidal and transcranial surgery. Experimental and Clinical Endocrinology & Diabetes. 112: 323–327 10.1055/s-2004-820914 [DOI] [PubMed] [Google Scholar]
- 10.Trivellin G & Korbonits M. 2011AIP and its interacting partners. Journal of Endocrinology. 210: 137–155 10.1530/JOE-11-0054 [DOI] [PubMed] [Google Scholar]
- 11.Tuominen I, Heliovaara E, Raitila A, Rautiainen MR, Mehine M, Katainen R, Donner I, Aittomaki V, Lehtonen HJ, Ahlsten Met al. 2012AIP inactivation leads to pituitary tumorigenesis through defective Gα-cAMP signaling. Oncogene. In press 10.1038/onc.2014.50 [DOI] [PubMed] [Google Scholar]
- 12.Linnert M, Haupt K, Lin YJ, Kissing S, Paschke AK, Fischer G, Weiwad M & Lücke CNMR assignments of the FKBP-type PPIase domain of the human aryl-hydrocarbon receptor-interacting protein (AIP). Biomolecular NMR Assignments. 6: 209–212 10.1007/s12104-012-9359-0 [DOI] [PubMed] [Google Scholar]
- 13.Linnert M, Lin YJ, Manns A, Haupt K, Paschke AK, Fischer G, Weiwad M & Lücke C. 2013The FKBP-type domain of the human aryl hydrocarbon receptor-interacting protein reveals an unusual Hsp90 interaction. Biochemistry. 52: 2097–2107 10.1021/bi301649m [DOI] [PubMed] [Google Scholar]
- 14.Jaffrain-Rea ML, Angelini M, Gargano D, Tichomirowa MA, Daly AF, Vanbellinghen JF, D'Innocenzo E, Barlier A, Giangaspero F, Esposito Vet al. 2009Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocrine-Related Cancer. 16: 1029–1043 10.1677/ERC-09-0094 [DOI] [PubMed] [Google Scholar]
- 15.Kasuki L, Vieira NL, Wildemberg LE, Colli LM, de Castro M, Takiya CM & Gadelha MR. 2012AIP expression in sporadic somatotropinomas is a predictor of the response to octreotide LAR therapy independent of SSTR2 expression. Endocrine-Related Cancer. 19: L25–L29 10.1530/ERC-12-0020 [DOI] [PubMed] [Google Scholar]
- 16.Morgan RM, Hernandez-Ramirez LC, Trivellin G, Zhou L, Roe SM, Korbonits M & Prodromou C. 2012Structure of the TPR domain of AIP: lack of client protein interaction with the C-terminal α-7 helix of the TPR domain of AIP is sufficient for pituitary adenoma predisposition. PLoS ONE. 7: e53339. 10.1371/journal.pone.0053339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toledo RA, Mendonca BB, Fragoso MC, Soares IC, Almeda MQ, Moraes MB, Laurenco DM Jr, Alves VA, Bronstein MD & Toledo SP. 2010Isolated familial somatotropinoma: 11q13-loh and gene/protein expression analysis suggests a possible involvement of aip also in non-pituitary tumorigenesis. Clinics. 65: 407–415 10.1590/S1807-59322010000400010 [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a