Abstract
Leptin released peripherally acts within the central nervous system (CNS) to modulate numerous physiological and behavioral functions. Histochemical identification of leptin-responsive CNS cells can reveal the specific cellular phenotypes and neural circuits through which leptin signaling modulates these functions. Leptin signaling elicits phosphorylation of signal transducer and activator of transcription 3 (pSTAT3), making pSTAT3-immunoreactivity (ir) a useful proxy for identifying leptin-responsive cells. Relatively low systemic doses of leptin (i.e., 10–130 μg/kg body wt) are sufficient to decrease food intake, inhibit gastric emptying, and increase sympathetic activity, but there are no histological reports of central pSTAT3-ir following leptin doses within this range. Considering this, we quantified central pSTAT3-ir in rats after intraperitoneal injections of leptin at doses ranging from 50 to 800 μg/kg body wt. Tissue sections were processed to identify pSTAT3-ir alone or in combination with immunolabeling for cocaine- and amphetamine-regulated transcript (CART), glucagon-like peptide-1 (GLP-1), prolactin-releasing peptide (PrRP), or dopamine-β-hydroxylase (DβH). Leptin doses as low as 50, 100, and 200 μg/kg body wt significantly increased the number of pSTAT3-ir cells in the arcuate nucleus of the hypothalamus (ARC), nucleus of the solitary tract (NTS), and ventromedial nucleus of the hypothalamus, respectively, and also led to robust pSTAT3 labeling in neural processes. The differential dose-dependent increases in pSTAT3-ir across brain regions provide new information regarding central leptin sensitivity. Within the ARC, CART-ir and pSTAT3-ir were often colocalized, consistent with evidence of leptin sensitivity in this neural population. Conversely, within the NTS, pSTAT3 only rarely colocalized with PrRP and/or DβH, and never with GLP-1.
Keywords: leptin, signal transducer and activator of transcription 3, arcuate nucleus of the hypothalamus, ventromedial nucleus of the hypothalamus, nucleus of the solitary tract, prolactin-releasing peptide, glucagon-like peptide-1, noradrenergic, cocaine- and amphetamine-regulated transcript
leptin, the peptide product of the obese (Ob) gene, is released from adipose tissue and the gastric epithelium to signal long-term and short-term caloric surfeit, respectively (4, 97). In this capacity, leptin acts as a hormonal feedback signal to promote negative energy balance (37), in part by increasing sensitivity to satiation signals (34, 74, 75, 77, 82) that decrease meal size (35, 36, 57, 59, 70). The strong influence of leptin signaling on energy balance, as well as reproductive, autonomic, and other physiological systems has been clearly demonstrated in rodents with recessive mutations in the gene encoding leptin [ob/ob mice (55)] or its receptor [db/db mice (50) and fa/fa rats (19, 54, 78)]. These rodent strains display marked obesity, hyperphagia, autonomic nervous system dysfunction, infertility, and disruption of hypothalamic-pituitary hormonal signaling (18, 27, 39, 50, 55, 96, 98, 99).
Leptin exerts its behavioral, metabolic, and hormonal effects, at least in part, via direct action within the central nervous system (CNS). Following peripheral release, leptin is unidirectionally transported into the CNS (8), where it can directly access leptin receptors expressed by cells within numerous CNS regions. Leptin receptors are abundantly expressed within the arcuate nucleus of the hypothalamus (ARC), the ventromedial nucleus of the hypothalamus (VMH), and the nucleus of the solitary tract (NTS) (33, 42, 83, 85), three regions that play integral roles in food intake, body weight regulation, glucose homeostasis, and energy metabolism (24, 41, 64, 68, 79, 84, 87). Indeed, acute central administration of leptin is sufficient to robustly decrease food intake and body weight (14, 42, 83), facilitate glucose homeostasis (68), and increase sympathetic nerve activity (17, 26). Furthermore, central leptin signaling is necessary for normal regulation of body weight and adiposity, since neuronal deletion of leptin receptors results in obesity (20), and CNS-specific rescue of leptin receptors in db/db mice ameliorates obesity (60).
Histological identification of leptin-responsive cells in the CNS can provide a wealth of information regarding the specific cellular phenotypes and neural circuits through which leptin signaling modulates physiology and behavior. The long form of the leptin receptor is a member of the cytokine receptor family and is coupled to the janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway (89). Leptin binding to this receptor results in phosphorylation of STAT 3 (pSTAT3) (92), making pSTAT3-immunoreactivity (ir) a useful proxy for identifying cells directly responsive to leptin. Centrally administered leptin increases pSTAT3 immunolabeling within the ARC, VMH, and NTS (49). However, given that endogenous leptin is secreted from peripheral tissues, the physiological relevance of centrally administered leptin is unclear.
A robust literature indicates that—similar to central leptin administration—systemic administration of leptin has pronounced effects on food intake, body weight, glucose homeostasis, reproductive function, and sympathetic activity (9, 25, 63, 73, 95). While most studies have used large intraperitoneal or intravenous doses of leptin (i.e., >1 mg/kg body wt) to observe these effects, systemic leptin doses ranging from 10 to 130 μg/kg body wt are sufficient to decrease food intake (10, 14, 23), inhibit gastric emptying (13), and increase sympathetic nervous system activity and lipolysis (86). Western blot and gel-shift assays indicate that peripheral doses of leptin as low as 50–100 μg/kg body wt are sufficient to increase STAT3 phosphorylation centrally (28, 92); however, these techniques do not permit identification of leptin-sensitive cellular populations or circuits. Studies that have identified central pSTAT3-positive cells following systemic leptin administration have used doses ranging from 1 to 15 mg/kg body wt (11, 12, 31, 43, 48, 51, 52, 62, 72, 80, 91), much higher than doses required for behavioral, metabolic, and hormonal responses. Central pSTAT3 immunolabeling following systemic leptin doses below 1 mg/kg body wt has not been reported. Considering this, we utilized immunohistochemical antigen retrieval techniques (11, 31, 62, 72) to reveal pSTAT3-ir within anatomically and phenotypically identified populations of CNS cells in rats after peripheral administration of leptin at doses ranging from 50 to 800 μg/kg body wt.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (Harlan, IN; 225–275 g body wt; n = 34) were housed singly in hanging stainless-steel wire mesh cages in a temperature-controlled room (20–22°C) on a 12:12-h light-dark cycle (lights on at 0700). Rats had ad libitum access to pelleted chow (Purina 5001) and water, except as noted. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Injections and Perfusions
Rats were weighed 1 day before leptin administration to determine proper dosage. Rats were deprived of food (but not water) for 16–18 h overnight before leptin or vehicle treatment, since food deprivation increases central responsiveness to systemically administered leptin in mice (11). On the day of the experiment, rats were removed from their home cages between 0830 and 1030 and injected intraperitoneally with 1.0 ml of sterile 0.15 M NaCl containing recombinant rat leptin (Sigma-Aldrich; L5037) as follows: 0 μg/kg body wt (n = 4), 50 μg/kg body wt (n = 3), 100 μg/kg body wt (n = 8), 200 μg/kg body wt (n = 6), 400 μg/kg body wt (n = 7), and 800 μg/kg body wt (n = 6). Leptin was dissolved in vehicle just before injection, and rats were returned to their home cages immediately after injection.
Ninety minutes after intraperitoneal injection, rats were deeply anesthetized with pentobarbital sodium (39 mg/1.0 ml ip, Fatal Plus Solution; Butler Schein) and perfused transcardially with a brief saline rinse followed by fixative (100 ml of 2% paraformaldehyde and 1.5% acrolein in 0.1 M phosphate buffer, followed by 100 ml of 2% paraformaldehyde alone) (65). Brains were postfixed in situ overnight at 4°C, then removed from the skull and cryoprotected for 24–48 h in 20% sucrose. Brains were blocked and sectioned coronally (35 μm) using a Leica freezing-stage sliding microtome. Sections were collected in six serial sets, and stored at −20°C in cryopreservant solution (94) until immunohistochemical processing.
Immunohistochemistry
pSTAT3 immunolabeling.
Immunohistochemical identification of pSTAT3 was used to measure direct leptin signaling in the brain, following a protocol adapted from those described previously (11, 12, 31, 53, 62, 72). Free-floating tissue sections were removed from cryoprotectant storage and rinsed thoroughly in 0.02 M phosphate buffer (PB). Importantly, our pilot studies indicate that this buffer molarity is essential for optimal pSTAT3-ir. Following rinses, tissue was treated with 0.5% sodium borohydride (20 min), 0.3% NaOH + 0.3% H2O2 (20 min), 0.3% glycine (10 min), and 0.03% SDS (10 min; all in 0.02 M PB). Nonspecific binding was prevented with a 20-min incubation in blocking solution (0.02 M PB containing 4% normal donkey serum, 0.4% Triton-X 100, and 1% BSA) before antibody incubation.
Primary and secondary antisera were diluted in blocking solution. Tissue sections were incubated in rabbit anti-pSTAT3 (1:1,000; Cell Signaling, D3A7). The specificity and selectivity of this commercially available antibody have been reported (16, 90). Tissue was incubated in biotinylated donkey anti-rabbit IgG (1:200; Jackson ImmunoResearch), treated with Elite Vectastain ABC reagents (Vector Laboratories), and reacted with diaminobenzidine (DAB) intensified with nickel sulfate to produce a blue-black reaction product.
ARC and NTS neural phenotypes.
To begin determining the chemical phenotypes of neurons demonstrating pSTAT3-ir following these relatively low doses of leptin, we assessed pSTAT3-ir within ARC neurons expressing the anorexigenic peptide, cocaine- and amphetamine-regulated transcript (CART) (61). We also assessed pSTAT3-ir within noradrenergic NTS neurons expressing dopamine-β-hydroxylase (DβH) alone or together with prolactin-releasing peptide (PrRP), and in NTS neurons expressing glucagon-like peptide-1 (GLP-1). CART, DβH/PrRP, and GLP-1 neurons were selected for examination because each population has been implicated in central control of body energy balance (61, 64, 79).
To identify pSTAT3-ir within neurons of the ARC that express CART, pSTAT3-labeled forebrain sections from some cases were subsequently incubated in rabbit anti-CART (1:10,000, Phoenix Pharmaceuticals, H-003-62) followed by Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:300; Jackson ImmunoResearch) to produce a green fluorescent cytoplasmic signal.
To localize pSTAT3 within DβH- and PrRP-positive neurons of the NTS, pSTAT3-labeled brain stem sections from some cases were incubated in a cocktail of mouse anti-DβH (1:5,000; Millipore, MAB308) and rabbit anti-PrRP (1:1,000; Phoenix Pharmaceuticals, H-008-52). After this, tissue sets were incubated in a cocktail of Cy3-conjugated donkey anti-rabbit IgG (1:300; Jackson ImmunoResearch) and Alexa Fluor 488-conjugated donkey anti-mouse IgG (1:300; Jackson ImmunoResearch) to produce red and green fluorescent cytoplasmic signals, respectively.
To localize pSTAT3 within NTS GLP-1-positive neurons, pSTAT3-labeled brain stem sections were incubated in rabbit anti-GLP-1 (1:10,000; Bachem, T-4363) followed by biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch), Elite Vectastain ABC reagents, and plain DAB to produce a brown cytoplasmic reaction product.
Imaging and Quantification
Quantification of pSTAT3-expressing cells in the ARC, dorsomedial (dm)VMH, and NTS.
pSTAT3-labeled tissue was visualized using a 20× objective on an Olympus microscope equipped for bright-field and fluorescent optics, and it was photographed using a digital camera (Hamamatsu Photonics, Hamamatsu, Japan). For all anatomical regions, pSTAT3-positive profiles were quantified on images using Adobe Photoshop CS4 image software. The criterion for counting a cell as pSTAT3-positive was the presence of visible blue-black nuclear immunolabeling, regardless of intensity.
pSTAT3-ir was quantified bilaterally at two rostrocaudal levels of the ARC (∼2.00 mm and 2.45 mm caudal to bregma), and bilateral counts were averaged per section. Within the dmVMH, pSTAT3-labeled profiles were quantified bilaterally at a single rostrocaudal level (∼2.45 mm caudal to bregma). This level represents the core of the nucleus and contained the most robust pSTAT3-ir after our highest peripheral dose of leptin. Within the NTS, pSTAT3-labeled cells were quantified bilaterally at two rostrocaudal levels (∼14.36 mm and 14.16 mm caudal to bregma; see Fig. 3) and bilateral counts were averaged per section. In all cases, distance from bregma was approximated on the basis of tissue comparison to a standard rat brain atlas (88).
Fig. 3.
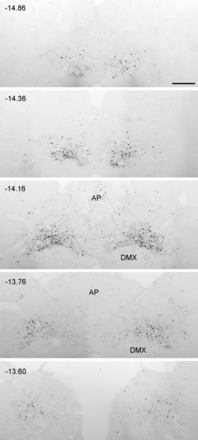
Images of pSTAT3 labeling through the rostrocaudal extent of the caudal nucleus of the solitary tract (NTS) in a representative rat after intraperitoneal leptin at a dose of 800 μg/kg body wt. pSTAT3 immunoreactivity was located primarily within the medial subnucleus of the caudal NTS. Numbers in each panel represent relative distance from bregma. AP, area postrema; DMX, dorsal motor nucleus of the vagus nerve. Scale bar = 200 μm, which applies to all panels.
Qualitative assessment of pSTAT3-ir in phenotypically identified ARC and NTS neurons.
pSTAT3-labeled sections colabeled for CART, DβH/PrRP, or GLP-1 were viewed on the Olympus photomicroscope described above. Using a 20× objective, we captured photographic images from a single selected rostrocaudal level of the ARC (∼2.00 mm caudal to bregma) or the NTS (∼14.36 mm caudal to bregma). Neurons were identified using Adobe Photoshop CS4 image software. Criteria for identifying a neuron as CART-, DβH/PrRP-, or GLP-1-positive included clear cytoplasmic labeling and a visible nucleus. Neurons were considered pSTAT3-positive if the nucleus contained pSTAT3 immunolabeling, regardless of intensity.
Statistics
Separate one-way ANOVAs were used to determine the effect of leptin dose (0, 50, 100, 200, 400, and 800 μg/kg body wt) on the number of pSTAT3-positive profiles within the ARC, dmVMH, and NTS. When F values indicated a significant effect, the ANOVA was followed by Fisher's least significant difference post hoc analyses. Differences were considered significant when P < 0.05.
Preparation of Images
Using Adobe Photoshop software, we adjusted photographic images for optimal brightness and contrast. Images that included both immunoperoxidase and immunofluorescence were altered to generate Fig. 5, B and C (i.e., blue-black NiDAB pSTAT3 labeling photographed in the green color channel was inverted, giving it the appearance of a fluorescent signal). The presence or absence of immunolabeling in images was not digitally manipulated.
Fig. 5.
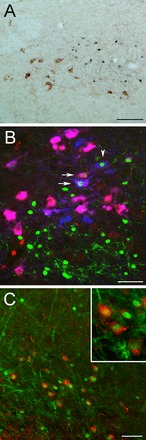
Representative color images of pSTAT3 and phenotypically identified neurons within the brain stem and hypothalamus. A: pSTAT3 (blue-black) is not colocalized in GLP-1-positive (brown) NTS neurons in rats after any dose of leptin (this image is from a rat treated with leptin at 200 μg/kg body wt; ∼14.36 mm caudal to bregma). B: pSTAT3 (green) is colocalized in some DβH-positive (blue) and DβH/PrRP-positive (purple-pink) neurons within the caudal NTS after leptin treatment (800 μg/kg body wt; ∼14.36 mm caudal to bregma). The majority of DβH- and DβH/PrRP-positive neurons did not colocalize pSTAT3, and vice versa. Arrows indicate pSTAT3+DβH/PrRP-positive neurons; the arrowhead indicates a pSTAT3+DβH-positive neuron that is not immunolabeled for PrRP. C: pSTAT3 (green) is colocalized with many CART-positive ARC neurons (red) after intraperitoneal leptin (400 μg/kg body wt; ∼2.45 mm caudal to bregma). Inset: higher-magnification view of several double-labeled ARC neurons. Scale bar in A = 100 μm. Scale bars in B and C = 50 μm.
RESULTS
The presence of leptin-induced pSTAT3-ir was assessed throughout the full rostrocaudal extent of the brain, from the upper cervical spinal cord through the prefrontal cortex. Systemically administered leptin elicited robust pSTAT3-ir within the ARC, dmVMH, and NTS. Other brain regions contained only scattered, sparse pSTAT3 labeling, even in rats that received the highest leptin dose.
pSTAT3 labeling within the ARC.
ANOVA indicated a significant effect of all leptin doses on the number of pSTAT3-positive profiles within the ARC [F (5,28) = 35.712, P < 0.001; Fig. 1D]. While some pSTAT3-ir was observed in the ARC of vehicle-treated rats, all doses of intraperitoneal leptin significantly increased pSTAT3-ir to the same degree (Fig. 1, A–C).
Fig. 1.
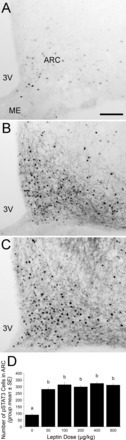
Representative images of pSTAT3 labeling within the arcuate nucleus (ARC) after intraperitoneal injection of saline vehicle (A), 200 μg/kg body wt leptin (B), or 800 μg/kg body wt leptin (C). Images depict the ARC ∼2.85 mm caudal to bregma. D: bar graph illustrating the number of pSTAT3-positive cells within the ARC bilaterally after intraperitoneal injection of leptin at doses from 0 to 800 μg/kg body wt. All doses significantly increased ARC pSTAT3 labeling compared with saline vehicle. Bars with different letters are significantly different (P < 0.05). 3V, third ventricle; ARC, arcuate nucleus of the hypothalamus; ME, median eminence. Scale bar in A = 100 μm, which applies also to B and C.
pSTAT3 labeling within the dmVMH.
In contrast to the binary pattern of STAT3 phosphorylation observed in the ARC (i.e., similar increases in pSTAT3-ir after all leptin doses), the dmVMH displayed a leptin dose-dependent increase in pSTAT3 labeling (Fig. 2, A–C). ANOVA indicated a significant effect of leptin dose on the number of pSTAT3-ir profiles within the dmVMH [F (5,28) = 78.057, P < 0.001]. pSTAT3-ir was negligible in vehicle-treated rats and in rats treated with 50 or 100 μg/kg body wt leptin, whereas higher doses elicited marked increases in the number of pSTAT3-ir profiles (Fig. 2D).
Fig. 2.
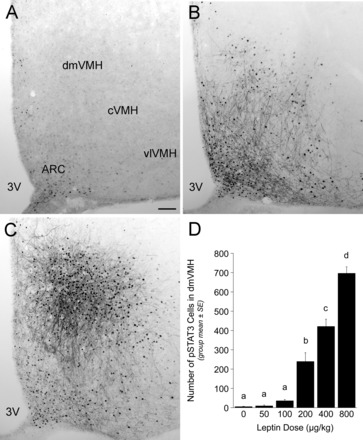
Representative images of pSTAT3 labeling within the dorsomedial subregion of the ventromedial hypothalamus (dmVMH) after intraperitoneal injection of saline vehicle (A), 200 μg/kg body wt leptin (B), or 800 μg/kg body wt leptin (C). Images depict the dmVMH ∼2.45 mm caudal to bregma. D: bar graph illustrating the number of pSTAT3-positive cells within the dmVMH after intraperitoneal injection of leptin at doses from 0–800 μg/kg body wt. Leptin dose-dependently increased dmVMH pSTAT3 labeling. Bars with different letters are significantly different (P < 0.05). 3V, third ventricle; ARC, arcuate nucleus of the hypothalamus; cVMH, central subregion of the ventromedial hypothalamus; vlVMH, ventrolateral subregion of the ventromedial hypothalamus. Scale bar in A = 100 μm, which applies also to B and C.
pSTAT3 labeling within the NTS.
The rostrocaudal extent of the NTS was examined for pSTAT3-ir to evaluate subregional effects of systemic leptin. Even in rats receiving the highest leptin dose (800 μg/kg body wt), pSTAT3-ir within the NTS was largely limited to the medial subnucleus of the caudal “visceral” NTS, with peak immunolabeling at the rostrocaudal level of the area postrema (Fig. 3). In each rat, STAT3 phosphorylation was quantified in two tissue sections through this level of the NTS (i.e., ∼14.36 and 14.16 mm caudal to bregma) (Fig. 3). ANOVA indicated a significant effect of leptin dose on the number of pSTAT3-ir profiles within the NTS at these rostrocaudal levels [F (5,28) = 11.282, P < 0.001]. pSTAT3-ir was negligible in vehicle-treated rats, whereas systemic leptin elicited a dose-dependent increase in pSTAT3-ir that reached a plateau at the 200 μg/kg body wt dose (Fig. 4).
Fig. 4.
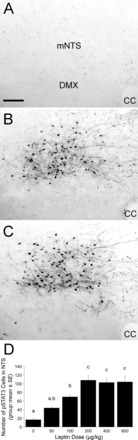
Representative images and summary data for pSTAT3 labeling within the NTS after intraperitoneal injection of saline vehicle (A), 200 μg/kg body wt leptin (B), or 800 μg/kg body wt leptin (C). Images depict the caudal NTS ∼14.36 mm caudal to bregma. D: bar graph illustrating the number of pSTAT3-positive cells within the NTS after intraperitoneal injection of leptin at doses from 0 to 800 μg/kg body wt. Leptin dose-dependently increased NTS pSTAT3 labeling. Bars with different letters are significantly different (P < 0.05). CC, central canal; mNTS, medial subnucleus of the nucleus of the solitary tract; DMX, dorsal motor nucleus of the vagus nerve. Scale bar in A = 100 μm, which applies also to B and C.
pSTAT3 immunolabeling in phenotypically identified neurons.
Consistent with a previous report using peripheral leptin administration in rats (51), pSTAT3 within the NTS was not colocalized in any GLP-1 neurons (Fig. 5A). pSTAT3-ir was colocalized in a subset of DβH- and DβH/PrRP-positive neurons of the caudal NTS (Fig. 5B). However, qualitative assessment indicated that the majority of DβH- and DβH/PrRP-positive neurons did not colocalize pSTAT3, and vice versa. Within the ARC, many CART-expressing neurons were also pSTAT3-positive (Fig. 5C), consistent with reports that peripheral leptin activates these neurons (21, 30).
pSTAT3 immunolabeling within neural processes.
We observed dose-dependent increases in cytoplasmic pSTAT3-ir within neural processes in the ARC, dmVMH, and NTS. Labeling within the ARC appeared to remain within the anatomic boundaries of the nucleus. Conversely, pSTAT3-positive dmVMH processes projected radially in all directions away from the core of the nucleus, with particularly prominent labeling located ventral to the dmVMH within the internuclear area separating the VMH from the ARC (Fig. 2C). Labeling of neural processes within the NTS was most prevalent within the medial subnucleus but was also observed within the commissural subnucleus and extending into the area postrema (Figs. 3 and 4, B and C).
DISCUSSION
Leptin plays a critical role in regulating energy balance and other physiological functions (15, 38, 71), and leptin signaling pathways that underlie these effects include direct activation of leptin receptors in brain stem and hypothalamic nuclei (45, 68, 81). To better understand how systemically released leptin acts on the brain to modulate physiology and behavior, it is necessary to localize and phenotypically identify leptin-responsive cells within the CNS. The present study is the first to immunohistochemically visualize pSTAT3 in rats after relatively low doses of systemic leptin. Doses used in the present study are known to elicit behavioral and physiological effects after systemic administration (13, 14, 86) and are much lower than systemic doses used previously to induce central pSTAT3 labeling. We report increased pSTAT3-ir after peripheral doses of leptin as low as 50 μg/kg body wt, well within the range of leptin doses that alter physiology and behavior (13, 14, 86). We also conducted the first reported dose-response assessment of leptin-induced pSTAT3 in several brain regions, providing new information regarding leptin sensitivity within and between central nuclei. Our results demonstrate that intraperitoneal leptin at doses ranging from 50 to 800 μg/kg body wt elicits unique and dose-dependent patterns of STAT3 phosphorylation in the ARC, dmVMH, and NTS.
Arcuate nucleus of the hypothalamus.
It is well established that the ARC plays a critical role in energy balance by transducing circulating signals, including the adiposity signal leptin, into neural responses (21, 22, 29). Leptin receptors are abundantly expressed within the ARC, and numerous studies have reported dense ARC pSTAT3-ir in rodents after high peripheral doses of leptin (11, 62, 91). However, our study is the first to assess leptin-induced pSTAT3-ir within the ARC after leptin doses lower than 1 mg/kg body wt. Our results demonstrate that the ARC displays moderate pSTAT3-ir in vehicle-treated rats, presumably the result of endogenous leptin or other signaling factors that phosphorylate STAT under these conditions. Interestingly, the number of pSTAT3-positive ARC cells increased to maximal levels following the lowest leptin dose used in the present study (50 μg/kg body wt), a dose 100–300 times lower than those previously shown to be effective (48, 62, 91). Higher leptin doses elicited no further increase in pSTAT3-ir. These results support the view that the ARC is unusually sensitive to systemically administered leptin, probably due to high rates of peripheral-to-central leptin transport at this site (7) and likely tied to the ARCs critical role in controlling body energy homeostasis.
Since leptin receptor activation within the ARC decreases food intake and body weight (81), we investigated the ability of peripheral leptin to phosphorylate STAT3 within ARC neurons that have a known anorexigenic role. ARC neurons that express both proopiomelanocortin (POMC) and CART (30) drive anorexigenic responses to leptin via downstream central targets (2, 5, 61). As predicted, many CART-positive ARC neurons colocalized pSTAT3 after 400 μg/kg body wt leptin. This finding is consistent with evidence that POMC/CART neurons contribute to the decreased food intake (14), inhibition of gastric motility (13), and increased sympathetic outflow (86) observed following peripheral doses of leptin similar to those used here.
Within the ARC, maximal pSTAT3-ir was observed in rats that received the lowest leptin dose (i.e., 50 μg/kg body wt). It will be important in future studies to identify the threshold dose for increased STAT3 phosphorylation within the ARC and to explore dose-related pSTAT3 responses among phenotypically identified neural populations.
Ventromedial nucleus of the hypothalamus.
The VMH participates in the control of feeding and energy balance (46), and recent evidence indicates that it does so, in part, through direct leptin signaling. The VMH expresses the leptin receptor (33, 83), and intra-VMH leptin injection facilitates blood glucose homeostasis (68), increases sympathetic outflow (81), and decreases food intake (56). Moreover, genetic deletion of the leptin receptor within a subset of VMH neurons that express steroidogenic factor-1 (SF-1) results in increased body weight and susceptibility to diet-induced obesity (24). The downstream targets through which VMH neurons modulate energy balance remain under investigation (32, 87).
Following peripheral administration of leptin, dose-dependent increases in pSTAT3-ir were observed within the VMH. pSTAT3-ir was present predominantly within the dorsomedial subregion of the VMH, although labeling was also observed in the central and ventrolateral subregions. Within the dmVMH, doses of leptin at or below 100 μg/kg body wt elicited negligible pSTAT3-ir, whereas dose-related increases in STAT3 phosphorylation were observed after administration of leptin at 200, 400, and 800 μg/kg body wt. The significant response to leptin at the 200 μg/kg dose is especially noteworthy, as this dose is 25 times lower than the lowest peripheral dose previously reported to increase pSTAT3-ir within the VMH (91). No plateau in pSTAT3-ir was observed within the VMH in the present study, suggesting that higher doses of leptin might induce additional STAT3 phosphorylation in this brain region.
The chemical phenotypes of VMH neurons sensitive to leptin at these doses are unclear, although they likely include SF-1 neurons that colocalize pituitary adenylate cyclase-activating polypeptide, based on evidence that these neurons mediate some of leptin's hypophagic effects (24, 44).
Nucleus of the solitary tract.
A growing literature has implicated neurons of the NTS in the changes in energy balance produced by central leptin signaling (for review, see Ref. 40). Recent studies show that leptin signaling in the NTS is not only sufficient to decrease food intake and body weight (42), but is necessary for maintenance of normal energy balance in freely feeding rats (45). Previous reports indicate that systemic leptin doses between 1 and 5 mg/kg body wt are sufficient to elicit STAT3 phosphorylation within the NTS (31, 51, 72, 91). No study, however, has investigated whether lower doses of leptin—sufficient to alter autonomic, physiological, and behavioral output (10, 13, 14, 86)—act directly on neurons of the NTS.
The present results demonstrate significantly elevated STAT3 phosphorylation within the NTS in rats after peripheral administration of leptin at doses as low as 100 μg/kg body wt, which is 10–50 times lower than doses used previously. pSTAT3-ir within the NTS displayed a leptin dose-response effect that was intermediate to effects observed within the ARC and the dmVMH. Some baseline pSTAT3-ir was present within the NTS in vehicle-treated rats, and the ability of leptin to increase pSTAT3-ir reached a plateau at the 200 μg/kg body wt dose. Increased pSTAT3-ir was confined to the caudal “visceral” NTS, a region known for its role in satiation and visceral sensory processing (64). Consistent with a previous report using systemic leptin administration (51), most of the pSTAT3-ir observed in the present study was located within the medial subnucleus (mNTS), which receives digestive related vagal sensory input (1, 69). Intra-mNTS leptin injections suppress food intake and motivation for food seeking (42, 58), while virus-mediated knockdown of mNTS leptin receptors increases food intake, meal size, body weight, and adiposity (45, 59). However, the neural circuits underlying these effects remain unclear.
The majority of pSTAT3-ir within the caudal NTS was localized in phenotypically unidentified neurons. We were somewhat surprised to observe relatively few DβH- and DβH/PrRP-positive neurons among those expressing pSTAT3-ir, because these neurons together comprise the A2 noradrenergic cell group, which contributes importantly to food intake and meal size control in rats (64, 79). GLP-1 neurons were never pSTAT3-positive, consistent with a previous report in rats, but in contrast to histological and electrophysiological results in mice (47, 51). This is not to say, however, that leptin does not affect A2 or GLP-1-expressing neurons in rats. Leptin exerts a strong depolarizing effect on glutamatergic vagal afferents that synapse directly onto neurons of the NTS (3, 74, 76), providing a potential route through which leptin receptors might modulate visceral afferent signaling to caudal NTS neurons, including A2 and GLP-1 neurons.
Dose-response summary.
To our knowledge, this report is the first to describe differential dose-related sensitivity of central pSTAT3-ir responses to leptin. The ARC displayed the greatest sensitivity, with maximal pSTAT3 labeling observed after the lowest leptin dose administered (i.e., 50 μg/kg body wt), while pSTAT3-ir within the NTS did not reach maximal levels until rats were dosed with leptin at 200 μg/kg body wt. Both the ARC and the NTS displayed leptin dose-related plateaus in pSTAT3-ir, evidence that higher doses would be unlikely to further increase pSTAT3-ir in these regions. Phosphorylation of STAT3 within the dmVMH, however, increased progressively through the highest dose administered (i.e., 800 μg/kg body wt) without reaching an evident plateau, suggesting that higher doses might produce additional increases in pSTAT3-ir within the VMH.
Cytoplasmic pSTAT3 immunoreactivity.
In addition to nuclear labeling, strong pSTAT3-ir was present within neural processes. Leptin receptors are expressed on both proximal and distal dendrites, where STAT3 phosphorylation occurs before translocation to the nucleus (6, 43, 93). Thus, neuritic labeling likely represents dendritic pSTAT3 that has yet to undergo nuclear translocation. Because pSTAT3 accumulates in the cell's nucleus but not in the cytoplasm, it is likely that visualization of dendritic pSTAT3-ir requires more sensitive immunohistochemical and optic techniques than are commonly used (43). Our report is not the first to show dendritic pSTAT3 labeling (43, 48, 49, 80), but immunolabeling in the present study is significantly more pronounced than previously documented.
Importantly, the dendritic pSTAT3 labeling in our material closely matches established dendritic patterns of neurons within each brain region. Golgi staining reveals that ARC neuronal dendrites remain largely within the anatomical boundaries of the ARC (66), whereas VMH neuronal dendrites radiate in all directions, including ventrally directed dendritic branches that extend into the internuclear area separating the ARC from VMH (66, 67). These dendritic patterns precisely match the pSTAT3 labeling we observed within the medial hypothalamus. Within the NTS, dendritic pSTAT3 immunolabeling was confined predominantly to the medial subnucleus but was also observed in processes extending medially into the commissural subnucleus and dorsally into the area postrema. This pattern of labeling reflects the organization of NTS neuronal dendrites (31) and is consistent with dendritic pSTAT3 labeling reported within the NTS of rats that received a much higher systemic dose of leptin (43, 48).
Perspectives and Significance
Since its discovery 20 years ago, much has been learned about leptin and leptin receptor-mediated regulation of physiology and behavior. However, much remains to be discovered regarding the structure and function of neural circuits through which leptin elicits its diverse effects. The present study documents STAT3 phosphorylation within the rat CNS after systemic doses of leptin that are up to 300 times lower than those used previously to generate pSTAT3-ir. Furthermore, this study provides the first dose-response assessment of leptin-induced STAT3 phosphorylation within the ARC, VMH, and NTS, providing new information regarding leptin sensitivity within and between these nuclei. Additional studies will be necessary to further characterize the neurochemical phenotypes and axonal projections of central neurons in which STAT3 is phosphorylated by systemic leptin. Further characterization of central leptin-sensitive neurons and circuits will lead to a better understanding of how peripherally derived leptin acts centrally to modulate energy balance and numerous other physiological functions.
GRANTS
This research was supported by National Institutes of Health Grant MH59911 to L. Rinaman.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.W.M. and L.R. conception and design of research; J.W.M. performed experiments; J.W.M. analyzed data; J.W.M. and L.R. interpreted results of experiments; J.W.M. prepared figures; J.W.M. drafted manuscript; J.W.M. and L.R. edited and revised manuscript; J.W.M. and L.R. approved final version of manuscript.
REFERENCES
- 1.Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14: 351–355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci 27: 13292–13302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature 394: 790–793, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Banks AS, Davis SM, Bates SH, Myers MG., Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab 278: E1158–E1165, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Banks WA, Kastin AJ, Huang WEA. Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305–311, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology 137: 3144–3147, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 94: 10455–10460, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becskei C, Lutz TA, Riediger T. Reduced fasting-induced activation of hypothalamic arcuate neurons is associated with hyperleptinemia and increased leptin sensitivity in obese mice. Am J Physiol Regul Integr Comp Physiol 299: R632–R641, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Bouret SG, Bates SH, Chen S, Myers MG, Jr, Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci 32: 1244–1252, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cakir B, Kasimay O, Devseren E, Yegen BC. Leptin inhibits gastric emptying in rats: role of CCK receptors and vagal afferent fibers. Physiol Res 56: 315–322, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269: 546–549, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Carlton ED, Demas GE, French SS. Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm Behav 62: 272–279, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol 518: 459–476, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Casto RM, VanNess JM, Overton JM. Effects of central leptin administration on blood pressure in normotensive rats. Neurosci Lett 246: 29–32, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12: 318–320, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271: 994–996, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108: 1113–1121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord 25: S63–S67, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Cowley MA, Cone R, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann NY Acad Sci 994: 175–186, 2003 [DOI] [PubMed] [Google Scholar]
- 23.de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology 151: 3589–3599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Dryden S, King P, Pickavance L, Doyle P, Williams G. Divergent effects of intracerebroventricular and peripheral leptin administration on feeding and hypothalamic neuropeptide Y in lean and obese (fa/fa) Zucker rats. Clin Sci 96: 307–312, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes 46: 2040–2043, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Durham HA, Truett GE. Development of insulin resistance and hyperphagia in Zucker fatty rats. Am J Physiol Regul Integr Comp Physiol 290: R652–R658, 2006 [DOI] [PubMed] [Google Scholar]
- 28.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol 2000: 2, 2000 [PubMed] [Google Scholar]
- 30.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Ellacott KLJ, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147: 3190–3195, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA 95: 741–746, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998 [PubMed] [Google Scholar]
- 34.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neuronal responsivity to CCK. Am J Physiol Regul Integr Comp Physiol 276: R1545–R1549, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Flynn MC, Plata-Salaman CR. Leptin (OB protein) and meal size. Nutrition 15: 508–509, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Flynn MC, Scott TR, Pritchard TC, Plata-Salaman CR. Mode of action of OB protein (leptin) on feeding. Am J Physiol Regul Integr Comp Physiol 275: R174–R179, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest 121: 2087–2093, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giachetti A. The functional state of sympathetic nerves in spontaneously diabetic mice. Diabetes 27: 969–974, 1978 [DOI] [PubMed] [Google Scholar]
- 40.Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Ha S, Baver S, Huo L, Gata A, Hairston J, Huntoon N, Li W, Zhang T, Benecchi EJ, Ericsson M, Hentges ST, Bjorbaek C. Somato-dendritic localization and signaling by leptin receptors in hypothalamic POMC and AgRP neurons. PLoS One 8: e77622, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci 29: 14828–14835, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec 78: 149–172, 1940 [Google Scholar]
- 47.Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like peptide 1 neurons. Diabetes 59: 1890–1898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y. Brain stem is a direct target for leptin's action in the central nervous system. Endocrinology 143: 3498–3504, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Hubschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci 21: 2413–2424, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science 153: 1127–1128, 1966 [DOI] [PubMed] [Google Scholar]
- 51.Huo L, Gamber KM, Grill HJ, Bjorbaek C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology 149: 492–497, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148: 2189–2197, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Huo L, Munzberg H, Nillni EA, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic trh gene expression by leptin. Endocrinology 145: 2516–2523, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Substitution at codon 269 (glutamine → proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun 224: 597–604, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered 41: 317–318, 1950 [DOI] [PubMed] [Google Scholar]
- 56.Jacob RJ, Dziura J, Medwick MB, Leone P, Caprio S, During M, Shulman GI, Sherwin RS. The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus. Diabetes 46: 150–152, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Kahler A, Geary N, Eckel LA, Campfield LA, Smith FJ, Langhans W. Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. Am J Physiol Regul Integr Comp Physiol 275: R180–R185, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Kanoski SE, Alhadeff AL, Fortin SM, Gilbert JR, Grill HJ. Leptin signaling in the medial nucleus tractus solitarius reduces food seeking and willingness to work for food. Neuropsychopharmacology 39: 605–613, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, Bence KK, Hayes MR, Grill HJ. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab 303: E496–E503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kowalski TJ, Liu SM, Leibel RL, Chua SC., Jr. Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes 50: 425–435, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393: 72–76, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Lin L, Martin R, Schaffhauser AO, York DA. Acute changes in the response to peripheral leptin with alteration in the diet composition. Am J Physiol Regul Integr Comp Physiol 280: R504–R509, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1. Front Neurosci 6: 199, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLean IW, Nakane PK. Periodate-lysine-paraformldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974 [DOI] [PubMed] [Google Scholar]
- 66.Millhouse OE. A golgi anatomy of the rodent hypothalamus. In: Handbook of the Hypothalamus, edited by Morgane PJ, Panksepp J. New York: Marcel Dekker, 1979, p. 221–265 [Google Scholar]
- 67.Millhouse OE. The organization of the ventromedial hypothalamic nucleus. Brain Res 55: 71–87, 1973 [PubMed] [Google Scholar]
- 68.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48: 287–291, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Miselis RR, Rinaman L, Altschuler SM, Bao X, Lynn RB. Medullary viscerotopic representation of the alimentary canal innervation in rat. In: Brain-Gut Interactions, edited by Tache Y, Wingate D. Boca Raton, FL: CRC Press, 1991, p. 3–17 [Google Scholar]
- 70.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril 77: 433–444, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144: 2121–2131, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Niimi M, Sato M, Yokote R, Tada S, Takahara J. Effects of central and peripheral injection of leptin on food intake and on brain Fos expression in the Otsuka Long-Evans Tokushima Fatty rat with hyperleptinaemia. J Neuroendocrinol 11: 605–611, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Peters JH, Karpiel AB, Ritter RC, Simasko SM. Cooperative activation of cultured vagal afferent neurons by leptin and cholecystokinin. Endocrinology 145: 3652–3657, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol 288: R879–R884, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol 290: R1544–R1549, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav 89: 477–485, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet 13: 18–19, 1996 [DOI] [PubMed] [Google Scholar]
- 79.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105: 7257–7262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K, Yoshimasa Y, Nishi S, Hosoda K, Nakao K. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci Lett 224: 149–152, 1997 [DOI] [PubMed] [Google Scholar]
- 82.Schwartz GJ, Moran TH. Leptin and neuropeptide Y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology 143: 3779–3784, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98: 1101–1106, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 85.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 514: 518–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett 416: 193–197, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH → arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci 8: 1356–1363, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Swanson L. Brain Maps III. Structure of the Rat Brain. Amsterdam: Elsevier, 2004 [Google Scholar]
- 89.Tartaglia LA. The leptin receptor. J Biol Chem 272: 6093–6096, 1997 [DOI] [PubMed] [Google Scholar]
- 90.Tripathi RB, McTigue DM. Chronically increased ciliary neurotrophic factor and fibroblast growth factor-2 expression after spinal contusion in rats. J Comp Neurol 510: 129–144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turek VF, Trevaskis JL, Levin BE, Dunn-Meynell AA, Irani B, Gu G, Wittmer C, Griffin PS, Vu C, Parkes DG, Roth JD. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology 151: 143–152, 2010 [DOI] [PubMed] [Google Scholar]
- 92.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97, 1996 [DOI] [PubMed] [Google Scholar]
- 93.Villanueva EC, Myers MG., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes 32 Suppl 7: S8–S12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watson RE, Wiegand ST, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7: 155–159, 1986 [DOI] [PubMed] [Google Scholar]
- 95.Wetzler S, Dumaz V, Goubern M, Tome D, Larue-Achagiotis C. Intraperitoneal leptin modifies macronutrient choice in self-selecting rats. Physiol Behav 83: 65–72, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Young JB, Landsberg L. Diminished sympathetic nervous system activity in genetically obese (ob/ob) mouse. Am J Physiol Endocrinol Metab 245: E148–E154, 1983 [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994 [DOI] [PubMed] [Google Scholar]
- 98.Zucker LMZT. Fatty, a new mutation in the rat. J Hered 52: 275–278, 1961 [Google Scholar]
- 99.Zucker TF, Zucker LM. Fat accretion and growth in the rat. J Nutr 80: 6–19, 1963 [DOI] [PubMed] [Google Scholar]


