Abstract
Endogenous acetylcarnitine is an indicator of acetyl-CoA synthesized by multiple metabolic pathways involving carbohydrates, amino acids, fatty acids, sterols, and ketone bodies, and utilized mainly by the tricarboxylic acid cycle. Acetylcarnitine supplementation has beneficial effects in elderly animals and humans, including restoration of mitochondrial content and function. These effects appear to be dose-dependent and occur even after short-term therapy. In order to set the stage for understanding the mechanism of action of acetylcarnitine, we review the metabolism and role of this compound. We suggest that acetylation of mitochondrial proteins leads to a specific increase in mitochondrial gene expression and mitochondrial protein synthesis. In the aged rat heart, this effect is translated to increased cytochrome b content, restoration of complex III activity, and oxidative phosphorylation, resulting in amelioration of the age-related mitochondrial defect.
Keywords: aging, mitochondrial metabolism, acetyl-CoA, electron transport chain complexes, mitochondrial proteins, mitochondrial biogenesis, complex III
1. Introduction
With people living longer, the number of aged individuals in the population in most industrialized countries is increasing and has important socio-economic and health consequences. Although medical progress has delayed death, improvements in alleviating the aging process lag behind; as a consequence, degenerative diseases, such as cardiovascular disease, Alzheimer, and cancer have increased [1]. The need for rational strategies to forestall the negative consequences of aging is one of the most important challenges for scientists in the 21st century.
Defects in oxidative phosphorylation during aging are now recognized as central players in impaired cellular and organ function (reviewed by Lesnefsky and Hoppel [2]). Impaired mitochondrial function not only affects energy production, but also increases the production of reactive oxygen species, further contributing to the aging process. Therapeutic agents targeting the mitochondrial defect constitute a meaningful way to fight aging.
Our review focuses on acetylcarnitine as a potential player in preventing age-related defects. Understanding the mechanism of action and the target of an agent that largely obviates age-related mitochondrial dysfunction is a rational approach for development of novel therapeutic agents. Such understanding leads to hope for improving health in the elderly. Why acetylcarnitine?— especially since this agent has failed in clinical trials for Alzheimer's disease [3], diabetic neuropathy [4, 5], and fatigue syndrome [6]. In fact, the literature contains little explanatory information concerning metabolism, metabolic effect, and potential mechanisms of the putative beneficial effect of acetylcarnitine in aging.
Herein we present acetylcarnitine as the avatar of metabolism, influenced by synthesis and utilization of acetyl-CoA through multiple metabolic pathways. By dissecting the effects of acetylcarnitine reported to occur in experimental studies, this review proposes a mechanism of action of the compound in the prevention of mitochondrial aging-related defects.
2. Endogenous acetylcarnitine
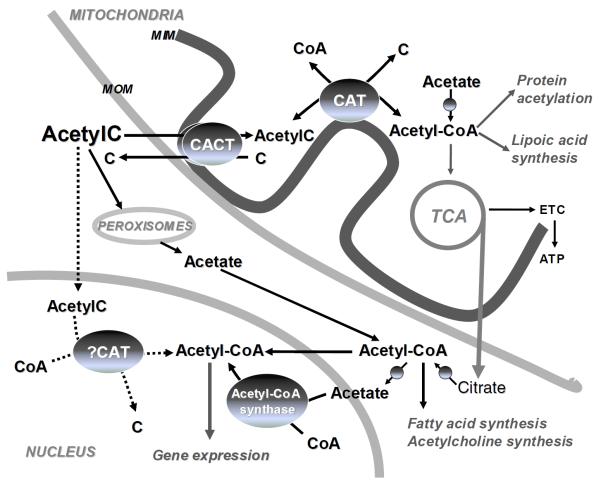
The equilibrium between acetyl-CoA (plus carnitine) and CoA (plus acetylcarnitine) (acetyl-CoA/CoA ratio) is crucial for mitochondrial metabolism. The mitochondrial content of endogenous acetylcarnitine is an indicator of mitochondrial metabolism of acetyl-CoA (Figure 1). Acetyl-CoA, derived from pyruvate, amino acids, and fatty acids, is reversibly converted to acetylcarnitine and CoA in the presence of carnitine by the carnitine acetyltransferase (CAT), a mitochondrial matrix enzyme attached to the inner membrane. This process regenerates free CoASH, which allows fatty acid oxidation and the tricarboxylic acid (TCA) cycle to proceed. Acetylcarnitine is transported to the cytosol through the mitochondrial inner membrane in exchange with carnitine by the antiport carnitine acylcarnitine translocase (CACT), thus providing acetyl groups for the synthesis of sterols, fatty acids, and ketone bodies.
Figure 1. Metabolic fate of acetyl-CoA derived from acetylcarnitine.
The supplemented acetylcarnitine (AcetylC) is transported into the mitochondria via the inner membrane carnitine acylcarnitine transferase (CACT). Acetyl-CoA formed through the mass-action of carnitine acetyltransferase (CAT) or synthesized de novo from acetate becomes available for tricarboxylic acid cycle (TCA), lipoic acid synthesis, and mitochondrial protein acetylation. Cytosolic acetyl-CoA is derived from transported mitochondrial citrate or peroxisomal acetate. Nuclear acetyl-CoA is either directly imported from the cytosol or synthesized from acetate by acetyl-CoA synthetase or via the potential nuclear CAT using acetylcarnitine. Nuclear acetyl-CoA controls gene expression via acetylation of histone and non-histone proteins.
Total carnitine and acetylcarnitine concentrations are maintained within normal ranges of 23–73 μmol/L and 3–14 μmol/L, respectively, in adult human plasma [7]. Plasma concentration and urinary excretion of acetylcarnitine vary with physiological and pathological states, reflecting mainly the degree of acetyl-CoA synthesis. In normal human subjects under fasting conditions, acetylcarnitine excretion in the urine increased to 78% of the excreted acylcarnitines [8]. In contrast, in obese human subjects under fasting conditions, the increase in urinary excretion of acetylcarnitine was markedly slower and at a lower level, suggesting a slower formation of acetyl-CoA derived from fat oxidation. In diabetic patients with ketosis, acetylcarnitine represented 61% of the total urine acylcarnitines, and decreased dramatically upon insulin treatment [8]. The content of acetylcarnitine in tissues depends upon the presence of the mitochondrial enzyme, CAT. Because of the limited amount of mitochondrial CAT, the transport of acetylcarnitine in liver is extremely restricted and the predominant transport of acetyl-CoA units out of hepatic mitochondria to the cytosol is as citrate (Figure 1).
3. Evidence for the protective effect of acetylcarnitine against the aging defect
Aging is accompanied by a progressive decline of physiological function, that leads to an increased rate of disease [9]. At the cellular level, aging is characterized by structural disorganization, disturbances in protein synthesis, decreased enzyme activity, and progressive impairment of the functions of cellular organelles [10]. The weight of the accumulating evidence indicates that the age-related damage is an ineluctable consequence of normal oxygen metabolism associated with a relentless formation of reactive oxygen species (ROS; [10]). Much of ROS production occurs in the mitochondria [11, 12], making this organelle both the source and the target of oxidative stress in advanced age [11–14]. Defects of mitochondrial metabolism in the elderly have been observed in heart [15–18], skeletal muscle [19, 20], liver [21–24], and brain [21, 25, 26], as reviewed by Lesnefsky and Hoppel [2].
3.1 The aging defect
3.1.1 Heart under standard and ischemic conditions
The mitochondrial defects in the aging heart are localised specifically in the interfibrillar mitochondrial (IFM) population [15], which is located between the myofibrils [27]. Mitochondrial oxidative phosphorylation in the IFM isolated from elderly rat heart decreased with substrates feeding electrons into complexes I (glutamate; [28]), III (duroquinol; [16, 28]), and IV (ascorbate and TMPD; [15, 28]). Because the aging defect was not relieved by uncoupling, the electron transport chain (ETC), rather than the phosphorylation system, was identified as the site of the defect. Furthermore, measurement of enzyme activities showed decreases in complexes III [16] and IV [15] and no change in complexes I and II. Defects in fatty acid oxidation [30] or Complex IV in a mixed population of mitochondria isolated from aging heart [31–33] were localised specifically to the IFM [15].
Electron flow through complex III involves the oxidation of ubiquinol followed by the simultaneous transfer of electrons to both cytochromes b and c1 in a bifurcated fashion mediated by motion of the iron-sulfur protein [34–36]. The subunit peptides (subunits VIII and X) and catalytic centers (cytochrome b, c1, and iron-sulfur protein) were not changed with aging in IFM [16]. Similarly, the content of cytochromes b and c1 is preserved in combined populations of heart mitochondria [25] as well as in individual populations [37] from aged rat compared to young rats. The aging defect resides within the myxothiazol-binding domain in the vicinity of heme bL of cytochrome b (Qo center) [18].
Mitochondrial dysfunction contributes to myocardial injury during ischemia/reperfusion injury [17, 38]. When exposed to a stress such as ischemia, mitochondria undergo damage, such as changes in ultrastructure [39] and functional impairment [40]. Cardiac ischemia results in damage to the mitochondrial ETC [37, 41, 42]. Short term ischemia (10–20 min) causes a decrease in complex I activity [41–43], as well as a reduction in phosphorylation activity involving complex V [42] and adenine nucleotide translocase [44, 45]. After a longer ischemic period, damage occurs at the level of complex III [46] and then complex IV [41]. It needs to be emphasized that the mitochondrial damage occurs during ischemia, rather than during reperfusion [17, 37, 47].
The aged heart exposed to ischemia sustains greater injury compared to the adult heart both in patients [48] and in animal models [49–52]. Compared to the adult heart, oxidative damage (oxidative protein modification [49]) and calcium-mediated damage [53] are increased, indicating mitochondria-driven mechanisms of injury. Ischemic-induced defects in complex III and IV are added to the aging-induced defect at the same complexes. Furthermore, because complex III is recognized as a source of ROS [54], the complex III defect in the aging heart provides an explanation for the enhanced oxidative damage occurring during ischemia [37]. By paramagnetic resonance signal, the ischemic defect was localized at the iron-sulfur protein in both populations of mitochondria, and is due to a functional decrease rather than a loss of subunit peptide [37].
To focus on the importance of complex III in the oxidative damage in mitochondria and during aging, we undertook studies to limit electron flow into complex III and thus decrease ROS production. The blockade of mitochondrial respiration with the irreversible inhibitor rotenone [55] or the reversible inhibitor amobarbital [56] at complex I before ischemia protects the distal ETC against ischemic damage. Protection of the ETC during ischemia by the reversible blockade of electron transport with amobarbital markedly decreases myocardial injury measured after reperfusion, supporting the premise that ischemic damage to mitochondria is a key factor in myocardial injury [57, 58]. The ischemic damage to the ETC increased both the capacity and the net production of H2O2 from complexes I and III and sets the stage for an increase in ROS production during reperfusion as a mechanism of cardiac injury [47].
In conclusion, at the onset of reperfusion in the aging heart, complex III in IFM exhibits the additive damage of aging and of ischemia; the combination is a likely mechanism of enhanced ischemic injury in the aging heart [37]. These defects act in concert to further slow electron flow within complex III, increase the reduction of cytochrome b, enhance production of ROS, and finally lead to cell death by necrosis or by apoptosis [17].
3.1.2 Skeletal muscle
In aged human and rat skeletal muscle, a reduction of muscle mass (sarcopenia; [59]) and performance occurs [60, 61]. The decreased mitochondrial content in muscle indicates an important role of mitochondrial biogenesis, defined as growth and division of pre-existing mitochondria [62], in the aging process. In the muscle from young animals, the mitochondrial content is maintained by production of new mitochondria. In elderly rats, in contrast, the content and the yield of total mitochondria decrease [19]. Additionally, an inverse relationship was shown between mitochondrial content and age [20]. Mitochondrial performance in aged skeletal muscle, however, is not affected, as shown by similar rates of OXPHOS with different substrates in young and old rats [19, 63] and in humans [64–66]. In contrast, other studies have reported defects in oxidative phosphorylation in the presence of substrates feeding electrons into complex I or complex II in skeletal muscle from aged humans [67] or mice [68]. Other studies have also shown aging-defects in activities of complexes I [69–71], II [70], III [70], IV [67, 69, 70], and V [70, 71].
3.1.3 Brain
In the brain from aged compared to young animals, the mitochondrial mass did not vary [21], but the function of mitochondria is affected. OXPHOS in the presence of substrates feeding electrons through complexes I and II decrease in the brain from aged compared to young rats [25]. The activities of complex I [25, 26, 72, 73], complex I+III [21, 74] and complex IV [21, 26, 72–78], and the content in cytochromes c and aa3 [25] also were reported to decrease during aging.
3.1.4 Liver
In the liver from aged compared to young animals, the mitochondrial mass is preserved but the function of mitochondria is affected [21]. The rate of OXPHOS in the presence of substrates feeding electron into complexes I, II, or III decreased in aged animals [22–24, 79, 80] and humans [81]. The decrease in OXPHOS in rat liver mitochondria was explained by a defect in complex III, whereas complex II [72, 79, 80], II+III [21], and IV [79, 80] were unaltered by age. An additional defect in complex IV activities was observed in the liver from aged mice [21, 72, 74].
3.2 Protection by acetylcarnitine: Experimental evidence
3.2.1 Organism, organ functions
The improvement of mitochondrial OXPHOS during aging has an important impact on preservation of organ function, as well as sensitivity of organs following a stress event, such as ischemia. With bolus administration of acetylcarnitine, three hours before an ischemic event, cardiac recovery improved in the aged heart [28]. The ischemic injury experienced by the aged heart treated with acetylcarnitine was neutralized to a level comparable to that in the adult heart in terms of myocardial damage and contractile recovery after ischemia. In the adult heart, acetylcarnitine treatment, however, does not alter the degree of injury occurring during ischemia; these data suggest that acetylcarnitine does not alter the basic mechanisms of ischemic damage common to adults and aged rat. From a central nervous system perspective, oral administration of acetylcarnitine in old rats has been shown to improve cognitive function [82] and ambulatory activity [83].
3.2.2 Mitochondrial structure
The improvement of cognitive function and ambulatory activity following acetylcarnitine supplementation [82], was related to restored mitochondrial cristae [83] and reduction of oxidized RNA, likely mitochondrial [84], in hypocampus neurons. A dose-response study showed that lower doses (0.5% in drinking water) ameliorated the age-related decline in mitochondrial cristae in the dentate gyrus of the hypocampus more effectively than did the higher doses (1.5%). In contrast to the lower doses, higher doses of acetylcarnitine did not decrease lipid peroxidation products in the brain, but caused an increase in protein carbonyl content [83]. These data suggest that an increase in oxidative stress in the brain is a side effect of high doses of acetylcarnitine.
3.2.3 Integrity of mitochondrial inner membrane lipid environment
The mitochondrial lipid environment contains the unique phospholipid, cardiolipin. The inner membrane tetra-linoleoyl cardiolipin (L4CL) preserves the physical properties of the mitochondrial inner membrane [85, 86], mitochondrial oxidative phosphorylation [87, 88], and activities of the mitochondrial transporters [89], ETC enzymes [33, 90–92], and the phosphorylation apparatus [93]. Additionally, cardiolipin is involved in the respirasome assembly of the mitochondrial complexes [94] and anchors cytochrome c to the inner mitochondrial membrane [95]. Cardiolipin depletion results in reduced complex I, III [90], and IV [96] activity along with the dissociation of subunits VIa and IVb from the complex IV.
Loss of cardiolipin has been purported as the mechanism for the aging defect in mitochondrial respiration and ETC activities (Table 1), and by restoring cardiolipin content, acetylcarnitine reverses the aging mitochondrial defect in rat heart [97, 98], skeletal muscle [99], and liver [100]. In contrast to these studies, we found that the content of total cardiolipin did not decrease and the molecular cardiolipin species did not change during aging [101]. Important differences in methodology may explain these discrepancies. The previously reported differences in cardiolipin content in adult versus aged rats were based on an HPLC-UV absorption method, without an internal standard. When using a balanced study approach to measure cardiolipin [101], the results showed that the content was not affected by aging. Since cardiolipin content is not decreased in aging, the mechanism by which acetylcarnitine improved mitochondrial function does not involve modification of cardiolipin content (Table 1).
Table 1.
Different proposed mechanisms of acetylcarnitine effect during supplementation for short term (ST) or long term (LT) periods, with experimental evidences supporting or rejecting the mechanisms.
| Pro | Contra |
|---|---|
|
Effect on integrity of the lipid environment of the mitochondrial inner membrane, principally the cardiolipin content (section 3.2.3)
| |
| ↑ cardiolipin content in rat heart mitochondria; LT [159] [160] | No change in cardiolipin total content, acyl group or individual molecular species in mitochondria from aged heart [101] |
|
| |
|
Control of mitochondrial protein synthesis (biogenesis) by acetylation/deacetylation regulating mt translational activity (section 3.2.4, 5.3, 5.4, 5.5)
| |
| ↑ OXPHOS and ETC activity in the aging heart; ST [28] | Need data on the specific protein acetylated |
| ↑ cytochrome b and aa3 in heart ST [28] and brain; LT [25] | |
| Preservation of nit content in skeletal muscle during aging [161] or inactive status [102], and in brain during aging; (LT) [103] | |
| ↑ mt RNA [162] | |
| ↑ nuclear transcripts for factors involved in nit biogenesis in skeletal muscle; LT [161] | |
| Prevention of unloading-induced downregulation of mRNA levels of kinases to transduce metabolic and neuronal stimuli into mt biogenesis in skeletal muscle; LT [161] | |
|
| |
|
Energy source (section 4.2.1)
| |
| ↑ fat utilization as a metabolic fuel and ↑ protein deposition [161] | No change in total energy expenditure; LT [161] |
|
| |
|
Dual effect on mitochondrial oxidative stress due to antioxidant properties of the compound (section 5.1)
| |
| ↓ ROS production in β-cells exposed to oleic-acid related with protection against mt dysfunction and maintenance of the glucose-stimulated insulin secretion [120] | None of the carnitine derivatives were able to scavenge peroxyl or superoxide radicals [130] |
| The effect may occur as a consequence of preservation of mitochondrial function rather than a direct effect of acetylcarnitine | |
| ↓ oxidized RNA in hypocampus neurons associated with improved cognitive function and mitochondrial cristae formation [83] | |
| ↑ oxidative stress at high doses in brain, consistent with antioxidants effect [83] | |
|
| |
|
Antiapototic effect (section 5.2)
| |
| Protect neurons [163] and hepatocytes [133] against methamphetamine-induced cellular death. | How can this increase mitochondrial proteins content or function? |
| Protect dopaminergic system against intraventricular injection of methamphetamine in rats [135] | The effect may occur as a consequence of preservation of mitochondrial function rather than a direct effect of acetylcarnitine |
ST: Hours, LT: weeks, mt: mitochondria
3.2.4 Mitochondrial function and content of mitochondrial proteins
Several reports showed that treatment with acetylcarnitine may have impact on mitochondrial biogenesis (Table 1). Mitochondrial biogenesis is indicated by the increase in mitochondrial size, number, and mass. Change in mitochondrial biogenesis may affect the content of mitochondrial proteins or function by an increase in specific functional units.
Treatment of the aged rat heart by intraperitoneal administration of acetylcarnitine restored the mitochondrial respiration through complexes III and IV in IFM to the level of the adult heart. It reversed the age-related decrease in the activity of complex III. The treatment also increased cytochrome b content in both IFM and SSM and cytochrome aa3 specifically in SSM from old rats [28]. In the aging heart that has been pretreated with acetylcarnitine and exposed to ischemia-reperfusion, the mitochondrial defect due to aging is eliminated, and only the defect due to ischemia is still present, improving the recovery after ischemia-reperfusion to the level of the adult heart [28]. Those effects were observed only three hours after administration of the acetylcarnitine. The supplementation with N-acetylcysteine also has been shown to prevent the decrease in cytochromes c and aa3 in the aging brain [25], suggesting that the effect of acetylcarnitine on the content of mitochondrial proteins is due to the acetyl portion rather than the carnitine portion.
Long term administration of acetylcarnitine (1 month, in drinking water) increases the mitochondrial content in rat skeletal muscle [99], suggesting that acetylcarnitine stimulates mitochondrial proliferation, even if the latter is not detected after a short time of supplementation. Acetylcarnitine also helps in preserving the mitochondrial content during a long period of inactivity of skeletal muscle [102] and during aging in the brain [103].
4. Supplemental acetylcarnitine: pharmacokinetics
4.1. Absorption, distribution, excretion
Orally supplemented acetylcarnitine is taken up from the gastrointestinal tract into the blood [104]. Acetylcarnitine is deacetylated during or immediately after its uptake into intestinal cells, and a portion of the newly formed intracellular free carnitine is re-acetylated [104]. Whereas high doses of acetylcarnitine are well tolerated, the absorption of orally administered acetylcarnitine is poor. In elderly humans with senile dementia, daily oral administration of 2 g for 50 days slightly raised the plasma concentration of acetylcarnitine. Carnitine concentration in plasma was unchanged whereas total carnitine (carnitine plus acylcarnitines) rose modestly [105] due to increased level of acylcarnitines. In contrast, in the adult human, intravenously administered acetylcarnitine as a bolus (500 mg) led to a rapid increase in plasma concentration followed by a progressive decline reaching the base value in 12 hours. The administered acetylcarnitine was mostly excreted in urine as carnitine and acetylcarnitine during the first 24 h after administration. Conversion of acetylcarnitine to carnitine was higher than the average renal clearance of acetylcarnitine, suggesting that a large proportion of the acetyl moieties of the exogenously administered acetylcarnitine is either rapidly used in biosynthesis and stored (in a form other than acetylcarnitine), or hydrolyzed [106].
The body distribution of acetylcarnitine is determined by systems transporting acetylcarnitine into cells against a concentration gradient. The uptake of acetylcarnitine by cardiomyocytes in culture is saturable and inhibited by carnitine [107], suggesting that both compounds share the same transporter. Ohashi et al. found that acetylcarnitine is transported by the organic cation transporter 2 (OCTN2), a homolog of the OCTN1, with high affinity (Km 8.50 μM) in a saturable manner [108]. The pH profile and Hill coefficient (0.989) of the OCTN2-mediated acetylcarnitine uptake were similar to those of carnitine transport. In heart, OCTN2 is highly expressed [109], suggesting a significant role in the uptake of acetylcarnitine in cardiomyocytes. A similar saturable sodium-dependent system exists at the blood-brain barrier; the transported acetylcarnitine is reported to improve neuronal energetics and to increase the synthesis of the neurotransmitter acetylcholine in the cholinergic system. The mechanism for acetylcarnitine export from the cell has not been characterized.
4.2. Effect of supplemental acetylcarnitine on metabolism
The question is: are the benefits of the supplemented acetylcarnitine due to 1) the acetyl unit as acetyl-CoA, 2) the carnitine derived from the parent compound, or 3) the intact acetylcarnitine?
4.2.1 Fate of acetyl-CoA
Exogenous acetylcarnitine is transported via CACT through the inner membrane to the mitochondrial matrix where it is exposed to CAT, which reversibly transforms acetylcarnitine to acetyl-CoA. The substrates used by the enzyme in one direction are competitive inhibitors of the reaction products obtained in that direction [110]. Therefore, if the intramitochondrial concentration of acetylcarnitine is higher than the Km of the enzyme (350 μM), one would expect that the exogenous acetylcarnitine facilitates the continuous generation of acetyl-CoA. An increased acetyl-CoA/CoA ratio decreases the activity of pyruvate dehydrogenase and inhibits mitochondrial fatty acid β-oxidation and the TCA cycle. Therefore, the increase in mitochondrial acetyl-CoA would have negative effects on mitochondrial metabolism. However, the mitochondrial content of acetyl-CoA during acetylcarnitine supplementation is the result of acetyl-CoA formation, due to the mass-action of CAT, and utilization of the former. The consumption of acetyl-CoA in irreversible metabolic pathways, i.e., lipoic acid and citrate synthesis within the mitochondria followed by biosynthetic processes within the cytosol (Figure 1), drives the process and maintains the mitochondrial content of acetyl-CoA within a safe range.
Catabolism of acetyl moieties via TCA cycle was suggested [111, 112] to stimulate energy production (Table 1). A Positron Emission Tomography study of the incorporation of acetate from acetylcarnitine in the brain indicated an accumulation and rapid metabolism of the acetate moiety of acetylcarnitine, but not of its carnitine part [113]. In mammalian sperm and in insect flight muscle, the endogenous cytosolic acetylcarnitine serves as an available source of acetyl-CoA used by the TCA for energy production [110]. In contrast, oral supplementation with acetylcarnitine for one month in old rats does not increase the total energy expenditure, despite the increase in fat utilization as metabolic fuel [99]. Coupled with the limited bioavailability of 10% of orally-supplemented acetylcarnitine, the administration of 2 g acetylcarnitine (10 mmoles) and its incorporation in the TCA cycle would give rise to 12 mmoles ATP per day. This value represents only 0.01% of the total amount of ATP turned over in an adult human organism per day (65 kg/day, 118 moles/day). Therefore, supplemental acetylcarnitine is not a major energy provider.
Anabolism of acetyl-CoA derived from the supplemented acetylcarnitine refers to the entrance of acetyl moieties into biosynthetic pathways. Acetylcarnitine provides acetyl-CoA for the synthesis of acetylcholine in the brain and fatty acids in lipogenic tissues that contain fatty acid synthase, i.e., brain, liver, and adipose tissue. Citrate generated by the condensation of acetyl-CoA and oxaloacetate in the TCA cycle is transported into the cytosol where it is cleaved by citrate lyase to re-generate cytosolic acetyl-CoA. One-third of the acetyl-CoA that reaches the cytosol via citrate in the brain is used for acetylcholine synthesis [114]. 13C NMR spectroscopy following 4 hour [(1,2-13C2)acetyl]-L-carnitine intravenous infusion showed that in the liver the acetyl groups enter the TCA cycle since 13C was found in liver glutamate, glutamine, and glutathione and was incorporated into cholesterol and 3-hydroxybutyrate [115]. Farrell et al. confirmed the incorporation of these acetyl groups into the TCA cycle since 14CO2 was a major product of IV injected [1-14C]-acetylcarnitine in rats [116]. Additionally, substantial radioactivity was found in fatty acids of phospholipids and triacylglycerols in the liver, with smaller amounts in the heart, brain, skeletal muscle and kidney [116]. Furthermore, the bulk of radioactivity was in fatty acids of phospholipids in the brain after intraventricular injection of [1-14C]-acetylcarnitine [112]. Similar results were obtained in cultured cells, where added acetylcarnitine (2 mM) contributed about 10% of the acetyl-CoA used for de novo synthesis and for elongation of fatty acids, as well as 6% for ketogenesis [117].
The concept that fatty acid synthesis is limited to the cytosol of lipogenic cells was recently revised. We recently provided evidence for the occurrence of fatty chain elongation in rat heart [118]. Also, several nuclear-encoded components of a putative mitochondrial pathway for de novo fatty acid synthesis were identified and characterized within mitochondria. Since lipoic acid synthase was found in mammalian mitochondria [119], and lipoic acid (LA) reversed age-associated mitochondrial decay [82, 120], it is reasonable to hypothesize that the supplemented acetylcarnitine exerts part of its protective effect via providing acetyl groups for LA synthesis within the mitochondria. Bovine heart mitochondria are able to elongate a C2 primer and synthesize de novo octanoyl precursor as the substrate for lipoic acid synthase, the enzyme that introduces the sulfur atoms into the octanoyl precursor for LA synthesis [121]. In addition to being an antioxidant, LA is an essential cofactor in mitochondrial metabolism. Acetylcarnitine in association with LA is a more effective supplemental regimen than acetylcarnitine alone to protect mitochondria [100].
4.2.2 Effect of carnitine
The metabolic effects of acetylcarnitine supplementation in aged animals might be due to carnitine. Aging is associated with carnitine insufficiency which may compromise function during stress conditions when carnitine is needed. A decrease in carnitine content has been described in the brain and myocardium of aged animals [122]. An age-related decrease in carnitine content in the brain and plasma is associated with an increase in carnitine content in the liver, possibly induced by an impaired transport of carnitine from the liver to the blood in old animals [83]. Chronic oral supplementation with acetylcarnitine in rats reverses the age-related decline in carnitine content in the brain, plasma [83], skeletal muscle, and heart [123]. Acetylcarnitine is reported to be better absorbed and to cross the blood-brain barrier more efficiently compared to carnitine [124, 125]. The recovery of brain, heart, and plasma carnitine content in the old animals treated with acetylcarnitine indicates that its intestinal absorption and tissue uptake are unchanged during aging.
By restoring tissue carnitine in the elderly, acetylcarnitine supplementation may facilitate the elimination of potentially toxic acyl-CoA metabolites derived from fatty acid oxidation. This would be required when acyl groups accumulate, i.e., increased fatty acid oxidation, and is accomplished by elimination of specific acylcarnitines [126].
4.2.3 Effect of intact acetylcarnitine
High concentrations of acetylcarnitine may normalize age-related abnormalities in the kinetic properties of CAT. An age-related decrease in CAT activity occurs in soleus, diaphragm, and heart from rat [122], and brain microvessels and cerebellum from humans with Alzheimer's disease [127]. Liu et al. [128] found a moderate decrease in CAT activity and a marked increase in the Km for both substrates, acetylcarnitine and CoA, in the brain of old rats due to alteration at the active site of CAT by malondialdehyde, a product of lipid peroxidation. Feeding old rats high amounts of acetylcarnitine restored brain CAT activity, as well as CAT-binding affinity for both substrates. In addition, the inhibitory effect of malondialdehyde on in vitro CAT activity was inhibited by acetylcarnitine, suggesting a competitive mechanism for the protective effect of acetylcarnitine.
5. New proposed protective mechanisms for an old compound
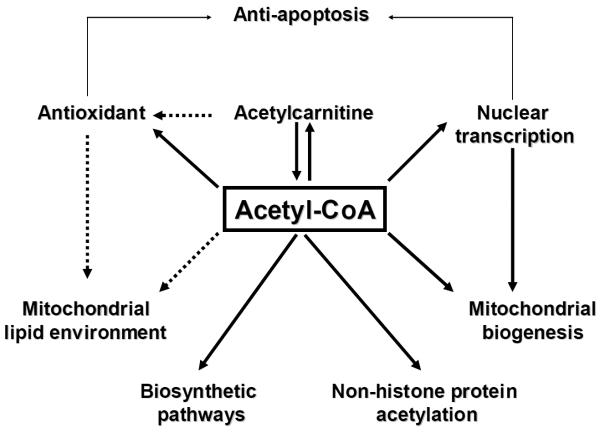
Acetylcarnitine has been considered a “mitochondrial nutrient” [120, 125], that reverses both aging-related mitochondrial dysfunction and the reaction of elderly mitochondria to challenge [28]. We propose that the rejuvenating effect of acetylcarnitine on mitochondria is through mechanisms in addition to the aforementioned metabolic effects. The next section reviews recent discoveries about the effects of acetylcarnitine on cellular signalling pathways, that may explain how elderly mitochondria are converted to a more youthful state (Figure 2).
Figure 2.
Signaling pathways for the protective effects of acetylcarnitine.
5.1. Dual effect on mitochondrial oxidative stress
The antioxidant properties of acetylcarnitine have been both popularized and advertized. Although unexpected from its chemical structure, the antioxidant properties of acetylcarnitine were reported in in vitro experiments (summarized in Table 1) and explained by its iron-chelating properties [129]. In contrast, when administered via the perfusate to ischemia-reperfused rat hearts, none of the carnitine derivatives were able to scavenge peroxyl or superoxide radicals [130].
In cell culture studies, the decrease in oxidative stress by acetylcarnitine occurred by 1) exerting a protective effect on mitochondrial structure and function, making the ETC less prone for electron leak and superoxide production, and 2) stimulating the endogenous cellular antioxidant defence mechanisms. The effect on mitochondria is supported by pretreatment of pancreatic β-cells with micromolar concentrations of acetylcarnitine, which protected the cells from oleic acid-induced mitochondrial dysfunction and decreasing ROS production [120]. Secondly, acetylcarnitine supplementation stimulated endogenous cellular antioxidant defence mechanisms. Treatment of astrocytes with acetylcarnitine (10–100 μM for 6 hours) increased the amount and activity of heme oxygenase-1 (HO-1) [131]. In addition, pre-incubation of astrocytes with acetylcarnitine before the initiation of a nitrosative stress with lipopolysacharide and interferon, prevented the decrease in complex IV activity, protein nitration and restored the reduced glutathione/oxidized glutathione ratio [131]. HO-1, the rate-limiting enzyme in the production of bilirubin, catalyzes the oxidative cleavage of the heme molecule to form biliverdin and carbon monoxide (CO). Therefore, the beneficial effect of HO-1 is due to both the antioxidant property of biliverdin/bilirubin and the increase in CO availability.
5.2. Antiapoptotic effect
High concentrations of acetylcarnitine (1 mM) protect neurons [132] and hepatocytes [133] against cellular death induced by methamphetamine, that is mediated via cardiolipin peroxidation, cytochrome c release, induction of mitochondrial transition pore and apoptosis [134]. Also, acetylcarnitine protected the dopaminergic system against the intraventricular injection of methamphetamine in rats [135] (Table 1).
The antiapoptotic effect of acetylcarnitine may be related to the overexpression and activation of HO-1, which increases the level of antiapoptotic bcl-2 protein and inactivates the pro-apoptotic transcription factor p53 in neurons [136]. It was suggested that co-localization of increased HO-1 with senile plaques [137] and neurofibrilar tangles [138] in human brains with Alzheimer disease reflects the adaptive reaction of neurons in order to limit neurodegeneration.
Orally-supplemented acetylcarnitine in rats was reported to decrease caspase activation by increasing the level of X-linked inhibitor of apoptosis protein (XIAP), thus limiting the mitochondrial-induced apoptosis in peripheral neurons [139]. Neither a protective effect on apoptosis induction nor a decrease in XIAP level was observed by these authors after carnitine administration, suggesting that the acetyl groups of acetylcarnitine have a fundamental role in protecting against mitochondrial-induced apoptosis.
5.3. Potential control of gene transcription by reversible lysine acetylation
The transcription of nuclear DNA recently has been linked to acetylation and deacetylation of core histone tails at lysine residues [140]; acetylated histone tails are associated with active chromatin, whereas histone deacetylation is associated with transcriptional repression of genes, because the removal of acetyl groups from lysine residues limits accessibility of the DNA for transcription [141]. Therefore, histone acetyl-transferases and deacetylases are transcriptional co-regulators. All known acetyltransferases use acetyl-CoA as a donor for acetylation. The existence of distinct mitochondrial and nucleo-cytosolic acetyl-CoA pools has been unambiguously shown; the nuclear concentration of acetyl-CoA is the limiting factor for histone acetylation [142]. Can acetylcarnitine increase the nuclear acetyl-CoA pool and increase the transcription of nuclear DNA (Table 1)?
The nuclear content of acetyl-CoA following acetylcarnitine supplementation was not determined. The possible existence of a nuclear CAT (referred by [111]) and the discovery of the nuclear acetyl-CoA synthetase may explain the contribution of supplemental acetylcarnitine to the nuclear acetyl-CoA. It was recently found that the global transcription in yeast, mainly under the control of the histone acetylase activity rather than the histone deacetylase activity, is regulated by the steady-state level of acetyl-CoA supplied by the nuclear acetyl-CoA synthetase [142]. Also, the free passage of acetyl-CoA through the nuclear pore complex facilitates the traffic of cytosolic acetyl-CoA to the nucleus [142]. Since cytoplasm does not contain CAT, the origin of the cytosolic acetyl-CoA generated from acetylcarnitine required contributions by mitochondria and peroxisomes. Mitochondrial acetyl-CoA derived from acetylcarnitine is incorporated into TCA cycle and forms citrate which further generates acetyl-CoA into the cytosol of lipogenic cells. Peroxisomal CAT reversibly transforms both endogenous (derived from peroxisomal fatty acid β-oxidation) and supplemented acetylcarnitine into acetyl-CoA. In the liver, acetyl-CoA is hydrolyzed into acetate by the respective acetyl-CoA hydrolases and then reactivated by the nuclear acetyl-CoA synthetase.
The transcription of specific nuclear genes controls the replication and transcription of the mitochondrial genome. Nuclear respiratory factor-1 (NRF-1) and mitochondrial transcription factor A (TFAM) are required for mitochondrial DNA (mtDNA) replication; together with mitochondrial transcription factors B (TFB1 and TFB2), they stimulate the transcription of both light and heavy chains of mtDNA [143]. In addition to acetylation of histone proteins, site-specific acetylation of non-histone proteins plays an important role in transcriptional regulation. In particular, high mobility group (HMG)-box proteins are acetylated [144]. TFAM contain two HMG-box-like domains. The total amount of TFAM increases in the liver, cerebellum and kidney with aging [145].
The acetylation status of histone and non-histone nuclear proteins was not determined following acetylcarnitine supplementation. As noted in section 4.1, acetylcarnitine up-regulates genes involved in cellular antioxidant capacity and repair [131]. Could this regulation by acetylcarnitine occur by changing the acetylation status of histone and non-histone nuclear proteins?
5.4. Control of the activity of mitochondrial enzymes by reversible lysine acetylation
The mitochondrial presence of several nicotinamide adenine nucleotide (NAD+)-dependent deacetylase silent information regulators (sirtuins, SIRT3, 4, and 5) recently has been described [146–148]. A proteomic survey of protein acetylation identified 388 acetylation sites on 195 proteins in mitochondria from HeLa cells and mouse liver, representing 20% of mitochondrial proteins; these proteins included those involved in the TCA cycle, fatty acid β-oxidation, amino acid and carbohydrate metabolism, membrane transport, and ETC [144]. We found that long-chain acyl-CoA synthetase 1 is acetylated at the lysine residue 633 in rat liver mitochondria [149]. Lysine acetylation neutralizes the positive charge and increases the hydrophobicity of the lysine side chain. While the functional consequence of this postranslational modification is unknown, these modifications should lead to changes in protein conformation and function. Mitochondrial matrix acetyl-CoA synthetase is reversibly acetylated at a lysine residue in the active site of the enzyme, and the SIRT3-induced deacetylation activates the enzyme [150]. Glutamate dehydrogenase is the known mitochondrial target for both SIRT3 [146] and SIRT4 [147] enzymatic activity and is inhibited upon deacetylation. A recent report shows that SIRT3 reversibly binds, decreases the acetylation status, and augments the activity of mitochondrial complex I [151]. These data clearly show that acetylation can control the activity of mitochondrial enzymes, and possibly de novo synthesis of acetyl-CoA in mitochondria. Since acetyl-CoA is the acetylation donor for all known acetyltransferases, the concentration of mitochondrial acetyl-CoA could be a limiting factor in the acetylation reaction; in fact, the Km for acetyl-CoA is high (330 μM) for the skeletal muscle mitochondrial acetyltransferase [152]). By increasing the acetyl-CoA content, supplemented acetylcarnitine should increase the acetylation status of mitochondrial proteins (Table 1).
5.5. Increase in mitochondrial biogenesis
Mitochondrial biogenesis relies on a spatiotemporally coordinated synthesis and import of approximately 1000 nuclear-encoded proteins, some of which are assembled with mitochondrial-encoded proteins within newly synthesized inner and outer mitochondrial phospholipid membranes. The replication of mitochondrial DNA, as well as the mitochondrial fusion and fission mechanisms, also must be synchronized with these processes.
Oral acetylcarnitine supplementation in rats increases soleus muscle mitochondrial content, nuclear transcripts of factors involved in mitochondrial biogenesis (PGC-1α, NRF-1, TFAM), as well as the level of mitochondrial transcripts (COX I, ATP6, ND6, 16 S rRNA), and prevents the unloading-induced downregulation of mRNA levels of kinases able to transduce metabolic (AMPK) and neuronal stimuli (CaMKIIβ) [153]. Acetylcarnitine enhances the activity and amount of HO-1 in cell culture in a dose- and time-dependent manner [131]. Further, HO-1 was shown to increase mitochondrial biogenesis in cardiomyocytes via the transcriptional control of the nuclear respiratory factor-1 (NRF-1) [154]. According to the model proposed by Piantadosi et al. [154], endogenous CO generated by HO-1 binds the reduced a3 heme in cytochrome c oxidase and increases the superoxide production from complex III [155]. Superoxide induces MnSOD overexpression and is dismutated to hydrogen peroxide, which activates protein kinase B (Akt) [156]. Akt deactivates glycogen synthase kinase-3β, allowing the nuclear translocation of Nrf2 [157]. Nuclear Nrf2 binds to antioxidant response elements in the HO-1, MnSOD, and NFR-1 gene promoters, thus amplifying the initial signal and driving the transcription of TFAM and other genes that have promoter binding sites for NRF-1. These genes are involved in the control of mitochondrial transcription and protein synthesis, mitochondrial protein import, and oxidative phosphorylation [154].
A study from our laboratory shows that acetylcarnitine reverses the age-related decrease in the activity of complex III and oxidative phosphorylation through complex III and IV, and increases the amount of cytochrome b and aa3 hemes in cardiac mitochondria isolated from old rats [28]. Of interest, both cytochrome b and aa3 proteins are encoded by the mitochondrial genome, suggesting that acetylcarnitine enhances either mtDNA transcription, the stability of mitochondrial mRNA, or mitochondrial protein synthesis. Since the transcription of mtDNA is polycistronic, other proteins encoded by mtDNA -subunits of complexes I, IV and V- would be expected to increase. Because the yield of mitochondria was unchanged, this suggests that mitochondrial replication of mtDNA, which is under similar nuclear control and parallels the polycistronic transcription of mtDNA, was not increased upon acetylcarnitine supplementation. These data suggest that short-term administration of acetylcarnitine enhances the stability of mitochondrial transcripts or mitochondrial protein synthesis (Table 1). These data also are supported by the observation that the decreased levels of a ribosomal RNA (12S rRNA) and a messenger RNA (mRNA for the subunit I of complex IV) were reversed in the brain and cardiac muscle of old rats one hour after acetylcarnitine administration [103]. Furthermore, the increase in the content of TFAM, which controls mitochondrial DNA transcription and translation, remains in skeletal muscle one month after the withdrawal from chronic acetylcarnitine supplementation, indicating the long-lasting effect of acetylcarnitine on mitochondria [158]. In the adult heart, similar acute acetylcarnitine treatment did not increase OXPHOS or the activity of ETC enzymes, suggesting that a selective age-related signalling pathway, possible initiated by the complex III-generated superoxide [18], is essential for the effect of acetylcarnitine in the aged heart.
6. Conclusion and future perspectives
Age-related decreases in mitochondrial oxidative capacity contribute to cardiac pathology in the elderly. Protecting mitochondria should forestall the decrease in age-related decay of these organelles (decrease in gene expression and mitochondrial biogenesis, oxidative stress, apoptosis) and consequent organ failure. We review acetylcarnitine as a therapeutic agent with the ability to reverse the age-associated mitochondrial defect and its impact on age-related diseases, such as cardiac ischemia.
Two common mechanisms have been proposed for improvement of mitochondrial function in aging. Although cardiolipin has been touted as the target of acetylcarnitine, because the content of mitochondrial cardiolipin does not decrease with aging, this proposed mechanism is not plausible. In contrast, the experimental evidence is compelling and convincing that acetylcarnitine reverses the age-related decrease in mitochondrial DNA transcription and translation in brain and cardiac muscle. In addition, in the heart, similar data support the increased content of the mitochondrial-encoded ETC subunits, normalization of ETC complex activities, and restoration of oxidative phosphorylation, indicating that acetylcarnitine acts by increasing mitochondrial protein synthesis.
Since 20% of the mitochondrial proteins are lysine-acetylated, the reversible acetylation of the mitochondrial proteome represents an underappreciated, but fundamental, mechanism to affect the activity of mitochondrial proteins. While the functional effect of acetylation/deacetylation has been shown for only three mitochondrial proteins, this mechanism of regulation has tremendous potential for linking the altered mitochondrial proteome with mitochondrial function. Such a link could lead to the mapping of mitochondrial metabolic pathways affected by acetylation, particularly in aging. Further studies are necessary to define if factors governing mitochondrial protein synthesis, i.e., mitochondrial ribosomal proteins, are acetylated following acetylcarnitine supplementation, and if this specific postranslational modification increases the mitochondrial translational activity and the content of mitochondrial-encoded ETC subunits. Deciphering the mitochondrial mechanisms of the protective effects of acetylcarnitine will set a stage to translate these events into therapeutic strategies to improve the clinical picture of the elderly.
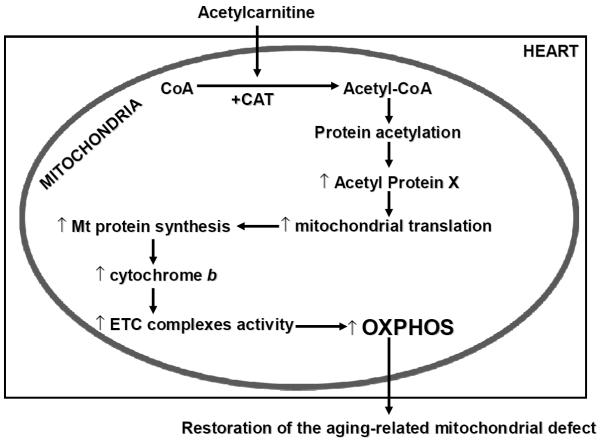
Figure 3. Proposed mechanism for the effect of acetylcarnitine on aged mitochondria.
The supplemented acetylcarnitine is transported into the cardiac mitochondria where the acetyl group favors the production of acetyl-CoA both directly and by activation of carnitine acetyltransferase (CAT), which is decreased in aging. The acetyl-CoA acts on the acetylation status of mitochondrial proteins, that increases mitochondrial transcription and protein synthesis. As a result, cytochrome b content increases, leading to increased activity of electron transport chain (ETC) complexes, and stimulating the oxidative phosphorylation (OXPHOS). This sequence of events leads to the restoration of the aging-related mitochondrial defect.
Acknowledgments
H.L. is supported by a fellowship from the Natural Sciences and Engineering Research Council of Canada. This work was supported by grants from the National Institutes of Health (P01 HL074237) and by the National Institute of Aging (P01 AG015885). Dr. Bernard Tandler provided editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brown G. Palgrave Macmillan. 2008. The living end: the future of death, aging and immortality. [Google Scholar]
- [2].Lesnefsky EJ, Hoppel CL. Oxidative phosphorylation and aging. Ageing Res. Rev. 2006;5:402–433. doi: 10.1016/j.arr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- [3].Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer's disease. Int. Clin. Psychopharmacol. 2003;18:61–71. doi: 10.1097/00004850-200303000-00001. [DOI] [PubMed] [Google Scholar]
- [4].Evans JD, Jacobs TF, Evans EW. Role of acetyl-L-carnitine in the treatment of diabetic peripheral neuropathy. Ann. Pharmacother. 2008;42:1686–1691. doi: 10.1345/aph.1L201. [DOI] [PubMed] [Google Scholar]
- [5].Sima AA. Acetyl-L-carnitine in diabetic polyneuropathy: experimental and clinical data. CNS Drugs. 2007;21:13–23. doi: 10.2165/00023210-200721001-00003. [DOI] [PubMed] [Google Scholar]
- [6].Iwase M, Ouchi Y, Okada H, Yokoyama C, Nobezawa S, Yoshikawa E, Tsukada H, Takeda M, Yamashita K, Takeda M, Yamaguti K, Kuratsune H, Shimizu A, Watanabe Y. Neural substrates of human facial expression of pleasant emotion induced by comic films: a PET Study. Neuroimage. 2002;17:758–768. [PubMed] [Google Scholar]
- [7].Minkler PE, Stoll MS, Ingalls ST, Yang S, Kerner J, Hoppel CL. Quantification of carnitine and acylcarnitines in biological matrices by HPLC electrospray ionization-mass spectrometry. Clin. Chem. 2008;54:1451–1462. doi: 10.1373/clinchem.2007.099226. [DOI] [PubMed] [Google Scholar]
- [8].Hoppel CL, Genuth SM. Urinary excretion of acetylcarnitine during human diabetic and fasting ketosis. Am. J. Physiol. 1982;243:E168–172. doi: 10.1152/ajpendo.1982.243.2.E168. [DOI] [PubMed] [Google Scholar]
- [9].Terman A, Brunk UT. Aging as a catabolic malfunction. Int. J. Biochem. Cell. Biol. 2004;36:2365–2375. doi: 10.1016/j.biocel.2004.03.009. [DOI] [PubMed] [Google Scholar]
- [10].Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- [11].Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- [12].Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int. J. Biochem. Cell. Biol. 1995;27:647–653. doi: 10.1016/1357-2725(95)00025-k. [DOI] [PubMed] [Google Scholar]
- [13].Salvioli S, Bonafè M, Capri M, Monti D, Franceschi C. Mitochondria, aging and longevity - a new perspective. FEBS Letters. 2001;492:9–13. doi: 10.1016/s0014-5793(01)02199-8. [DOI] [PubMed] [Google Scholar]
- [14].Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- [15].Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectivity decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch. Biochem. Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- [16].Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Trukaly PJ, Hoppel CL. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J. Mol. Cell. Cardiol. 2001;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- [17].Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: Ischemia-reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- [18].Moghaddas S, Hoppel CL, Lesnefsky EJ. Aging defect at the QO site of complex III augments oxyradical production in rat heart interfibrillar mitochondria. Arch. Biochem. Biophys. 2003;414:59–66. doi: 10.1016/s0003-9861(03)00166-8. [DOI] [PubMed] [Google Scholar]
- [19].Kerner J, Turkaly PJ, Minkler PE, Hoppel CL. Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am. J. Physiol.-Endocrinol. Metab. 2001;281:E1054–E1062. doi: 10.1152/ajpendo.2001.281.5.E1054. [DOI] [PubMed] [Google Scholar]
- [20].Huang JH, Hood DA. Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life. 2009;61:201–214. doi: 10.1002/iub.164. [DOI] [PubMed] [Google Scholar]
- [21].Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1244–R1249. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- [22].Darnold JR, Vorbeck ML, Martin AP. Effect of aging on the oxidative phosphorylation pathway. Mech. Ageing Dev. 1990;53:157–167. doi: 10.1016/0047-6374(90)90067-p. [DOI] [PubMed] [Google Scholar]
- [23].Genova ML, Castelluccio C, Fato R, Parenti Castelli G, Merlo Pich M, Formiggini G, Bovina C, Marchetti M, Lenaz G. Major changes in complex I activity in mitochondria from aged rats may not be detected by direct assay of NADH:coenzyme Q reductase. Biochem. J. 1995;311:105–109. doi: 10.1042/bj3110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y. Hepatic mitochondrial dysfunction in senescence-accelerated mice: correction by long-term, orally administered physiological levels of melatonin. J. Pineal. Res. 2002;33:127–133. doi: 10.1034/j.1600-079x.2002.02109.x. [DOI] [PubMed] [Google Scholar]
- [25].Cocco T, Sgobbo P, Clemente M, Lopriore B, Grattagliano I, Di Paola M, Villani G. Tissue-specific changes of mitochondrial functions in aged rats: effect of a long-term dietary treatment with N-acetylcysteine. Free Radic. Biol. Med. 2005;38:796–805. doi: 10.1016/j.freeradbiomed.2004.11.034. [DOI] [PubMed] [Google Scholar]
- [26].Navarro A, López-Cepero JM, Bández MJ, Sánchez-Pino MJ, Gómez C, Cadenas E, Boveris A. Hippocampal mitochondrial dysfunction in rat aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294(2008):R501–R509. doi: 10.1152/ajpregu.00492.2007. [DOI] [PubMed] [Google Scholar]
- [27].Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- [28].Lesnefsky EJ, He D, Moghaddas S, Hoppel CL. Reversal of mitochondrial defects before ischemia protects the aged heart. Faseb J. 2006;20:1543–1545. doi: 10.1096/fj.05-4535fje. [DOI] [PubMed] [Google Scholar]
- [29].Hoppel CL, Tandler B, Parland W, Turkay JS, Albers LD. Hamster cardiomyopathy. A defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J. Biol. Chem. 1982;257:1540–1548. [PubMed] [Google Scholar]
- [30].Hansford RG. Lipid oxidation by heart mitochondria from young adult and senescent rats. Biochemical Journal. 1978;170:285–295. doi: 10.1042/bj1700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Paradies G, Ruggiero FM, Dinoi P, Petrosillo G, Quagliariello E. Decreased cytochrome oxidase activity and changes in phospholipids in heart mitochondria from hypothyroid rats. Arch. Biochem. Biophys. 1993;307:91–95. doi: 10.1006/abbi.1993.1565. [DOI] [PubMed] [Google Scholar]
- [32].Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Enhanced cytochrome oxidase activity and modification of lipids in heart mitochondria from hyperthyroid rats. Biochimica and Biophysica Acta. 1994;1225:165–170. doi: 10.1016/0925-4439(94)90074-4. [DOI] [PubMed] [Google Scholar]
- [33].Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: Role of cardiolipin. FEBS Lett. 1997;406:136–138. doi: 10.1016/s0014-5793(97)00264-0. [DOI] [PubMed] [Google Scholar]
- [34].Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J. Biol. Chem. 1990;265:11409–11412. [PubMed] [Google Scholar]
- [35].Crofts AR, Barquera B, Gennis RB, Kuras R, Guergova-Kuras M, Berry EA. Mechanism of ubiquinol oxidation by the bc(1) complex: different domains of the quinol binding pocket and their role in the mechanism and binding of inhibitors. Biochemistry. 1999;38:15807–15826. doi: 10.1021/bi990962m. [DOI] [PubMed] [Google Scholar]
- [36].Crofts AR, Guergova-Kuras M, Huang L, Kuras R, Zhang Z, Berry EA. Mechanism of ubiquinol oxidation by the bc(1) complex: role of the iron sulfur protein and its mobility. Biochemistry. 1999;38:15791–15806. doi: 10.1021/bi990961u. [DOI] [PubMed] [Google Scholar]
- [37].Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: Damage to the iron-sulfur protein subunit of electron transport complex III. Arch. Biochem. Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- [38].Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am. J. Physiol. Cell. Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- [39].Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ. Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- [40].Flameng W, Andres J, Ferdinande P, Mattheussen M, Van Belle H. Mitochondrial function in myocardial stunning. J. Mol. Cell. Cardiol. 1991;23:1–11. doi: 10.1016/0022-2828(91)90034-j. [DOI] [PubMed] [Google Scholar]
- [41].Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am. J. Physiol. 1997;273:H1544–1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- [42].Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am. J. Physiol. 1983;244:H743–H748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- [43].Piper HM, Sezer O, Schleyer M, Schwartz P, Hütter JF, Spieckermann PG. Development of ischemia-induced damage in defined mitochondrial subpopulations. J. Mol. Cell. Cardiol. 1985;17:885–896. doi: 10.1016/s0022-2828(85)80102-4. [DOI] [PubMed] [Google Scholar]
- [44].Asimakis GK, Conti VR. Myocardial ischemia: correlation of mitochondrial adenine nucleotide and respiratory function. Journal of Molecular and Cellular cardiology. 1984;16:439–448. doi: 10.1016/s0022-2828(84)80615-x. [DOI] [PubMed] [Google Scholar]
- [45].Duan J, Karmazyn M. Relationship between oxidative phosphorylation and adenine nucleotide translocase activity of two populations of cardiac mitochondria and mechanical recovery of ischemic hearts following reperfusion. Can. J. Physiol. Pharmacol. 1989;67:704–709. doi: 10.1139/y89-114. [DOI] [PubMed] [Google Scholar]
- [46].Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- [47].Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am. J. Physiol. Cell. Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- [48].Lesnefsky EJ, Lundergan CF, Hodgson JM, Nair R, Reiner JS, Greenhouse SW, Califf RM, Ross AM. Increased left ventricular dysfunction in elderly patients despite successful thrombolysis: the GUSTO-I angiographic experience. J. Am. Coll. Cardiol. 1996;28:331–337. doi: 10.1016/0735-1097(96)00148-9. [DOI] [PubMed] [Google Scholar]
- [49].Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc. Natl. Acad. Sci. U.S.A. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lesnefsky EJ, Gallo DS, Ye J, Whittingham TS, Lust WD. Aging increases ischemia-reperfusion injury in the isolated, buffer-perfused heart. J. Lab. Clin. Med. 1994;124:843–851. [PubMed] [Google Scholar]
- [51].Frolkis VV, Frolkis RA, Mkhitarian LS, Fraifeld VE. Age-dependent effects of ischemia and reperfusion on cardiac function and Ca2+ transport in myocardium. Gerontology. 1991;37:233–239. doi: 10.1159/000213266. [DOI] [PubMed] [Google Scholar]
- [52].Liu L, Azhar G, Gao W, Zhang X, Wei JY. Bcl-2 and Bax expression in adult rat hearts after coronary occlusion: age-associated differences. Am. J. Physiol. 1998;275:R315–R322. doi: 10.1152/ajpregu.1998.275.1.R315. [DOI] [PubMed] [Google Scholar]
- [53].Ataka K, Chen D, Levitsky S, Jimenez E, Feinberg H. Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation. 1992;86:II371–II376. [PubMed] [Google Scholar]
- [54].Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- [55].Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J. Biol. Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- [56].Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J. Pharmacol. Exp. Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- [57].Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc. Res. 2008;77:406–415. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [58].Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J. Pharmacol. Exp. Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- [59].Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J. Lab. Clin. Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- [60].Lee CM, Aspnes LE, Chung SS, Weindruch R, Aiken JM. Influences of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and mice. Ann. N. Y. Acad. Sci. 1998;854:182–191. doi: 10.1111/j.1749-6632.1998.tb09901.x. [DOI] [PubMed] [Google Scholar]
- [61].Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J. Appl. Physiol. 2005;99:1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- [62].Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc. Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- [63].Beyer RE, Starnes JW, Edington DW, Lipton RJ, Compton R.T.r., Kwasman MA. Exercise-induced reversal of age-related declines of oxidative reactions, mitochondrial yield, and flavins in skeletal muscle of the rat. Mech. Ageing Dev. 1984;24:309–323. doi: 10.1016/0047-6374(84)90116-7. [DOI] [PubMed] [Google Scholar]
- [64].Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of aging - A study of isolated human skeletal muscle mitochondria. Exp. Gerontol. 2003;38:877–886. doi: 10.1016/s0531-5565(03)00092-5. [DOI] [PubMed] [Google Scholar]
- [65].Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Arch. 2003;446:270–278. doi: 10.1007/s00424-003-1022-2. [DOI] [PubMed] [Google Scholar]
- [66].Barrientos A, Casademont J, Rötig A, Miró O, Urbano-Márquez A, Rustin P, Cardellach F. Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem. Biophys. Res. Commun. 1996 doi: 10.1006/bbrc.1996.1839. [DOI] [PubMed] [Google Scholar]
- [67].Tonkonogi M, Fernström M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- [68].Figueiredo PA, Powers SK, Ferreira RM, Appell HJ, Duarte JA. Aging impairs skeletal muscle mitochondrial bioenergetic function. J. Gerontol. A. Biol. Sci. Med. Sci. 2009;64:21–33. doi: 10.1093/gerona/gln048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sugiyama S, Takasawa M, Hayakawa M, Ozawa T. Changes in skeletal muscle, heart and liver mitochondrial electron transport activities in rats and dogs of various ages. Biochem. Mol. Biol. Int. 1993;30:937–944. [PubMed] [Google Scholar]
- [70].Choksi KB, Nuss JE, Deford JH, Papaconstantinou J. Age-related alterations in oxidatively damaged proteins of mouse skeletal muscle mitochondrial electron transport chain complexes. Free Radic. Biol. Med. 2008;45:826–838. doi: 10.1016/j.freeradbiomed.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech. Ageing Dev. 2006;127:298–306. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- [72].Navarro A, Sánchez-Pino MJ, Gómez C, Bández MJ, Cadenas E, Boveris A. Dietary thioproline decreases spontaneous food intake and increases survival and neurological function in mice. Antioxid. Redox. Signal. 2007;9:131–141. doi: 10.1089/ars.2007.9.131. [DOI] [PubMed] [Google Scholar]
- [73].Boveris A, Navarro A. Brain mitochondrial dysfunction in aging. IUBMB Life. 2008;60:308–314. doi: 10.1002/iub.46. [DOI] [PubMed] [Google Scholar]
- [74].Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- [75].Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E. Mitochondrial respiratory chain activity in the human brain as a function of age. Mech. Ageing Dev. 1999;111:39–47. doi: 10.1016/s0047-6374(99)00071-8. [DOI] [PubMed] [Google Scholar]
- [76].Itoh K, Weis S, Mehraein P, Müller-Höcker J. Cytochrome c oxidase defects of the human substantia nigra in normal aging. Neurobiol. Aging. 1996;17:843–848. doi: 10.1016/s0197-4580(96)00168-6. [DOI] [PubMed] [Google Scholar]
- [77].Tian L, Cai Q, Wei H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic. Biol. Med. 1998;24:1477–1484. doi: 10.1016/s0891-5849(98)00025-2. [DOI] [PubMed] [Google Scholar]
- [78].Haripriya D, Devi MA, Kokilavani V, Sangeetha P, Panneerselvam C. Age-dependent alterations in mitochondrial enzymes in cortex, striatum and hippocampus of rat brain - potential role of L-Carnitine. Biogerontology. 2004;5:355–364. doi: 10.1007/s10522-004-2575-y. [DOI] [PubMed] [Google Scholar]
- [79].Vazquez EJ, Hoppel CL. Aging decreases the activity of complex III in rat hepatic mitochondria. FASEB J. 2004;18 [Google Scholar]
- [80].Vazquez EJ, Hoppel CL. Aging decreases mitochondrial electron transport at complex III. FASEB J. 2005;19 [Google Scholar]
- [81].Castelluccio C, Baracca A, Fato R, Pallotti F, Maranesi M, Barzanti V, Gorini A, Villa RF, Castelli GP, Marchetti M, Lenaz G. Mitochondrial activities of rat heart during ageing. Mech. Ageing Dev. 1994;76:73–88. doi: 10.1016/0047-6374(94)91583-0. [DOI] [PubMed] [Google Scholar]
- [82].Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann. N. Y. Acad. Sci. 2002;959:133–166. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- [84].Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J. Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J. Bioenerg. Biomembr. 1993;25:153–163. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- [86].Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- [87].Hoch FL. Cardiolipins and biomembrane function. Biochim. Biophys. Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- [88].Hoch FL. Cardiolipins and mitochondrial proton-selective leakage. J. Bioenerg. Biomembr. 1998;30:511–532. doi: 10.1023/a:1020576315771. [DOI] [PubMed] [Google Scholar]
- [89].Nalecz KA, Bolli R, Wojtczak L, Azzi A. The monocarboxylate carrier from bovine heart mitochondria: partial purification and its substrate-transporting properties in a reconstituted system. Biochim. Biophys. Acta. 1986;851:29–37. doi: 10.1016/0005-2728(86)90245-8. [DOI] [PubMed] [Google Scholar]
- [90].Fry M, Green DE. Cardiolipin requirement by cytochrome oxidase and the catalytic role of phospholipid. Biochem. Biophys. Res. Commun. 1980;93:1238–1246. doi: 10.1016/0006-291x(80)90622-1. [DOI] [PubMed] [Google Scholar]
- [91].Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J. Biol. Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- [92].Paradies G, Petrosillo G, Ruggiero FM. Cardiolipin-dependent decrease of cytochrome c oxidase activity in heart mitochondria from hypothyroid rats. Biochim. Biophys. Acta. 1997;1319:5–8. doi: 10.1016/s0005-2728(97)00012-1. [DOI] [PubMed] [Google Scholar]
- [93].Beyer K, Nuscher B. Specific cardiolipin binding interferes with labeling of sulfhydryl residues in the adenosine diphosphate/adenosine triphosphate carrier protein from beef heart mitochondria. Biochemistry. 1996;35:15784–15790. doi: 10.1021/bi9610055. [DOI] [PubMed] [Google Scholar]
- [94].Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- [95].Tuominen EK, Wallace CJ, Kinnunen PK. Phospholipid-cytochrome c interaction: evidence for the extended lipid anchorage. J. Biol. Chem. 2002;277:8822–8826. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- [96].Sedlak E, Panda M, Dale MP, Weintraub ST, Robinson NC. Photolabeling of cardiolipin binding subunits within bovine heart cytochrome c oxidase. Biochemistry. 2006;45:746–754. doi: 10.1021/bi050870z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Paradies G, Ruggiero FM, Gadaleta MN, Quagliariello E. The effect of aging and acetyl-L-carnitine on the activity of the phosphate carrier and on the phospholipid composition in rat heart mitochondria. Biochim. Biophys. Acta. 1992;1103:324–326. doi: 10.1016/0005-2736(92)90103-s. [DOI] [PubMed] [Google Scholar]
- [98].Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, Quagliariello E. Effect of aging and acetyl-L-carnitine on the activity of cytochrome oxidase and adenine nucleotide translocase in rat heart mitochondria. FEBS Lett. 1994;350:213–215. doi: 10.1016/0014-5793(94)00763-2. [DOI] [PubMed] [Google Scholar]
- [99].Iossa S, Mollica MP, Lionetti L, Crescenzo R, Botta M, Barletta A, Liverini G. Acetyl-L-carnitine supplementation differently influences nutrient partitioning, serum leptin concentration and skeletal muscle mitochondrial respiration in young and old rats. J. Nutr. 2002;132:636–642. doi: 10.1093/jn/132.4.636. [DOI] [PubMed] [Google Scholar]
- [100].Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song MH, Ames BN. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9562–9566. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Moghaddas S, Stoll MS, Minkler PE, Salomon RG, Hoppel CL, Lesnefsky EJ. Preservation of cardiolipin content during aging in rat heart interfibrillar mitochondria. J. Gerontol. A. Biol. Sci. Med. Sci. 2002;57:B22–28. doi: 10.1093/gerona/57.1.b22. [DOI] [PubMed] [Google Scholar]
- [102].Moriggi M, Cassano P, Vasso M, Capitanio D, Fania C, Musicco C, Pesce V, Gadaleta MN, Gelfi C. A DIGE approach for the assessment of rat soleus muscle changes during unloading: effect of acetyl-L-carnitine supplementation. Proteomics. 2008;8:3588–3604. doi: 10.1002/pmic.200701176. [DOI] [PubMed] [Google Scholar]
- [103].Gadaleta MN, Petruzzella V, Renis M, Fracasso F, Cantatore P. Reduced transcription of mitochondrial DNA in the senescent rat. Tissue dependence and effect of L-carnitine. Eur. J. Biochem. 1990;187:501–506. doi: 10.1111/j.1432-1033.1990.tb15331.x. [DOI] [PubMed] [Google Scholar]
- [104].Gross CJ, Henderson LM, Savaiano DA. Uptake of L-carnitine, D-carnitine and acetyl-L-carnitine by isolated guinea-pig enterocytes. Biochim. Biophys. Acta. 1986;886:425–433. doi: 10.1016/0167-4889(86)90178-3. [DOI] [PubMed] [Google Scholar]
- [105].Parnetti L, Gaiti A, Mecocci P, Cadini D, Senin U. Pharmacokinetics of IV and oral acetyl-L-carnitine in a multiple dose regimen in patients with senile dementia of Alzheimer type. Eur. J. Clin. Pharmacol. 1992;42:89–93. doi: 10.1007/BF00314926. [DOI] [PubMed] [Google Scholar]
- [106].Marzo A, Arrigoni Martelli E, Urso R, Rocchetti M, Rizza V, Kelly JG. Metabolism and disposition of intravenously administered acetyl-L-carnitine in healthy volunteers. Eur. J. Clin. Pharmacol. 1989;37:59–63. doi: 10.1007/BF00609426. [DOI] [PubMed] [Google Scholar]
- [107].Molstad P, Bohmer T, Eiklid K. Specificity and characteristics of the carnitine transport in human heart cells (CCL 27) in culture. Biochim. Biophys. Acta. 1977;471:296–304. doi: 10.1016/0005-2736(77)90257-7. [DOI] [PubMed] [Google Scholar]
- [108].Ohashi R, Tamai I, Yabuuchi H, Nezu JI, Oku A, Sai Y, Shimane M, Tsuji A. Na(+)-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J. Pharmacol. Exp. Ther. 1999;291:778–784. [PubMed] [Google Scholar]
- [109].Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- [110].Bremer J. Carnitine--metabolism and functions. Physiol. Rev. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- [111].Ramsay RR, Zammit VA. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol. Aspects Med. 2004;25:475–493. doi: 10.1016/j.mam.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [112].Ricciolini R, Scalibastri M, Kelleher JK, Carminati P, Calvani M, Arduini A. Role of acetyl-L-carnitine in rat brain lipogenesis: implications for polyunsaturated fatty acid biosynthesis. J. Neurochem. 1998;71:2510–2517. doi: 10.1046/j.1471-4159.1998.71062510.x. [DOI] [PubMed] [Google Scholar]
- [113].Kuratsune H, Watanabe Y, Yamaguti K, Jacobsson G, Takahashi M, Machii T, Onoe H, Onoe K, Matsumura K, Valind S, Kitani T, Langstrom B. High uptake of [2–11C]acetyl-L-carnitine into the brain: a PET study. Biochem. Biophys. Res. Commun. 1997;231:488–493. doi: 10.1006/bbrc.1996.5919. [DOI] [PubMed] [Google Scholar]
- [114].Tucek S. Short-term control of the synthesis of acetylcholine. Prog. Biophys. Mol. Biol. 1993;60:59–69. doi: 10.1016/0079-6107(93)90013-a. [DOI] [PubMed] [Google Scholar]
- [115].Aureli T, Puccetti C, Di Cocco ME, Arduini A, Ricciolini R, Scalibastri M, Manetti C, Conti F. Entry of [(1,2-13C2)acetyl]-L-carnitine in liver tricarboxylic acid cycle and lipogenesis: a study by 13C NMR spectroscopy in conscious, freely moving rats. Eur. J. Biochem. 1999;263:287–293. doi: 10.1046/j.1432-1327.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- [116].Farrell S, Vogel J, Bieber LL. Entry of acetyl-L-carnitine into biosynthetic pathways. Biochim. Biophys. Acta. 1986;876:175–177. doi: 10.1016/0005-2760(86)90332-2. [DOI] [PubMed] [Google Scholar]
- [117].Lligona-Trulla L, Arduini A, Aldaghlas TA, Calvani M, Kelleher JK. Acetyl-L-carnitine flux to lipids in cells estimated using isotopomer spectral analysis. J. Lipid Res. 1997;38:1454–1462. [PubMed] [Google Scholar]
- [118].Kerner J, Minkler PE, Lesnefsky EJ, Hoppel CL. Fatty acid chain-elongation in perfused rat heart: synthesis of stearoylcarnitine from perfused palmitate. FEBS Lett. 2007;581:4491–4494. doi: 10.1016/j.febslet.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Morikawa T, Yasuno R, Wada H. Do mammalian cells synthesize lipoic acid? Identification of a mouse cDNA encoding a lipoic acid synthase located in mitochondria. FEBS Lett. 2001;498:16–21. doi: 10.1016/s0014-5793(01)02469-3. [DOI] [PubMed] [Google Scholar]
- [120].Shen W, Liu K, Tian C, Yang L, Li X, Ren J, Packer L, Head E, Sharman E, Liu J. Protective effects of R-alpha-lipoic acid and acetyl-L-carnitine in MIN6 and isolated rat islet cells chronically exposed to oleic acid. J. Cell. Biochem. 2008;104:1232–1243. doi: 10.1002/jcb.21701. [DOI] [PubMed] [Google Scholar]
- [121].Witkowski A, Joshi AK, Smith S. Coupling of the de novo fatty acid biosynthesis and lipoylation pathways in mammalian mitochondria. J. Biol. Chem. 2007;282:14178–14185. doi: 10.1074/jbc.M701486200. [DOI] [PubMed] [Google Scholar]
- [122].Hansford RG, Castro F. Age-linked changes in the activity of enzymes of the tricarboxylate cycle and lipid oxidation, and of carnitine content, in muscles of the rat. Mech. Ageing Dev. 1982;19:191–200. doi: 10.1016/0047-6374(82)90010-0. [DOI] [PubMed] [Google Scholar]
- [123].Maccari F, Arseni A, Chiodi P, Ramacci MT, Angelucci L. Levels of carnitines in brain and other tissues of rats of different ages: effect of acetyl-L-carnitine administration. Exp. Gerontol. 1990;25:127–134. doi: 10.1016/0531-5565(90)90043-2. [DOI] [PubMed] [Google Scholar]
- [124].Rebouche CJ. Carnitine function and requirements during the life cycle. Faseb J. 1992;6:3379–3386. [PubMed] [Google Scholar]
- [125].Liu J, Ames BN. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer's disease, and Parkinson's disease. Nutr. Neurosci. 2005;8:67–89. doi: 10.1080/10284150500047161. [DOI] [PubMed] [Google Scholar]
- [126].Minkler PE, Anderson VE, Maiti NC, Kerner J, Hoppel CL. Isolation and identification of two isomeric forms of malonyl-coenzyme A in commercial malonyl-coenzyme A. Anal. Biochem. 2004;328:203–209. doi: 10.1016/j.ab.2004.01.015. [DOI] [PubMed] [Google Scholar]
- [127].Makar TK, Cooper AJ, Tofel-Grehl B, Thaler HT, Blass JP. Carnitine, carnitine acetyltransferase, and glutathione in Alzheimer brain. Neurochem. Res. 1995;20:705–711. doi: 10.1007/BF01705539. [DOI] [PubMed] [Google Scholar]
- [128].Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha -lipoic acid. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Schinetti ML, Rossini D, Greco R, Bertelli A. Protective action of acetylcarnitine on NADPH-induced lipid peroxidation of cardiac microsomes. Drugs Exp. Clin. Res. 1987;13:509–515. [PubMed] [Google Scholar]
- [130].Reznick AZ, Kagan VE, Ramsey R, Tsuchiya M, Khwaja S, Serbinova EA, Packer L. Antiradical effects in L-propionyl carnitine protection of the heart against ischemia-reperfusion injury: the possible role of iron chelation. Arch. Biochem. Biophys. 1992;296:394–401. doi: 10.1016/0003-9861(92)90589-o. [DOI] [PubMed] [Google Scholar]
- [131].Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J. Neurosci. Res. 2005;79:509–521. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- [132].Virmani A, Gaetani F, Binienda Z, Xu A, Duhart H, Ali SF. Role of mitochondrial dysfunction in neurotoxicity of MPP+: partial protection of PC12 cells by acetyl-L-carnitine. Ann. N. Y. Acad. Sci. 2004;1025:267–273. doi: 10.1196/annals.1316.033. [DOI] [PubMed] [Google Scholar]
- [133].Snyder JW, Kyle ME, Ferraro TN. L-carnitine delays the killing of cultured hepatocytes by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Arch. Biochem. Biophys. 1990;276:132–138. doi: 10.1016/0003-9861(90)90019-u. [DOI] [PubMed] [Google Scholar]
- [134].Petrosillo G, Casanova G, Matera M, Ruggiero FM, Paradies G. Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett. 2006;580:6311–6316. doi: 10.1016/j.febslet.2006.10.036. [DOI] [PubMed] [Google Scholar]
- [135].Steffen V, Santiago M, de la Cruz CP, Revilla E, Machado A, Cano J. Effect of intraventricular injection of 1-methyl-4-phenylpyridinium: protection by acetyl-L-carnitine. Hum. Exp. Toxicol. 1995;14:865–871. doi: 10.1177/096032719501401102. [DOI] [PubMed] [Google Scholar]
- [136].Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J. Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- [137].Schipper HM. Heme oxygenase-1: role in brain aging and neurodegeneration. Exp. Gerontol. 2000;35:821–830. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- [138].Takeda A, Perry G, Abraham NG, Dwyer BE, Kutty RK, Laitinen JT, Petersen RB, Smith MA. Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. J. Biol. Chem. 2000;275:5395–5399. doi: 10.1074/jbc.275.8.5395. [DOI] [PubMed] [Google Scholar]
- [139].Di Cesare Mannelli L, Ghelardini C, Calvani M, Nicolai R, Mosconi L, Vivoli E, Pacini A, Bartolini A. Protective effect of acetyl-L-carnitine on the apoptotic pathway of peripheral neuropathy. Eur. J. Neurosci. 2007;26:820–827. doi: 10.1111/j.1460-9568.2007.05722.x. [DOI] [PubMed] [Google Scholar]
- [140].Lee TI, Causton HC, Holstege FC, Shen WC, Hannett N, Jennings EG, Winston F, Green MR, Young RA. Redundant roles for the TFIID and SAGA complexes in global transcription. Nature. 2000;405:701–704. doi: 10.1038/35015104. [DOI] [PubMed] [Google Scholar]
- [141].Kim DH, Kim M, Kwon HJ. Histone deacetylase in carcinogenesis and its inhibitors as anti-cancer agents. J. Biochem. Mol. Biol. 2003;36:110–119. doi: 10.5483/bmbrep.2003.36.1.110. [DOI] [PubMed] [Google Scholar]
- [142].Takahashi H, McCaffery JM, Irizarry RA, Boeke JD. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol. Cell. 2006;23:207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- [143].Alonso-Montes C, Castro MG, Reguero JR, Perrot A, Ozcelik C, Geier C, Posch MG, Moris C, Alvarez V, Ruiz-Ortega M, Coto E. Mitochondrial transcription factors TFA, TFB1 and TFB2: a search for DNA variants/haplotypes and the risk of cardiac hypertrophy. Dis. Markers. 2008;25:131–139. doi: 10.1155/2008/575323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- [145].Dinardo MM, Musicco C, Fracasso F, Milella F, Gadaleta MN, Gadaleta G, Cantatore P. Acetylation and level of mitochondrial transcription factor A in several organs of young and old rats. Biochem. Biophys. Res. Commun. 2003;301:187–191. doi: 10.1016/s0006-291x(02)03008-5. [DOI] [PubMed] [Google Scholar]
- [146].Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr., Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- [148].Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Distler AM, Kerner J, Hoppel CL. Post-translational modifications of rat liver mitochondrial outer membrane proteins identified by mass spectrometry. Biochimica et biophysica acta. 2007;1774:628–636. doi: 10.1016/j.bbapap.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Edwards YH, Chase JF, Edwards MR, Tubbs PK. Carnitine acetyltransferase: the question of multiple forms. Eur. J. Biochem. 1974;46:209–215. doi: 10.1111/j.1432-1033.1974.tb03613.x. [DOI] [PubMed] [Google Scholar]
- [153].Cassano P, Sciancalepore AG, Pesce V, Fluck M, Hoppeler H, Calvani M, Mosconi L, Cantatore P, Gadaleta MN. Acetyl-L-carnitine feeding to unloaded rats triggers in soleus muscle the coordinated expression of genes involved in mitochondrial biogenesis. Biochim. Biophys. Acta. 2006;1757:1421–1428. doi: 10.1016/j.bbabio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- [154].Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Zhang J, Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J. Clin. Invest. 1992;90:1193–1199. doi: 10.1172/JCI115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J. Cell. Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- [157].Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- [158].Pesce V, Fracasso F, Musicco C, Lezza AM, Cantatore P, Gadaleta MN. Acetyl-L-carnitine dietary supplementation to old rats increases mitochondrial transcription factor A content in rat hindlimb skeletal muscles. Ann. N. Y. Acad. Sci. 2004;1019:430–433. doi: 10.1196/annals.1297.077. [DOI] [PubMed] [Google Scholar]
- [159].Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decrease in the cytochrome c oxidase activity and changes in phospholipids in rat heart mitochondria. Arch. Gerontol. Geriatr. 1993;16:263–272. doi: 10.1016/0167-4943(93)90037-i. [DOI] [PubMed] [Google Scholar]
- [160].Vik SB, Capaldi RA. Lipid requirements for cytochrome c oxidase activity. Biochemistry. 1977;16:5755–5759. doi: 10.1021/bi00645a016. [DOI] [PubMed] [Google Scholar]
- [161].Iossa S, Mollica MP, Lionetti L, Crescenzo R, Botta M, Liverini G. Skeletal muscle oxidative capacity in rats fed high-fat diet. Internernational Journal of Obesity. 2002;26:65–72. doi: 10.1038/sj.ijo.0801844. [DOI] [PubMed] [Google Scholar]
- [162].Gadaleta MN, Petruzzella V, Daddabbo L, Olivieri C, Fracasso F, Loguercio Polosa P, Cantatore P. Mitochondrial DNA transcription and translation in aged rat. Effect of acetyl-L-carnitine. Ann. N. Y. Acad. Sci. 1994;717:150–160. doi: 10.1111/j.1749-6632.1994.tb12082.x. [DOI] [PubMed] [Google Scholar]
- [163].Virmani A, Gaetani F, Binienda Z, Xu A, Duhart H, Ali SF. Role of mitochondrial dysfunction in neurotoxicity of MPP+: partial protection of PC12 cells by acetyl-L-carnitine. Ann. N. Y. Acad. Sci. 2004;1025:267–273. doi: 10.1196/annals.1316.033. [DOI] [PubMed] [Google Scholar]