Abstract
The very strong relationship between suicide, depressive disorders and substance use disorders is well recognized. Certain pain syndromes are significantly associated with suicide, irrespective of co-occurring medical or psychiatric diagnosis. Chronic pain, depression, substance use disorders and suicide appear to involve overlapping neural pathways and brain regions that function in the processing of emotional and physical pain, as well as maintaining reward and anti-reward circuitry. In this article, we employ a clinical case to illustrate how various stressors disrupted the balance between pain and opioid-facilitated analgesia. This disruption resulted in excessive use of short-acting opioids to treat pain with ensuing allostatic overload and culmination in chronic suicidal ideation with a suicide attempt. Sublingual buprenorphine was selected to treat the opioid use disorder. We propose that the unique pharmacodynamics of this drug served to stabilize dysregulated neural circuits, neurotransmitters and neuropeptides, allowing the mitigation of pain, assuaging opioid cravings, easing depression and resolving suicidal ideation. To our knowledge, this is the first case report to describe the possible anti-suicidal effect of sublingual buprenorphine.
Index Words: buprenorphine, suicidality, pain, depression
INTRODUCTION
In this report, we present a case of chronic suicidal ideation in the context of treatment-resistant depression, chronic back pain, and severe opioid use disorder. Suicidal ideation rapidly resolved with the initiation of buprenorphine.
CASE
Ms. S. is a 61 year-old widowed Caucasian woman with treatment-resistant major depression, chronic hepatitis C, hypertension, migraines, renal calculi status post stenting, chronic back pain, and obesity status post gastric bypass with gastric dumping syndrome. Her primary care provider managed her chronic pain with opioids. She was referred to our opioid replacement clinic after repeated urine drug screens returned positive for opiates, oxycodone, methadone and benzodiazepines.
On intake interview, Ms. S. described daily injection heroin use during her late teens and twenties. She entered a 12-Step Program in her late 20s and remained abstinent from opioids, but began drinking alcohol socially after seven years. During her late 50s, she became increasingly depressed in the context of her husband’s terminal illness, financial difficulties and chronic pain. She was treated with a succession of antidepressants that did little to ameliorate her depression. Over time, suicidal ideation emerged and became chronic.
A change in primary care providers in conjunction with her urine toxicology results resulted in a decision to cease opioid treatment of chronic pain. Ms. S. began obtaining prescription opioids illicitly to treat her pain and would intermittently purchase heroin. She injected heroin for pain relief and “energy”. Then, in the context of ongoing depression and undertreated pain, she attempted to end her life by driving her car off a bridge and was psychiatrically hospitalized. A change in her antidepressant served to diminish depressive symptoms; yet, she continued to be plagued with thoughts of ending her life.
When suicidal thoughts were discussed, she stated, “I think of suicide every day. It’s usually thoughts such as, ‘Damn, I’m tired…’” She cited reasons to live as her children and grandchildren. Suicide risk assessment showed numerous risk factors for suicide, including previous suicide attempt, chronic suicidal ideation, chronic pain, multiple medical comorbidities, depression, substance abuse, unemployment and bereavement.
Ms. S. met DSM-V criteria for opioid use disorder, severe. She was deemed to be a candidate for office-based buprenorphine treatment due to her desire to stop using illicit opioids, her stable living situation and the support she received from her daughters. She was inducted successfully, and her dose was titrated to 16/4 mg of buprenorphine/naloxone daily. This checked cravings and managed her pain.
On return visit one week after induction, when asked about suicidal thoughts, Ms. S. paused and replied that they had completely disappeared. She affirmed that she hadn’t thought of ending her life in several days. In terms of cause, she cited a resolution of her depression, more energy, decrease in her pain, increased motivation and the ability to complete daily tasks. “I finally planted the flowers that I bought three months ago. That’s a big deal!” she exclaimed.
On follow-up one month after buprenorphine/naloxone initiation, Ms. S. remained feeling well, with positive mood. Suicidal ideation remained absent. Three months post-induction, suicidality had not re-emerged. To date, all subsequent urine drug screens have been positive only for buprenorphine.
DISCUSSION
The very strong relationship between suicide, depressive disorders and substance use disorders is well-recognized, with depression and substance use disorder comprising the top risk factors for completed suicides [1]. Of all pain diagnoses, back pain, migraine and psychogenic pain are significantly associated with suicide, irrespective of co-occurring medical or psychiatric diagnosis [2]. Evidence-based treatments independently exist for depression, chronic pain and substance use disorder. However, no treatment has been found to be efficacious in all three disorders simultaneously.
Chronic pain, depression, substance use disorders and suicide appear to involve overlapping neural pathways and brain regions that function in the processing of emotional and physical pain, as well as maintaining reward and anti-reward circuitry [3-5]. The lateral habenula is an example of one of these regions, in that it is a junction for pain-carrying spinothalamic neurons, as well as connecting to three major neurotransmitter systems – dopaminergic, serotonergic and adrenergic.
This case provides a clinical illustration of the developing theories elucidating the complex neurocircuitry of pain, mood, addiction and reward. In this case, we see the presence of multiple circuits, neurotransmitters and neuropeptides with a tragic culmination – chronic suicidal ideation. We speculate that the cycle began with opioid-induced analgesia and a hyper-dopaminergic euphoria, followed by hyperalgesia and hypo-dopaminergic dysphoria after withdrawal from short-acting opioids. In our patient, this dysphoria may have motivated repeated use of opioids to treat poor mood, amotivation and lack of energy. Repeated use resulted in the development of tolerance, as well as alteration of this individual’s homeostatic set point [3-6]. This alteration in set point likely induced a chronic stress, impacting the hypothalamic-pituitary-adrenal (HPA)-axis with resulting dysregulation of the noradrenergic system. Dysregulation of the noradrenergic system has been implicated in depression and suicide in humans [7].
A number of neuropeptides, including corticotropin releasing factor (CRF) and dynorphin, have been found to be involved in stress, depression and suicidal behavior [8-12]. Dynorphin is an endogenous opioid peptide with a variety of actions including analgesia, which acts through kappa opioid agonism. CRF is released from the hypothalamus in response to stress with a number of downstream effects including the secretion of cortisol. The CRF system appears to play a strong part in maintaining the negative mood states that drive addictive processes [13]. Land et al (2008) first elucidated the relationship between CRF and dynorphin by demonstrating that the stress-induced release of CRF stimulated dynorphin resulted in dysphoria [10]. Dynorphin immunoreactivity is increased in the nucleus accumbens and hippocampus in pre-clinical models of stress and depression, with kappa antagonism producing an antidepressant effect [14]. This literature highlights the connection between stress, CRF, dynorphin and the kappa opioid system, and places this important circuit within the larger reward and anti-reward circuits described above.
Buprenorphine, a thebaine derivative, is a partial agonist at mu-opioid receptors and opioid receptor-like receptors with primarily antagonist actions at kappa and delta opioid receptors. The parental form was approved as an analgesic in 1981; however, the sublingual form is approved only for treatment of opioid dependence. As illustrated in Figure 1, short acting prescription opioids were initially able to provide Ms. S with appropriate analgesia. As stressors increased, this homeostasis was disrupted, resulting in allostatic overload with predominance of anti-reward circuitry. We speculate that treating Ms. S’s opioid dependence with sublingual buprenorphine reinstated the balance between the reward and anti-reward circuits. Buprenorphine action at the mu-opioid receptor in conjunction with its long half-life, regular dosing and ultra-stable receptor occupancy terminated the cycle of analgesia/euphoria and withdrawal/hyperalgesia/dysphoria. This resulted in treatment of pain, as well as stabilization of the dopaminergic and adrenergic systems. Buprenorphine’s antagonism of the kappa-opioid receptor mediated tolerance. We hypothesize that buprenorphine’s kappa antagonist effect resulted in the blockade of acute dynorphin signaling, as well as functioning to reverse stress-induced neural adaptions to chronic kappa agonism via CRF. By acting on multiple receptors, buprenorphine stabilized multiple circuits mitigating pain, assuaging opioid cravings, easing depression and resolving suicidal ideation.
FIGURE 1.
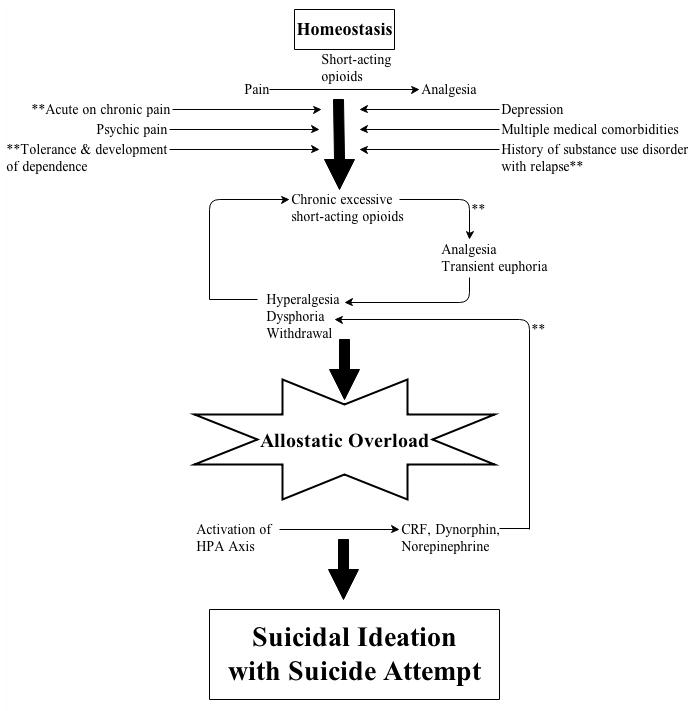
Illustration of potential sites of buprenorphine action on anti-reward and acute stress-induced dynorphin signaling.
** = Potential site of buprenorphine action
The rapid antidepressant effect of buprenorphine has previously been described [15, 16]. To our knowledge, this is the first case report describing a possible anti-suicidal effect of buprenorphine. While still unclear, the mechanism of other drugs with anti-suicidal effects – lithium and clozapine – is thought to be related to action on serotonergic neurotransmission. However, buprenorphine is without robust direct effect on this neurotransmitter system. Further observation is warranted to determine if this anti-suicidal effect is indeed present and, if so, to further elucidate the mechanism.
Acknowledgments
Dr. Striebel is currently funded by the ACGME-accredited Fellowship in Addiction Psychiatry at the University of California, San Francisco. Dr. Kalapatapu is currently funded by K23DA034883.
Footnotes
CONFLICTS OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Yoshimasu K, Kiyohara C, Miyashita K. Suicidal risk factors and completed suicide: meta-analyses based on psychological autopsy studies. Environmental Health and Preventive Medicine. 2008;13:243–256. doi: 10.1007/s12199-008-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilgen MA, Kleinberg F, Ignacio RV, Bohnert AS, Valenstein M, McCarthy JF, Blow FC, Katz IR. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70:692–697. doi: 10.1001/jamapsychiatry.2013.908. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elman I, Borsook D, Volkow ND. Pain and suicidality: Insights from reward and addiction neuroscience. Progress in Neurobiology. 2013;109:1–27. doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neuroscience and Biobehavioral Reviews. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garland EL, Froeliger B, Zeidan F, Suveg K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience and Biobehavioral Reviews. 2013 doi: 10.1016/j.neubiorev.2013.08.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandley MJ, Ordway GA. Noradrenergic Dysfunction in Depression and Suicide. In: Yogesh Dwivedi., editor. The Neurobiological Basis of Suicide. Chapter 3. Boca Raton, FL: CRC Press; 2012. [PubMed] [Google Scholar]
- 8.Serafini G, Pompili M, Lindqvist D, Dwivedi Y, Girardi P. The role of neuropeptides in suicidal behavior: a systematic review. BioMed Research International. 2013;2013:687575. doi: 10.1155/2013/687575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 10.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. The Journal of Neuroscience. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoll AT, Carlezon WA. Dynorphin, stress, and depression. Brain Research. 2010;1314c:56. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropinreleasing factor. Current Opinion in Investigational Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- 14.Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. Journal of Neurochemistry. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 15.Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. Journal of Clinical Psychopharmacology. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Emrich HM, Vogt P, Herz A, Kissling W. Antidepressant effects of buprenorphine. Lancet. 1982;2:709. doi: 10.1016/s0140-6736(82)90727-9. [DOI] [PubMed] [Google Scholar]


