Abstract
Genome instability contributes to cancer development and accelerates age-related pathologies as evidenced by a variety of congenital cancer susceptibility and progeroid syndromes that are caused by defects in genome maintenance mechanisms. DNA damage response pathways that are mediated through the tumor suppressor p53 play an important role in the cell intrinsic responses to genome instability, including a transient cell cycle arrest, senescence and apoptosis. Both senescence and apoptosis are powerful tumor suppressive pathways preventing the uncontrolled proliferation of transformed cells. However, both pathways can potentially deplete stem and progenitor cell pools, thus promoting tissue degeneration and organ failure, which are both hallmarks of aging. p53 signaling is also involved in mediating non-cell autonomous interactions with the innate immune system and in the systemic adjustments during the aging process. The network of p53 target genes thus functions as an important regulator of cancer prevention and the physiology of aging.
p53 at the crossroad of cancer and aging
Preservation of genomic integrity is crucial for the survival and reproduction of all life on earth. Genome integrity is constantly threatened by a variety of genotoxic insults, with tens of thousands damaging events occurring in every single cell on a daily basis 1. Erroneous repair can result in mutations potentially leading to altered gene function, which in turn can give rise to cancer development. Persistence of lesions can lead to cellular senescence and apoptosis eventually resulting in tissue degeneration 2. Specifically cellular senescence has been shown to serve a barrier function against malignant transformation of premalignant lesions 3-5. As DNA damage gradually accumulates during lifetime, both the likelihood of oncogenic transformation as well as tissue dysfunction and degeneration increases with age. Cellular responses to DNA damage are mediated through highly conserved DNA damage checkpoint mechanisms that are important for tumor suppression by arresting cell cycle progression, or evoking cellular senescence and apoptosis 6. p53 is a key player in the tumor suppressive DNA damage response (DDR) and is mutationally inactivated in approximately 50% of human cancers 7. Recent evidence suggests that p53 not only antagonizes oncogenic transformation, but also orchestrates non-cell autonomous responses to DNA damage by mediating clearance of damaged cells through the innate immune system 8-10. On an organismal level, p53 activity has been implicated in driving tissue degeneration and aging 11, 12. In contrast to its role in contributing to the functional decline of tissues in aging, p53 also regulates genes that are associated with lifespan extension 13. To understand the outcome of p53 activity in cancer and aging it is crucial to understand how p53 mediates distinct outcomes of DNA damage signaling in the context of cells and tissues. Here, we draw a picture integrating cell autonomous and non-cell autonomous p53-mediated DNA damage responses to cancer prevention and lifespan regulation.
DNA repair defects lead to cancer susceptibility, developmental abnormalities and premature aging
The genome of every cell is constantly threatened by intrinsic and extrinsic genotoxic insults, such as metabolic byproducts and radiation, respectively 1, 14. Genotoxic agents can lead to a plethora of different modifications in the physicochemical structure of DNA. This large variety of DNA lesions is recognized by highly specialized DNA repair systems 15. Congenital defects in DNA repair systems underlie a variety of complex genetic disorders that are characterized by cancer susceptibility, developmental abnormalities, and accelerated tissue degeneration 16, 17. While the causal links between DNA repair defects and cancer susceptibility are rather well-established, how genome instability leads to complex pathologies during development and aging remains less well-understood. DNA repair defects can lead to enhanced mutation rates, which when occurring in tumor suppressors or oncogenes can lead to cancer development as for instance in mismatch repair defects 18. Likewise defects in DNA damage checkpoints can fuel tumorigenesis by abrogating cellular senescence and apoptosis programs as seen for instance in highly cancer prone Li Fraumeni or ataxia telangectasia patients 19, 20. However, when DNA damage persists and interferes with replication or transcription as observed in transcription-coupled repair (TCR)-deficient Cockayne syndrome patients, ensuing high levels of apoptosis and cellular senescence can accelerate tissue dysfunction and degeneration 21.
The DDR effector p53 - tipping the balance between cancer and aging
Chronic DNA damage, as that observed in TCR defects, appears to be associated with constitutive DDR signaling and premature aging. On the other hand loss of downstream DDR effectors appears to be primarily linked to a tumor-prone phenotype. Indeed, mice lacking the critical DDR gene TP53, encoding for the p53 protein, are highly tumor-prone and regularly succumb to neoplastic disease 22. Patients suffering from Li-Fraumeni syndrome, caused by germline mutations in the TP53 gene, are characterized by the development of multiple tumors early in life 19. The consequences of defective DDR activity in causing cancer and accelerating aging are particularly well-illustrated in mice carrying activated and impaired TP53alleles. p53 is a transcription factor that exerts tumor suppressive functions by inducing the expression of effector genes (Figure 1). Mutational inactivation of p53 allows the uncontrolled proliferation of damaged cells 23. In contrast, the expression of dominant active forms of p53 leading to constitutive expression of downstream genes, results in degenerative phenotypes and premature aging12, 24. It is likely that constitutive activation of p53 annihilates stem cell compartments thus impairing tissue regeneration. Interestingly, keeping a fine balance between p53 activation sufficient for tumor surveillance but insufficient to trigger stem cell depletion, seems to result in beneficial effects both for cancer protection and longevity as demonstrated by the expression of extraneous wt alleles of TP53 in mice 25 (see below).
Figure 1. p53 is activated downstream of the proximal DNA damage checkpoint signaling cascade in response to genotoxic damage or oncogene-induced replication stress.
The depicted checkpoint kinase complexes DNA-PK, ATM, ATR, CHK1, CHK2 and MK2 mediate DNA damage signaling, which funnels into activation of p53. Upon activation, p53 transcriptionally induces a host of target genes, which promote cell cycle arrest, allowing time for DNA repair, senescence or apoptosis leading to cell loss and ultimately contributing to tissue degeneration in aging. The fine balance between these different p53-mediated cellular outcomes translates into physiological consequences between cancer protection and aging. DSB, DNA double strand break; SSB, DNA single strand break.
p53 activity has been linked to the regulation of a complex cellular DDR network (Figure 1) and more recently also to non-cell autonomous interactions between damaged cells and the innate immune system (see below). The cellular DDR has initially been characterized in single cell yeast, where it functions to arrest the cell cycle allowing time for DNA repair 26. DNA damage checkpoint mechanisms in yeast are highly conserved in mammals where they became part of a complex network that balances the DNA damage outcomes between transient cell cycle arrest, senescence and apoptosis. While yeast is devoid of a TP53 homolog, the nematode Caenorhabditis elegans harbors a functional TP53 homolog that mediates apoptosis in response to double strand breaks during meiosis through transcriptional induction of pro-apoptotic Bcl-2 homology (BH)3-only proteins 27-30. While this mechanism of p53-mediated checkpoint responses is highly conserved, mammalian p53 is involved in a more complex signaling network 31 and a variety of target genes that are transcriptionally controlled by p53 have been identified (Figure 1). The induction of p21 and BH3-only proteins is probably the physiologically best understood p53-mediated checkpoint response 31, 32. The cell cycle inhibitor p21 binds and inhibits cyclin dependent kinases (CDKs), thus preventing progression through the cell cycle. In contrast, BH3-only proteins such as p53 upregulated modulator of apoptosis (PUMA) as well as the BH domain proteins Bcl-2-associated protein X (Bax) and Bcl-2 antagonist/killer (Bak), are potent activators of apoptosis. Both, senescence and apoptosis are potent tumor suppressive mechanisms that ultimately prevent the (neoplastic) proliferation of damaged cells. On the other hand, both pathways have the potential to deplete proliferation-competent stem and progenitor cell pools, thus leading to impaired tissue renewal ultimately impairing organ homeostasis – a hallmark of aging. How these two outcomes of p53 activation are balanced is still poorly understood. One important factor is certainly a cell type specificity of the p53 response. For example, such a cell type-specific DDR is apparent in thymocytes that typically undergo apoptosis in response to genotoxic stress 33. In contrast, fibroblasts rather initiate cellular senescence, when exposed to DNA damage 34. This might relate to their physiological function where thymocytes are rapidly replenished while fibroblasts function in connective tissues, which require physical integrity.
While it is widely accepted that impaired p53 activity reduces organismal lifespan through uncontrolled proliferation of cancerous cells, loss of p53 activity should also lead to repression of catastrophic cell fate decisions, such as senescence and apoptosis, which result in aging phenotypes as tissue and organ degeneration (Figure 2). In fact, there is recent evidence from mouse models suggesting that p53 is indeed involved in regulating the aging process 35. The first hint that p53 activity might drive organismal aging came from experiments with mice in which a spontaneous recombination event deleted a stretch of DNA containing the 5′ sequence of the TP53 gene. This mutant allele, which was termed TP53m, contained exons 7-11 and transcription was driven from a promoter of an upstream gene. Experiments performed in mouse embryonic fibroblasts (MEFs) derived from TP53m/+ animals revealed that the TP53m gene product augmented stability and transactivation capacity of wildtype p53. In keeping with the idea that increased p53 activity protects from cancer development, TP53m/+ animals displayed robust cancer resistance. However, this cancer resistance came at the cost of a statistically significant lifespan reduction of the TP53m/+ animals, which was accompanied by signs of premature aging. Some of the aging phenotypes observed in the TP53m/+ mutant animals might be the result of reduced stem cell proliferation in these animals compared to wildtype controls 36. Additional support for a role of p53 function in organismal aging came from transgenic (tg) mice overexpressing a naturally occurring p53 isoform termed p44 24. Translation of the short isoform initiates at codon 41 in exon 4 producing a 44kD protein, lacking large parts of the transactivation domain 37. p44tg/tg animals, like TP53m/+ mutant mice, were cancer resistant and displayed signs of premature aging. These p44-mediated effects were dependent on p53, suggesting that p44 overexpression increased wildtype p53 activity.
Figure 2. Activation modes of p53 determine the physiological outcome.
(a) In wildtype cells and tissues, p53 exhibits low baseline activity through tight regulation of p53 protein levels. Only upon the appropriate cellular stress signals, such as those evoked by genotoxic lesions, p53 is activated to prevent oncogenic transformation of cells. (b) Increased tonic p53 activity, such as that induced by the expression of short p53 isoforms, promotes cancer resistance. However, the increased baseline activity of p53 appears to prevent tissue regeneration and to accelerate the aging process. (c) Low baseline levels with strong p53 activity only in response to appropriate stress stimuli, such as DNA damage, lead to extraordinary cancer protection with normal lifespan. Interestingly, recent data suggest that the physiological p53 response following exposure to ionizing radiation (IR) occurs in a non-linear oscillating fashion in repeating pulses with fixed amplitude and duration 83, 84. The observation of these oscillations became possible with recent advances in single-cell imaging techniques 85. This pulsatile behavior of p53 is induced by activation of p53 through ataxia telangectasia mutated (ATM) subsequently resulting in transactivation of Mouse double minute (Mdm)2 and Wild-type p53 induced phosphatase (Wip)1, two negative regulators of p53 85. This induction of Mdm2 and Wip1 results in downregulation of p53 through a negative feedback loop. Such pulsatile waves of p53 activation occur in 4- to 7hr intervals at similar intensity until the DNA is fully repaired. In contrast to the IR response, UV irradiation causes a dose-dependent sustained induction of p53 that does not appear to result in oscillating waves 85. The role of IR-induced p53 pulses in determining cell fate remains to be elucidated.
While both of the short isoforms of p53 promote cancer resistance, their expression was associated with decreased lifespan. In contrast to the short isoforms, where p53 mutants are expressed outside of the natural genomic context of the TP53 gene, the so-called ‘super p53′ mouse was generated using BAC transgenics, allowing the expression of wildtype p53 from within its natural genomic surrounding 25. As has been observed in the p44tg/tg and TP53m/+ mice, the ‘super p53′ mouse, carrying one or two TP53tg alleles in addition to the two endogenous copies is highly cancer resistant. However, in stark contrast to mice expressing the short isoforms of p53, the ‘super p53′ mouse did not display any signs of premature aging. While p53 expression and activity in ‘super p53′ cells were not elevated in the absence of stress, p53 activity was enhanced in response to genotoxic stress 25. Despite enhanced p53-dependent apoptosis in response to genotoxic stress, ‘super p53′ mice had a normal lifespan and displayed no signs of premature aging. In an extension of this work, ‘super p53/ARF’ mice have been generated that not only carry an extra copy of p53, but also carry an extra allele of p19 alternative reading frame protein (ARF) 38. Like the ‘super p53′ mice, the ‘super p53/ARF’ animals are cancer-resistant. In addition, the ‘super p53/ARF’ animals showed increased resistance to oxidative stress and a significantly increased lifespan. Thus, a picture is emerging where chronically elevated p53 expression is providing protection against cancer development at the cost of a reduced lifespan. However, a similar cancer resistance can be brought about when additional copies of wildtype p53 are available to increase the magnitude of p53 expression and activity only in response to appropriate stimuli (Figure 2).
p53 interacts with the immune system
While the different p53-dependent cellular outcomes are largely the result of cell-autonomous signaling cascades, p53 signaling also involves non-cell autonomous and systemic mechanisms (Figure 3). This is illustrated by observations made with mice, in which p53 could be re-activated in initially p53-deficient tumors. Reactivation of functional p53 in pre-existing p53-deficient tumors resulted in widespread apoptosis or senescence, where lymphomas appeared to be particularly prone to apoptosis, while senescence was the primary response in sarcomas and liver carcinomas 8-10. Of particular interest are experiments performed with mice carrying liver carcinomas 10 that revealed an intimate relationship between p53 signaling and recruitment and activation of the innate immune system. Specifically, p53 reactivation-induced senescence in liver carcinomas was associated with upregulation of a number of pro-inflammatory cytokines including Colony-stimulating factor (Csf)1 and Monocyte chemotactic protein (Mcp)1, Chemokine CXC motif ligand (Cxcl)1, and Interleukin (IL)-15 that attract macrophages, neutrophils, and natural killer cells, respectively 10. These mRNAs were also induced by p53 reactivation in cultured hepatoma cells, indicating that they are expressed specifically in tumor cells. Further experiments depleting macrophages, neutrophils or natural killer cells, suggested that innate immune cells were indeed required for tumor clearance 10. Each antagonist efficiently depleted the targeted immune cell population, but did not impact on p53 reactivation-mediated cancer cell senescence, indicating that induction of p53-dependent tumor cell senescence and the evoked immune attack cooperate to promote tumor clearance.
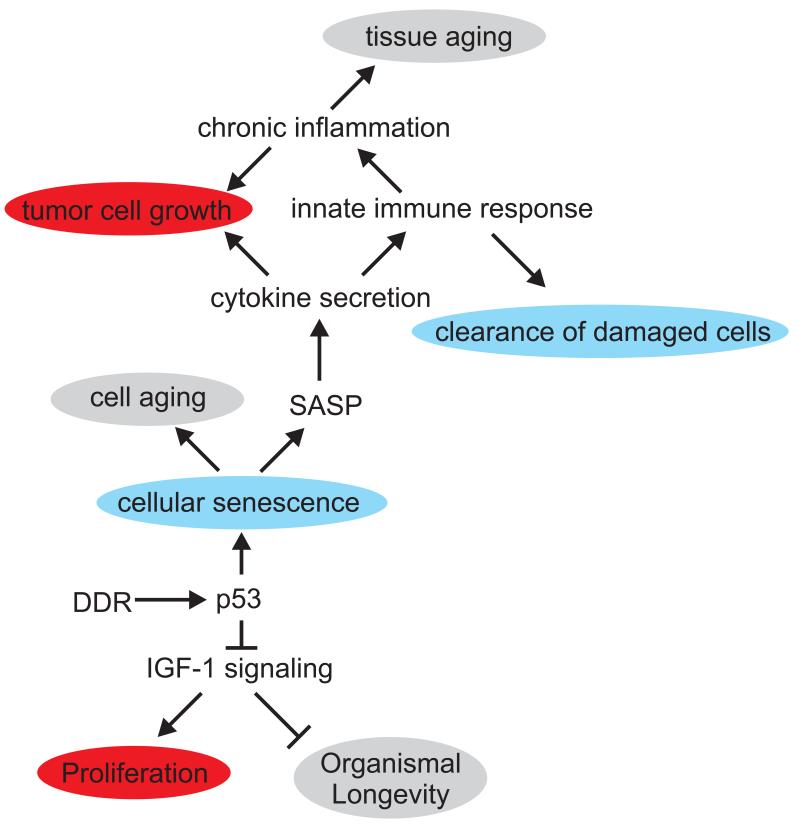
Figure 3. Non-cell autonomous consequences of p53 signaling in aging (grey) and cancer (red) associated processes.
p53 induces several negative regulators of the cell intrinsic Insulin-like growth factor (IGF)-1 receptor signaling pathway as well as systemically acting IGF-BP3, the major antagonistic IGF-1 carrier in the circulation. IGF-1 signaling induces cell proliferation, while reduced IGF-1 signaling is associated with extended lifespan in species ranging from nematode worms to mammals. p53-mediated cellular senescence, in contrast, promotes cellular aging, while senescent cells modify their tissue environment through the senescence associated secretory phenotype (SASP) resulting in cytokine secretion that activates the innate immune system. The innate immune system in turn can act tumor suppressive (blue) by clearing cells that have become senescent as result of oncogene activation or chronic DNA damage. However, SASP might also have tumor promoting consequences on surrounding cells as cytokine signaling and chronic inflammation can promote proliferation of tumor cells. In addition, chronic inflammation is also associated with tissue aging.
In a different line of experimentation the impact of p53 signaling on ionizing radiation (IR)-mediated lymphomagenesis was directly investigated either in transgenic mice that expressed p53 fused to a tamoxifen-sensitive estrogen receptor allowing control of p53 activity through administration of 4-hydroxy-tamoxifen (4-OHT) 39 or in a conditional knockout model where p53 was deleted before, concurrent with, or after IR-induced DNA damage 40. When p53 was kept active at the time of irradiation, typical radiation toxicity could be observed, including widespread apoptosis in proliferative tissues, such as the gastro intestinal tract, bone marrow and lymphoid system. Following recovery from acute radiation toxicity, p53 was inactivated through 4-OHT withdrawal and lymphoma incidence was measured compared to animals in which p53 was kept inactive at the time of irradiation. Counterintuitively, the animals in which p53 was made available at the time of irradiation showed no detectable lymphoma protection. In contrast, p53 reactivation after IR prevented acute radio-toxicity and provided tumor protection. These findings suggest that rather than the intensity of acute p53-driven apoptosis in response to irradiation injury, a delayed function of p53 appears to have the determining impact on p53-mediated tumor suppression in this tumor model. Interestingly, the tumor suppressive effect of delayed p53 re-activation was dependent on p19ARF, which has been suggested as a critical mediator of oncogene-driven p53 activation leading to cellular senescence 39. However, these experiments did not directly test the acute role of proximal DDR components in preventing lymphomagenesis. This could be achieved through deletion of ATM or Chk2 in this system. Furthermore, it is very likely that there is a qualitative difference between acute radiation-induced DNA damage as a trigger for lymphomagenesis and sustained oncogene-induced replication stress. In fact, the observation that delayed p53 activation was protective in the above-mentioned experiments might argue that it is indeed oncogene-induced DNA damage that occurs after IR-induced mutational oncogene activation that is the relevant source of p53-activating DNA damage in this system. However this possibility has so far not been addressed. Lastly, whether such a delayed p53 effect on IR-induced tumorigenesis can also be observed in other models, specifically those mirroring solid tumors, remains to be elucidated. Together these data suggest that activation of the canonical DDR is critical to prevent the transformation of premalignant lesions facing oncogene-induced genotoxic stress.
Senescent cells and their environment
Senescence of somatic cells has already been proposed in the late 19th century when August Weissmann realized that in contrast to the germ line, somatic cells have a finite lifespan 41. This replicative senescence was experimentally observed in the 1960s by Leonard Hayflick when growing human primary fibroblasts in culture 42. A plethora of senescence-promoting stimuli have since been identified, including telomere erosion, which causes a permanent DDR, non-telomeric genomic DNA damage and oncogenic stress, which also leads to an activation of a persistent DDR through misfired replication origins and replication fork collapse 43. As senescence is defined as an irreversible cell cycle arrest, it is obvious that senescence serves as a potent tumor-suppressive function through preventing the outgrowth of genomically damaged incipient cancer cells. Indeed, cellular senescence has recently been shown to act as a powerful barrier preventing malignant transformation of precancerous lesions 4, 5, 44. The importance of cellular senescence as a tumor-suppressive mechanism is underscored by the observation that most human tumors display inactivating mutations in the p53 and/or p16 inhibitor of cyclin-dependent kinase (INK)4A/ retinoblastoma (Rb) pathways, which are critical molecular constituents of the senescence response 45, 46. Studies on human tumor material have shown that senescent cells are enriched in premalignant lesions, while the malignant lesions ultimately arising from these precursors are essentially devoid of senescent cells, suggesting that senescence suppresses tumor development through an arrest at a benign state 3, 47. Interestingly, forced induction of senescence in malignant tumors through re-expression of p53 does not only halt tumor growth, but has been shown to result in tumor regression in mice. The senescent tumors were shown to be invaded by cells of the innate immune system, which ultimately cleared the senescent cells 9, 10. These observations suggest that senescence does not only act as a cell autonomous mechanism, but also involves a non-cell autonomous component, likely involving the induction of a local pro-inflammatory response (Figure 3). Thus, in addition to p53-dependent apoptosis, senescence has emerged as a powerful tumor suppressive mechanism downstream of p53. However, recent data are challenging this model of senescence as a simple failsafe mechanism redundant to p53-driven apoptosis. It has been shown that senescent cells do not just simply withdraw from the cell cycle, but that they induce the secretion of a variety of growth-promoting pro-inflammatory factors, including Il-6, Il-8, Cotton rat growth-regulated protein (GRO)α, Vascular endothelial growth factor (VEGF) and amphiregulin 48-54. The secretion of these growth factors, which appears to be at least partially dependent on the activity of the DDR kinase ATM54, is commonly referred to as senescence-associated secretory phenotype (SASP).
Cellular senescence in the aging organism
Intriguingly, the number of senescent cells increases with age in the majority of mitotically active mammalian tissues 55-61. The causal contribution of cellular senescence to aging has been recently demonstrated in a progeroid mouse model expressing hypomorphic (H/H) budding uninhibited by benzimidazoles (BubR)1, a key mediator of the mitotic checkpoint. BubR1H/Hmice display highly elevated levels of p16Ink4a-mediated cellular senescence. When senescent cells were eliminated by expressing an inducible Caspase-8 transgene under the control of the p16Ink4a promoter age-related pathology in the BubR1H/Hmice was alleviated 62. It will be highly interesting to determine whether ablation of senescent cells might also antagonize normal aging. While it remains unclear whether the accumulation of senescent cells in aging organisms is the result of increased generation, reduced clearance or both, senescent cells, by way of the SASP, might collectively contribute to the sterile inflammation that is frequently observed in aged tissues. Through the secretion of pro-inflammatory cytokines, senescent cells could create a local inflammatory reaction. In fact, there is a strong correlation between chronic organ inflammation and development of neoplasias within that particular organ 63. Intriguingly, several studies have shown that low-grade systemic inflammation is a characteristic feature of the aging organism and that molecular markers of inflammation, such as IL-6 and Tumor necrosis factor (TNF)-α, are significant predictors of mortality in aging humans 63, 64. Various molecular mechanisms mediate the link between an inflammatory microenvironment and tumor initiation and progression. Pro-inflammatory leukocytes, which might be recruited in response to factors secreted by resident senescent cells 9, 10, release genotoxic reactive oxygen and nitrogen species, which lead to genomic instability and growth-promoting alterations of the genome of the surrounding incipient cancer cells. In addition, activated leukocytes also secrete a number of growth-promoting cytokines, including IL-4, IL-6, IL-10, transforming growth factor (TGF)-β and TNF-α, further fueling the oncogenic process through various mechanisms, including direct stimulation of epithelial cancer cells, as well as activation of angiogenesis. Furthermore, established tumors have also been shown to maintain an inflammatory microenvironment further promoting malignant transformation and tumor maintenance 65. There is mounting evidence suggesting that senescent cells might indeed promote the transformation of premalignant epithelial cells. This is exemplified by the observation that co-injection of senescent fibroblasts with premalignant epithelial cells resulted in the successful formation of xenograft tumors 66. In addition, co-injection of senescent cells with malignant tumor cells increases the take rate in xenograft models 49, 66-69.
p53 impacts the IGF-1 system, a key regulator of Aging and Cancer
In contrast to its role in promoting cellular aging through mediating cellular senescence, p53 might also exert longevity assurance functions by impacting on the conserved insulin-like growth factor (IGF)-1 signaling network. p53 was shown to antagonize mitogenic signaling, energy metabolism, autophagy and cellular survival signaling through inhibiting IGF-1 and target of rapamycin (mTOR)-mediated signaling pathways at multiple levels (Figure 3) 13. p53 transcriptionally induces a number of inhibitors of IGF-1 and mTOR signaling including the PIP3 phosphatase PTEN, the antagonistic IGF-binding protein IGF-BP3, AMP-activated protein kinase (AMPK)-β, Tuberous sclerosis (Tsc)2 and Sestrin1/2 and p53 expression was also found to lead to reduced transcription of the IGF-1 receptor (IGF-1R) 70-74. IGF-1 receptor-mediated signaling is frequently hyperactivated in tumor cells providing proliferative and pro-survival signals 75. While activation of IGF-1 signaling promotes cellular growth and tumorigensis, reduced IGF-1 signaling in various model systems including nematodes, fruit flies and mammals delays aging and extends lifespan 76. mTOR functions as a downstream effector of IGF-1 signaling and regulates translation, autophagy, metabolism, cell growth and stress resistance 77. Consistent with the conserved longevity assurance effect of dampened IGF-1 signaling, inhibition of mTOR by administrating rapamycin led to lifespan extension in mice 78. By regulating genes that control IGF-1 signaling activity both cell intrinsically as well as systemically through induction of the antagonistic circulating IGF-1 carrier IGFBP3, p53 might thus play an important role in the physiological adjustments during the aging process. The concept that DNA damage signaling dampens the IGF-1 mediated somatic growth axis resulting in a shift from growth to cellular preservation as DNA damage accumulates with aging has been based on the observation of reduced IGF-1 levels in DNA repair defective progeroid mice (see box 1) 21. Likewise, aging humans display lower circulating levels of IGF-1 and growth hormone (GH) 79 and an association between polymorphisms in the IGF-1 receptor and human longevity has been reported 80. However, while p53 transcriptionally induces negative regulators of the IGF-1 signaling network, mice that express the hyperactive p44tg/tg allele display elevated IGF-1 signaling activity and age prematurely 11. It is conceivable that hyperactive p53 might lead to depletion of stem cell compartments by inducing extraneous levels of apoptosis and cellular senescence while IGF-1 mediated cellular growth factor signaling might function to compensate for the cell intrinsic growth arrest in this model. Taken together, these data suggest that p53 can exert both pro- and anti-aging functions. It will be highly important to determine how the physiological outcome of p53 activity is mediated through the differential transcriptional regulation of apoptotic, senescence and longevity target genes.
Box 1. Systemic DNA damage responses in tumor suppression and aging.
Recent evidence in progeroid (“premature aging-like”) mouse models have revealed links between DNA damage accumulation and the regulation of genetic longevity pathways. DNA damage responses were shown to dampen somatotropic signaling in mice that prematurely aged as result of defects in Sirt6, a deacetylase linked to base excision repair (BER) and double strand break (DSB) repair, or impairment of nucleotide excision repair (NER) 86-89. Transcriptome analysis and endocrine measurements in Csbm/m/Xpa−/− mouse mutants that model Cockayne syndrome (CS) as well as in mice carrying defects in the downstream NER components ERCC1 have suggested that IGF-1 secretion, circulation and signaling is reduced 87, 88. Moreover, it was shown that DNA lesions leading to blockage of RNA Polymerase II induce a growth suppressive response 90. In particular, IGF-1 receptor signaling is reduced in response to even low amounts of persistent transcription-blocking lesions. It is thus conceivable that part of the tumor suppressive phenotypes observed in CS patients might be due to the growth factor-deprived endocrine environment. In fact, a large number of tumors have been shown to critically depend on IGF-1 signaling 91.
As aging humans and mice, as well as progeroid mice with DNA repair defects dampen the IGF-1-mediated somatotropic axis, it is conceivable that cellular responses to DNA damage accumulation in aging orchestrate the shift from a growth permissive endocrine environment to tissue maintenance and enhanced systemic tumor surveillance. There is evidence from various model systems to support this hypothesis. Experiments performed in the early 1970s have shown that aging of murine mammary tissue, measured by reduced ductal elongation capacity throughout serial transplantations, is not consistently influenced by the age of the donor animal. In fact, mammary grafts from young and old mice showed similar growth kinetics and overall lifespan when transplanted into a young recipient. In marked contrast, old host animals did not support the growth of either old or young transplants 92. Likewise, transplantation of muscle tissue from old rats into young recipients resulted in successful regeneration, while transplantation of young muscle grafts into old hosts did not 93, 94. It has been shown that Notch signaling is critical for the proliferation and myogenic lineage progression of satellite cells, which are essential for muscle regeneration 95. The age-associated reduced regenerative potential of muscle tissue is at least in part due to an inability of this pathway to be activated 96. Intriguingly, exposure of old murine muscle tissue to a circulation of young mice in the context of parabiosis experiments, resulted in a restoration of Notch signaling, regenerative potential and proliferation of satellite cells within the aged parabiosed partner, suggesting that the age-related decline of tissue progenitor cell activity can be modulated and restored through exposure to circulating factors present in younger organisms 82. A similar correlation between host age and growth rate could be observed when mammary tumor growth rates were assessed in women 97. In these studies, serial mammography was used to estimate the age-dependent tumor volume doubling time in primary breast cancer. The median volume doubling time in women between 50 and 70 years of age was almost twice that of breast cancers diagnosed in females younger than 50 years. These observations are supported by other reports that found significantly less tumors with short doubling times in patients over the age of 60 years than in those below the age of 60 98. One might argue that breast cancer growth, at least partially, depends on the endocrine status of the host organism and that passage through menopause might result in decreased hormonal growth support of both primary breast epithelium, as described in the experiments discussed above 92,97. However, a recent study examining tumor volume doubling times in hereditary (BRAC1/2 mutation carriers) and familial breast cancer patients using multivariate analysis showed that only age, but not risk group or menopausal status correlated significantly with tumor volume doubling times 99. In addition, CT-guided tumor growth rate analysis in stage I lung cancer, as well as renal carcinoma, irrespective of the histologic subtype, revealed that age at diagnosis was negatively correlated with tumor volume doubling time of these hormone-independent cancers 100, 101. Together, these observations from mouse models and different human tumor entities support the hypothesis that tumor growth is attenuated in an aged host environment and that responses to accumulating DNA damage with aging mediate this shift from cellular growth to tissue preservation.
Concluding remarks: Targeting the p53 balance
p53-mediated apoptosis and cellular senescence in combination with the systemic dampening of the IGF-1 growth axis might be crucial to prevent tumor growth, an inevitable requirement given the accumulation of potentially harmful mutations resulting from DNA damage accrual during aging. One consequence of growth factor deprivation might be increased frailty as for instance reduced GH levels have also been linked to osteoporosis 81. In addition, the renewal capacity of tissues might be compromised by a combination of increasingly activated p53 in stem and progenitor cells and a less growth permissive environment in the aging organism. Parabiosis experiments demonstrating that a circulation of a young mouse can reactivate stem cells and consequently tissue repair in old animals 82 have raised the question under which circumstances hormone replacement therapy could be beneficial to avert age-related pathologies. Such stem cell niche-reactivating therapies might be beneficial at late stages of life when frailty becomes a major health concern. However, it is likely to come at the cost of enhanced tumor initiation and progression. Somatotropic mutants, in contrast, have suggested that a long-term reduction of IGF-1 signaling can have beneficial effects on lifespan and tumor suppression. In addition, a moderately enhanced expression of p53 in combination with Ink4a/ARF using BAC transgenic mice resulted in tumor free life extension 25. In order to develop “health span” promoting therapeutic strategies that avert cancer development while maintaining tissue function and renewal, it will be crucial to further understand how the intricately complex network of p53 target genes regulates the very fine balance between tissue physiology, maintenance, repair and renewal and tumor suppression in the aging organism.
Acknowledgement
We apologize to our colleagues for the omission of many seminal contributions to the field, and their references, owing to space constraints. We are grateful to members of the Schumacher and Reinhardt laboratories for critically reading the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (RE2246/1-1, RE2246/2-1, to H.C.R., SFB-832 to H.C.R., SFB-829 to H.C.R. and B.S., and CECAD to H.C.R. and B.S.) the Ministerium für Innovation, Wissenschaft, Forschung und Technologie des Landes Nordrhein-Westfalen (313-005-0910-0102 to H.C.R.) the European Research Council (ERC Starting grant 260383 to B.S.), Marie Curie (European Reintegration Grant 239330 to B.S.), the German-Israeli Foundation (GIF, YIG 2213 to B.S.), the Deutsche Krebshilfe (109453 to B.S.) and the Bundesministerium für Bildung und Forschung (BMBF Gerontosys-SyBaCol to B.S.).
References
- 1.Lindahl T, Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- 2.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 4.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 5.Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 6.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petitjean A, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 8.Martins CP, et al. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 10.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyner SD, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 13.Feng Z, Levine AJ. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010;20:427–434. doi: 10.1016/j.tcb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb LA, Harris CC. Advances in chemical carcinogenesis: a historical review and prospective. Cancer Res. 2008;68:6863–6872. doi: 10.1158/0008-5472.CAN-08-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez CL, et al. Human progeroid syndromes, aging and cancer: new genetic and epigenetic insights into old questions. Cell Mol Life Sci. 2007;64:155–170. doi: 10.1007/s00018-006-6349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombard DB, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Poulogiannis G, et al. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology. 2010;56:167–179. doi: 10.1111/j.1365-2559.2009.03392.x. [DOI] [PubMed] [Google Scholar]
- 19.Malkin D, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 20.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher B, et al. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Jacks T, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 23.Lowe SW, et al. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 24.Maier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Cao I, et al. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinert T, Hartwell L. Control of G2 delay by the rad9 gene of Saccharomyces cerevisiae. J Cell Sci Suppl. 1989;12:145–148. doi: 10.1242/jcs.1989.supplement_12.12. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann ER, et al. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr Biol. 2002;12:1908–1918. doi: 10.1016/s0960-9822(02)01262-9. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher B, et al. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr Biol. 2001;11:1722–1727. doi: 10.1016/s0960-9822(01)00534-6. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher B, et al. C. elegans ced-13 can promote apoptosis and is induced in response to DNA damage. Cell Death Differ. 2005;12:153–161. doi: 10.1038/sj.cdd.4401539. [DOI] [PubMed] [Google Scholar]
- 30.Derry WB, et al. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science. 2001;294:591–595. doi: 10.1126/science.1065486. [DOI] [PubMed] [Google Scholar]
- 31.Vogelstein B, et al. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Zhang L. No PUMA, no death: implications for p53-dependent apoptosis. Cancer Cell. 2003;4:248–249. doi: 10.1016/s1535-6108(03)00249-6. [DOI] [PubMed] [Google Scholar]
- 33.Clarke AR, et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 34.Di Leonardo A, et al. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 35.Rodier F, et al. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35:7475–7484. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumble M, et al. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109:1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rovinski B, et al. Deletion of 5′-coding sequences of the cellular p53 gene in mouse erythroleukemia: a novel mechanism of oncogene regulation. Mol Cell Biol. 1987;7:847–853. doi: 10.1128/mcb.7.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matheu A, et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 39.Christophorou MA, et al. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 40.Hinkal G, et al. Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression. PLoS One. 2009;4:e6654. doi: 10.1371/journal.pone.0006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkwood TB, Cremer T. Cytogerontology since 1881: a reappraisal of August Weismann and a review of modern progress. Hum Genet. 1982;60:101–121. doi: 10.1007/BF00569695. [DOI] [PubMed] [Google Scholar]
- 42.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 43.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 45.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 46.Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- 47.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 48.Bavik C, et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 49.Coppe JP, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ksiazek K, et al. Senescence induces a proangiogenic switch in human peritoneal mesothelial cells. Rejuvenation Res. 2008;11:681–683. doi: 10.1089/rej.2008.0736. [DOI] [PubMed] [Google Scholar]
- 52.Millis AJ, et al. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- 53.Kang MK, et al. Senescence-associated genes in normal human oral keratinocytes. Exp Cell Res. 2003;287:272–281. doi: 10.1016/s0014-4827(03)00061-2. [DOI] [PubMed] [Google Scholar]
- 54.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeyapalan JC, et al. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C, et al. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 57.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005;40:634–642. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Melk A, et al. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63:2134–2143. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 59.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paradis V, et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 61.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Provinciali M, et al. Inflammation, aging, and cancer vaccines. Biogerontology. 2010;11:615–626. doi: 10.1007/s10522-010-9280-9. [DOI] [PubMed] [Google Scholar]
- 64.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 66.Krtolica A, et al. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 68.Bartholomew JN, et al. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 2009;69:2878–2886. doi: 10.1158/0008-5472.CAN-08-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhatia B, et al. Evidence that senescent human prostate epithelial cells enhance tumorigenicity: cell fusion as a potential mechanism and inhibition by p16INK4a and hTERT. Int J Cancer. 2008;122:1483–1495. doi: 10.1002/ijc.23222. [DOI] [PubMed] [Google Scholar]
- 70.Kavurma MM, et al. Oxidative stress regulates IGF1R expression in vascular smooth-muscle cells via p53 and HDAC recruitment. Biochem J. 2007;407:79–87. doi: 10.1042/BJ20070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng Z, et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 73.Feng Z, et al. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stambolic V, et al. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 75.Bruchim I, et al. Targeting the IGF1 axis in cancer proliferation. Expert Opin Ther Targets. 2009;13:1179–1192. doi: 10.1517/14728220903201702. [DOI] [PubMed] [Google Scholar]
- 76.Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 77.Kapahi P, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009 doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carter CS, et al. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- 80.Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perrini S, et al. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 82.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 83.Batchelor E, et al. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol Cell. 2008;30:277–289. doi: 10.1016/j.molcel.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lahav G, et al. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet. 2004;36:147–150. doi: 10.1038/ng1293. [DOI] [PubMed] [Google Scholar]
- 85.Batchelor E, et al. The ups and downs of p53: understanding protein dynamics in single cells. Nat Rev Cancer. 2009;9:371–377. doi: 10.1038/nrc2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mostoslavsky R, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 87.Niedernhofer LJ, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 88.van der Pluijm I, et al. Impaired genome maintenance suppresses the growth hormone--insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 2006;5:e2. doi: 10.1371/journal.pbio.0050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaidi A, et al. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science. 2010;329:1348–1353. doi: 10.1126/science.1192049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Garinis GA, et al. Persistent transcription-blocking DNA lesions trigger somatic growth attenuation associated with longevity. Nat Cell Biol. 2009;11:604–615. doi: 10.1038/ncb1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 92.Young LJ, et al. The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp Gerontol. 1971;6:49–56. doi: 10.1016/0531-5565(71)90048-9. [DOI] [PubMed] [Google Scholar]
- 93.Carlson BM, et al. Skeletal muscle regeneration in very old rats. J Gerontol A Biol Sci Med Sci. 2001;56:B224–233. doi: 10.1093/gerona/56.5.b224. [DOI] [PubMed] [Google Scholar]
- 94.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 95.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 96.Conboy IM, et al. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 97.Peer PG, et al. Age-dependent growth rate of primary breast cancer. Cancer. 1993;71:3547–3551. doi: 10.1002/1097-0142(19930601)71:11<3547::aid-cncr2820711114>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 98.Kusama S, et al. The cross rates of growth of human mammary carcinoma. Cancer. 1972;30:594–599. doi: 10.1002/1097-0142(197208)30:2<594::aid-cncr2820300241>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 99.Tilanus-Linthorst MM, et al. Hereditary breast cancer growth rates and its impact on screening policy. Eur J Cancer. 2005;41:1610–1617. doi: 10.1016/j.ejca.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 100.Jennings SG, et al. Distribution of stage I lung cancer growth rates determined with serial volumetric CT measurements. Radiology. 2006;241:554–563. doi: 10.1148/radiol.2412051185. [DOI] [PubMed] [Google Scholar]
- 101.Zhang J, et al. Distribution of renal tumor growth rates determined by using serial volumetric CT measurements. Radiology. 2009;250:137–144. doi: 10.1148/radiol.2501071712. [DOI] [PubMed] [Google Scholar]