Abstract
Right ventricular outflow tract surgery was originally confined to transannular patching, in the belief that pulmonary regurgitation was well tolerated. Because follow-up evaluations revealed the deleterious effects of pulmonary regurgitation, surgery today aims to spare or replace the valve. Available replacement devices have short lifetimes, considering growth mismatch in children. We hypothesize that oversizing the right infundibulum anticipates growth and that a squeezed prosthesis can complete the expansion process.
The No-React® Injectable BioPulmonic Valve is designed for right infundibular surgery in adults, and hundreds of implants have shown promising results. We used this device for surgery in babies, with the addition of an innovative oversizing technique. This study evaluates our preliminary results and investigates whether such a technique might reduce growth mismatch.
From September 2010 through July 2012, we implanted 11 injectable pulmonic valves. The median age of our patients was 23 months. After opening the right infundibulum, we enlarged it as much as possible with a wide patch. Before completing the patch suture, we injected an oversized valve.
No problems occurred during surgery. No major insufficiency or leak was observed. We conclude that prostheses can be quite oversized and perform well even when not completely expanded.
Oversized injectable pulmonic valves, shrunken to a smaller diameter, enabled the implantation of a device wider than otherwise possible, without affecting performance. Moreover, the prosthesis tended to return to its original size following growth, thereby reducing growth mismatch. Longer follow-up and larger numbers of patients are needed for verification.
Keywords: Bioprosthesis; cardiac surgical procedures/methods/pediatric; child; constriction, pathologic; heart defects, congenital; heart valve prosthesis implantation; infant; prostheses and implants; pulmonary regurgitation; pulmonary valve/abnormalities/surgery; pulmonary valve insufficiency; right ventricular outflow tract; time factors; treatment outcome; ventricular outflow obstructions
Because congenital anomalies of the right side of the heart are often characterized by a wide spectrum of right ventricular outflow tract (RVOT) and pulmonary valve (PV) alterations, the RVOT and PV are frequently involved in pediatric cardiac surgery. Nevertheless, the PV remained neglected for a very long time; it has gained consideration only in recent years.
The first surgical corrections involved ventricular septal defect (VSD) closure and RVOT enlargement with a simple transannular patch, without attention to the PV. Emphasis on short-term results led incorrectly to the idea that the PV had no significance. The belief that “no valve is better than a stenotic valve” induced surgeons to leave many RVOTs unvalved.1–3 When long-term follow-up results became available, it became clear that unvalved patients could develop pulmonary artery dilation and right ventricular (RV) failure,4–7 which led to additional valve procedures. A new idea—that “a working valve is better than no valve”—then arose, and valve preservation gradually became a must in tetrology of Fallot (TOF) treatment. For this reason, much surgical effort in dealing with an altered RVOT is today directed toward sparing the valve whenever possible.3,8,9
Unfortunately, valve-sparing is often difficult and sometimes impossible. When the valve is atretic or very narrow, there is no doubt that the only solution is a transannular patch.10 On the other hand, when pulmonary diameter is acceptable or good, the valve often presents alterations: it is bicuspid, has leaflet tethering, leaflet thickening, commissural fusion, etc.11,12 Repairing the valve is not as easy as it sounds. When the anatomic defects are substantial, the results of repair can be unsatisfactory or outright null.13,14 So, what should the surgeon do when the PV cannot be adequately preserved? Although several solutions have been applied (for example, the creation of a monocusp or a valved conduit, or the implantation of a valvular prosthesis), the results of these measures are debatable, and all studies agree that their usefulness is shortlived.15–19 This is especially true in small children, in whom a valvular prosthesis cannot be used (the available diameters are too large) and in whom most other prostheses should not be used because of the size mismatch between a device that does not grow and a patient who grows rapidly. In recent years, the development of self-expanding prostheses has enabled the birth of several alternatives (percutaneous or surgical) to traditional surgery. Already, these have been used with optimal results in hundreds of cases all over the world and have become an important option for secondary PV placement in adults.20,21
The characteristics of these new devices have led us to believe that they can be used in performing primary repair in children. Therefore, we recently started applying these prostheses to RVOT surgery in babies, using an oversizing technique. This study examines our preliminary results in an effort to determine whether the characteristics of these devices can be useful in reducing growth mismatch.
Patients and Methods
From the beginning of September 2010 through the end of July 2012, we used No-React® Injectable BioPulmonic™ (NRIP) prostheses (BioIntegral Surgical, Inc.; Mississauga, Ontario, Canada) for RVOT surgery in 11 patients. The group consisted of 4 boys and 7 girls. The median age was 23 months (range, 2 mo–8 yr), with 4 patients less than one year of age. The median weight was 9.4 kg and the median height 80 cm. The diagnoses were TOF (6 patients), pulmonary atresia (2 patients), transposition of the great arteries (TGA) with VSD and aortic coarctation (1 case), truncus arteriosus (1 case), and double-outlet RV (DORV) + TGA (1 case).
The study was performed in compliance with U.S. Food and Drug Administration guidelines and with the human-study guidelines of the authors' institution. Informed written consent was obtained from the subjects' parents after the nature of the procedure was explained.
Primary RVOT surgery was performed in 7 patients (Patients 1, 2, 3, 4, 5, 8, and 9; see Table I), 4 of whom had undergone palliation 2 to 9 months earlier.
TABLE I.
Summary of Patient Characteristics
Patient 6—the baby with TGA, VSD, and aortic coarctation—had undergone decoarctation and pulmonary banding at birth and, 10 days later, had undergone an arterial switch operation with VSD closure. That child's present surgery (revalving at 15.7 months of age) was needed to alleviate critical stenosis of the RVOT, which was insufficiently developed.
In none of those 8 patients had a valve-sparing procedure been possible, due to a very narrow pulmonary annulus and severe valvular defects. Use of the NRIP was indicated to replace the missing PV, thereby correcting the regurgitation present since the earlier surgery.
Because of severe pulmonary regurgitation or stenosis with substantial pulmonary hypertension, the remaining 3 patients (Table I) needed pulmonary revalving 2, 4, or 6 years after undergoing initial correction (at a neonatal age) with a monocusp, a valved conduit, or a reparation endoventriculaire (REV) procedure.2,4
The most distinctive aspect of the surgical technique was our oversizing both the RVOT and the prosthesis. In accordance with studies by Kirklin and others,22–25 we determined the expected RVOT diameter on the basis of each patient's weight and body surface area (BSA). We made a transvalvular RVOT incision from the pulmonary bifurcation to the infundibulum. The infundibular opening was between 15 and 20 mm long, enough to enable the elimination of RVOT hypertrophy while avoiding extensive muscular resection. Next we examined the dimensions and other characteristics of the patient's chest and heart and decided what amount of RVOT oversizing was prudent, in order to widen the RVOT as much as possible. We then tailored an enlargement patch and sutured it onto the RVOT with the help of a Hegar dilator, starting from the pulmonary bifurcation. Bovine pericardium was used in 9 instances. An extracellular matrix patch (Cormatrix; Roswell, Ga) was used in 2 patients because they had previously shown a marked reaction to other biological tissues. Once the pulmonary annulus was reached, we tied and stabilized the suture lines. An oversized NRIP (with a circumference wider than that of the enlarged RVOT) was then chosen and injected under direct vision. The valve expanded immediately, but, because of oversizing, it did not deploy in the RVOT to its full extent. We then checked the prosthesis and adjusted it if needed, paying close attention to avoid obstructing the right pulmonary branch (Fig. 1). When the positioning was considered optimal, we anchored the prosthesis with 4 stitches: 2 on the lower wall of the RVOT and 2 from outside the patch. The surgical procedure was terminated upon completion of the suture of the patch on the infundibular portion of the RVOT.
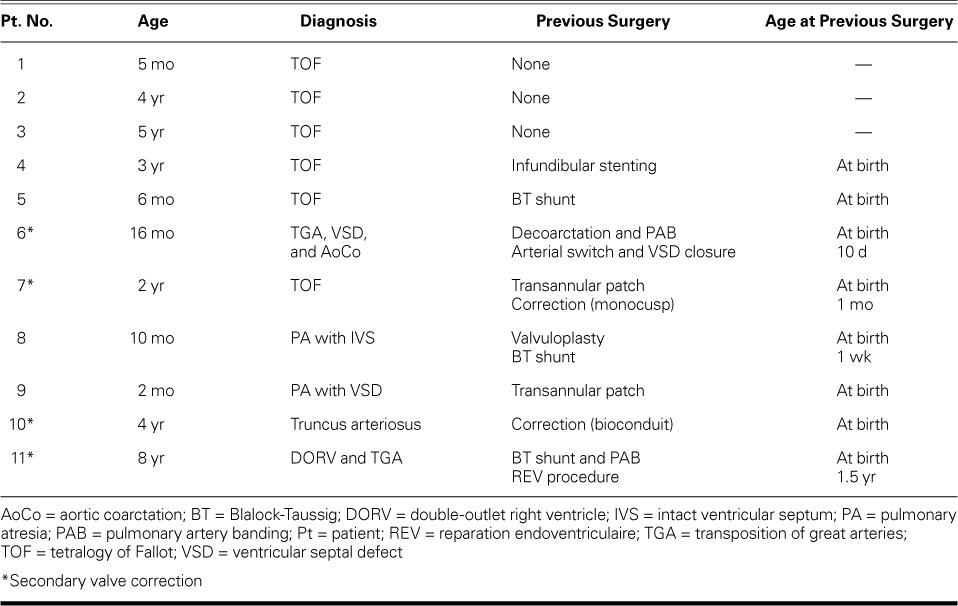
Fig. 1.
A) Intraoperative photograph shows the BioPulmonic valve injected into the right ventricular outflow tract. The enlarged tract is seen from the cephalic perspective. The enlargement patch has been sutured onto the pulmonary trunk, and the infundibular suture is yet to be completed. B) The same valve is shown from the caudal perspective. The prosthetic cusps are closed.
In only 1 patient was the technique slightly different: the RVOT was enlarged (not reduced), all the while maintaining the concept of oversizing. The patient was an 8-year-old girl affected by DORV + TGA, which had been corrected with REV during her infancy. Severe pulmonary regurgitation had led to RV failure, together with substantial RVOT dilation (22 mm). After removing the infundibular portion of the REV patch, we externally plicated its pulmonary portion, to reduce the pulmonary diameter to 17 mm. A 19-mm prosthesis was then injected, and the infundibular opening was sutured directly.
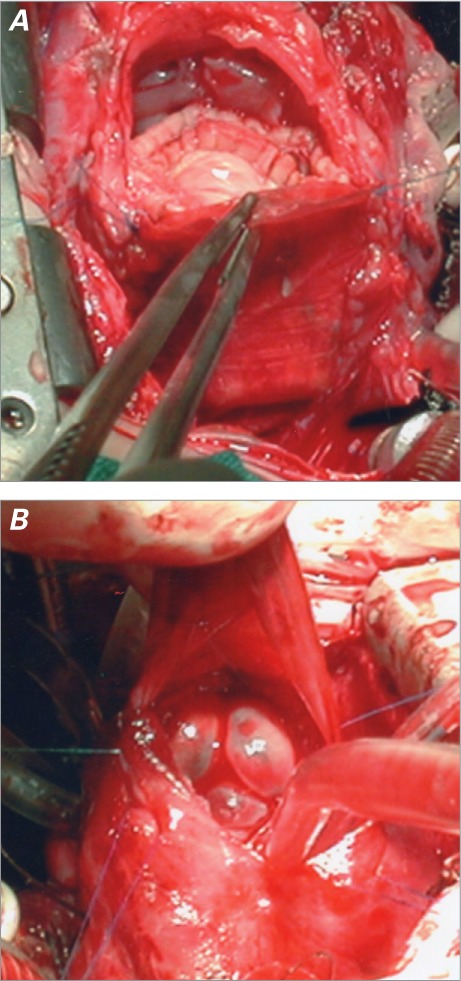
The Injectable BioPulmonic™ Prosthesis is a biological porcine valve inserted into a nitinol stent, which is covered with porcine pericardium. Stent hooks lock the device by grasping the surrounding tissue. Both the heart valve and the pericardium are affixed with glutaraldehyde and then detoxified with No-React®, a specific treatment that eliminates any free residual aldehyde from surfaces. Available device diameters range from 15 to 31 mm. Devices between 15 and 19 mm in diameter are 15 mm in length; these are said to be of “small diameter” and are used mainly in children. Wider devices are 26 mm in length and are most often used in adults. A special introducer, developed for injection, is made of a trocar barrel and a plunger. The valve is crimped and inserted into the trocar; the plunger is positioned behind the valve and pushed until the valve reaches the tip of the trocar. The trocar is then inserted into the RVOT until its tip reaches the desired site, then the plunger is pushed forward, at which point the valve shoots out and immediately self-expands. Stent hooks lock the device into position and no suture, in theory, is needed (Fig. 2).
Fig. 2.
A) Schematic drawing of the BioPulmonic valve. Porcine aortic cusps have been sewn inside a porcine pericardium conduit. The device is completed by a nitinol self-expanding stent, located outside the conduit. B) Schematic drawing of the introducer, which consists of a trocar and a plunger. After the trocar has received the crimped BioPulmonic valve, the plunger is inserted and pushed to eject the valve from the trocar tip.
Surgical results and prosthesis performance were examined with use of echocardiography, both intraoperatively and during the postoperative hospital stay. Oral anticoagulation with warfarin was administered for 3 months only, to keep the international normalized ratio between 2 and 2.5.
To evaluate RV function, we studied both tricuspid annular plane systolic elevation (TAPSE) and fractional area change (FAC). A TAPSE <18 mm or a FAC <32% were considered markers of ventricular dysfunction.
Prosthetic function was evaluated by searching both for stenosis (mean gradient, >20 mmHg) and for regurgitation (diastolic jet size and deceleration rate).
In 6 patients in whom anatomy of the RVOT or measurement of prosthetic diameter could not be evaluated with accuracy at echocardiography, we obtained computed tomograms with 3-dimensional reconstruction, one year after the operation. In 6 patients, postoperative cardiac catheterization was needed to relieve pulmonary-branch stenosis.
Results
Follow-up (mean duration, 16.05 ± 8.19 mo; range, 2 wk–26.77 mo) is now 100% complete. All patients have been monitored by means of periodic echocardiograms.
No technical difficulties were encountered during surgery. The RVOT diameter was increased to a mean of 15.27 ± 1.62 mm (range, 12–17 mm). Given the body characteristics and expected dimensions, this amounts to a mean oversizing of 4.63 ± 2.5 mm (range, 0.5–7.8 mm) in accordance with weight and of 3.86 ± 1.88 mm (range, 0.5–6.3 mm) in accordance with BSA. The prosthetic sizes in use were 15, 16, 17, and 19 mm, with mean diameter of 17.45 ± 1.37 mm and mean oversizing of 2.18 ± 1.17 mm (range, 0–3 mm).
No problems occurred during weaning from cardiopulmonary bypass or thereafter. Right ventricular function was normal in all patients at intraoperative echocardiography (TAPSE >20 mm and FAC >40%). No prosthetic malfunction, regurgitation, or stenosis was observed. In 2 patients, a perivalvular leak was seen immediately after operation, but these did not have relevant hemodynamic consequences. The right-to-left ventricular pressure ratio ranged from 0.35 to 0.74 (mean, 0.55 ± 0.12). The mean RVOT pressure gradient was 17.27 ± 8.09 mmHg (range, 10–40 mmHg). A 40-mmHg gradient was measured in only one patient and was not prosthesis dependent, for it occurred at a subvalvular site and decreased rapidly to 20 mmHg by the time of discharge. We observed no sign of compression on other intrathoracic structures by the oversized RVOT and prosthesis. The mean hospital stay was 11.64 ± 6.8 days (range, 6–27 d), and no NRIP-related complication occurred.
At the postoperative follow-up evaluation, all patients were leading normal lives, with regular growth and no symptoms. Furthermore, compliance with oral anticoagulation was optimal during the entire 3 postoperative months. Right ventricular function was preserved (TAPSE >22 mm and FAC >41%) in all patients, and no prosthetic malfunction or regurgitation occurred. Only one perivalvular leak was still present, this in the patient who had a moderate leak immediately after operation; that leak is now reduced to mild. The mean RVOT pressure gradient was unmodified at 17.71 ± 10.08 mmHg (range, 10–40 mmHg). In one patient, an intraprosthetic stenosis had developed at 15 postoperative months, because of neointimal proliferation (Table II).
TABLE II.
Postoperative and Short-Term Surgical Results

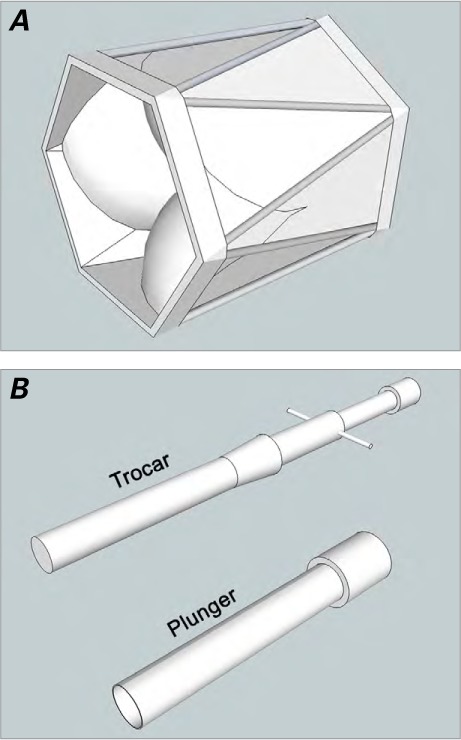
In all 6 patients studied by means of computed tomography, we observed a normally positioned prosthesis, with no alteration or distortion of any anatomic structure. The prosthetic diameter was measured by both echocardiography and computed tomography (Fig. 3).
Fig. 3.
Axial computed tomogram at 18-month follow-up shows the stent of a 16-mm BioPulmonic valve in the enlarged right ventricular outflow tract and a completely expanded prosthesis.
Upon follow-up, 4 prostheses appeared to be substantially expanded since the time of implantation, and 3 of those had returned to their original size (Fig. 4). In the remaining 6 patients, the prostheses had a diameter identical to or slightly wider than the one measured after the operation.
Fig. 4.
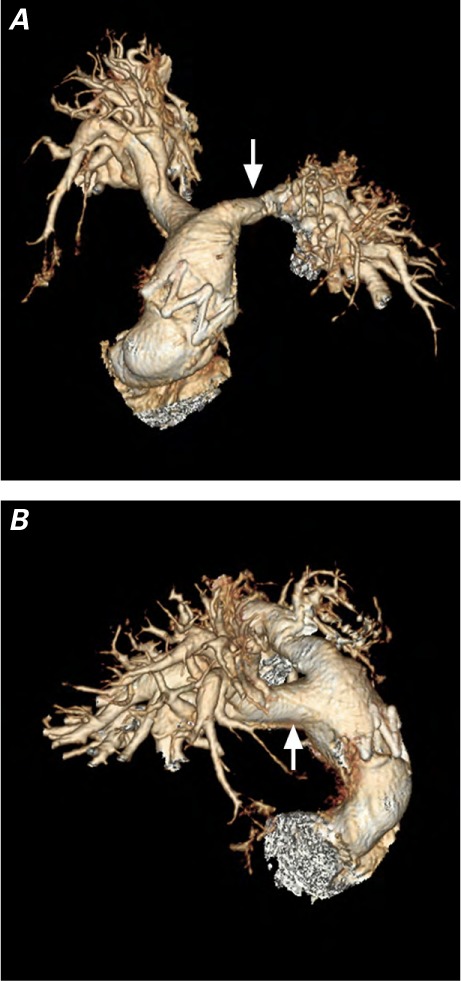
Three-dimensional computed tomograms of the pulmonary artery. A) This anterior view, obtained at 17 months of follow-up, shows the stent of a 16-mm BioPulmonic valve in the enlarged right ventricular outflow tract. The suture line of the patch on the left pulmonary branch is evident (arrow). B) The right lateral view shows the suture line of the patch on the right pulmonary branch (arrow).
Discussion
The technique of implanting an NRIP is easy and does not entail a significant lengthening of surgical time. The prosthesis is usually inserted at the level of the pulmonary annulus, but it deploys normally even if it is a little displaced in the distal portion of the infundibulum. Such a position is mandatory when the pulmonary trunk is short, in which case the valve would obstruct the origin of the right pulmonary branch. Even if we preferred to anchor the device with 4 stitches, the NRIP is self-locking and does not need to be sutured, especially when oversized.
No problems seem to occur with oversizing; RVOT enlargement should be suited to chest and heart dimensions, but usually a prosthetic size increase of 5 or 6 mm in diameter can easily be achieved, does not occupy a substantial amount of space, and does not compress other structures. Because repeated operative procedures are probable, some kind of RVOT “protection” should be put in place before sternal closure. In our center, we usually apply a Gore-Tex® membrane (W.L. Gore & Associates, Inc.; Flagstaff, Ariz) before closing the chest whenever a repeated procedure cannot be excluded. A prosthesis that is 2 or 3 mm wider than the final diameter of the enlarged RVOT can easily be injected and performs normally even if not completely expanded.
In the 8-year-old girl with DORV + TGA, the surgical technique deserved particular consideration. Because of the previous REV procedure, the RVOT was located anteriorly and was compressed between the sternum and the aorta. Preoperative magnetic resonance showed its elliptical section, with an anteroposterior diameter of 16 to 17 mm and a transverse diameter of 30 to 31 mm—not suitable for direct valvular insertion. At operation, the RVOT diameter was 22 mm. According to the patient's weight and BSA, respectively, we had anticipated a 15- or 14.3-mm RVOT. To avoid compression against the aorta, the RVOT was reduced to 17 mm (the maximum retrosternal space available—and actually oversized). A 19-mm NRIP was then injected.
The immediate postoperative results were satisfactory: both the RVOT gradient and the RV pressure appeared to be unaffected by the presence of an incompletely expanded prosthesis. We measured a mean gradient of 17.5 ± 8.5 mmHg (range, 10–40 mmHg). If the only patient with a value of 40 mmHg (consequent to subvalvular residual stenosis) is removed from the overall calculation, the mean RVOT gradient drops to 15 ± 3.09 mmHg (range, 10–20 mmHg).
Oral anticoagulation was well tolerated and we did not observe any thromboembolic event. No prosthetic malfunction or regurgitation had been observed at the time of this writing. Mild or moderate perivalvular leaks appear to be quite common immediately after implantation, but in this set of patients they did not affect hemodynamic status and tended to close spontaneously in a few months.
In only one patient did we observe a significant intra-prosthetic stenosis, due to neointimal proliferation, 15 months after operation. Since his birth, this baby had shown a very poor anatomy and an aggressive reaction to foreign bodies; consequently, he had needed repeated balloon dilations at the pulmonary bifurcation. Moreover, he was one of the first patients in this series—therefore, he was treated at the very beginning of our learning curve. His mean RVOT pressure gradient at follow-up was 17.71 ± 10.08 mmHg (range, 10–40 mmHg). Excluding this patient lowers the mean gradient of our series to 14 ± 10.45 mmHg (range, 10–16 mmHg).
No problems with patient growth have occurred. The prostheses appear to expand with time, tending to return to their original diameter. Our hypothesis is that a wider-than-expected RVOT anticipates growth and that an incompletely dilated NRIP can gradually complete its expansion process until its original diameter is reached. However, NRIP expansion appears to be related to many different factors, which show complex reciprocal interactions.
The first factor is the patient's growth, which determines a progressive increase in RVOT dimensions. This process is typically very slow, taking place over the course of years.
The second factor is RVOT adjustment at the level of the patch and of the suture lines, which is due to intrapulmonary pressure and to the self-expanding valve itself. This phenomenon is much faster and occurs over weeks, sometimes months. The RVOT adjustment is strongly dependent on the amount of oversizing performed during operation. In other words, when oversizing is not considered to be very important, some “residual space” remains in the patient's chest around the RVOT, enabling NRIP expansion to occur easily and rapidly. On the contrary, when oversizing is pushed to the limits of chest dimensions, RVOT adjustment is limited by adjacent structures.
Another factor is the degree of prosthetic oversizing: the residual expansion needed to reach its original diameter. This is what we have named the “distance to the finish line.” The greater this distance, the longer is the expansion time.
Unfortunately, considering these various factors, 11 patients are too few, both RVOT and NRIP oversizing vary too much between patients, and a 16- to 17- month follow-up period is too short a time to yield firm conclusions. Therefore, no substantive inference is now available: we need longer follow-up and more patients.
What we can reasonably say is that the RVOT can easily be oversized 1.5 times the expected diameter, and an NRIP ≤3 mm wider than the enlarged RVOT can easily be injected, without prosthetic malfunction or compression of surrounding structures.
The median age of our patients was 23 months, the median weight 9.4 kg, the median height 80 cm, and the median BSA 0.45 m2. According to curves of normal cardiac dimensions, a 10.65- or 11.41-mm RVOT was expected (when weight or BSA, respectively, was considered). We enlarged the RVOT to a mean diameter of 15.27 ± 1.62 mm and implanted valves with a mean diameter of 17.45 ± 1.37 mm. In this manner, RVOT oversizing (obtained RVOT vs expected RVOT) was 4.63 ± 2.5 mm or 3.86 ± 1.88 mm, valvular oversizing (prosthesis vs obtained RVOT) was 2.18 ± 1.17 mm, and total oversizing (the prosthetic diameter vs expected RVOT) was between 6.81 ± 2.13 mm (range, 3.5–9.5 mm) and 6.05 ± 1.52 mm (range, 3.5–8.6 mm). What is interesting is that 17.45 mm is the normal diameter of a 1.5-m2 BSA, which is the BSA of a 160-cm, 50-kg man—actually a small adult. This means that a child with such a prosthesis can in theory reach adulthood without growth mismatch.
In examining the patients with the smallest and the largest implanted prostheses (15 and 19 mm, respectively), we got similar interesting results (Table III).
TABLE III.
Smallest and Widest Prosthetic Diameters Used
The surgical procedure is easy. Using an oversized NRIP squeezed to a diameter appropriate for age and dimensions enables the implanting of a device much wider in diameter than would otherwise be possible, without affecting prosthetic performance. Moreover, as the RVOT adjusts or grows, the prosthesis appears to complete its expansion to its maximal size. This could potentially reduce growth mismatch and the number of interventions the patient will need during his or her life. Obviously, considering the small number of cases, the short follow-up period, and the variability between patients' characteristics and the oversizing factor, further investigations are needed to reach more significant conclusions.
References
- 1.Katz NM, Blackstone EH, Kirklin JW, Pacifico AD, Bargeron LM., Jr. Late survival and symptoms after repair of tetralogy of Fallot. Circulation. 1982;65(2):403–10. doi: 10.1161/01.cir.65.2.403. [DOI] [PubMed] [Google Scholar]
- 2.Bacha EA, Scheule AM, Zurakowski D, Erickson LC, Hung J, Lang P et al. Long-term results after early primary repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001;122(1):154–61. doi: 10.1067/mtc.2001.115156. [DOI] [PubMed] [Google Scholar]
- 3.Bacha E. Valve-sparing options in tetralogy of Fallot surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15(1):24–6. doi: 10.1053/j.pcsu.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Bouzas B, Kilner PJ, Gatzoulis MA. Pulmonary regurgitation: not a benign lesion. Eur Heart J. 2005;26(5):433–9. doi: 10.1093/eurheartj/ehi091. [DOI] [PubMed] [Google Scholar]
- 5.Frigiola A, Tsang V, Nordmeyer J, Lurz P, van Doorn C, Taylor AM et al. Current approaches to pulmonary regurgitation. Eur J Cardiothorac Surg. 2008;34(3):576–82. doi: 10.1016/j.ejcts.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 6.Geva T, Gauvreau K, Powell AJ, Cecchin F, Rhodes J, Geva J, del Nido P. Randomized trial of pulmonary valve replacement with and without right ventricular remodeling surgery. Circulation. 2010;122(11 Suppl):S201–8. doi: 10.1161/CIRCULATIONAHA.110.951178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickey EJ, Veldtman G, Bradley TJ, Gengsakul A, Manlhiot C, Williams WG et al. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg. 2009;35(1):156–64. doi: 10.1016/j.ejcts.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 8.Boni L, Garcia E, Galletti L, Perez A, Herrera D, Ramos V et al. Current strategies in tetralogy of Fallot repair: pulmonary valve sparing and evolution of right ventricle/left ventricle pressures ratio. Eur J Cardiothorac Surg. 2009;35(5):885–90. doi: 10.1016/j.ejcts.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Geva T. Tetralogy of Fallot repair: ready for a new paradigm. J Thorac Cardiovasc Surg. 2012;143(6):1305–6. doi: 10.1016/j.jtcvs.2012.01.076. [DOI] [PubMed] [Google Scholar]
- 10.Awori MN, Leong W, Artrip JH, O'Donnell C. Tetralogy of Fallot repair: optimal z-score use for transannular patch insertion. Eur J Cardiothorac Surg. 2013;43(3):483–6. doi: 10.1093/ejcts/ezs372. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostopoulos P, Azakie A, Natarajan S, Alphonso N, Brook MM, Karl TR. Pulmonary valve cusp augmentation with autologous pericardium may improve early outcome for tetralogy of Fallot. J Thorac Cardiovasc Surg. 2007;133(3):640–7. doi: 10.1016/j.jtcvs.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Hua Z, Li S, Wang L, Hu S, Wang D. A new pulmonary valve cusp plasty technique markedly decreases transannular patch rate and improves midterm outcomes of tetralogy of Fallot repair. Eur J Cardiothorac Surg. 2011;40(5):1221–6. doi: 10.1016/j.ejcts.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Robinson JD, Rathod RH, Brown DW, Del Nido PJ, Lock JE, McElhinney DB et al. The evolving role of intraoperative balloon pulmonary valvuloplasty in valve-sparing repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2011;142(6):1367–73. doi: 10.1016/j.jtcvs.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 14.Stewart RD, Backer CL, Young L, Mavroudis C. Tetralogy of Fallot: results of a pulmonary valve-sparing strategy. Ann Thorac Surg. 2005;80(4):1431–9. doi: 10.1016/j.athoracsur.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Bigras JL, Boutin C, McCrindle BW, Rebeyka IM. Short-term effect of monocuspid valves on pulmonary insufficiency and clinical outcome after surgical repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 1996;112(1):33–7. doi: 10.1016/s0022-5223(96)70175-7. [DOI] [PubMed] [Google Scholar]
- 16.Jun TG, Park PW, Park KH, Chae H, Kang IS, Lee HJ. Homologous monocuspid valve patch in right ventricular outflow tract reconstruction. J Cardiovasc Surg (Torino) 2001;42(1):17–21. [PubMed] [Google Scholar]
- 17.Holmes AA, Co S, Human DG, Leblanc JG, Campbell AI. The Contegra conduit: late outcomes in right ventricular outflow tract reconstruction. Ann Pediatr Cardiol. 2012;5(1):27–33. doi: 10.4103/0974-2069.93706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boethig D, Schreiber C, Hazekamp M, Blanz U, Pretre R, Asfour B et al. Risk factors for distal Contegra stenosis: results of a prospective European multicentre study. Thorac Cardiovasc Surg. 2012;60(3):195–204. doi: 10.1055/s-0031-1298062. [DOI] [PubMed] [Google Scholar]
- 19.Yuan SM, Mishaly D, Shinfeld A, Raanani E. Right ventricular outflow tract reconstruction: valved conduit of choice and clinical outcomes. J Cardiovasc Med (Hagerstown) 2008;9(4):327–37. doi: 10.2459/JCM.0b013e32821626ce. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Turner M, Caputo M, Stoica S, Marianeschi S, Parry A. Pulmonary valve implantation using self-expanding tissue valve without cardiopulmonary bypass reduces operation time and blood product use. J Thorac Cardiovasc Surg. 2013;145(4):1040–5. doi: 10.1016/j.jtcvs.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber C, Horer J, Vogt M, Fratz S, Kunze M, Galm C et al. A new treatment option for pulmonary valvar insufficiency: first experiences with implantation of a self-expanding stented valve without use of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2007;31(1):26–30. doi: 10.1016/j.ejcts.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Kirklin JW, Barratt-Boyes BG, editors. Cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results, and indication. 2nd ed. New York: Churchill Livingstone; 1993. pp. 31–56. p. [Google Scholar]
- 23.Rowlatt JF, Rimoldi JHA, Lev M. The quantitative anatomy of the normal child's heart. Pediatr Clin North Am. 1963;10(2):499–588. [Google Scholar]
- 24.Sievers HH, Onnasch DG, Lange PE, Bernhard A, Heintzen PH. Dimensions of the great arteries, semilunar valve roots, and right ventricular outflow tract during growth: normative angiocardiographic data. Pediatr Cardiol. 1983;4(3):189–96. doi: 10.1007/BF02242254. [DOI] [PubMed] [Google Scholar]
- 25.Snider AR, Enderlein MA, Teitel DF, Juster RP. Two-dimensional echocardiographic determination of aortic and pulmonary artery sizes from infancy to adulthood in normal subjects. Am J Cardiol. 1984;53(1):218–24. doi: 10.1016/0002-9149(84)90715-x. [DOI] [PubMed] [Google Scholar]


