Abstract
Nontyphoidal Salmonella, especially Salmonella enterica, is a rare cause of endocarditis and pericarditis that carries a high mortality rate. Proposed predisposing conditions include immunodeficiency states, congenital heart defects, and cardiac valve diseases. We present 2 cases of cardiovascular salmonellosis. The first case is that of a 73-year-old woman with mechanical mitral and bioprosthetic aortic valves who died from sequelae of nontyphoidal Salmonella mitral valve vegetation, aortic valve abscess, and sepsis. The second case is that of a 62-year-old man with a recent systemic lupus erythematosus exacerbation treated with oral steroids, who presented with obstructive features of tamponade and sepsis secondary to a large S. enteritidis purulent pericardial cyst. He recovered after emergent pericardial drainage and antibiotic therapy. Identifying patients at risk of cardiovascular salmonellosis is important for early diagnosis and treatment to minimize sequelae and death. We reviewed the literature to identify the predisposing risk factors of nontyphoidal Salmonella cardiac infection.
Keywords: Autoimmune diseases/complications, bacteremia, endocarditis, immunocompromised host, nontyphoidal Salmonella, pericarditis, prosthesis-related infections, Salmonella enteritidis, Salmonella infections/epidemiology/etiology/mortality/transmission
The Centers for Disease Control and Prevention estimates that among nontyphoidal Salmonella (NTS) species, Salmonella enterica causes 1 million cases of foodborne disease in the United States annually.1 Salmonella enteritidis is the most common serotype, presumably as a consequence of frequent chicken and egg contamination.1,2 Most cases of salmonellosis are limited to gastroenteritis, but bacteremia occurs in 3% to 8% of infections; cardiovascular infections, including pericarditis and endocarditis, develop in 1% to 5% of patients.3,4 The pathogenesis of cardiac salmonellosis is related to the quantity of bacteria ingested, the characteristic ability of Salmonella to adhere to damaged endothelium, the strength of the host's immune response, and the host's previous exposure. The sequelae of Salmonella endocarditis, including valve perforation, valve ring abscess, atrioventricular wall perforation, and cusp rupture, result in an estimated mortality rate of up to 75%.5,6 Identifying patients at risk of cardiovascular salmonellosis is important for early diagnosis and treatment.
Case Reports
Patient 1
A 73-year-old woman presented after 3 days of progressive nausea, diarrhea, fevers, and weakness. Her medical history was significant for asthma, hypertension, and replacements (4 years earlier) of both mechanical mitral and bioprosthetic aortic valves.
The patient's initial temperature was 101 °F, her blood pressure was 59/31 mmHg, and her heart rate was 74 beats/min. An electrocardiogram (ECG) showed sinus rhythm. Her white blood cell count was 8,200 K/μL; blood urea-nitrogen level, 42 mg/dL; creatinine level, 2.04 mg/dL; and initial venous lactate level, 1.3 mmol/L. Urinalysis showed 26 to 100 white cells per high-power field, and a chest radiograph showed bilateral pulmonary infiltrates. She was stabilized, started on intravascular levofloxacin (0.75 mg/kg/d), and admitted to the intensive care unit, where she developed rigors and respiratory distress that necessitated endotracheal intubation and mechanical ventilation. A repeat ECG showed a new pattern of left bundle-branch block. An echocardiogram showed heavy mitral annular calcification, which produced a dense artifact around the sewing ring that suggested endocarditis.
On the 2nd day of hospitalization, blood-culture results were positive for gram-negative rods. Transesophageal echocardiography revealed an aortic valve abscess and a 1.1 × 0.5-cm vegetation (with a central cavitation) on her mechanical mitral valve (Fig. 1). The presumed source of the endocarditis was the urinary tract infection, and intravascular gentamicin (0.6 mg/kg 3 times daily) and aztreonam (20 mg/kg 3 times daily) were initiated. On the following day, the bacterium was serotyped as S. enteritidis, and cardiovascular surgery was planned. Before the patient could undergo surgery, she developed refractory cardiac arrhythmias and progressive hemodynamic instability. After discussion with the person who held the patient's power of attorney, we took palliative measures, and she died shortly after extubation.
Fig. 1.
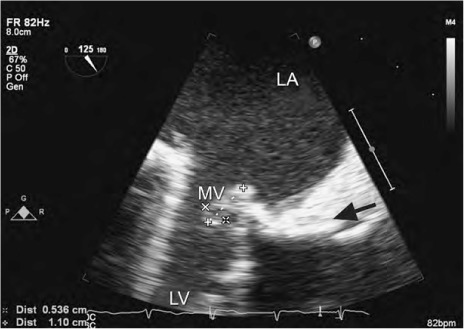
Patient 1. Transesophageal echocardiogram shows a 1.1 × 0.5-cm vegetation (arrow) attached to a mechanical mitral valve.
LA = left atrium; LV = left ventricle; MV = mitral valve
Patient 2
A 62-year-old man presented after 3 days of rapid heart rate. His medical history included hypertension, intermittent atrial fibrillation, and drug-induced systemic lupus erythematosus (SLE). He had been treated recently for an SLE flare-up for 3 weeks with oral prednisolone (0.1 mg/kg). On presentation, his heart rate was 126 beats/min, his heart rhythm irregular, his blood pressure 126/84 mmHg and his temperature 98.5 °F. Auscultation revealed distant heart sounds. His white blood cell count was 14.8 K/μL and his creatinine level 1.22 mg/dL. An ECG revealed atrial fibrillation with a nonspecific T-wave abnormality, and a chest radiograph showed bibasilar linear opacity.
He was admitted to our medical center for the investigation of atrial fibrillation with rapid ventricular rate. The initial blood cultures were negative. Transesophageal echocardiograms revealed a 5.6 × 7-cm extracardiac mass inferior and posterior to the right atrium and right ventricle (RV), compressing the right-sided cardiac chambers and mildly obstructing the RV inflow tract (Figs. 2 and 3). Cardiac magnetic resonance images confirmed that the mass was confined between the parietal layers of the pericardium (Fig. 4). Two small satellite lesions of similar characteristics were also noted.
Fig. 2.
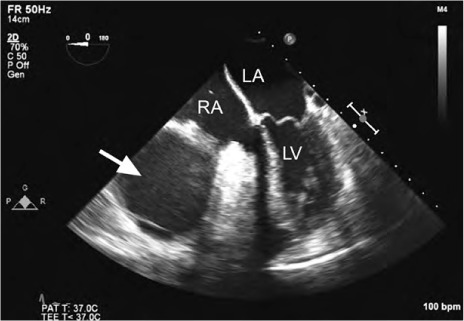
Patient 2. Transesophageal echocardiogram (4-chamber view) shows a homogeneous echogenic mass (arrow) compressing the right atrium and the right ventricle.
LA = left atrium; LV = left ventricle; RA = right atrium
Fig. 3.
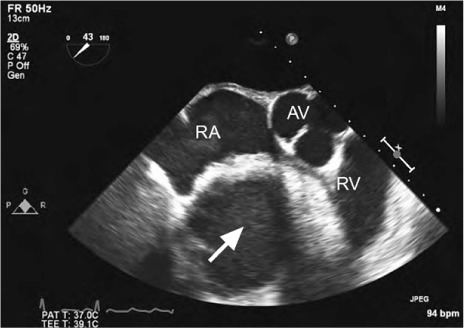
Patient 2. Transesophageal echocardiogram (short-axis view) at the level of the aortic valve shows a large, homogeneous, echogenic mass (arrow) compressing the right atrium and right ventricle.
AV = aortic valve; RA = right atrium; RV = right ventricle
Fig. 4.
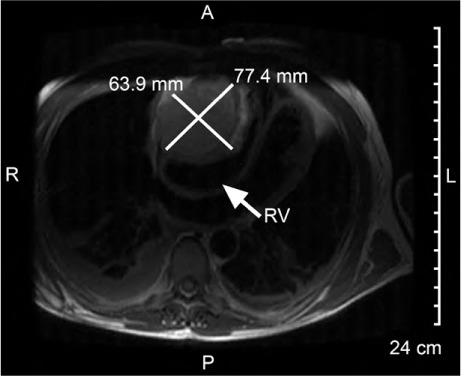
Patient 2. Magnetic resonance image shows a large cardiac cystic mass with significant mass effect on the right ventricle (RV) (arrow).
The pericardial cyst was at first thought to be an inflammatory sequela of SLE, and the patient was treated medically with intravascular methylprednisolone (2.9 mg/kg/d) and twice daily with oral colchicine (0.007 mg/kg), mycophenolate (2.9 mg/kg), and hydroxychloroquine (2.3 mg/kg).
On the 9th day of hospitalization, the patient became febrile and progressively unstable hemodynamically. Repeat echocardiography showed physical characteristics consistent with cardiac tamponade. He underwent emergent creation of a subxiphoid pericardial window, from which we drained 900 mL of foul-smelling, grossly purulent pericardial fluid. Cultures obtained from the pericardial fluid yielded S. enteritidis subspecies I. The patient was placed on intravascular ceftriaxone (23.2 g/kg/d). He recovered well and, after 26 days, was discharged from the hospital on a 6-week regimen of oral levofloxacin (8.7 mg/kg). He continued to experience drainage from the pericardium and ultimately underwent a right atrial and RV pericardiectomy, with no further sequelae.
Discussion
Broad media coverage of Salmonella outbreaks in the past few years, including the largest domestic outbreak ever in the United States (from contaminated eggs in 2010), has increased public awareness of food safety.1 Annually, S. enterica alone causes an estimated 155,000 deaths worldwide and at least 350 deaths in the U.S.; it is responsible for up to 78% of NTS endocarditis cases and 57% of NTS pericarditis cases.4,7 Despite increasing awareness of epidemic NTS and S. enterica infections, clinicians rarely consider them potential causes of endovascular and cardiac infections. Fever (100%), heart murmurs (57%), and heart failure (71.4%) are frequently observed in association with NTS endovascular infections, but antecedent diarrhea is rare.8 In NTS pericarditis, which has a mortality rate of nearly 16%, dyspnea has been reported in 73% of cases, followed by fever (47%), chest pain or discomfort (31%), and cardiac palpitations (21%). Advanced age, underlying valve disease, and immunodeficiency have for some time been recognized as predisposing conditions for NTS cardiac infection, but recent case reports and larger studies have proposed additional risk factors for NTS endocarditis and pericarditis.9,10
Pericarditis
We found 19 cases of NTS pericarditis reported from 1993 through 2014 (Table I).2,5,11–25 The most common organism was S. enteritidis (58%; 11 cases) followed by S. typhimurium (26%; 5 cases). The white blood cell count was significantly elevated on initial presentation in 58% of cases (11), and approximately half of patients presented with pericardial tamponade (53%; 10 cases). All survivors underwent pericardiocentesis or thoracentesis. Men accounted for 78% of patients, and the average age at presentation was 53 years. The only prior review of NTS pericarditis, by Pace and colleagues in 2002, encompassed 11 cases of Salmonella pericarditis; the median age of the patients was 40.8 years.2 Part of the reason for the shift in median age at presentation is that we found only one reported pediatric case in the past 20 years, whereas children accounted for 17% of the cases in the review by Pace and colleagues.2
TABLE I.
Reports of Nontyphoidal Salmonella Pericarditis Cases, 1993–2014
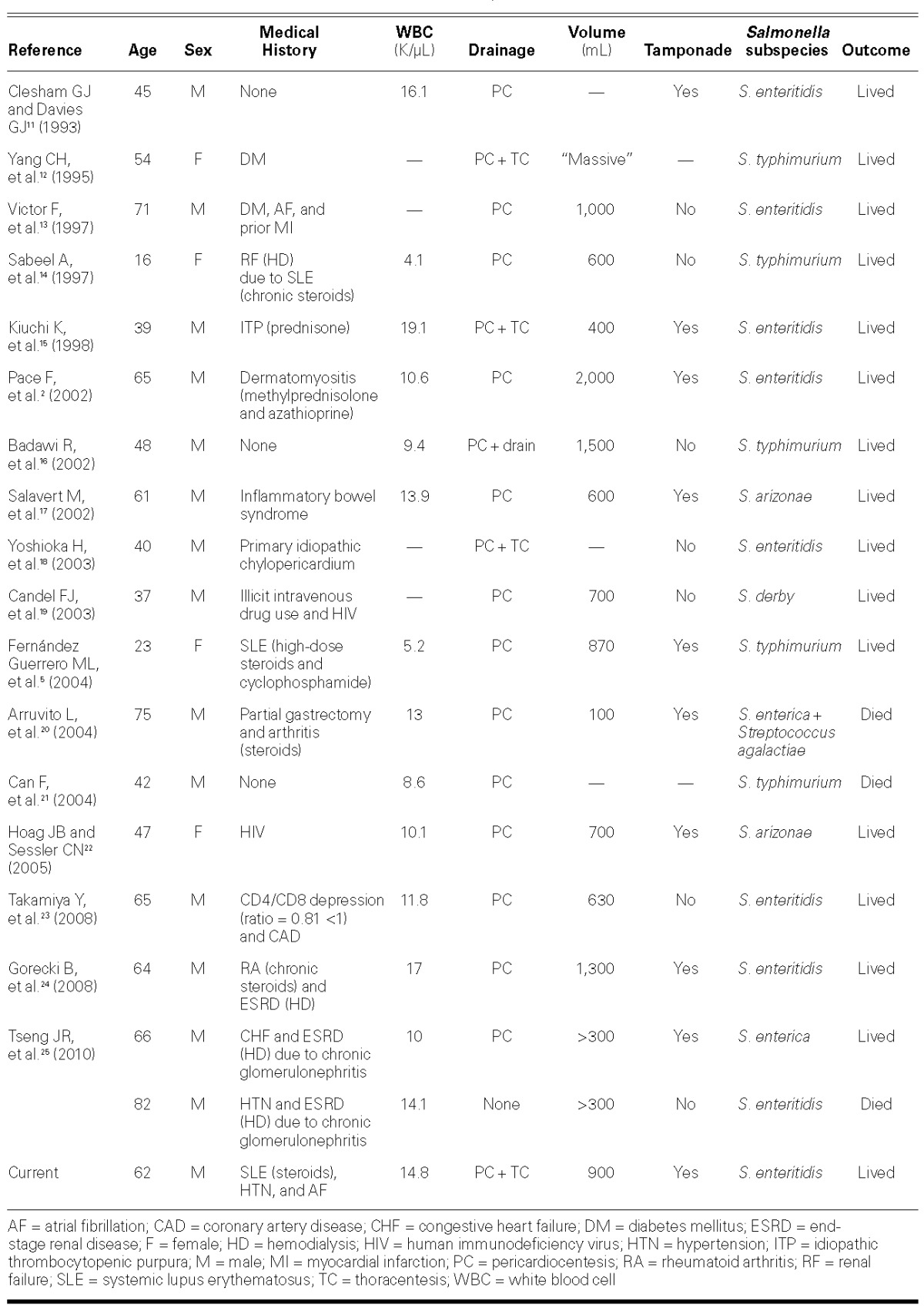
Thirteen of the 19 patients (68%) found in our literature search had an identifiable immunosuppressed state. The most frequently identified risk factor for NTS pericarditis was chronic corticosteroid therapy (37%; 7 patients) for treatment of autoimmune disease, including SLE, rheumatoid arthritis, idiopathic thrombocytopenia purpura, and dermatomyositis. Four patients (21%) had renal disease managed with hemodialysis, and 2 of those were also receiving steroids for autoimmune disease (11%). Three of the 19 patients (16%) had SLE and were on immunosuppressant therapy. The chronic pericardial effusions seen in SLE and uremic renal failure might serve as a nidus for infection.14,25 The diagnostic challenge that SLE presents lies in differentiating pericardial effusions intrinsic to the disease from those arising from infectious or other causes.26
Endocarditis
Cardiac NTS infection was previously considered a disease of youth, but it is increasingly associated with advanced age and medical comorbidities.5,27 The earliest published review of Salmonella endocarditis, in 1978, reported a mean age at diagnosis of 49 years; however, more recent studies have raised that mean to 59–64 years.5,9,10 In a large Taiwanese study of 121 cases of NTS bacteremia, including 26 with endovascular infection, age served as a more reliable predictor of endovascular infection than did immunodeficiency, SLE, or malignancy.27 It is important to note that increased age does not predict risk for developing NTS endocarditis in patients who have a valve prosthesis.6
Immunodeficiency
Patients with immunodeficiencies such as chronic corticosteroid therapy and human immunodeficiency virus (HIV) are predisposed to NTS cardiac infections.6,9,27 Alterations in local intestinal mucosal immunity—specifically, decreased numbers of lymphocytes and immunoglobulin A-secreting plasma cells—can contribute to the systemic spread of salmonellae from the gut.5 In patients with NTS, diarrhea is a result of bacterial type III effector protein's activation of proinflammatory caspase-1 in the intestinal mucosa and to the subsequent secretion of the cytokine IL-1β.28 Because diarrhea is a defense mechanism that depends on a healthy immune system, NTS bacteremia not associated with diarrhea quite possibly warrants an immunodeficiency evaluation.27,29
Recurrent Salmonella septicemia is an acquired immunodeficiency syndrome-defining illness. Fernández Guerrero and colleagues8 found that NTS septicemia results in extraintestinal infections in 25% of human immunodeficiency virus (HIV) patients; 8.5% of those infections were cardiac in nature. Other investigators found that NTS was the most frequent cause of endocarditis in HIV patients without intravenous drug abuse.30,31 In the absence of another clearly identified cause of immunocompromise, all patients with NTS endocarditis should be tested for HIV.9,11,29
Systemic Lupus Erythematosus
Hypocomplementemia, failing opsonization, renal failure, cardiac valve abnormalities (occurring in more than a third of patients with SLE), and immunosuppressive treatment have all been proposed as mechanisms for the increased incidence of NTS bacteremia and endocarditis in patients with SLE.6,8,9,26,27,32 Investigators in one NTS–SLE study determined that 83% of Salmonella infections occurred during periods of active SLE, but it was unclear if this was due to the SLE itself or to the more aggressive immunosuppressant therapy given to those affected.33 Patients with SLE who have been treated with prednisolone, cyclophosphamide, azathioprine, or methotrexate are more predisposed to NTS septicemia and endocarditis than are patients who do not receive immunoregulatory medication.6–8,33
Structural Heart Disease
In the previously mentioned 1978 review,10 83% of Salmonella endocarditis patients younger than age 50 had underlying valvular disease with predominantly mural (rather than valvular) lesions. In a more recent study, 85.7% of cardiovascular NTS patients had underlying heart conditions that included valve disease, prosthetic valves, and ventricular aneurysms.6 Another study of 17 patients, who in this instance had prosthetic-valve NTS endocarditis, found that aortic valve prostheses were more frequently affected than were mitral valve prostheses.6,33 Other valvulopathies associated with NTS endocarditis include rheumatic valve disease, myxoid mitral valve disease, and degenerative valve disease. Postinfarction ventricular aneurysms also predispose patients to mural endocarditis, which is characterized by extensive infections spreading from endocardium to pericardium—with pseudoaneurysm and abscess formation.6
Other Risk Factors
Other proposed risk factors for NTS endocarditis include colon cancer, chronic gastritis, and cimetidine therapy (acid suppression is thought to reduce the stomach's ability to destroy enteric pathogens), but these contentions remain mostly anecdotal.5 Cirrhosis, with its attendant humoral and cell-mediated immunity deficiencies, increases the risk of NTS bacteremia, but it has not been determined to increase the risk of cardiac infection.9 Surprisingly, diabetes mellitus does not appear to increase the risk of cardiovascular NTS.2,6,9
Conclusions
Bacteremia and cardiac infections are known sequelae of S. enterica infections. As the population ages and more people receive immunomodulating drugs, it is possible that NTS bacteremia and cardiac infections will become more common. Structural heart disease predisposes individuals to NTS endocarditis, but not to NTS pericarditis. Patients with diseases that predispose them to chronic pericardial effusion, including SLE, chronic renal failure, and collagen vascular disease, might be at increased risk for NTS pericarditis. Because patients with NTS bacteremia frequently do not have typical gastrointestinal symptoms, that condition is often unsuspected. Early diagnosis, important in reducing morbidity and death, hinges on maintaining a high degree of suspicion in high-risk populations, especially when there are known Salmonella outbreaks. Empiric echocardiography should be considered for patients with NTS bacteremia who have risk factors for progression to endocarditis or pericarditis.
Acknowledgments
The authors acknowledge Joe Grundle, Katie Klein, Jennifer Pfaff, Brian J. Miller, and Brian Schurrer for their help in preparing the manuscript.
References
- 1.Chai SJ, White PL, Lathrop SL, Solghan SM, Medus C, McGlinchey BM et al. Salmonella enterica serotype Enteritidis: increasing incidence of domestically acquired infections. Clin Infect Dis. 2012;54(Suppl 5):S488–97. doi: 10.1093/cid/cis231. [DOI] [PubMed] [Google Scholar]
- 2.Pace F, Fanfarillo F, Giorgino F, Baratta L. Salmonella enteritidis pericarditis: case report and review of the literature. Ann Ital Med Int. 2002;17(3):189–92. [PubMed] [Google Scholar]
- 3.Huang DB, DuPont HL. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect Dis. 2005;5(6):341–8. doi: 10.1016/S1473-3099(05)70138-9. [DOI] [PubMed] [Google Scholar]
- 4.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–9. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez Guerrero ML, Aguado JM, Arribas A, Lumbreras C, de Gorgolas M. The spectrum of cardiovascular infections due to Salmonella enterica: a review of clinical features and factors determining outcome. Medicine (Baltimore) 2004;83(2):123–38. doi: 10.1097/01.md.0000125652.75260.cf. [DOI] [PubMed] [Google Scholar]
- 6.Gorki H, Nicolay NH, Loulmet DF, Patel NC, Ciuffol GB, Subramanian VA, Lessnau KD. Non-typhoid Salmonellae and prosthetic valve endocarditis: more than a rare coincidence? A review of the literature. J Heart Valve Dis. 2009;18(4):401–10. [PubMed] [Google Scholar]
- 7.Shimoni Z, Pitlik S, Leibovici L, Samra Z, Konigsberger H, Drucker M et al. Nontyphoid Salmonella bacteremia: age-related differences in clinical presentation, bacteriology, and outcome. Clin Infect Dis. 1999;28(4):822–7. doi: 10.1086/515186. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez Guerrero ML, Ramos JM, Nunez A, Cuenca M, de Gorgolas M. Focal infections due to non-typhi Salmonella in patients with AIDS: report of 10 cases and review. Clin Infect Dis. 1997;25(3):690–7. doi: 10.1086/513747. [DOI] [PubMed] [Google Scholar]
- 9.Hsu RB, Lin FY. Risk factors for bacteraemia and endovascular infection due to non-typhoid salmonella: a reappraisal. QJM. 2005;98(11):821–7. doi: 10.1093/qjmed/hci126. [DOI] [PubMed] [Google Scholar]
- 10.Cohen PS, O'Brien TF, Schoenbaum SC, Medeiros AA. The risk of endothelial infection in adults with salmonella bacteremia. Ann Intern Med. 1978;89(6):931–2. doi: 10.7326/0003-4819-89-6-931. [DOI] [PubMed] [Google Scholar]
- 11.Clesham GJ, Davies GJ. Bacterial pericarditis caused by Salmonella enteritidis phage type 1. Int J Cardiol. 1993;41(3):241–3. doi: 10.1016/0167-5273(93)90122-w. [DOI] [PubMed] [Google Scholar]
- 12.Yang CH, Chen KJ, Tseng HH, Yang CJ, Liu JD. Salmonella pericarditis and empyema: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1995;56(3):199–204. [PubMed] [Google Scholar]
- 13.Victor F, Gras D, Le Breton H, Gras S, Amelot J, Pony JC. Salmonella enteritidis pericarditis. Apropos of a case and review of the literature [in French] Arch Mal Coeur Vaiss. 1997;90(2):301–3. [PubMed] [Google Scholar]
- 14.Sabeel A, Alrajhi A, Alfurayh O. Salmonella pericarditis and pericardial effusion in a patient with systemic lupus erythematosus on haemodialysis. Nephrol Dial Transplant. 1997;12(10):2177–8. doi: 10.1093/ndt/12.10.2177. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi K, Endo T, Nejima J, Okamatsu K, Takayama M, Takano T, Hayakawa H. Purulent pericarditis with tamponade caused by Salmonella enteritidis. Jpn Circ J. 1998;62(2):139–41. doi: 10.1253/jcj.62.139. [DOI] [PubMed] [Google Scholar]
- 16.Badawi R, Nageh T, Walker D, Wray R. Nontyphoidal salmonella pericarditis: a case of Salmonella typhimurium phage type 2 pericarditis. Int J Cardiol. 2002;82(2):187–9. doi: 10.1016/s0167-5273(01)00617-9. [DOI] [PubMed] [Google Scholar]
- 17.Salavert M, Navarro V, Roig P. Purulent pericarditis due to Salmonella enterica subsp. arizonae [in Spanish] Enferm Infecc Microbiol Clin. 2002;20(1):47–9. [PubMed] [Google Scholar]
- 18.Yoshioka H, Shigemitsu K, Takeuchi M, Mori S, Imaizumi M, Ueda Y. Salmonella pericarditis in a patient with primary idiopathic chylopericardium. Jpn J Thorac Cardiovasc Surg. 2003;51(1):16–7. doi: 10.1007/s11748-003-0059-7. [DOI] [PubMed] [Google Scholar]
- 19.Candel FJ, Roca-Arbones V, Nunez MJ, Arroyo C, Valdivia A, Tellez MJ, Picazo JJ. A rare cause of pericarditis. Clin Microbiol Infect. 2003;9(12):1261–3. doi: 10.1111/j.1469-0691.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 20.Arruvito L, Ber MG, Martinez Martinez JA. Purulent pericarditis with pericardial tamponade caused by Streptococcus agalactiae and Salmonella enterica no typhi [in Spanish] Medicina (B Aires) 2004;64(4):340–2. [PubMed] [Google Scholar]
- 21.Can F, Demirbilek M, Erdem B, Ciftci U, Tunaoglu M, Laleli Y. A purulent pericarditis caused by Salmonella typhimurium. J Med Microbiol. 2004;53(Pt 10):1051–2. doi: 10.1099/jmm.0.05449-0. [DOI] [PubMed] [Google Scholar]
- 22.Hoag JB, Sessler CN. A comprehensive review of disseminated Salmonella arizona infection with an illustrative case presentation. South Med J. 2005;98(11):1123–9. doi: 10.1097/01.smj.0000177346.07719.00. [DOI] [PubMed] [Google Scholar]
- 23.Takamiya Y, Shirai K, Fujino M, Miller N, Tsuchiya Y, Okabe M, Saku K. Purulent pericarditis with Salmonella enteritidis in a patient with CD4/CD8 depression. J Cardiol. 2008;51(3):201–4. doi: 10.1016/j.jjcc.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Gorecki B, Flasinski J, Gorski J. Patient with purulent pericarditis caused by Salmonella enteritidis complicated by tamponade [in Polish] Kardiol Pol. 2008;66(6):664–8. [PubMed] [Google Scholar]
- 25.Tseng JR, Lee MJ, Lin JL, Yen TH. Definite and probable septic pericarditis in hemodialysis. Ren Fail. 2010;32(10):1177–82. doi: 10.3109/0886022X.2010.516858. [DOI] [PubMed] [Google Scholar]
- 26.Bourre-Tessier J, Huynh T, Clarke AE, Bernatsky S, Joseph L, Belisle P, Pineau CA. Features associated with cardiac abnormalities in systemic lupus erythematosus. Lupus. 2011;20(14):1518–25. doi: 10.1177/0961203311420318. [DOI] [PubMed] [Google Scholar]
- 27.Hsu RB, Tsay YG, Chen RJ, Chu SH. Risk factors for primary bacteremia and endovascular infection in patients without acquired immunodeficiency syndrome who have nontyphoid salmonellosis. Clin Infect Dis. 2003;36(7):829–34. doi: 10.1086/367932. [DOI] [PubMed] [Google Scholar]
- 28.Muller AJ, Hoffmann C, Galle M, Van Den Broeke A, Heikenwalder M, Falter L et al. The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe. 2009;6(2):125–36. doi: 10.1016/j.chom.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Brown M, Eykyn SJ. Non-typhoidal Salmonella bacteraemia without gastroenteritis: a marker of underlying immunosuppression. Review of cases at St. Thomas' Hospital 1970–1999. J Infect. 2000;41(3):256–9. doi: 10.1053/jinf.2000.0750. [DOI] [PubMed] [Google Scholar]
- 30.Bestetti RB, Figueiredo JF, Da Costa JC. Salmonella tricuspid endocarditis in an intravenous drug abuser with human immunodeficiency virus infection. Int J Cardiol. 1991;30(3):361–2. doi: 10.1016/0167-5273(91)90019-l. [DOI] [PubMed] [Google Scholar]
- 31.Losa JE, Miro JM, Del Rio A, Moreno-Camacho A, Garcia F, Claramonte X et al. Infective endocarditis not related to intravenous drug abuse in HIV-1-infected patients: report of eight cases and review of the literature. Clin Microbiol Infect. 2003;9(1):45–54. doi: 10.1046/j.1469-0691.2003.00505.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsao CH, Chen CY, Ou LS, Huang JL. Risk factors of mortality for salmonella infection in systemic lupus erythematosus. J Rheumatol. 2002;29(6):1214–8. [PubMed] [Google Scholar]
- 33.Lim E, Koh WH, Loh SF, Lam MS, Howe HS. Non-typhoidal salmonellosis in patients with systemic lupus erythematosus. A study of fifty patients and a review of the literature. Lupus. 2001;10(2):87–92. doi: 10.1191/096120301675973164. [DOI] [PubMed] [Google Scholar]


