Abstract
Primary tumors of the aorta are rare entities. We report the unusual manifestation of an aortic intimal sarcoma that presented as a brain metastasis in a 56-year-old, otherwise healthy woman. After the brain mass had been resected, multiple imaging methods revealed pseudocoarctation and the primary tumor in the aortic arch. To our knowledge, this is the first report of the diagnosis of an aortic intimal sarcoma with use of real-time, 3-dimensional transesophageal echocardiography.
Keywords: Aorta, thoracic/pathology/radiography; diagnostic imaging/methods; hemangiosarcoma/complications/diagnosis; tunica intima/pathology; vascular neoplasms/diagnosis
Primary tumors of the aorta are rare. We describe the case of a 56-year-old woman who presented with neurologic symptoms from a brain mass and was subsequently discovered to have a primary aortic sarcoma metastatic to the brain and causing partial obstruction of the descending thoracic aorta. We discuss our diagnosis of the tumor with use of multiple imaging methods.
Case Report
In August 2012, a 56-year-old, previously healthy woman presented with a 2-week history of headache and progressive right-sided weakness. Magnetic resonance images (MRI) of the brain showed a 4-cm, left parafalx, gadolinium-enhanced lesion (Fig. 1), which we first presumed to be a meningioma. The patient was immediately treated with intravenous dexamethasone and underwent bilateral frontal craniotomy for resection of the mass. Histopathologic examination of the excised specimen revealed a malignant, high-grade sarcoma that stained positive for vimentin. Immunohistochemical staining for Ki-67 proliferation was positive in up to 75% of the tumor cells. Other tissue-specific immunohistochemical staining was negative.
Fig. 1.
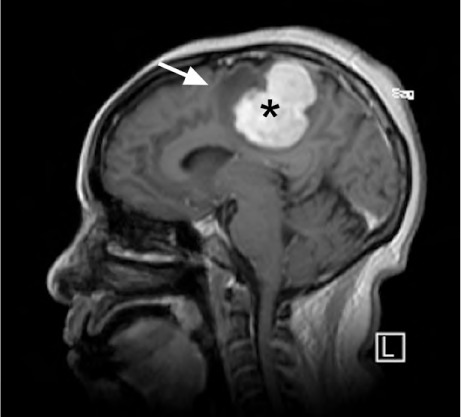
Magnetic resonance image shows a 4.3 × 3.8 × 4.3-cm, dural-based, left parafalx gadolinium-enhanced mass (asterisk) with surrounding edema (arrow) and mass effect.
Contrast-enhanced computed tomograms of the patient's chest showed a large intraluminal mass in the aortic arch. The mass extended into the brachiocephalic artery, the left subclavian artery, and the proximal descending aorta (Fig. 2). Chest MRI showed that the mass involved the aortic arch and extended into the arch vessels (Fig. 3). Its radiographic appearance was thought to be consistent with a thrombus, because of the lack of contrast enhancement. A transthoracic echocardiogram (TTE) revealed an abnormal spectral Doppler flow-velocity pattern in the aortic arch and the descending thoracic aorta: the elevated peak systolic velocity rose as high as 2.7 m/s (normal rate, ~1 m/s), and abnormal antegrade flow persisted throughout diastole (Fig. 4). The findings of substantial flow obstruction indicated acquired coarctation of the aorta, caused by the intraluminal mass. Real-time 3-dimensional transesophageal echocardiograms (TEE) showed a large, fungating, highly mobile intraluminal mass, extending from the aortic arch to the proximal descending thoracic aorta and almost completely obliterating the aortic lumen (Fig. 5).
Fig. 2.
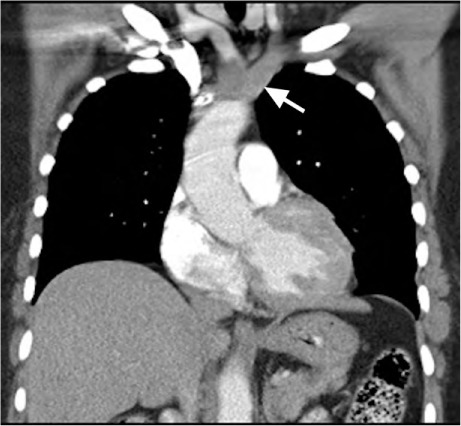
Computed tomogram (coronal view) with intravenous contrast medium shows a large aortic intimal sarcoma that was initially mistaken for thrombus. The tumor involves most of the aortic arch, extending superiorly into the proximal portions of the brachiocephalic trunk and left subclavian artery (arrow) and into the proximal descending aorta.
Supplemental motion image (4MB, m1v) is available for Fig. 2.
Fig. 3.
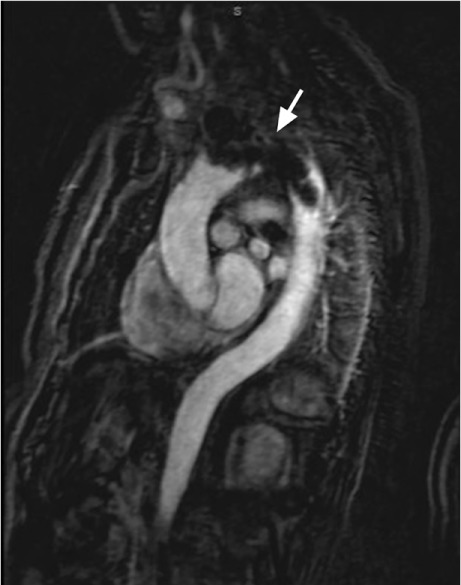
Magnetic resonance image of the chest (sagittal view) shows a large, irregular mass extending into the arch and the proximal descending thoracic aorta (arrow). The mass appeared to be a thrombus.
Fig. 4.
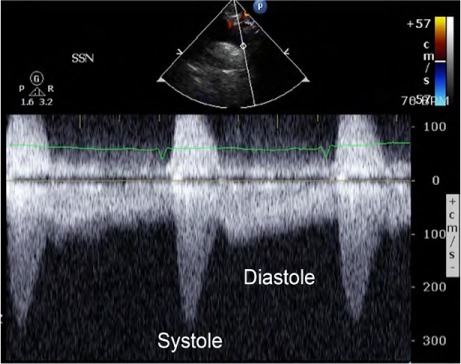
Continuous-wave spectral Doppler image from the suprasternal notch shows abnormal coarctation-like flow in the descending thoracic aorta during systole and diastole.
Fig. 5.
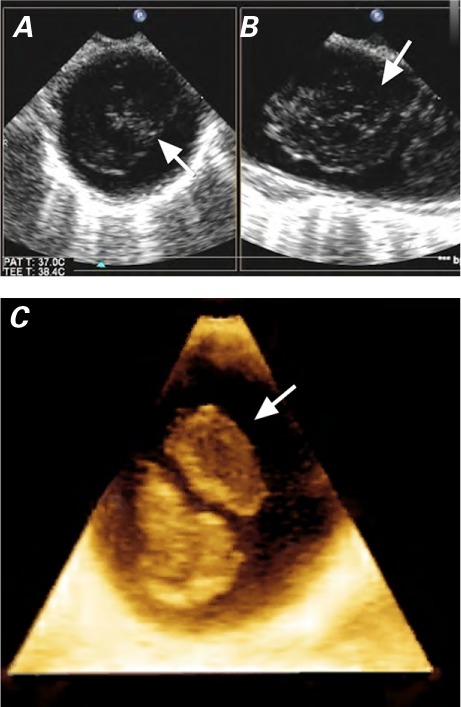
Two-dimensional transesophageal echocardiograms in A) short-axis and B) long-axis views show nearly complete obliteration of the descending thoracic aorta by the mass (arrows), just distal to the left subclavian artery. C) Three-dimensional transesophageal echocardiogram shows the mass in the descending thoracic aorta (arrow).
Supplemental motion images are available for Figs. 5A–B and for Fig. 5C.
Intraoperatively, we found extensive invasion of the aortic arch and all 3 aortic arch branches. A palliative resection of the mass was performed. Histopathologic analysis of the aortic mass yielded a pleomorphic, high-grade sarcoma that stained positive for vimentin. The morphologic appearance of this tumor was similar to that of the previously excised brain tumor and characteristic of a poorly differentiated aortic intimal sarcoma (Fig. 6).
Fig. 6.
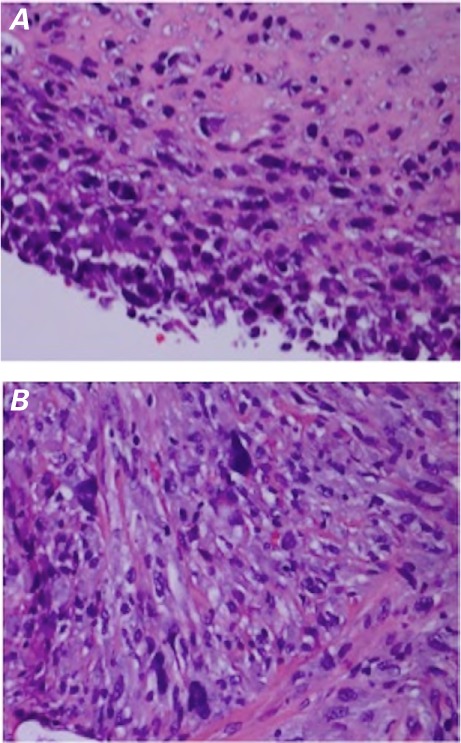
Photomicrographs (H & E, orig. × 400). A) The aortic intimal sarcoma displays hyperchromatic, occasionally spindled cells, poorly differentiated to undifferentiated. B) The metastatic brain lesion displays hyperchromatic, pleomorphic cells similar to those of the primary sarcoma.
The overall findings were consistent with a primary intimal sarcoma of the aortic arch that had metastasized to the left side of the brain. The patient was transferred for palliative care and later died.
Discussion
To our knowledge, this is the first report of the diagnosis of an aortic intimal sarcoma with the use of real-time, 3-dimensional TEE.
Primary tumors of the aorta are rarely reported. Sarcoma, the histologic type most often identified, constituted approximately one quarter of all primary aortic tumors in a retrospective analysis of 100 cases.1 Aortic sarcomas can be differentiated into intimal angiosarcomas (of endothelial origin), intimal myofibroblastic sarcomas (of mesenchymal origin), and mural sarcomas of the aorta.2 Our patient's tumor was most likely an angiosarcoma.
In a case series of 21 aortic intimal sarcomas,2 the median age of patients at presentation was 62 years; the clinical manifestations were chiefly due to tumor embolization or metastatic sequelae, rather than to the primary tumor itself. On noncontrast radiographic images, aortic intimal sarcomas can be difficult to differentiate from atherosclerotic disease; the administration of intravenous contrast material might help distinguish between the two, if the lesion displays any contrast enhancement.3,4 However, in our patient, it was nearly impossible to differentiate the tumor from thrombus on the computed tomograms or MRI, because the mass did not enhance with contrast.
Three-dimensional echocardiography has been found to enable the live acquisition of full volumes at sufficient resolution to characterize the features and spatial relationships of cardiac tumors.5 We used 3-dimensional TEE to obtain the best characterization of our patient's tumor.
For aortic intimal sarcoma, radical surgical resection is rarely curative, because most such tumors present late and already exhibit evidence of embolic or metastatic disease. Radiation therapy and adjuvant chemotherapy with doxorubicin and ifosfamide is associated with a response rate of up to 20%.6 Overall, the prognosis for aortic intima sarcoma is quite poor, with a mean survival period of about one year.2
Supplementary Material
References
- 1.Bohner H, Luther B, Braunstein S, Beer S, Sandmann W. Primary malignant tumors of the aorta: clinical presentation, treatment, and course of different entities. J Vasc Surg. 2003;38(6):1430–3. doi: 10.1016/s0741-5214(03)00935-2. [DOI] [PubMed] [Google Scholar]
- 2.Thalheimer A, Fein M, Geissinger E, Franke S. Intimal angiosarcoma of the aorta: report of a case and review of the literature. J Vasc Surg. 2004;40(3):548–53. doi: 10.1016/j.jvs.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Mohsen NA, Haber M, Urrutia VC, Nunes LW. Intimal sarcoma of the aorta. AJR Am J Roentgenol. 2000;175(5):1289–90. doi: 10.2214/ajr.175.5.1751289. [DOI] [PubMed] [Google Scholar]
- 4.Salhab KF, Said SM, Sundt TM., 3rd. Pseudocoarctation of the aorta secondary to aortic intimal sarcoma. Ann Thorac Surg. 2012;94(1):279–81. doi: 10.1016/j.athoracsur.2011.08.088. [DOI] [PubMed] [Google Scholar]
- 5.Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261–2. [PMC free article] [PubMed] [Google Scholar]
- 6.Keohan ML, Taub RN. Chemotherapy for advanced sarcoma: therapeutic decisions and modalities. Semin Oncol. 1997;24(5):572–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


