Abstract
Catalytic FeCl3 in the presence of 4Å molecular sieves has been shown to effect highly diastereoselective tandem Prins and Friedel-Crafts cyclization of substituted (E/Z)-6-phenylhex-3-en-1-ol and a variety of aldehydes to provide a range of polycyclic compounds in good to excellent yields. The reaction of an enantioenriched alcohol with an aldehyde provided the cyclization product without loss of optical activity. Furthermore, a Lewis acid catalyzed ring opening resulted in functionalized tetralin derivatives with multiple chiral centers.
Keywords: FeCl3 catalysis, Isochromene, Prins cyclization, Friedel-Crafts reaction, CBS reduction
Iron salts are inexpensive, widely abundant, and considerably less toxic than other transition-metal catalysts. In recent years, there has been significant effort towards the development of iron-based catalysts for carbon-carbon and carbon-heteroatom bond formation.1,2 In our continuing interest in the synthesis of substituted tetrahydropyran rings, we have recently developed Cu(OTf)2 and bisphosphine catalyzed reactions that provided a range of tetrahydropyran derivatives through a sequential olefin migration and Prins-type cyclization.3 A number of other effective methods have also been developed for the synthesis of such functionalized tetrahydropyrans using Prins cyclizations.4,5 These methods have been widely employed in the synthesis of natural products and other bioactive compounds.6,7 Furthermore, combination of Prins and Friedel-Crafts reactions in an intermolecular sense provided convenient access to 4-aryl tetrahydropyran derivatives.8,9 The potential of the Prins and Friedel-Crafts cyclization in an intramolecular pathway has been reported by trapping a carbocation with an aromatic nucleophile.10 These reactions were carried out in the presence of Sc(OTf)3 and p-TsOH to provide heterocyclic and polycyclic ring systems. An InBr3-catalyzed Prins and Friedel-Crafts cyclizations have also shown to afford polycyclic ring compounds.11 This methodology was utilized in the synthesis of moluccanic acid methyl ester.
The availability of substituted alkenols and aldehydes makes this synthesis of polycyclic ring structures very attractive. However, current catalytic systems require both Brϕnsted and Lewis acids, or expensive metal triflates and high catalyst loading. In addition, shorter reaction time, high diastereoselectivity, and access to these polycyclic structures in optically active form would greatly enhance the utility of this tandem cyclization. Herein, we report a FeCl3-catalyzed sequential Prins and Friedel-Crafts cyclization of E/Z-substituted alkenols with a variety of aldehydes to provide a range of cis and trans-fused tricyclic derivatives with high diastereoselectivity and good to excellent isolated yields. Reaction of an enantioenriched alkenol with an aldehyde generated the corresponding tricyclic derivative in optically active form. Furthermore, Cu(OTf)2-catalyzed opening of the tetrahydropyran ring provided access to functionalized tetralin derivatives with three contiguous chiral centers.
For an initial survey of suitable catalysts and reaction conditions, we carried out a cascade cyclization of (Z)-6-phenylhex-3-enol 1-(Z)12 with p-nitrobenzaldehyde (2a) as shown in Scheme 1. The results are summarized in Table 1. As can be seen, both Dy(OTf)3 and Cu(OTf)2 in the presence of 2 equivalents of aldehyde did not provide the desired cyclization product (entries 1 and 2). However, reaction with Cu(OTf)2 (20 mol %) in combination with commercially available (Z)-1,2- bis(diphenylphosphino)ethylene (20 mol%) in CH2Cl2 at 23 °C for 24 h furnished desired cyclization product 4a as the only isolable product in 81% yield. Both 1H-NMR and 13C-NMR of the crude product did not show the other diastereomer. In this particular reaction, after 24 h, the reaction mixture showed the presence of around 6% starting alkenol by 1H- NMR analysis (entry 3). We then surveyed the feasibility of FeCl3 for the Prins and Friedel- Crafts cyclization. Reaction of alkenol 1-(Z) and 1.2 equivalents of aldehyde 2a in the presence of 20 mol% FeCl3 in CH2Cl2 provided cyclization product 4a as the only isolable product in 68% yield. The reaction was completed within 2 h and the 1H-NMR of the crude reaction mixture showed the presence of 4a as the sole diastereomer (entry 4). The corresponding reaction with 10 mol% FeCl3 provided an improved yield of 4a (entry 5). This reaction in the presence of molecular sieves afforded 4a in 89% yield within 1 h (entry 6). Thus, the presence of molecular sieves reduced the reaction time and slightly improved the yield. The reaction with Fe(OTf)3, however, resulted in no product 4a (entry 7). We then investigated the scope and utility of these catalytic conditions using both 1-(E)- and 1-(Z)-alkenols12,13 and a number of aromatic and aliphatic aldehydes.
Scheme 1.

Prins and Friedel Crafts tandem cyclization.
Table 1.
Preliminary survey of catalysts and conditions.
| Entry | Aldehyde | Conditions | Time (h) | Yield |
|---|---|---|---|---|
| 1 | 2 equiv | Dy(OTf)3 (20 mol %) | 24 | NR[a] |
| 2 | 2 equiv | Cu(OTf)2 (20 mol %) | 24 | NR[a] |
| 3 | 2 equiv | Cu(OTf)2 (20 mol %) + L | 24 | 81%[b] |
| 4 | 1.2 equiv | FeCl3 (20 mol%) | 2 | 68%[c] |
| 5 | 1.2 equiv | FeCl3 (10 mol%) | 4 | 87%[c] |
| 6 | 1.2 equiv | FeCl3 (10 mol%) + 4Å MS | 1 | 89%[c] |
| 7 | 1.2 equiv | Fe(OTf)3 (10 mol %) | 24 | NR%[a] |
No Reaction;
L = (Z-1,2-bis(diphenylphosphino)-ethylene (20 mol%), around 6% starting material remained;
dr = 20:1 by crude 1H NMR. All reactions were carried out at 23 °C
As shown in Table 2, reaction of alkenol 1-(E) and p-nitrobenzaldehyde provided 71% yield of the cyclization product 5a as a single isomer by 1H-NMR and 13C-NMR analysis. The yield of product 5a was reduced compared to reaction with alkenol 1-(Z). Reactions of 1-(E/Z) with electron-rich 4-methoxybenzaldehyde 2b and piperonal 2c have shown similar yields (entries 3-6). Furthermore, reaction of benzaldehyde 2d and conjugated aldehyde 2e proceeded well to provide the corresponding cyclization products in good to excellent yields (entries 7-10). Reactions of aliphatic aldehydes such as isovaleraldehyde 2f and benzyloxypropionaldehyde 2g also furnished good to excellent yields and excellent diastereoselectivity (entries 11-14). Stereochemical assignments of products were made based upon 1H-NMR NOESY experiments of the tricyclic products. Also, the X-ray crystal structure of 5b supports our stereochemical assignment (Figure 1).14,15 While a wide range of functionalities are tolerated for these cyclization conditions, it should be noted that reactions with benzyloxyacetaldehyde or tosyloxyacetaldehyde did not provide the desired tricyclic products.
Table 2.
Substrate scope and structures of cyclization products
| Entry | Aldehyde | Alkene | Product[a],[b] | Yield (%) |
|---|---|---|---|---|
| 1 |

|
1-(Z) |
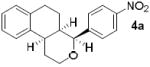
|
89 |
| 2 | 2a | 1-(E) |
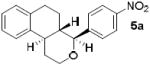
|
71 |
| 3 |

|
1-(E) |
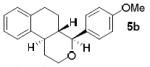
|
68 |
| 4 | 2b | 1-(Z) |

|
86 |
| 5 |

|
1-(E) |

|
77 |
| 6 | 2c | 1-(Z) |

|
85 |
| 7 |

|
1-(E) |

|
71 |
| 8 | 2d | 1-(Z) |

|
73 |
| 9 |

|
1-(E) |
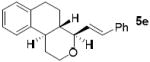
|
68 |
| 10 | 2e | 1-(Z) |
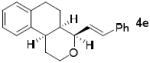
|
89 |
| 11 |
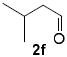
|
1-(E) |

|
91 |
| 12 | 2f | 1-(Z) |

|
77 |
| 13 |

|
1-(E) |

|
77 |
| 14 | 2g | 1-(Z) |

|
76 |
Conditions: 1 (1 equiv), aldehyde (1.2 equiv), FeCl3 (10 mol %), 4 Å MS, CH2Cl2, 23°C, 1-2 h.
Diastereomeric ratio 20:1 by 1H-NMR analysis.
Figure 1.

ORTEP drawing of compound 5b
We have also investigated this FeCl3-based cyclization protocol using three secondary alkenols 6a-c in combination with a variety of aldehydes. As can be seen in Table 3, both electron-poor and electron-rich aromatic aldehydes provided excellent yields with methyl, phenyl and benzyl substituted alkenols (compounds 7a-7e). However, reaction of phenyl substituted alkenol 6c with 4-methoxybenzaldehyde and acetaldehyde provided the cyclization products 7f and 7g, respectively, with substantial reduction in yields. Reaction of 6a-c with isovaleraldehyde 2f, piperonal 2c and benzaldehyde 2d provided excellent yield of cyclization products 7h-7m with excellent diastereoselectivity. The reaction of sterically demanding 2-methoxybenzaldehyde with alkenol 6c provided cyclization product 7n as the only isolable product in 70% yield. Stereochemical assignment of these products were made by 1H-NMR NOESY studies. The X-ray structure of cyclization product 7e further corroborated our stereochemical assignment (Figure 2).14,16
Table 3.
Product structures resulting from substituted alkenols[a]

|
Only isolated product is shown. Diastereomeric ratio 20:1 by 1H NMR analysis.
Figure 2.
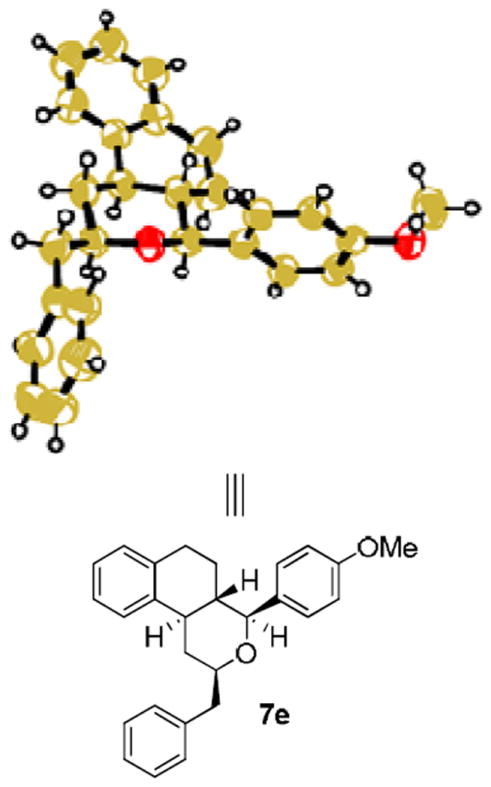
ORTEP drawing of compound 7e
To further examine whether this cyclization reaction involved an oxonia-Cope rearrangement,17 we have carried out the cascade cyclization using an optically active alcohol 9. As shown in Scheme 2, reaction of known Weinreb amide 818 with Grignard reagent derived from m-trifluoromethylphenyl bromide provided the corresponding ketone in 74% yield. The resulting ketone was subjected to Corey-Bakshi-Shibata reduction19 to provide enantioenriched alcohol 9 in 92% yield. Enantiopurity of 9 (82% ee) was determined by HPLC with a chiral column after forming the corresponding p-nitrobenzoate derivative.20
Scheme 2.
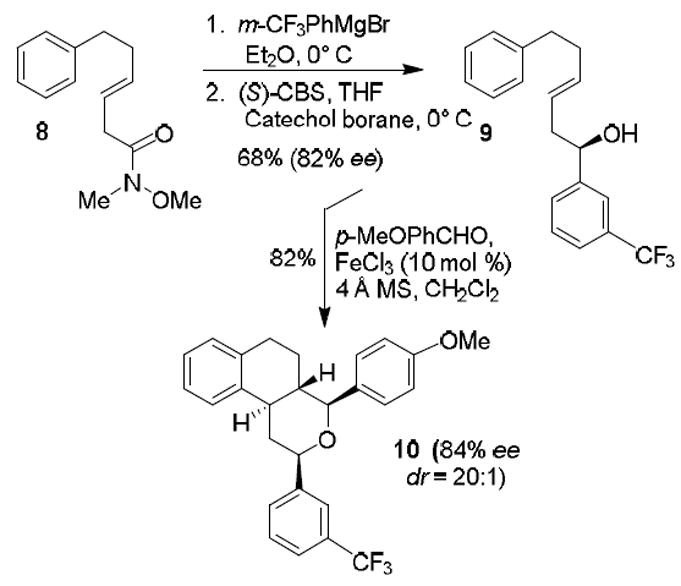
Prins and Friedel-Crafts Cyclization of optically active alcohol
The cyclization reaction of alcohol 9 with 4-methoxybenzaldehyde afforded tricyclic derivative 10 with high diastereoselectivity (dr = 20:1) and in 82% isolated yield. HPLC analysis using a chiral column showed that enantiopurity of compound 10 was 84% ee. This result indicated that the cascade cyclization may not have involved an oxonia-Cope rearrangement4b,c,17 which was described by Rychnovski and co-workers in the context of the racemization of Prins cyclizations.
We have investigated chemoselective acyloxycarbenium ion-mediated ring opening of the tetrahydropyran rings.21 Our initial attempts at ring opening of 7g using 5 mol% Zn(OTf)2 proved to be inefficient as large amounts of the starting material remained after 24 h. However, reaction of 7g with 5 mol% Cu(OTf)2 and 20 equivalents of Ac2O in toluene at reflux resulted in the corresponding ring opened product within 1 h (Scheme 3). Saponification of the resulting acetate derivative then afforded alcohol 11g in 79% isolated yield in two steps. Furthermore, we have applied these reaction conditions to tricyclic ring compound 7i with bulky substituents. However, this ring opening required higher temperature and xylene was used as the solvent. The corresponding ring opened product was saponified to provide alcohol 11i in moderate yield.
Scheme 3.
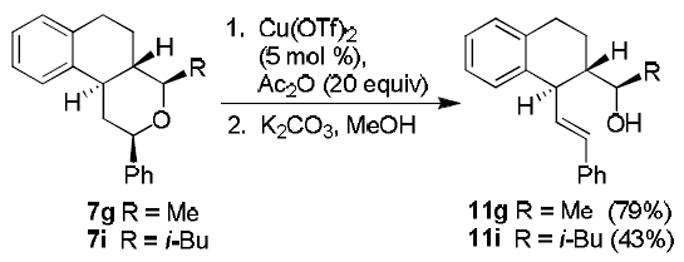
Chemoselective opening of tetrahydropyran rings
In conclusion, we have demonstrated an inexpensive FeCl3 catalyzed (10 mol%) Prins-Friedel Crafts cascade cyclization of (E)- and (Z)-6-phenylhex-3-en-1-ols with a variety of aliphatic andaromatic aldehydes. This FeCl3-based reaction protocol provided convenient access to tricyclic substituted hexahydro-1H-benzo[f]isochromene derivatives with excellent diastereoselectivity and good to excellent isolated yields. Using an optically active alkenol, we have shown that the present cyclization pathway does not involve an oxonia-Cope process. Furthermore, treatment of the tricyclic derivatives with Cu(OTf)2 and acetic anhydride provided the corresponding ring opened tetralin derivatives. Application of these substituted cyclic derivatives in synthesis is ongoing.
Supplementary Material
Acknowledgments
Financial support by the National Institutes of Health and Purdue University is gratefully acknowledged.
Footnotes
Supplementary Data
Supplementary data associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Enthaler S, Junge K, Beller M. Angew Chem Int Ed. 2008;47:3317–3321. doi: 10.1002/anie.200800012. [DOI] [PubMed] [Google Scholar]; (b) Bolm C, Legros J, Le Paih J, Zani L. Chem Rev. 2004;104:6217–6254. doi: 10.1021/cr040664h. [DOI] [PubMed] [Google Scholar]
- 2.(a) Sherry BD, Furstner A. Acc Chem Res. 2008;41:1500–1511. doi: 10.1021/ar800039x. [DOI] [PubMed] [Google Scholar]; (b) Chen MS, White MC. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh AK, Nicponski DR. Org Lett. 2011;13:4328–4331. doi: 10.1021/ol2016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Barry CS, Crosby SR, Harding JR, Hughes RA, King CD, Parker GD, Willis CL. Org Lett. 2003;5:2429–2432. doi: 10.1021/ol0346180. [DOI] [PubMed] [Google Scholar]; (b) Dalgard JE, Rychnovsky SD. J Am Chem Soc. 2004;126:15662–15663. doi: 10.1021/ja044736y. [DOI] [PubMed] [Google Scholar]; (c) Hu YQ, Skalitzky DJ, Rychnovsky SD. Tetrahedron Lett. 1996;37:8679–8682. [Google Scholar]
- 5.(a) Olier C, Kaafarani M, Gastaldi S, Bertrand MP. Tetrahedron. 2010;66:413–445. [Google Scholar]; (b) Cloninger MJ, Overman LE. J Am Chem Soc. 1999;121:1092–1093. [Google Scholar]
- 6.(a) Cossey KN, Funk RL. J Am Chem Soc. 2004;126:12216–12217. doi: 10.1021/ja046940r. [DOI] [PubMed] [Google Scholar]; (b) Custar DW, Zabawa TP, Scheidt KA. J Am Chem Soc. 2008;130:804–805. doi: 10.1021/ja710080q. [DOI] [PubMed] [Google Scholar]; (c) Kwon MS, Woo SK, Na SW, Lee E. Angew Chem Int Ed. 2008;47:1733–1735. doi: 10.1002/anie.200705018. [DOI] [PubMed] [Google Scholar]
- 7.(a) Crane EA, Scheidt KA. Angew Chem Int Ed. 2010;49:8316–8326. doi: 10.1002/anie.201002809. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wender PA, Dechristopher BA, Schrier AJ. J Am Chem Soc. 2008;130:6658–6659. doi: 10.1021/ja8015632. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Spivey AC, Laraia L, Bayly AR, Rzepa HS, White AJ. Org Lett. 2010;12:900–903. doi: 10.1021/ol9024259. [DOI] [PubMed] [Google Scholar]
- 8.(a) Reddy UC, Bondalapati S, Saikia AK. Eur J Org Chem. 2009;10:1625–1629. doi: 10.1021/jo802531h. [DOI] [PubMed] [Google Scholar]; (b) Reddy UC, Bondalapati S, Saikia AK. J Org Chem. 2009;74:2605–2608. doi: 10.1021/jo802531h. [DOI] [PubMed] [Google Scholar]
- 9.Yang XF, Wang MW, Zhang YH, Li CJ. Synlett. 2005;12:1912–1916. [Google Scholar]
- 10.Reddy BV, Borkar P, Yadav JS, Sridhar B, Gree R. J Org Chem. 2011;76:7677–7690. doi: 10.1021/jo201027u. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Lai Y-C, Zhao Y, Wong Y-H, Shen Z-L, Loh T-P. Angew Chem. 2012;124:10771–10775. [Google Scholar]
- 12.Dijikink J, Speckamp W. Tetrahedron. 1977;34:173–178. [Google Scholar]
- 13.Brown CA, Ahuja V. J Org Chem. 1973;38:2226–2230. [Google Scholar]
- 14.Single-Crystal X-Ray analysis was performed in-house. Dr. Phil Fanwick, X-Ray Crystallography laboratory, Department of Chemistry, Purdue University; West Lafayette, IN 47907: [Google Scholar]
- 15.CCDC 997772 contains the supplementary crystallographic data for Compound 5b. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- 16.CCDC 997771 contains the supplementary crystallographic data for Compound 7e These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- 17.Jasti R, Rychnovsky SD. J Am Chem Soc. 2006;128:13640–13648. doi: 10.1021/ja064783l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, Uteuliyev M, Takacs J. Chem Comm. 2011;47:7812–7814. doi: 10.1039/c1cc11746g. [DOI] [PubMed] [Google Scholar]
- 19.Corey EJ, Bakshi RK, Shibata S. J Am Chem Soc. 1987;109:5551–5553. [Google Scholar]
- 20.For details, please see supplementary data.
- 21.(a) Ghosh AK, Kass J. Org Lett. 2012;14:510–512. doi: 10.1021/ol203093g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghosh AK, Shurrush K, Kulkarni S. J Org Chem. 2009;74:4508–4518. doi: 10.1021/jo900642f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ghosh AK, Swanson L. J Org Chem. 2003;68:9823–9826. doi: 10.1021/jo035077v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


