Abstract
In the title compound, C22H19Cl2NO3, the central six-membered ring is in a twist-boat conformation. The two aryl groups are in equatorial positions, trans to each other and with a dihedral angle of 77.50 (2)° between them. One of the least hindered –CH2– groups and one of the aryl-substituted C atoms, with its axial H atom, are in the flagpole positions. The ethoxycarbonyl group is in an equatorial position and is cis to the second aryl group. In the crystal, molecules are linked via weak C—H⋯O hydrogen bonds, forming chains along [010].
Keywords: crystal structure
Related literature
For the synthesis, see: Zhang et al. (2010 ▶); Tan et al. (2009 ▶). For related structures, see: Rowland & Gill (1988 ▶); Aleman et al. (2009 ▶); Wu et al. (2011 ▶); Li et al. (2011 ▶). For other [5 + 1] annulation reactions, see: Bi et al. (2005 ▶); Zhao et al. (2006 ▶); Fu et al. (2009 ▶); Xu et al. (2012 ▶).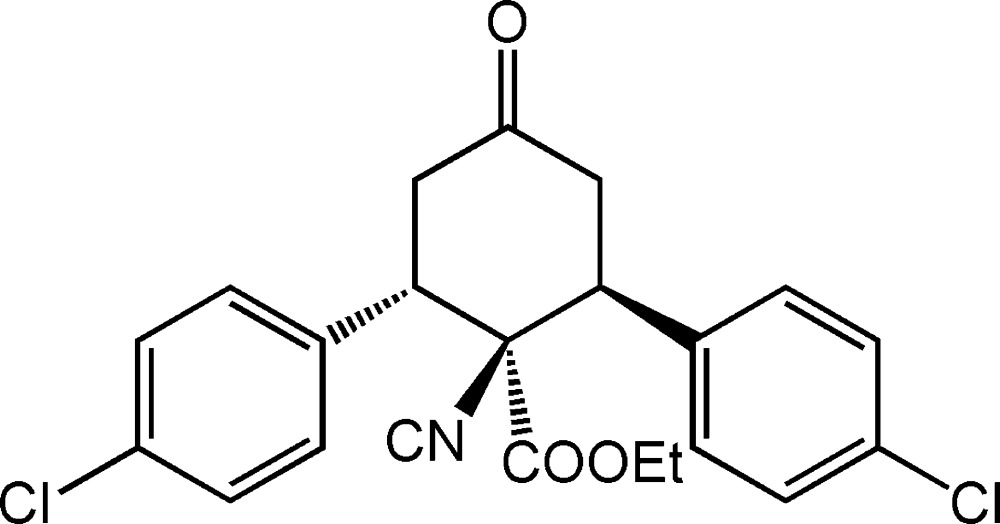
Experimental
Crystal data
C22H19Cl2NO3
M r = 416.28
Monoclinic,

a = 21.6980 (17) Å
b = 11.0770 (19) Å
c = 17.515 (3) Å
β = 104.535 (2)°
V = 4075.0 (10) Å3
Z = 8
Mo Kα radiation
μ = 0.34 mm−1
T = 293 K
0.21 × 0.19 × 0.15 mm
Data collection
Bruker SMART APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.932, T max = 0.951
9983 measured reflections
3602 independent reflections
2584 reflections with I > 2σ(I)
R int = 0.027
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.117
S = 1.01
3602 reflections
253 parameters
H-atom parameters constrained
Δρmax = 0.37 e Å−3
Δρmin = −0.35 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S160053681401383X/lr2127sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681401383X/lr2127Isup2.hkl
Supporting information file. DOI: 10.1107/S160053681401383X/lr2127Isup3.cml
CCDC reference: 1008201
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11⋯O1i | 0.98 | 2.57 | 3.218 (3) | 123 |
Symmetry code: (i)  .
.
Acknowledgments
Financial support of this research by the Science and Technology Development Program Foundation of Jilin Province (No. 20140204022NY) and the Interdisciplinary Innovation Fund of Jilin University (No. 450060481143) is gratefully acknowledged.
supplementary crystallographic information
S1. Introduction
S2. Experimental
S2.1. Synthesis and crystallization
To the mixture of 1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one (303 mg, 1.0 mmol) and ethyl isocyanoacetate (0.132 mL, 1.2 mmol) in DMF (5 mL) was added 1,8-diazabicyclo [5.4.0]undec-7-ene (DBU) (0.015 mL, 0.1 mmol) in one portion at room temperature. The reaction mixture was stirred at room temperature, and the reaction mixture was monitored by TLC. After the substrate 1,5-bis(4-chlorophenyl)penta-1,4-dien-3-one was consumed, the resulting mixture was poured into ice-water (30 mL) under stirring. The precipitated solid was collected by filtration, washed with water (3 × 10 mL), and dried under vacuum to afford the crude product, which was purified by flash chromatography (silica gel, petroleum ether : diethyl ether = 3:1, V/V) to give ethyl 2,6-bis(4-chlorophenyl)-1-isocyano-4-oxocyclohexanecarboxylate (387 mg, 93%). The material was recrystallized from a mixture of petroleum ether and diethyl ether to provide a crystalline solid.
S2.2. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1.Hydrogen atoms were generated in idealized positions (according to the sp2 or sp3 geometries of their parent carbon), and then refined using a riding model with fixed C—H distances (C—H = 0.95–1.00 Å) and with Uiso(H) = 1.2Ueq(C).
S3. Results and discussion
[5+1] annulation is a novel strategy for the construction of six-membered cyclic compounds and total synthesis of natural products (Rowland & Gill, 1988; Wu et al., 2011; Li et al., 2011). The regiospecific [5+1] annulation reactions have drawn much attentions and both the five-carbon 1,5-bielectrophiles and the one-atom nucleophiles been explored extensively (Bi et al., 2005; Zhao et al., 2006; Fu et al., 2009; Xu et al., 2012). We have been dealing with functionalized ketene dithioacetals for several years and have succeeded in the preparation of six-membered aromatic and heterocyclic compounds based on [5C+1X] annulations (Zhang et al., 2010; Tan et al., 2009). The aromatic cyclic compounds are analogues of phenylalanine (Phe) which are potential moieties for the synthesis of peptide analogues with controlled fold in the backbone. The constrained ring systems play important roles in restricting torsional angle χ1 and in peptide receptor recognition processes (Aleman et al., 2009).
The crystal structure of title compound, a phenyl substituted highly constrained cyclohexane analogue of Ph, is reported in this paper. Due to the steric hindrance, the oxocyclohexane is in a twist-boat conformation (Fig. 1). The ethoxyl carbonyl and the two aryl groups are located in equatorial positions. The dihedral angle between two aromatic rings is 77.495 (20)°. The C7 axial hydrogen and the CH2 bonded to C10 are on the flagpole positions of the boat conformation, which give the least torsional strain. The equatorial ethoxyl carbonyl on C18 and the equatorial aryl group on C11 also lead the formation of a comparable stable boat conformation of this compound.
Figures
Fig. 1.

View of the molecular structure of the title compound with labeling and displacement ellipsoids drawn at the 30% probability level.
Crystal data
| C22H19Cl2NO3 | F(000) = 1728 |
| Mr = 416.28 | Dx = 1.357 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71069 Å |
| Hall symbol: -C 2yc | Cell parameters from 106 reflections |
| a = 21.6980 (17) Å | θ = 1.3–26.0° |
| b = 11.0770 (19) Å | µ = 0.34 mm−1 |
| c = 17.515 (3) Å | T = 293 K |
| β = 104.535 (2)° | BLOCK, colorless |
| V = 4075.0 (10) Å3 | 0.21 × 0.19 × 0.15 mm |
| Z = 8 |
Data collection
| Bruker SMART APEXII CCD area-detector diffractometer | 3602 independent reflections |
| Radiation source: fine-focus sealed tube | 2584 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.027 |
| ω scans | θmax = 25.0°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −25→25 |
| Tmin = 0.932, Tmax = 0.951 | k = −13→13 |
| 9983 measured reflections | l = −20→9 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.041 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.117 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0514P)2 + 2.7869P] where P = (Fo2 + 2Fc2)/3 |
| 3602 reflections | (Δ/σ)max < 0.001 |
| 253 parameters | Δρmax = 0.37 e Å−3 |
| 0 restraints | Δρmin = −0.35 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl2 | −0.01505 (3) | 0.07074 (6) | 0.62309 (5) | 0.0780 (2) | |
| Cl1 | 0.59500 (4) | 0.06816 (9) | 0.99930 (6) | 0.1128 (4) | |
| O2 | 0.30900 (7) | 0.21848 (13) | 0.84990 (9) | 0.0528 (4) | |
| C12 | 0.18929 (9) | 0.19357 (17) | 0.66846 (12) | 0.0443 (5) | |
| C18 | 0.30971 (9) | 0.15687 (17) | 0.72324 (12) | 0.0435 (5) | |
| N1 | 0.30407 (9) | 0.04712 (16) | 0.67773 (12) | 0.0524 (5) | |
| C11 | 0.25594 (9) | 0.24575 (18) | 0.68262 (12) | 0.0424 (5) | |
| H11 | 0.2578 | 0.3129 | 0.7196 | 0.051* | |
| C4 | 0.42949 (9) | 0.17517 (19) | 0.79764 (14) | 0.0492 (5) | |
| O1 | 0.28944 (9) | 0.02289 (15) | 0.82275 (11) | 0.0759 (5) | |
| C7 | 0.37764 (9) | 0.21213 (18) | 0.72628 (13) | 0.0477 (5) | |
| H7 | 0.3901 | 0.1767 | 0.6811 | 0.057* | |
| C19 | 0.30148 (9) | 0.12206 (19) | 0.80460 (13) | 0.0475 (5) | |
| C9 | 0.33050 (11) | 0.3749 (2) | 0.63092 (15) | 0.0552 (6) | |
| C17 | 0.15361 (10) | 0.2176 (2) | 0.72194 (14) | 0.0561 (6) | |
| H17 | 0.1719 | 0.2608 | 0.7676 | 0.067* | |
| O3 | 0.34259 (9) | 0.45029 (17) | 0.58723 (12) | 0.0832 (6) | |
| C14 | 0.09922 (11) | 0.0868 (2) | 0.58857 (14) | 0.0567 (6) | |
| H14 | 0.0811 | 0.0413 | 0.5440 | 0.068* | |
| C10 | 0.27117 (10) | 0.2997 (2) | 0.60953 (13) | 0.0517 (5) | |
| H10A | 0.2767 | 0.2352 | 0.5744 | 0.062* | |
| H10B | 0.2358 | 0.3494 | 0.5818 | 0.062* | |
| C15 | 0.06450 (10) | 0.1152 (2) | 0.64196 (15) | 0.0551 (6) | |
| C8 | 0.37399 (10) | 0.34828 (19) | 0.71039 (14) | 0.0542 (6) | |
| H8A | 0.3584 | 0.3888 | 0.7509 | 0.065* | |
| H8B | 0.4162 | 0.3791 | 0.7125 | 0.065* | |
| C13 | 0.16142 (10) | 0.12688 (19) | 0.60210 (13) | 0.0526 (6) | |
| H13 | 0.1849 | 0.1086 | 0.5659 | 0.063* | |
| C20 | 0.30188 (13) | 0.2027 (3) | 0.93036 (14) | 0.0705 (7) | |
| H20A | 0.2595 | 0.2272 | 0.9327 | 0.085* | |
| H20B | 0.3074 | 0.1183 | 0.9452 | 0.085* | |
| C16 | 0.09146 (11) | 0.1789 (2) | 0.70886 (16) | 0.0646 (7) | |
| H16 | 0.0680 | 0.1961 | 0.7453 | 0.078* | |
| C3 | 0.46190 (11) | 0.2575 (2) | 0.85150 (16) | 0.0690 (7) | |
| H3 | 0.4496 | 0.3381 | 0.8459 | 0.083* | |
| C5 | 0.44867 (12) | 0.0562 (2) | 0.80921 (17) | 0.0694 (7) | |
| H5 | 0.4275 | −0.0025 | 0.7744 | 0.083* | |
| C1 | 0.53022 (11) | 0.1084 (3) | 0.92278 (17) | 0.0717 (7) | |
| C6 | 0.49881 (14) | 0.0224 (3) | 0.87177 (19) | 0.0829 (9) | |
| H6 | 0.5109 | −0.0582 | 0.8789 | 0.099* | |
| C22 | 0.30053 (14) | −0.0380 (2) | 0.63980 (18) | 0.0765 (8) | |
| C2 | 0.51188 (12) | 0.2252 (3) | 0.91352 (18) | 0.0793 (8) | |
| H2 | 0.5329 | 0.2834 | 0.9489 | 0.095* | |
| C21 | 0.34862 (18) | 0.2745 (3) | 0.98441 (18) | 0.1104 (12) | |
| H21A | 0.3437 | 0.2640 | 1.0370 | 0.166* | |
| H21B | 0.3428 | 0.3581 | 0.9698 | 0.166* | |
| H21C | 0.3905 | 0.2493 | 0.9825 | 0.166* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl2 | 0.0464 (3) | 0.0811 (5) | 0.1010 (6) | −0.0096 (3) | 0.0083 (3) | −0.0033 (4) |
| Cl1 | 0.0699 (5) | 0.1466 (8) | 0.1075 (7) | 0.0144 (5) | −0.0045 (4) | 0.0360 (6) |
| O2 | 0.0637 (9) | 0.0523 (9) | 0.0461 (9) | −0.0020 (7) | 0.0207 (7) | 0.0016 (7) |
| C12 | 0.0474 (11) | 0.0384 (10) | 0.0454 (12) | 0.0012 (9) | 0.0087 (10) | 0.0009 (9) |
| C18 | 0.0492 (11) | 0.0345 (10) | 0.0487 (13) | −0.0035 (8) | 0.0159 (10) | −0.0030 (9) |
| N1 | 0.0580 (11) | 0.0386 (10) | 0.0609 (12) | −0.0003 (8) | 0.0153 (9) | −0.0051 (9) |
| C11 | 0.0472 (11) | 0.0377 (10) | 0.0428 (12) | −0.0030 (8) | 0.0122 (9) | −0.0017 (9) |
| C4 | 0.0417 (11) | 0.0520 (12) | 0.0586 (14) | −0.0016 (9) | 0.0216 (10) | 0.0030 (11) |
| O1 | 0.1060 (14) | 0.0511 (10) | 0.0770 (13) | −0.0162 (9) | 0.0350 (11) | 0.0125 (9) |
| C7 | 0.0480 (12) | 0.0463 (12) | 0.0534 (13) | −0.0034 (9) | 0.0210 (10) | −0.0009 (10) |
| C19 | 0.0430 (11) | 0.0458 (12) | 0.0543 (14) | −0.0024 (9) | 0.0136 (10) | 0.0049 (11) |
| C9 | 0.0647 (14) | 0.0492 (12) | 0.0582 (15) | −0.0031 (11) | 0.0275 (12) | 0.0072 (12) |
| C17 | 0.0511 (13) | 0.0594 (14) | 0.0583 (15) | −0.0059 (10) | 0.0147 (11) | −0.0144 (12) |
| O3 | 0.0888 (13) | 0.0857 (13) | 0.0778 (13) | −0.0170 (10) | 0.0257 (11) | 0.0318 (11) |
| C14 | 0.0580 (14) | 0.0522 (13) | 0.0528 (14) | −0.0055 (10) | 0.0009 (11) | −0.0060 (11) |
| C10 | 0.0611 (13) | 0.0484 (12) | 0.0456 (13) | 0.0006 (10) | 0.0133 (11) | 0.0013 (10) |
| C15 | 0.0456 (12) | 0.0486 (12) | 0.0674 (16) | −0.0023 (10) | 0.0076 (11) | 0.0037 (12) |
| C8 | 0.0538 (12) | 0.0498 (13) | 0.0609 (15) | −0.0114 (10) | 0.0179 (11) | 0.0043 (11) |
| C13 | 0.0548 (13) | 0.0538 (13) | 0.0491 (14) | −0.0020 (10) | 0.0130 (11) | −0.0039 (11) |
| C20 | 0.0799 (17) | 0.0865 (19) | 0.0499 (15) | 0.0054 (14) | 0.0254 (14) | 0.0099 (14) |
| C16 | 0.0535 (13) | 0.0734 (16) | 0.0714 (17) | −0.0050 (12) | 0.0240 (13) | −0.0131 (14) |
| C3 | 0.0583 (14) | 0.0608 (15) | 0.0810 (19) | 0.0014 (12) | 0.0046 (14) | −0.0045 (14) |
| C5 | 0.0659 (15) | 0.0577 (15) | 0.0817 (19) | 0.0030 (12) | 0.0130 (14) | 0.0013 (14) |
| C1 | 0.0472 (13) | 0.095 (2) | 0.0732 (18) | 0.0015 (13) | 0.0148 (13) | 0.0141 (16) |
| C6 | 0.0760 (18) | 0.0686 (17) | 0.102 (2) | 0.0186 (15) | 0.0177 (17) | 0.0211 (17) |
| C22 | 0.0902 (19) | 0.0528 (15) | 0.083 (2) | 0.0022 (14) | 0.0155 (16) | −0.0109 (15) |
| C2 | 0.0608 (16) | 0.087 (2) | 0.080 (2) | −0.0032 (14) | −0.0005 (15) | −0.0080 (16) |
| C21 | 0.143 (3) | 0.130 (3) | 0.0617 (19) | −0.051 (2) | 0.033 (2) | −0.021 (2) |
Geometric parameters (Å, º)
| Cl2—C15 | 1.745 (2) | C14—C15 | 1.377 (3) |
| Cl1—C1 | 1.739 (3) | C14—C13 | 1.383 (3) |
| O2—C19 | 1.316 (3) | C14—H14 | 0.9300 |
| O2—C20 | 1.466 (3) | C10—H10A | 0.9700 |
| C12—C13 | 1.382 (3) | C10—H10B | 0.9700 |
| C12—C17 | 1.383 (3) | C15—C16 | 1.367 (3) |
| C12—C11 | 1.519 (3) | C8—H8A | 0.9700 |
| C18—N1 | 1.442 (3) | C8—H8B | 0.9700 |
| C18—C19 | 1.529 (3) | C13—H13 | 0.9300 |
| C18—C11 | 1.556 (3) | C20—C21 | 1.441 (4) |
| C18—C7 | 1.584 (3) | C20—H20A | 0.9700 |
| N1—C22 | 1.144 (3) | C20—H20B | 0.9700 |
| C11—C10 | 1.523 (3) | C16—H16 | 0.9300 |
| C11—H11 | 0.9800 | C3—C2 | 1.376 (4) |
| C4—C3 | 1.373 (3) | C3—H3 | 0.9300 |
| C4—C5 | 1.382 (3) | C5—C6 | 1.388 (4) |
| C4—C7 | 1.513 (3) | C5—H5 | 0.9300 |
| O1—C19 | 1.191 (3) | C1—C2 | 1.352 (4) |
| C7—C8 | 1.532 (3) | C1—C6 | 1.365 (4) |
| C7—H7 | 0.9800 | C6—H6 | 0.9300 |
| C9—O3 | 1.205 (3) | C2—H2 | 0.9300 |
| C9—C10 | 1.500 (3) | C21—H21A | 0.9600 |
| C9—C8 | 1.502 (3) | C21—H21B | 0.9600 |
| C17—C16 | 1.378 (3) | C21—H21C | 0.9600 |
| C17—H17 | 0.9300 | ||
| C19—O2—C20 | 117.11 (18) | H10A—C10—H10B | 108.0 |
| C13—C12—C17 | 118.1 (2) | C16—C15—C14 | 120.7 (2) |
| C13—C12—C11 | 122.58 (19) | C16—C15—Cl2 | 119.88 (19) |
| C17—C12—C11 | 119.21 (19) | C14—C15—Cl2 | 119.39 (18) |
| N1—C18—C19 | 106.80 (16) | C9—C8—C7 | 110.72 (19) |
| N1—C18—C11 | 109.33 (17) | C9—C8—H8A | 109.5 |
| C19—C18—C11 | 109.64 (16) | C7—C8—H8A | 109.5 |
| N1—C18—C7 | 107.07 (16) | C9—C8—H8B | 109.5 |
| C19—C18—C7 | 113.03 (17) | C7—C8—H8B | 109.5 |
| C11—C18—C7 | 110.82 (15) | H8A—C8—H8B | 108.1 |
| C22—N1—C18 | 177.6 (2) | C12—C13—C14 | 121.2 (2) |
| C12—C11—C10 | 114.26 (17) | C12—C13—H13 | 119.4 |
| C12—C11—C18 | 114.07 (16) | C14—C13—H13 | 119.4 |
| C10—C11—C18 | 109.69 (16) | C21—C20—O2 | 109.8 (2) |
| C12—C11—H11 | 106.0 | C21—C20—H20A | 109.7 |
| C10—C11—H11 | 106.0 | O2—C20—H20A | 109.7 |
| C18—C11—H11 | 106.0 | C21—C20—H20B | 109.7 |
| C3—C4—C5 | 116.7 (2) | O2—C20—H20B | 109.7 |
| C3—C4—C7 | 122.3 (2) | H20A—C20—H20B | 108.2 |
| C5—C4—C7 | 120.9 (2) | C15—C16—C17 | 119.6 (2) |
| C4—C7—C8 | 114.22 (18) | C15—C16—H16 | 120.2 |
| C4—C7—C18 | 114.57 (17) | C17—C16—H16 | 120.2 |
| C8—C7—C18 | 111.65 (16) | C4—C3—C2 | 122.4 (3) |
| C4—C7—H7 | 105.1 | C4—C3—H3 | 118.8 |
| C8—C7—H7 | 105.1 | C2—C3—H3 | 118.8 |
| C18—C7—H7 | 105.1 | C4—C5—C6 | 121.4 (3) |
| O1—C19—O2 | 126.2 (2) | C4—C5—H5 | 119.3 |
| O1—C19—C18 | 124.5 (2) | C6—C5—H5 | 119.3 |
| O2—C19—C18 | 109.36 (17) | C2—C1—C6 | 120.4 (3) |
| O3—C9—C10 | 122.4 (2) | C2—C1—Cl1 | 119.6 (2) |
| O3—C9—C8 | 122.6 (2) | C6—C1—Cl1 | 120.0 (2) |
| C10—C9—C8 | 114.99 (18) | C1—C6—C5 | 119.5 (3) |
| C16—C17—C12 | 121.2 (2) | C1—C6—H6 | 120.2 |
| C16—C17—H17 | 119.4 | C5—C6—H6 | 120.2 |
| C12—C17—H17 | 119.4 | C1—C2—C3 | 119.6 (3) |
| C15—C14—C13 | 119.1 (2) | C1—C2—H2 | 120.2 |
| C15—C14—H14 | 120.4 | C3—C2—H2 | 120.2 |
| C13—C14—H14 | 120.4 | C20—C21—H21A | 109.5 |
| C9—C10—C11 | 111.22 (18) | C20—C21—H21B | 109.5 |
| C9—C10—H10A | 109.4 | H21A—C21—H21B | 109.5 |
| C11—C10—H10A | 109.4 | C20—C21—H21C | 109.5 |
| C9—C10—H10B | 109.4 | H21A—C21—H21C | 109.5 |
| C11—C10—H10B | 109.4 | H21B—C21—H21C | 109.5 |
| C19—C18—N1—C22 | −164 (6) | C7—C18—C19—O2 | 59.8 (2) |
| C11—C18—N1—C22 | 78 (6) | C13—C12—C17—C16 | −1.5 (3) |
| C7—C18—N1—C22 | −43 (6) | C11—C12—C17—C16 | 175.7 (2) |
| C13—C12—C11—C10 | 41.3 (3) | O3—C9—C10—C11 | −160.1 (2) |
| C17—C12—C11—C10 | −135.7 (2) | C8—C9—C10—C11 | 20.6 (3) |
| C13—C12—C11—C18 | −86.0 (2) | C12—C11—C10—C9 | 166.34 (18) |
| C17—C12—C11—C18 | 96.9 (2) | C18—C11—C10—C9 | −64.1 (2) |
| N1—C18—C11—C12 | 55.2 (2) | C13—C14—C15—C16 | −2.0 (4) |
| C19—C18—C11—C12 | −61.5 (2) | C13—C14—C15—Cl2 | 177.18 (17) |
| C7—C18—C11—C12 | 173.01 (17) | O3—C9—C8—C7 | −139.3 (2) |
| N1—C18—C11—C10 | −74.4 (2) | C10—C9—C8—C7 | 40.1 (3) |
| C19—C18—C11—C10 | 168.84 (17) | C4—C7—C8—C9 | 169.06 (18) |
| C7—C18—C11—C10 | 43.4 (2) | C18—C7—C8—C9 | −58.9 (2) |
| C3—C4—C7—C8 | 11.8 (3) | C17—C12—C13—C14 | 1.1 (3) |
| C5—C4—C7—C8 | −164.7 (2) | C11—C12—C13—C14 | −176.0 (2) |
| C3—C4—C7—C18 | −118.7 (2) | C15—C14—C13—C12 | 0.7 (3) |
| C5—C4—C7—C18 | 64.7 (3) | C19—O2—C20—C21 | 140.9 (3) |
| N1—C18—C7—C4 | −93.2 (2) | C14—C15—C16—C17 | 1.6 (4) |
| C19—C18—C7—C4 | 24.1 (2) | Cl2—C15—C16—C17 | −177.61 (19) |
| C11—C18—C7—C4 | 147.63 (18) | C12—C17—C16—C15 | 0.2 (4) |
| N1—C18—C7—C8 | 134.96 (19) | C5—C4—C3—C2 | 1.1 (4) |
| C19—C18—C7—C8 | −107.7 (2) | C7—C4—C3—C2 | −175.6 (2) |
| C11—C18—C7—C8 | 15.8 (2) | C3—C4—C5—C6 | −0.8 (4) |
| C20—O2—C19—O1 | −0.1 (3) | C7—C4—C5—C6 | 176.0 (2) |
| C20—O2—C19—C18 | 179.17 (17) | C2—C1—C6—C5 | 1.3 (4) |
| N1—C18—C19—O1 | −3.5 (3) | Cl1—C1—C6—C5 | −177.6 (2) |
| C11—C18—C19—O1 | 114.8 (2) | C4—C5—C6—C1 | −0.4 (4) |
| C7—C18—C19—O1 | −121.0 (2) | C6—C1—C2—C3 | −1.0 (4) |
| N1—C18—C19—O2 | 177.26 (16) | Cl1—C1—C2—C3 | 178.0 (2) |
| C11—C18—C19—O2 | −64.4 (2) | C4—C3—C2—C1 | −0.2 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11···O1i | 0.98 | 2.57 | 3.218 (3) | 123 |
Symmetry code: (i) −x+1/2, y+1/2, −z+3/2.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: LR2127).
References
- Aleman, C., Jiménez, A. I., Cativiela, C., Nussinov, R. & Casanovas, J. (2009). J. Org. Chem. 74, 7834–7843. [DOI] [PMC free article] [PubMed]
- Bi, X., Dong, D., Liu, Q., Pan, W., Zhao, L. & Li, B. (2005). J. Am. Chem. Soc. 127, 4578–4579. [DOI] [PubMed]
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Fu, Z., Wang, M., Dong, Y., Liu, J. & Liu, Q. (2009). J. Org. Chem. 74, 6105–6110. [DOI] [PubMed]
- Li, Y., Xu, X., Tan, J., Xia, C., Zhang, D. & Liu, Q. (2011). J. Am. Chem. Soc. 133, 1775–1777. [DOI] [PubMed]
- Rowland, A. T. & Gill, B. C. (1988). J. Org. Chem. 53, 434–437.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tan, J., Xu, X., Zhang, L., Li, Y. & Liu, Q. (2009). Angew. Chem. Int. Ed. 48, 2868–2872. [DOI] [PubMed]
- Wu, B., Liu, G., Li, M., Zhang, Y., Zhang, S., Qiu, J., Xu, X., Ji, S. & Wang, X. (2011). Chem. Commun. 47, 3992–3994. [DOI] [PubMed]
- Xu, X., Liu, Y. & Park, C. (2012). Angew. Chem. Int. Ed. 51, 9372–9376. [DOI] [PubMed]
- Zhang, D., Xu, X. & &Liu, Q. (2010). Synlett, 6, 917–920.
- Zhao, L., Liang, F., Bi, X., Sun, S. & Liu, Q. (2006). J. Org. Chem. 71, 1094–1098. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S160053681401383X/lr2127sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681401383X/lr2127Isup2.hkl
Supporting information file. DOI: 10.1107/S160053681401383X/lr2127Isup3.cml
CCDC reference: 1008201
Additional supporting information: crystallographic information; 3D view; checkCIF report


