Abstract
Working memory scanning and motor response speeds were assessed in chronically sleep restricted participants using the Sternberg item recognition paradigm (SIRP). Twenty-two healthy volunteers (ages 21–30) living in a controlled hospital environment were allowed either 4 h of sleep opportunity (50% of habitual sleep) or 8 h of sleep opportunity (100% of habitual sleep) for 12 days. Working memory scanning efficiency (time taken to access an item in working memory) was tested for the first 9 days of sleep restriction and improved over time in participants permitted an 8 h sleep period, but did not change significantly in participants permitted a 4 h sleep period. Speed of motor response (reaction time independent of cognitive processing) did not change significantly in either group. These results indicate that the efficiency of working memory scanning can improve with repeated practice given sufficient sleep, and that prolonged sleep restriction to 50% of habitual sleep prevents this improvement.
Keywords: Sleep restriction, Working memory, Short-term memory
1. Introduction
The contributions of sleep to human performance have been demonstrated through both performance decrements that occur when sleep is reduced (for reviews, see Bonnet, 2000; Broughton and Ogilvie, 1992; Dinges and Kribbs, 1991; Kleitman, 1963) and performance improvements that occur given an intervening period of sleep (for reviews, see Robertson et al., 2004; Walker, 2005). The effects of sleep reduction are most often measured during periods of total sleep deprivation (TSD), where sleep is prevented for up to 88 h. The effects of sleep reduction can also be assessed by restricting the number of hours of sleep per night to some percentage of habitual sleep. Though less frequently employed, sleep restriction is perhaps more widely applicable as moderate reductions in the number of hours slept per night are quite common. The current study assesses the effects of chronic partial sleep restriction on working memory scanning speed, where working memory is defined as a short-term store for holding and manipulating information (Baddeley, 1990).
Studies of TSD demonstrate performance deficits in measures of memory, speed of cognitive processing, attention, and task switching. Measures of working memory during TSD for 24–88 h have most frequently demonstrated decreases in performance accuracy (Elkin and Murray, 1974; Habeck et al., 2004; Van Dongen et al., 2003; Williams et al., 1959, 1965). A recent report by Habeck et al. also demonstrated deficits in working memory scanning speed following 48 h of TSD.
Carefully controlled experimental studies of prolonged partial sleep restriction (Belenky et al., 2003; Van Dongen et al., 2003) have shown a decreased capacity to sustain attention as reflected by an increased number of lapses (response times > 500 ms) on a reaction time test. Van Dongen et al. further demonstrated that restricting sleep to as little as 75% of habitual sleep for 14 days decreased the number of correct responses on a working memory task and a mental arithmetic task. These deficits were equivalent to those produced by two nights of TSD.
The Van Dongen et al. (2003) study mentioned above illustrates that chronic partial sleep restriction has an effect on working memory accuracy, but it does not provide evidence for deficits in working memory speed. To our knowledge, no study has demonstrated the effects of chronic partial sleep restriction on the speed of working memory scanning. This may be because it is difficult to dissociate speed and accuracy components of working memory performance.
The Sternberg item recognition paradigm (SIRP) is a standard measure of working memory scanning speed with low rates of error, making it a useful measure of memory scanning speed. Furthermore, the SIRP allows for an at least partial dissociation of memory scanning and motor components of response time (for a review, see Sternberg, 1975). Research participants are presented with one or more digits that they must keep in memory. Participants are subsequently presented with a series of individual digits and must indicate which digits were part of the positive set by making yes/no responses. Sternberg (1969) demonstrated that the time it takes participants to recognize a digit in the positive set will increase linearly with the number of digits they are asked to remember. The slope of this linear function provides a measure of working memory scanning speed, while the y-intercept of the function provides a measure of motor speed.
In the current study, SIRP performance was assessed in participants who normally slept approximately 8 h per night, and were randomized into groups where they were permitted a sleep period duration of either 4 or 8 h per night for 12 nights. The effects of chronic sleep restriction on the memory scanning and the motor components of this task were assessed independently. It was hypothesized that the time taken to recognize an item in working memory would increase as a result of chronic partial sleep restriction, but would remain stable in non-sleep restricted participants.
2. Method
2.1. Participants
Participants were 10 females and 12 males between the ages of 21 and 30 (M = 24.27, S.D. = 2.75) who gave written informed consent to participate in a 16-day inpatient study of chronic sleep restriction at the Beth Israel Deaconess Medical Center. All participants were physically and psychologically healthy with no history of sleep disorders or substance abuse. Participants regularly slept between 7 and 9 h per day as verified by sleep logs collected for at least 2 weeks during the screening phase. Participants were instructed to sleep between 11:00 p.m. and 7:00 a.m. the week before they entered the study.
2.2. Procedure
Study participants were admitted to the Harvard–Thorndike General Clinical Research Center. All participants were given a sleep period duration (time designated for sleeping, with lights out) of 8 h, scheduled between 11:00 p.m. and 7:00 a.m., for the first 2 days of the study. On day 3, participants were randomly assigned to either the sleep restricted (n = 11) or the non-sleep restricted (n = 11) condition. The sleep period for participants in the sleep restricted condition was between 11:00 p.m. and 3:00 a.m. (4 h) on nights 3 through 12, while participants in the non-sleep restricted condition continued to receive sleep periods between 11:00 p.m. and 7:00 a.m. (8 h). Participants in the sleep restricted condition were kept in a semi-recumbent position, with light levels below 40 lx, from 3:00 a.m. to 7:00 a.m. Participants were not allowed out of bed, with the exception of brief toilet visits, until 7:00 a.m. each morning. Participants were accompanied by experimenters throughout wake periods for both conditions. Experimenters helped participants maintain wakefulness by playing board games, talking, and watching video taped movies. The SIRP was administered at 10:00 a.m. each day.
2.3. Sternberg item recognition paradigm
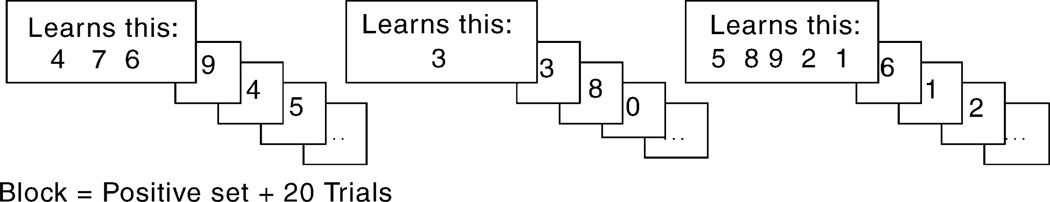
Participants completed a self-paced practice task followed by a test task each day, requiring a total of 20 min. During both tasks, participants were visually presented with positive sets of one, three, or five digits. Each positive set was presented for 5 s, and digits within each positive set were pseudo-randomized so that no digit appeared more than once in the same positive set. Immediately after the presentation of a positive set, 20 single digit trials were presented for up to 2 s and the participant made yes/no responses to indicate whether each digit was included in the positive set (probe) or was not (foil). The presentation of the positive set along with the 20 following single-digit trials composed a single block (see Fig. 1). The practice task included three blocks (one of each set size) and the test program included six blocks (two of each set size). The task was administered using Superlab Experimental Laboratory Software (version 2.0, Cedrus Corporation, San Pedro, CA, USA) on a desktop PC. Digits were 2.5 cm in height and participants were seated approximately 50 cm from the computer monitor. Responses were made with the dominant hand on a four-button response box (RB-420, Cedrus Corporation).
Fig. 1.
Sternberg item recognition paradigm. Participants are asked to learn one, three, or five digits (memory set) and then indicate which of the 20 trial digits were in the memory set by making yes/no responses.
Twelve participants, seven sleep restricted and five non-sleep restricted, were reinforced with a tone when an incorrect response was made and were informed of their average response times and error rates before each testing session. This reinforcement was introduced because preliminary analyses for the first 10 study participants indicated that non-sleep restricted participants exhibited an improvement in the slope across sessions, contrary to our expectations, and all participants had larger motor response times than usually observed with the SIRP. Reinforcement was introduced to encourage the remaining study participants to respond as quickly and accurately as possible, reducing lack of reinforcement as a potential confound to the Sternberg effect (Sternberg, personal communication, October 10, 2002).
2.4. Statistical analyses
Data from the test task at baseline (day 3) and across the 9 days following implementation of the sleep restriction protocol (days 4–12) were analyzed using SAS (version 9.1.2, SAS Institute, Cary, NC, USA). Testing on day 3 was completed before sleep restriction, and was therefore used as a baseline measure of performance. To allow time for participants to adjust to the study routine, testing on days 1 and 2 were not included in the analyses. Data from the practice tasks that preceded each testing session were not included in the analyses. Incorrect responses (2% of trials) and responses under 150 ms (<0.1% of trials) were also excluded from analyses.
Median response times were calculated for each block, based on 20 trials. Thus, as there were two blocks at each of three set sizes, a total of six median response times were calculated for each subject each day. Individual linear regressions, using set size as a predictor variable and median response time as a response variable, were performed for each subject on each day. The slope and y-intercept from each regression provided measures of memory scanning and motor response times, respectively.
The effects of prolonged sleep restriction on memory scanning and motor response times were assessed using random coefficients models. The model for memory scanning response time included group as a fixed factor and participant as a random factor. An identical model was used to assess motor response time.
t-Tests were performed to determine whether there was a difference in memory scanning or motor response time between groups at baseline testing. MANOVA was performed to determine whether the introduction of reinforcement had a differential effect on memory scanning or motor response times between groups.
3. Results
3.1. Sleep parameters
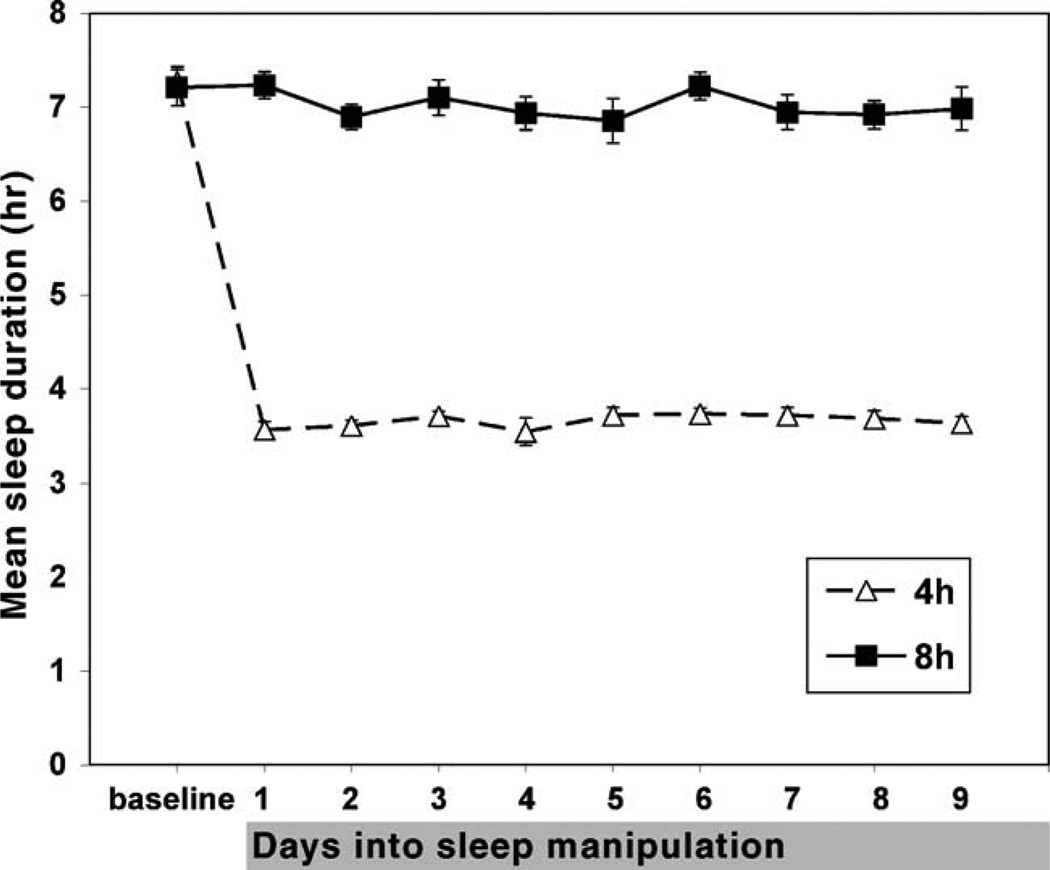
Based on sleep log data collected during the screening phase, subjects slept an average of 7 h and 52 min (S.D. = 32 min) per night. Average sleep duration determined by sleep log data was significantly longer than that found by baseline measurements using actigraphy, F(1,17) = 21.63, p < .001 (available in full for all but three subjects who had data missing due to technical failure). Average sleep duration at baseline was 7 h and 16 min (S.D. = 29 min) and 7 h and 12 min (S.D. = 32 min) respectively for the sleep restricted and non-sleep restricted groups, and there was no significant difference between these sleep amounts, F(1,17) = 0.26, p > .05. Through the nights of sleep manipulation which followed, the average sleep duration for participants in the sleep restricted and non-sleep restricted groups was 3 h and 39 min (S.D. = 10 min) and 7 h (S.D. = 24 min), respectively (actigraphy data are shown in Fig. 2).
Fig. 2.
Actigraphic estimates of mean sleep duration (±standard error) for sleep restricted and non-sleep restricted participants at baseline and on each day of sleep manipulation.
3.2. Memory scanning response time
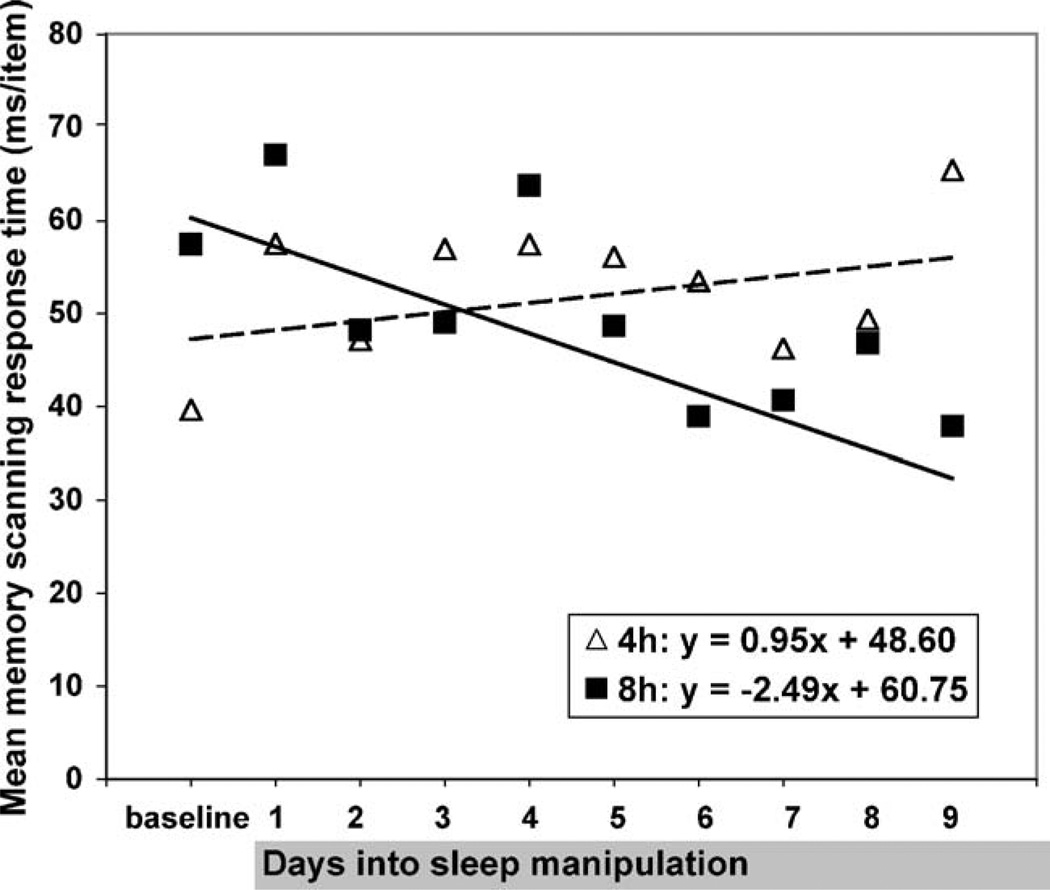
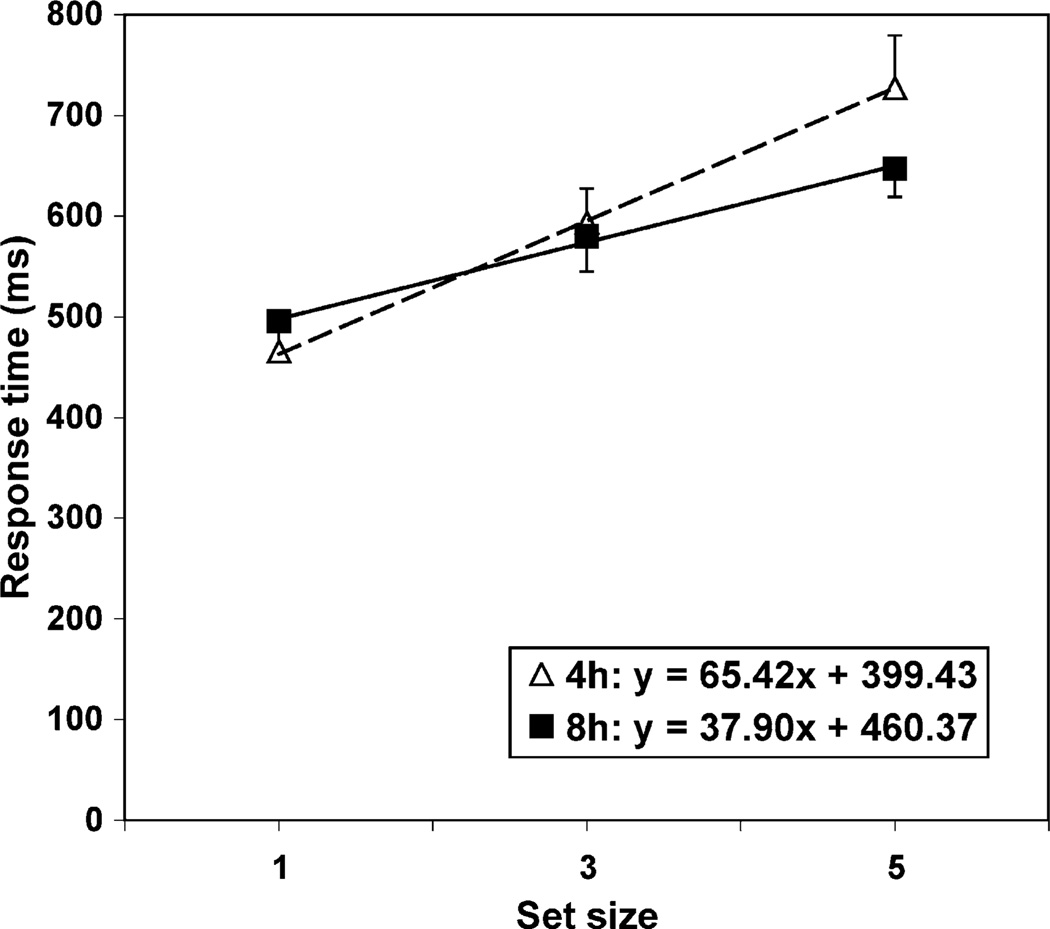
A simple contrast indicated no significant effect of sleep condition on baseline memory scanning speed, t(20) = −1.42, p > .05, confirming that randomization was successful. Random coefficient modeling indicated a significant decrease in memory scanning response time across days in the non-sleep restricted group, t(20) = −3.56, p < .01 (see Table 1, Fig. 3). The memory scanning speed of the sleep restricted group did not change significantly across days, t(20) = 1.40, p > .05. Furthermore, the change in non-sleep restricted participants’ memory scanning speed was significantly different from that of the sleep restricted group, t(20) = 3.52, p < .01. Average response times at each set size for each group on the last day of testing are shown in Fig. 4.
Table 1.
Mean speed of working memory scanning (±standard error) for sleep restricted and non-sleep restricted participants at baseline and on each day of sleep manipulation
| 4 h | 8 h | |
|---|---|---|
| Baseline | 39.67 (±6.99) | 57.42 (±10.33) |
| 1 | 57.49 (±13.32) | 67.04 (±13.32) |
| 2 | 47.19 (±9.05) | 48.19 (±12.46) |
| 3 | 56.90 (±12.19) | 48.98 (±9.97) |
| 4 | 57.43 (±9.13) | 63.72 (±13.28) |
| 5 | 56.07 (±10.66) | 48.64 (±10.15) |
| 6 | 53.48 (±6.71) | 38.88 (±5.58) |
| 7 | 46.23 (±6.36) | 40.66 (±6.32) |
| 8 | 49.33 (±9.63) | 46.81 (±8.50) |
| 9 | 65.42 (±13.22) | 37.90 (±6.61) |
Fig. 3.
Mean speed of working memory scanning for sleep restricted and non-sleep restricted participants at baseline and on each day of sleep manipulation.
Fig. 4.
Average of median reaction times (±standard error) at each set size for sleep restricted and non-sleep restricted participants following the last night of sleep manipulation.
An ANOVA indicated that the introduction of reinforcement resulted in a decrease in working memory scanning speed across sleep conditions, F(3,212) = 27.95, p < .0001, where participants who were told their average response times and error rates each day had faster working memory scanning (M = 41.83 ms/item, S.D. = 21.75) than those who were not reinforced (M = 63.24 ms/item, S.D. = 39.20). However, providing reinforcement did not differentially affect memory scanning speed between sleep conditions, F(3,212) = 0.21, p > .05.
3.3. Motor response time
A simple contrast indicated no effect of sleep condition on baseline motor response time, t(20) = 0.23, p > .05. Random coefficient modeling indicated no significant differences in motor response time across days in either the non-sleep restricted group, t(20) = 0.28, p > .05, or the sleep restricted group, t(20) = −1.58, p > .05.
4. Discussion
Working memory scanning speed improved over a 9 day period in participants who were given an 8 h sleep period per night, and this differed from participants who were permitted only 4 h of sleep per night and failed to show any change. The working memory scanning speed of non-sleep restricted participants on the last day of testing was 58% faster than that of participants limited to 4 h of sleep per night, or approximately 50% of their normal sleep amount. This effect of sleep condition was unexpected; sleep restriction prevented improvements in the speed of working memory scanning, but did not produce performance deficits relative to baseline.
Though improvements in working memory scanning in non-sleep restricted participants were not expected at the outset of this study, additional evidence for this phenomenon was presented in a recent paper by Verhaegen et al. (2004). Verhaegen et al. measured the effects of extended practice on the n-back working memory task, and found significant improvements in participants’ working memory capacities after the completion of ten 1 h sessions. Furthermore, n-back results indicated a linear increase in response time as the number of items in working memory increased, and a decrease in the slope of this function following practice. Verhaegen et al. indicate that practice facilitates parallel scanning of items held in working memory when the number of items to be remembered is less than five.
The results of Verhaegen et al. (2004) indicate that there is a significant learning component to working memory scanning skill, and the current study demonstrates that chronically restricting sleep to a 4 h period per night may prevent either the acquisition of working memory scanning skill or the expression of skill improvements. Evidence exists for both sleep dependent skill acquisition and skill expression (for reviews, see Robertson et al., 2004; Stickgold et al., 2001), but this is most often observed in tests of procedural skill rather than declarative working memory tasks. The improvement of non-sleep restricted participants on the SIRP, a test of declarative working memory scanning, provides evidence that sleep may also aid declarative task acquisition.
It would be of interest to determine whether improvements in declarative memory function reflect proceduralization of working memory scanning. The visual presentation of the positive set may allow participants to either (1) decrease the amount of time taken to recognize each item in working memory where working memory scanning is conducted serially or (2) decrease the amount of time taken to recognize each item in working memory by learning to scan items more efficiently in parallel. Evidence for serial or parallel processes of working memory scanning remains inconclusive (for review, see Sternberg, 1975).
It is worth noting that the effects of practice on working memory scanning have only been observed when the information to be remembered is presented as a visual array. Previous studies have not demonstrated an effect of practice on SIRP performance when the items to be remembered were presented auditorally (Sternberg, 1967; Kristofferson, 1972). The presentation of the positive set as a visual array in the current study, and the serial auditory presentation of the positive set in studies by Sternberg and Kristofferson, may facilitate two different mechanisms of working memory scanning. The neural and cognitive bases for the practice-related improvements in working memory scanning seen here remain unclear.
In summary, this is the first study we know of to examine the effects of chronic partial sleep restriction on working memory scanning speed. Results indicate both that working memory scanning speed can improve in non-sleep restricted participants, and that chronically restricting sleep to approximately 50% of habitual sleep (4 h per night) prevents improvements in working memory scanning. Additionally, performance improvements in working memory scanning speed were maintained even after the introduction of reinforcement for rapid and accurate responses, a step taken specifically to eliminate effort as a cause of the effect. Further research is needed to determine the mechanisms responsible for improvements in working memory scanning, and how different degrees of sleep restriction and time-of-day influences might affect the development of working memory scanning skill.
Acknowledgements
The study was supported by NIH grants MH 60641 (JMM) and RR 01032 (Beth Israel Deaconess Medical Center General Clinical Research Center) and additional support from NIH grant MH 65434 (DZP). We thank the study participants, the sleep laboratory behavioral monitors, Saul Sternberg for comments on preliminary data, Edwin M. Robertson for comments on the data analyses and the manuscript, Monika Haack for help in analyzing the actigraphy data, and Kathy Welch at the University of Michigan Center for Statistical Consultation and Research for statistical assistance.
Footnotes
Portions of this research were presented at the Annual Meeting for the Society for Neuroscience (2003) and the annual Harvard Medical School Division of Sleep Medicine Poster Session (2003).
References
- Baddeley A. Human Memory: Theory and Practice. East Sussex, UK: Lawrence Erlbaum Associates Ltd.; 1990. [Google Scholar]
- Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose–response study. Journal of Sleep Research. 2003;12(1):1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. third ed. Philadelphia, PA: W.B. Saunders; 2000. pp. 53–71. [Google Scholar]
- Broughton RJ, Ogilvie RD, editors. Sleep, Arousal, and Performance. Boston, MA: Birkhauser; 1992. [Google Scholar]
- Dinges DF, Kribbs NB. Performing while sleepy: effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, NY: Wiley; 1991. pp. 97–128. [Google Scholar]
- Elkin AJ, Murray DJ. The effects of sleep loss on short-term recognition memory. Canadian Journal of Psychology. 1974;28(2):192–198. [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Cognitive Brain Research. 2004;18:306–321. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Sleep and Wakefulness. Chicago, IL: University of Chicago Press; 1963. [Google Scholar]
- Kristofferson MW. Effects of practice on character-classification performance. Canadian Journal of Psychology. 1972;26(1):54–60. [Google Scholar]
- Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nature Reviews Neuroscience. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Two operations in character recognition: some evidence from reaction-time measurements. Perception and Psychophysics. 1967;2:45–53. [Google Scholar]
- Sternberg S. Memory scanning: mental processes revealed by reaction-time experiments. American Scientist. 1969;57:421–457. [PubMed] [Google Scholar]
- Sternberg S. Memory scanning: new findings and current controversies. Quarterly Journal of Experimental Psychology. 1975;27:1–32. [Google Scholar]
- Stickgold R, Hobson JA, Rosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294(5544):1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose–response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Verhaegen P, Cerella J, Basak C. A working memory workout: how to expand the focus of serial attention from one to four items in 10 h or less. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30(6):1322–1337. doi: 10.1037/0278-7393.30.6.1322. [DOI] [PubMed] [Google Scholar]
- Walker MP. A refined model of sleep and the time course of memory formation. Behavioral and Brain Sciences. 2005;28(1):51–64. doi: 10.1017/s0140525x05000026. [DOI] [PubMed] [Google Scholar]
- Williams HL, Kearney OF, Lubin A. Signal uncertainty and sleep loss. Journal of Experimental Psychology. 1965;69:401–407. doi: 10.1037/h0021755. [DOI] [PubMed] [Google Scholar]
- Williams HD, Lubin A, Goodnow JJ. Impaired performance with acute sleep loss. Psychological Monographs: General and Applied. 1959;73:1–26. [Google Scholar]