Abstract
Purpose
To evaluate, in a phase 2 study, the safety and efficacy of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer (BRPC or LAPC, respectively).
Methods and Materials
Patients received gemcitabine and oxaliplatin chemotherapy repeated every 14 days for 6 cycles, combined with weekly cetuximab. Patients were then restaged; “downstaged” patients with resectable disease underwent attempted resection. Remaining patients were treated with chemoradiation consisting of intensity modulated radiation therapy (54 Gy) and concurrent capecitabine; patients with borderline resectable disease or better at restaging underwent attempted resection.
Results
A total of 39 patients were enrolled, of whom 37 were evaluable. Protocol treatment was generally well tolerated. Median follow-up for all patients was 11.9 months. Overall, 29.7% of patients underwent R0 surgical resection (69.2% of patients with BRPC; 8.3% of patients with LAPC). Overall 6-month progression-free survival (PFS) was 62%, and median PFS was 10.4 months. Median overall survival (OS) was 11.8 months. In patients with LAPC, median OS was 9.3 months; in patients with BRPC, median OS was 24.1 months. In the group of patients who underwent R0 resection (all of which were R0 resections), median survival had not yet been reached at the time of analysis.
Conclusions
This regimen was well tolerated in patients with BRPC or LAPC, and almost one-third of patients underwent R0 resection. Although OS for the entire cohort was comparable to that in historical controls, PFS and OS in patients with BRPC and/or who underwent R0 resection was markedly improved.
Introduction
It is estimated that pancreatic cancer accounted for 43,920 cancer cases and 37,390 cancer deaths in 2010 (1). The overall 5-year survival rate among patients with pancreatic cancer is approximately 5%, and only 10%-20% of patients are candidates for curative surgery (2). Approximately 40% of patients present with borderline resectable or unresectable locally advanced pancreatic cancer (BRPC or LAPC, respectively) secondary to local tumor involvement of the adjacent vasculature (2). These patients are at high risk for an incomplete resection, which is associated with poor outcome (3). Furthermore, recent studies using routine staging laparoscopy in patients with nonmetastatic “locally advanced” pancreatic cancer have reported rates of occult, intraabdominal metastases ranging from 24% to 37% (4-7).
A potential strategy to treat patients with BRPC or LAPC is to sequence systemic chemotherapy before chemoradiation, to treat systemic disease upfront and optimize selection of candidates for consolidation chemoradiation and/or resection. We designed a phase 2 study to evaluate the safety and efficacy of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with BRPC or LAPC. The combination of gemcitabine with another, more active chemotherapeutic agent (oxaliplatin) and a second agent targeting other molecular pathways involved in tumorigenesis and metastasis (cetuximab) was selected to optimize treatment of potential, occult metastatic disease at presentation, minimize disease progression, maximize radiologic response rate (and the rate of complete surgical resection), and enhance progression-free and overall survival (PFS and OS, respectively). Chemoradiation was used selectively in patients with persistent vascular involvement after induction chemotherapy to minimize the risk of a positive pathologic margin at the time of attempted resection.
Methods and Materials
Eligibility criteria and initial patient evaluation
Patients (aged 18 years or older) with biopsy-proven, measurable (by Response Evaluation Criteria In Solid Tumors [RECIST] criteria) BRPC or LAPC of the pancreatic head, body, or tail with Eastern Cooperative Oncology Group performance status 0-2 were eligible. Chest computed tomography (CT), pancreas-protocol CT or magnetic resonance imaging scan (MRI), and endoscopic ultrasound were performed in all patients. Patients were deemed as having BRPC or LAPC according to CT or MRI findings. Patients with encasement (≥180° or ≥50% of the vessel circumference) of the celiac axis, common hepatic artery (CHA), superior mesenteric artery (SMA), and/or extensive encasement/occlusion of the superior mesenteric vein–portal vein (SMV-PV) confluence were categorized as having LAPC. All patients were independently evaluated by a surgical oncologist, a medical oncologist, and a radiation oncologist and deemed medically fit for chemotherapy, chemoradiation, and surgical resection before enrollment. Endobiliary stenting to relieve obstructive jaundice was performed (as needed), but no prior therapy for pancreatic cancer was allowed. Patients were required to have adequate hepatic, renal, and hematopoietic function, and for women of childbearing potential, a negative pregnancy test within 7 days of starting therapy.
Patients were excluded from enrollment in the study if they had active hepatitis, known human immunodeficiency virus infection, an active or uncontrolled infection, a significant history of uncontrolled heart disease, prior anti-endothelial growth factor receptor therapy, prior severe infusion reaction to a monoclonal antibody, a concurrent second malignancy (other than nonmelanoma skin cancer), a history of deep venous thrombosis/bleeding diathesis/coagulopathy, recent/current use of anticoagulants, an open biopsy/major surgical procedure within 28 days of initiation of therapy, or any prior radiation therapy or chemotherapy.
All eligible patients signed an informed consent form, and the study was approved and monitored by the institutional review board at our institution. The trial was registered with clinicaltrials.gov.
Study design and treatment plan
All patients were started on induction chemotherapy consisting of gemcitabine (1000 mg/m2 given intravenously [IV] over 100 minutes on day 1) and oxaliplatin (100 mg/m2 given IV over 120 minutes on day 2) repeated every 14 days for 6 cycles combined with weekly cetuximab (400 mg/m2 given IV over 120 minutes on day 1 of week 1, followed by 11 weekly infusions of 250 mg/m2 given IV over 60 minutes on day 1 of each subsequent week). Patients then restaged at 2 to 4 weeks after completion of induction chemotherapy with a chest CT and pancreas-protocol CT (or MRI) and endoscopic ultrasound, and each case was reviewed/discussed at the gastrointestinal multidisciplinary tumor board. Patients with evidence of radiologic response and resectable disease by CT or MRI criteria (ie, no persistent abutment/encasement of the adjacent celiac axis, CHA, SMA, and/or the SMV-PV confluence) underwent attempted surgical resection. Patients with stable disease went on to chemoradiation, whereas patients with evidence of disease progression were removed from the protocol (but followed) and subsequently treated at their treating physician's discretion.
Chemoradiation consisted of intensity modulated radiation therapy delivered to 45.9 Gy at 1.53 Gy per fraction to the elective nodal regions while simultaneously delivering 54 Gy at 1.8 Gy per fraction (30 fractions) to the gross disease with concurrent weekly capecitabine (800 mg/m2 orally twice daily on days of radiation therapy). Details of radiation therapy planning and delivery have been reported previously (8). Normal tissue and target planning objectives are listed in Table 1.
Table 1. Radiation therapy treatment planning objectives.
| Volume | Description | Criteria 1 | Criteria 2 | Criteria 3 |
|---|---|---|---|---|
| PTV | Planning target volume | V100% ≥95% | V93% ≥99% | Dmax (0.1 cm3) ≤106% |
| Liver | Volume of liver less any CTV in liver | V20 Gy ≤67% | V30 Gy ≤40% | |
| Kidneys | Contoured separately and expanded by 0.5 cm | “Hot” kidney V18 Gy ≤75% | “Cool” kidney V18 Gy ≤25% | |
| Small bowel | Entire small bowel volume as a compartment | Dmean ≤13 Gy | V30Gy ≤20% | V45 Gy ≤10% |
| Stomach | Volume of stomach less any CTV | V30 Gy ≤ 50% | V45 Gy ≤ 10% |
Abbreviations: CTV = clinical target volume; Dmean = mean dose; PTV = planning target volume; Vx% = volume receiving at least x% of the prescribed dose; VyGy = volume receiving at least y Gy.
Four to 8 weeks after completion of chemoradiation, patients were restaged and reviewed/discussed, as above. Patients with evidence of radiologic response or stable disease (ie, localized or borderline resectable disease) underwent attempted surgical resection; as above, patients with evidence of disease progression were removed from the protocol (but followed) and subsequently treated at their treating physician's discretion.
Monitoring during induction chemotherapy and chemoradiation
Patients were evaluated by a medical oncologist every 2 weeks during induction chemotherapy and by a radiation oncologist every week during chemoradiation. Serum chemistries were evaluated every 2 weeks during therapy; white blood cell counts, hemoglobin/hematocrit levels, and platelet counts were evaluated every 2 weeks during chemotherapy and weekly during chemoradiation.
Treatment toxicities were coded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Gemcitabine and oxaliplatin were reduced to 800 mg/m2 and 85 mg/m2 (respectively) for grade 4 hematologic toxicity, grade 3 stomatitis/dysphagia, and grade 3-4 diarrhea. Protocol therapy was discontinued permanently for grade 4 stomatitis/dysphagia, grade 3-4 pulmonary toxicity, or any toxicity above grade 1 that persisted beyond 2 weeks off therapy. Oxaliplatin was reduced to 85 mg/m2 for grade 2 neurologic toxicity that persisted between cycles or grade 3 neurologic toxicity that lasted more than 7 days. Protocol therapy was discontinued permanently for grade 3 neurologic toxicity that persisted between cycles, grade 4 neurologic toxicity, or grade 3-4 acute hypersensitivity or anaphylactic reactions. In patients who experienced a grade 1-2 infusion reaction, the dose of cetuximab was decreased by 50%; in patients who experienced a grade 3-4 reaction, cetuximab was permanently discontinued. Cetuximab dose was reduced to 200 mg/m2 and 150 mg/m2 after the second and third occurrences (respectively) of grade 3 acneiform rash; cetuximab therapy was permanently discontinued if the rash failed to resolve after 2 weeks or after the fourth occurrence. Patients continued to receive gemcitabine and oxaliplatin on study if cetuximab was held and/or discontinued. Capecitabine dose was reduced to 600 mg/m2 (or 400 mg/m2) for mild hematologic toxicity, grade 1-2 gastrointestinal toxicity, or grade 3 hand-and-foot syndrome. Capecitabine was held for up to 1-2 weeks for severe hematologic toxicity, grade 3-4 gastrointestinal toxicity, or grade 3-4 hepatic toxicity; capecitabine was permanently discontinued for persistent toxicity off therapy. Radiation therapy was continued during weeks when capecitabine was held (except in the case of persistent grade 3-4 gastrointestinal toxicity, in which case all protocol therapy was discontinued permanently).
Follow-up studies
During the first year after completion of treatment, patients were followed with history and physical examination, repeat CA19-9 levels, and surveillance CT scans every 3 months. Beyond the first year, patients were followed and evaluated at the discretion of the treating physician.
Statistical design
The primary objective of this single-arm, phase 2 study was to determine the rate of PFS at 6 months. Secondary objectives included determining the tolerance and toxicity, radiologic response rate, R0 (negative microscopic margin) resection rate, and OS associated with this regimen. Progression-free survival was defined as the time from treatment initiation to the first indication of disease progression according to CT/MRI scan RECIST criteria, clinical deterioration requiring the stoppage of protocol therapy, or death. Patients who had not progressed at the time of analysis were censored at the date of their last scan at which they were found to be progression-free. Overall survival was defined as the time from treatment initiation to death. Patients who withdrew early from the study were censored at the time of their withdrawal.
A single-stage design was used to test the null hypothesis that the 6-month PFS was 50% versus the alternative hypothesis that the 6-month PFS was 70% (corresponding to an approximate doubling of the median PFS time from 6 to 12 months). Thirty-seven patients were required for the study design; 39 were enrolled to adjust for early dropout. If ≥23 of 37 evaluable patients were disease-free at 6 months the null hypothesis was rejected. Using an exact binomial test and a 1-sided significance level of P<.05, the design provided an 80% probability of rejecting the null hypothesis if the true 6-month PFS is 70%.
Kaplan-Meier estimation and curves were used to demonstrate OS and PFS distributions and to estimate median PFS and OS and their 95% confidence intervals (CIs). Fisher exact test (1-sided) was used to estimate the P value for testing the 6-month PFS rate. Exact CIs were estimated for the 6-month PFS rate.
Results
Patient characteristics
From March 2006 through November 2008, 39 patients were enrolled (Table 2). The intention-to-treat analysis of safety and efficacy included 37 evaluable patients; 1 patient with a nonfunctioning, pancreatic neuroendocrine tumor (mistakenly classified as pancreatic adenocarcinoma at enrollment) was excluded, as well as another patient who received less than 1 dose of induction chemotherapy. Thirteen patients had BRPC, and 24 had LAPC. Overall median follow-up was 11.9 months for all patients and 46.3 months for living patients.
Table 2. Patient and tumor characteristics (n=37).
| Age (y) | |
| Median | 60 |
| Range | 28-78 |
| Sex | |
| Male | 20 (54) |
| Female | 17 (46) |
| Race | |
| White | 29 (78) |
| African American | 8 (22) |
| Tumor extent | |
| BRPC | 13 (35.1) |
| LAPC | 24 (64.9) |
| Tumor location | |
| Head | 29 (78.4) |
| Head/neck | 4 (10.8) |
| Head/neck/body | 1 (2.7) |
| Neck | 1 (2.7) |
| Neck/body | 1 (2.7) |
| Body | 1 (2.7) |
Abbreviations: BRPC = borderline resectable pancreatic cancer; LAPC = locally advanced pancreatic cancer.
Values are number (percentage) unless otherwise noted.
Treatments received
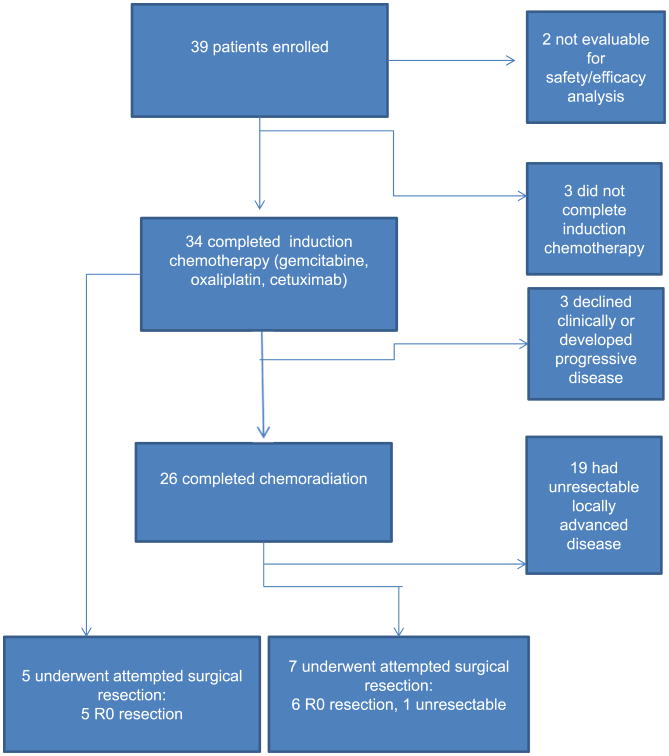
Thirty-four of 37 patients (92%) completed induction chemotherapy (Fig. 1). Reasons for not completing induction chemotherapy included oxaliplatin infusion reaction (n=1), cetuximab infusion reaction/anaphylaxis (n=1), and clinical deterioration (n=1). Of the 34 patients who completed induction chemotherapy, none had a radiographic complete response (CR) and 6 (17.6%) had a partial response (PR) by RECIST criteria at initial restaging. Twenty-two (64.7%) of the 34 patients had stable disease (SD), and 6 (17.6%) showed evidence of progressive disease.
Fig. 1.
Flow of patients through the study and treatment received.
Five patients (14.7%) underwent exploration and attempted surgical resection after completion of induction chemotherapy; the remaining 29 patients were eligible to receive subsequent chemoradiation. Three of these 29 patients did not proceed to chemoradiation as planned owing to rapid tumor progression (n=1) or clinical decline (n=2). Twenty-one of the 26 patients that received chemoradiation were treated at our institution, whereas the remaining 5 patients received chemoradiation elsewhere. Of the 26 patients who received chemoradiation, all but 1 underwent the subsequent imaging required to assess radiologic response after chemoradiation. One patient had a radiographic CR by RECIST criteria, 18 patients had SD, and 6 patients had progressive disease; no patients showed evidence of a PR. After completion of chemoradiation, 7 patients underwent exploration and attempted surgical resection.
In total, 12 (32.4%) of the 37 evaluable study patients underwent surgical exploration after protocol therapy. Overall, 11 patients (29.7%) underwent successful surgical resection, including 9 (69.2%) of the 13 patients with BRPC and 2 (8.3%) of 24 patients with LAPC. All 5 patients who were explored after induction chemotherapy underwent R0 resections. Six patients who underwent resection after chemoradiation had R0 resections, including 1 patient with LAPC who completed all protocol therapy and was found to have a pathologic CR at resection. One patient who underwent exploration after chemoradiation was found to have occult encasement of a large segment of CHA and was deemed unresectable. All resected patients underwent pancreaticoduodenectomies; 4 patients required venous resection/reconstruction, and 1 patient required arterial resection/reconstruction alone.
Toxicity
Thirty-one (83.8%) of the 37 evaluable patients completed planned induction chemotherapy, including 5 patients who underwent surgical resection after completion of induction chemotherapy and 26 patients who completed induction chemotherapy and chemoradiation. Twenty-six of 37 patients (70.3%) had no grade 3 or higher toxicity during induction chemotherapy (toxicities experienced by the remaining patients are listed in Table 3). There was 1 grade 3 infusion reaction to oxaliplatin. In addition, there were 2 grade 3 cetuximab-related acneiform rashes and 1 grade 4 cetuximab-related infusion reaction (anaphylaxis). There were 9 grade 3 and 2 grade 4 hematologic toxicities. Finally, there were 9 grade 3-4 electrolyte disturbances during induction chemotherapy.
Table 3. Toxicity (grade 3 or greater per CTCAE, version 3.0) during induction chemotherapy (n=37).
| Toxicity | Grade 3 | Grade 4 | Grade 3-4 |
|---|---|---|---|
| Oxaliplatin infusion reaction | 1 (2.7) | — | 1 (2.7) |
| Cetuximab acneiform rash | 2 (5.4) | — | 2 (5.4) |
| Cetuximab infusion reaction | — | 1 (2.7) | 1 (2.7) |
| Leukopenia | 5 (13.5) | — | 5 (13.5) |
| Neutropenia | 4 (10.8) | — | 4 (10.8) |
| Anemia | — | 2 (5.4) | 2 (5.4) |
| Hypokalemia | 3 (8.1) | 1 (2.7) | 4 (10.8) |
| Hypomagnesemia | — | 1 (2.7) | 1 (2.7) |
| Hypophosphatemia | 1 (2.7) | 1 (2.7) | 2 (5.4) |
| Hypernatremia | — | 1 (2.7) | 1 (2.7) |
| Hypocalcemia | 1 (2.7) | — | 1 (2.7) |
Abbreviation: CTCAE = Common Terminology Criteria for Adverse Events.
Values are number (percentage).
There were 21 patients who underwent subsequent chemoradiation at our institution and were therefore evaluable for chemoradiation toxicity assessment. Nineteen (90.5%) had no grade 3 or higher toxicity during chemoradiation. Two patients (9.5%) experienced grade 3 gastrointestinal toxicities (eg, nausea, vomiting, and/or diarrhea) that required intravenous fluids but not hospitalization.
Outcomes
Twenty-three of 37 patients were progression free at 6 months, for a 6-month PFS rate of 62% (1-sided 90% CI 0.50-1.00). The 1-sided P value testing that the PFS rate was >50% was .09. Kaplan-Meier curves for PFS and OS are shown in Figure 2. Overall, the median PFS was 10.4 months (95% CI 7.2-16.2 months), and the median OS was 11.8 months (95% CI 9.2-20.4 months). In patients who presented with LAPC at diagnosis, the median OS was 9.3 months (95% CI 8.6-13.1 months); in patients with BRPC, the median overall survival was 24.1 months (95% CI 12.2-∞). In the group of patients who underwent resection (all of which were R0 resections), median OS had not yet been reached (95% CI: 27.1-∞) at the time of analysis.
Fig. 2.
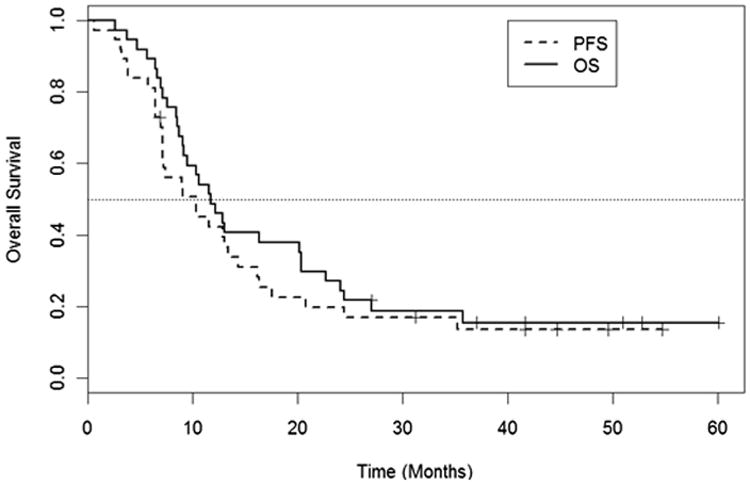
Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) for the evaluable study cohort (n=37).
With a minimum follow-up of 27 months for the 6 patients (16%) still living (median follow-up 46.3 months), 5 of these 6 patients had no evidence of cancer at the time of last follow-up. All of these patients underwent curative resection, and 2 of these patients had LAPC at presentation, including the patient who completed all protocol therapy and was found to have a pathologic CR at resection.
Discussion
In this phase 2 study, patients with BRPC or LAPC were treated with induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation. In general, protocol therapy was well tolerated. Overall, 6-month PFS and OS were not significantly improved compared with historical controls. Although the majority of patients had stable disease, almost one-third of patients underwent R0 resections. The majority of these patients had BRPC at presentation, and a significant proportion had sufficient “downstaging” after induction chemotherapy alone to allow surgical resection. In patients who underwent R0 resection, median survival had not been reached at a median follow-up of 11.9 months.
Recently, other investigators have evaluated the safety and efficacy benefit of gemcitabine/oxaliplatin-based induction chemotherapy with or without chemoradiation in patients with “locally advanced” pancreatic cancer (Table 4) (9-12). Crane et al (11) reported the results of a phase 2 trial of induction gemcitabine, oxaliplatin, and cetuximab (for 8 weeks) followed by capecitabine and cetuximab-based chemoradiation in 69 patients with LAPC. The majority (n=51, 73.9%) of patients had LAPC; 16 patients (23.1%) had BRPC due to vascular abutment, whereas 2 patients (2.9%) had borderline resectable disease only on the basis of advanced regional adenopathy. Median follow-up was 16.3 months for all patients and 20.9 months for living patients; median PFS and OS were 12.5 and 19.2 months, respectively. Nine BRPC patients (56.3%) underwent R0 resection; 2 were ultimately found to have nonpancreatic periampullary tumors, and another 2 died of perioperative complications. One of the 5 remaining resected patients was alive without evidence of disease at time of follow-up. Leone et al (12) reported the results of a trial of induction gemcitabine and oxaliplatin (for 8 weeks) followed by gemcitabine-based chemoradiation in 15 patients with BRPC and 24 patients with LAPC. At a median follow-up of 13 months, the median PFS and OS were 10.2 months and 16.7 months, respectively. Fourteen patients underwent surgical exploration, of whom 9 underwent R0 resection; 2 patients underwent R1 resection, and 3 were deemed unresectable at exploration. Given the relatively modest sample sizes and varying definitions of “locally advanced” (vs borderline resectable vs unresectable) pancreatic cancer used in these phase 2 trials, it is difficult to compare the efficacies of their respective regimens.
Table 4.
Selected trials of chemotherapy, with or without chemoradiation, for “locally advanced” pancreatic cancer.
| Study | Definition of “locally advanced” pancreatic cancer | Sample size | Induction chemo agents | RCT chemo agents | Radiation therapy total dose/dose per fraction (Gy) | Median PFS (mo) | Median OS (mo) | R0 resection rate | Patients alive and NED after R0 resection |
|---|---|---|---|---|---|---|---|---|---|
| Louvet (9) | Locally advanced, NOS | 32 | Gem, Ox | — | — | 7.4 | 10.3 | 2 of 32 (6.3%) | 1 of 2 |
| Merchan (10) | Locally advanced, unresectable, NOS | 21 | Gem, Ox, Cet | — | — | 12.4 | 15.7 | 2 of 21 (9.5%) | N/R |
| Crane (11) | Borderline resectable: <180° of the SMA or involvement of the CHA within 1 cm of the CA or advanced regional adenopathy (n =18, 26%) Unresectable, NOS (n =51, 74%) | 69 | Gem, Ox, Cet | Cet, Cap | 50.4/1.8 | 12.5 | 19.2 | 9 of 69 (13%) | 0 of 9 |
| Leone (12) | Borderline resectable: abutment <50% in the circumference of the SMA or CA; short segment encasement of the CHA; segmental PV/SMV stenosis; or occlusion amenable to vascular resection (n=15, 38.5%) Unresectable: encasement of the SMA or CA or with occlusion of the PV or SMV that precluded vascular resection (n=24, 61.5%) |
39 | Gem, Ox | Gem | 50.4/1.8 | 10.2 | 16.7 | 9 of 39 (23.1%) | 1 of 9 |
| Present study | Borderline resectable: abutment (≤180° or ≤50% of the vessel circumference) and/or unilateral narrowing of the celiac axis, CHA, SMA, and/or abutment/narrowing/short-segment encasement or occlusion of the SMV-PV confluence (n= 15, 40.5%) Unresectable: encasement (≥180° or ≥50% of the vessel circumference) of the celiac axis, CHA, SMA, and/or extensive encasement/occlusion of the SMV-PV confluence (n =22, 59.5%) |
37 | Gem, Ox, Cet | Cap | 54/1.8 | 10.4 | 11.8 | 11 of 37 (29.7%) | 5 of 11 |
Abbreviations: CA = celiac axis; Cap = capecitabine; Cet = cetuximab; CHA = common hepatic artery; Gem = gemcitabine; LAPC = locally advanced pancreatic cancer; N/A = not applicable; NED = no evidence of disease; NOS = not otherwise specified; N/R = not reported; OS = overall survival; Ox = oxaliplatin; PFS = progression-free survival; PV = portal vein; RCT = chemoradiation; SMA = superior mesenteric artery; SMV = superior mesenteric vein.
The radiologic criteria used in the present study to determine eligibility and classify patients as having either BRPC or LAPC were consistent with consensus panel recommendations (later refined by investigators at Fox Chase Cancer Center) (13, 14). Unlike the previous studies, however, patients received 6 cycles (instead of 4 cycles) of gemcitabine/oxaliplatin-based induction chemotherapy, and radiologic response after induction chemotherapy was used to direct patients toward attempted resection versus selective chemoradiation. It is notable that all 5 patients who had a PR and were explored after induction chemotherapy alone underwent R0 resections. Even though no patients experienced a radiologic response after chemoradiation, it was well tolerated, and 6 additional patients ultimately underwent R0 resection. Although overall PFS and OS were not significantly improved in our cohort compared with historical controls, the R0 resection rate was excellent, and the PFS and OS in patients who underwent R0 resection were markedly improved (compared with historical controls).
Although targeting the endothelial growth factor receptor (which is overexpressed in the majority of pancreatic tumors) had intuitive appeal at the time the present study was designed, the addition of cetuximab to gemcitabine monotherapy (in patients with advanced pancreatic carcinoma) or gemcitabine/oxaliplatin has not been shown to improve survival (these trials were published after enrollment was completed on this study). Given that the addition of cetuximab to gemcitabine failed to improve objective response rates in a recent phase 3 trial, it is unclear to what extent (if any) the inclusion of cetuximab in our induction chemotherapy regimen contributed to the observed radiologic response and resection rates. Unlike in colorectal cancer, there is persistent controversy as to the role of KRAS mutations in response to cetuximab therapy in patients with pancreatic cancer (15).
Our study suggests that induction gemcitabine, oxaliplatin, and cetuximab (with surgical exploration in patients who “downstaged”) followed by selective capecitabine-based chemoradiation (in patients with SD) is well tolerated in patients with BRPC or LAPC and may enhance R0 resection rates compared with historical controls. The longer course of induction chemotherapy was associated with modest toxicity but may have enhanced our ability to select patients for attempted surgical resection versus chemoradiation. Thorough radiologic restaging after induction chemotherapy allowed for identification of patients who were candidates for attempted R0 resection, obviating the need for routine preoperative chemoradiation in all patients. Our ability to achieve R0 resections in 6 additional patients who had SD after chemoradiation supports observations by others that radiologic evidence of persistent vascular involvement (in the absence of disease progression elsewhere) in patients with BRPC should not be considered a contraindication for attempted complete resection after induction therapy (16). Although this regimen resulted in a very favorable R0 resection rate, it unfortunately did not improve PFS and OS for the entire cohort. Future prospective studies will determine whether more aggressive neoadjuvant chemotherapy regimens (such as FOLFIRINOX, with or without chemoradiation) will prove equally safe, maintain or improve upon these R0 resection rates, and ultimately improve survival outcomes in this challenging patient population (17, 18).
Summary.
This phase 2 study evaluated the safety and efficacy of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer. This regimen seems relatively effective, allowing complete surgical resections in almost one-third of patients. Survival in resected patients was markedly prolonged.
Acknowledgments
Supported in part by the Biostatistics and Clinical Trials Office Shared Resources, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313). Cetuximab provided by Bristol Myers Squibb. Oxaliplatin and financial support provided by Sanofi Aventis.
Footnotes
Conflicts of interest: none.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Tumor staging and results. Ann Surg. 1984;199:418–425. doi: 10.1097/00000658-198404000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisters PW, Lee JE, Vauthey JN, et al. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88:325–337. doi: 10.1046/j.1365-2168.2001.01695.x. [DOI] [PubMed] [Google Scholar]
- 5.White RR, Paulson EK, Freed KS, et al. Staging of pancreatic cancer before and after neoadjuvant chemoradiation. J Gastrointest Surg. 2001;5:626–633. doi: 10.1016/s1091-255x(01)80105-0. [DOI] [PubMed] [Google Scholar]
- 6.Liu RC, Traverso LW. Diagnostic laparoscopy improves staging of pancreatic cancer deemed locally unresectable by computed tomography. Surg Endosc. 2005;19:638–642. doi: 10.1007/s00464-004-8165-x. [DOI] [PubMed] [Google Scholar]
- 7.Shoup M, Winston C, Brennan MF, et al. Is there a role for staging laparoscopy in patients with locally advanced, unresectable pancreatic adenocarcinoma? J Gastrointest Surg. 2004;8:1068–1071. doi: 10.1016/j.gassur.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Taylor R, Opfermann KJ, Jones BD, et al. Comparison of radiation treatment delivery for pancreatic cancer: Linac IMRT versus helical tomotherapy. J Med Imaging Radiat Oncol. 2012;56:332–337. doi: 10.1111/j.1754-9485.2012.02373.x. [DOI] [PubMed] [Google Scholar]
- 9.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Merchan JR, Ferrell A, Macintyre J, et al. Phase II study of gemcitabine, oxaliplatin, and cetuximab in advanced pancreatic cancer. Am J Clin Oncol. 2011;35:446–450. doi: 10.1097/COC.0b013e31821862fb. [DOI] [PubMed] [Google Scholar]
- 11.Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: Correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–3043. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leone F, Gatti M, Massucco P, et al. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: A single institutional experience. Cancer. 2013;119:277–284. doi: 10.1002/cncr.27736. [DOI] [PubMed] [Google Scholar]
- 13.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 14.Chun YS, Milestone BN, Watson JC, et al. Defining venous involvement in borderline resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2832–2838. doi: 10.1245/s10434-010-1284-9. [DOI] [PubMed] [Google Scholar]
- 15.Kullmann F, Hartmann A, Stohr R, et al. KRAS mutation in metastatic pancreatic ductal adenocarcinoma: Results of a multicenter phase II study evaluating efficacy of cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first-line therapy. Oncology. 2001;81:3–8. doi: 10.1159/000330194. [DOI] [PubMed] [Google Scholar]
- 16.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 17.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 18.Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer. 2012;12:199. doi: 10.1186/1471-2407-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]