Abstract
Hydrogen peroxide (H2O2) acts as a signaling molecule and modulates various aspects of cell functions in a wide variety of cells including mammalian germ cells. We examined whether a decreased level of intra-oocyte cyclic 3′,5′-adenosine monophosphate (cAMP) leads to accumulation of H2O2, and if so, whether a moderate increase of H2O2 inactivates maturation promoting factor (MPF) during spontaneous resumption of meiosis in rat oocytes cultured in vitro. Removal of cumulus cells and culture of denuded oocytes in vitro significantly decreased oocyte cAMP level and led to spontaneous meiotic resumption from diplotene arrest. The reduced oocyte cAMP level was associated with an increased oocyte H2O2 level and reduced catalase activity. Exogenous supplementation of H2O2 induced meiotic resumption from diplotene arrest in a concentration- and time-dependent manner in oocytes treated with 0.1 mM of 3-isobutyl-1-methylxanthine, while dibutyryl-cAMP and 3-t-butyl-4-hydroxyanisole inhibited the stimulatory effect of exogenous H2O2. The increased intra-oocyte H2O2 level induced Thr-14/Tyr-15 phosphorylation of CDK1, while Thr-161 phosphorylated CDK1 and cyclin B1 levels were reduced significantly. These results suggest that a decreased level of intra-oocyte cAMP is associated with an increased level of H2O2. The increased level of H2O2 was associated with high phosphorylation of Thr-14/Tyr-15 and dephosphorylation of the Thr-161 residue of CDK1 and reduced the cyclin B1 level, which eventually inactivated MPF. The MPF inactivation triggered spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro.
Key words: : cAMP, hydrogen peroxide, meiotic resumption from diplotene arrest, MPF, rat oocytes
Introduction
Meiotic cell cycle in mammalian oocytes is a complex process that involves several stop and go channels. It starts during fetal life and gets arrested at the diplotene stage of the first meiotic prophase. The diplotene stage oocytes are morphologically characterized by the presence of germinal vesicle and nucleolus inside the oocytes' cytoplasm, and this arrest may last for several months to several years depending on the mammalian species.1–3 Meiotic resumption from diplotene arrest may occur in response to a gonadotropin surge in vivo. The removal of follicular oocytes from ovary and their culture in vitro also induce meiotic resumption from diplotene arrest, so-called spontaneous oocyte maturation.4 This is the crucial period when oocytes achieve meiotic competence, and it determines oocytes quality, which directly affects reproductive outcome in most mammalian species, including human.5–8
It is well established that intra-oocyte cyclic 3′,5′-adenosine monophosphate (cAMP) plays an important role in the maintenance of meiotic arrest at the diplotene stage.3 The continuous transfer of cAMP through gap junctions from cumulus granulosa cells to the oocyte results in the maintenance of a high level of intra-oocyte cAMP level.5,7,9,10 This increased level of intra-oocyte cAMP maintains meiotic arrest at diplotene arrest for a long time in follicular oocytes inside the follicular microenvironment.10,11 Existing evidence suggests that the oocyte is capable of generating a sufficient amount of cAMP required for the maintenance of meiotic arrest.10,12 On the other hand, disruption in the gap junctions between cumulus cells and oocytes or removal of encircling cumulus cells from oocytes reduces intra-oocyte cAMP level and leads to spontaneous resumption of meiosis from diplotene arrest under in vitro culture conditions.4,6,13
Removal of cumulus cells from oocytes and culture of diplotene-arrested oocytes under in vitro culture conditions may generate reactive oxygen species (ROS). Encircling granulosa cells protect oocytes from oxidative stress damage14 because granulosa cells have their own enzymatic antioxidant system that regulates ROS levels during in vitro maturation of oocytes.15 The initial decrease of oocytes' cAMP can also modulate several cascades of events including generation of hydrogen peroxide (H2O2), which triggers meiotic resumption from diplotene arrest.16 Further, cAMP reduces the accumulation of ROS,17 particularly H2O2 in mammalian somatic cells,18 and increased level of ROS plays a beneficial role during maturation of mouse and rat oocytes cultured in vitro.8,19–21 On the other hand, antioxidants inhibit meiotic resumption from diplotene arrest in mammalian oocytes cultured in vitro.22,23
A growing body of evidence suggests that a moderate increase of H2O2 in the physiological range acts as a signaling molecule and modulates the phosphorylation status of several kinases in various somatic cell types.24–27 The maturation promoting factor (MPF) is a heterodimer of an enzymatic subunit of cyclin-dependent kinase 1 (CDK1), a catalytic subunit of MPF and regulatory subunit cyclin B1.7,28 High MPF activity is required for the maintenance of meiotic arrest.29 The dissociation of cyclin B1 from the MPF heterodimer followed by its degradation reduces MPF activity, which triggers meiotic resumption.29,30 Recent studies suggest that MPF inactivation does not solely dependent on cyclin B1 degradation.30 The phosphorylation at Thr-14/Tyr-15 and/or dephosphorylation at Thr-161 residues of CDK1 make MPF inactive.31,32 It has been reported that H2O2 induces tyrosine phosphorylation of CDK1 and thereby capacitation in human spermatozoa.33 However, it remains unclear whether an increase of the intra-oocyte H2O2 level could inactivate MPF by inducing phosphorylation at Thr-14/Tyr-15 and/or dephosphorylation at Thr-161 residues of CDK1 and cyclin B1 degradation during spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. Therefore, in the present study in vitro effects of dibutyryl-cAMP (db-cAMP), H2O2 and 3-t-butyl-4-hydroxyanisole (BHA), catalase activity, and intra-oocyte levels of cAMP and H2O2, phosphorylation at Thr-14/Tyr-15, Thr-161 phosphorylated CDK1 and cyclin B1 levels were analyzed during meiotic resumption from diplotene arrest in rat oocytes cultured in vitro.
Materials and Methods
Chemicals and culture media
All chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO) unless stated otherwise. The M2 culture medium (M5910; Sigma) has widely been used to handle mammalian oocytes and embryos under in vitro culture conditions. Hence, in the present study we used M2 media (AL142) purchased from HiMedia Laboratories (Mumbai, India), which has the exact formulation as the M2 culture medium from Sigma. This medium was HEPES buffered and contained lactic acid and sodium bicarbonate. The osmolarity of the liquid medium was 280±10 mOsm and pH 7.0±0.20, as per the company manual data sheet.
Animals
Sexually immature female albino rats (Rattus norvegicus) of Charles Foster strain (23–25 days old, 45±5 g body weight) were housed in air-conditioned, light-controlled rooms, with food and water available ad libitum. All procedures conformed to the stipulations of the Animal Ethical Committee, Faculty of Science (wide letter number F.Sc./ IAEC/ 2013-14/0341/2199, dated: September 23, 2013) Banaras Hindu University, Varanasi.
Collection of diplotene stage oocytes
To obtain diplotene stage oocytes that had germinal vesicle (GV) and nucleolus, rats were given a single subcutaneous injection of 20 IU pregnant mare's serum gonadotropin (PMSG) in 100 μL of sterile normal saline to promote the growth of a cohort of healthy antral follicles. Forty-eight hours after PMSG injection, rats were euthanized; ovaries were removed and transferred to a 35-mm petri dish containing 2 mL of sterile medium. Ovarian follicles (0.8-mm diameter) were punctured with a sterile 26-gauge needle attached to a 1-mL syringe in prewarmed medium. Cumulus oocyte complexes were isolated in prewarmed medium containing 0.1 mM of 3-isobutyl-1-methylxanthine (IBMX) to inhibit spontaneous meiotic resumption22 and then denuded using 0.01% (w/v) hyaluronidase in medium followed by repeated pipetting through a narrow-bore pipette in culture medium. The denuded diplotene-arrested oocytes (showing germinal vesicle and nucleolus) were washed at least three times with fresh plain M2 medium to remove IBMX as well as hyaluronidase from the culture medium. The average time for isolation and preparation of culture for denuded oocytes was 6±2 min. Denuded oocytes were quickly used for all in vitro studies.
Quantitative analysis of cAMP concentration
The intra-oocyte cAMP concentration was analyzed using cAMP assay kit purchased from R&D Systems (Minneapolis, MN). Approximately 200 to 220 diplotene-arrested oocytes were collected and cultured in plain M2 medium for 3 h. The diplotene-arrested cells as well as those with meiotic resumption from the diplotene stage were sorted out under a Nikon (Model C-DS, Tokyo, Japan) microscope. The 100 oocytes that were either arrested at the diplotene stage or had resumed meiosis from diplotene arrest were transferred to a microcentrifuge tube containing 100 μL of hypotonic lysis buffer (5 mM Tris, 20 mM EDTA, 0.5% TritonX-100, pH 8) for 1 h on ice for lysis. The lysates were centrifuged at 10,000×g at 4°C for 15 min and clear supernatant was used for the quantitative estimation of cAMP concentration by colorimetric assay as per company manual protocols. Reagents, samples, and standards were prepared according to instruction manual. The 50 μL of primary antibody solution was added to each well excluding nonspecific binding (NSB) wells and then incubated for 1 h at room temperature. Wells were aspirated and washed four times with wash buffer and then 100 μL of standard, lysates obtained by lysing diplotene-arrested oocytes and rate oocytes that had resumed meiosis after diplotene arrest were added to the appropriate wells. Further, 100 μL of diluent was added to NSB and zero standard wells. Fifty microliters of cAMP conjugate was added to all wells and incubated for 2 h at room temperature. Thereafter, plates were aspirated and washed four times with wash buffer. Two hundred microliters of substrate solution was added to each well and incubated for 30 min at room temperature. Finally, 100 μL of stop solution was added to each well, and readings were taken using a microplate reader (Micro Scan MS5608A, ECIL, Hyderabad, India) set at 450 nm within 10 min. Three independent samples were run in one assay to avoid interassay variation, and intra-assay variation was found to be 1.9%.
Quantitative analysis of H2O2 concentrations in oocytes
The intra-oocyte H2O2 concentration was analyzed using a H2O2 assay kit purchased from BioVision (Milpitas, CA). Oocyte lysates were prepared as described for the quantitative analysis of cAMP and immediately used for the quantitative estimation of H2O2 concentration by colorimetric assay as per company manual protocols. The optical density was determined using a microplate reader (Micro Scan MS5608A) set at 560 nm for H2O2. Three independent samples were run in triplicate to avoid inter-assay, and intra-assay variation was found to be 2.1%.
Measurement of intra-oocyte H2O2 level using DCF fluorescence dye
The intra-oocyte H2O2 level was detected using 2,7-dichlorodihydrofluorescein diacetate (DCF-DA) following a previously published protocol34 with some minor modifications. In brief, slides containing 20–22 oocytes arrested at diplotene stage or ones that had resumed meiosis after the diplotene stage were washed with fresh M2 medium and further incubated in a 3.5-mm petri dish containing 2 mL of sterile medium containing DCF-DA (10 mM) for 15 min at 37°C in a humidified BOD Incubator (Yorco BOD Incubator Automate 10; York Scientific Industries, New Delhi, India). After 15 min of incubation oocytes were washed five times with prewarmed phosphate-buffered saline (PBS), mounted with VECTASHIELD fluorescence mounting media (Vector Laboratories, Burlingame, CA) for preventing photo bleaching, and then observed under fluorescence microscope (Nikon, model Ni-U, Nikon Eclipse). DCF fluorescence was measured at 485 nm excitation/520 nm emission, monitored by fluorescence microscopy. A total of 36–42 oocytes from three independent experiments were used for the measurement of fluorescence intensity, and representative photographs are shown in the Results section. The corrected total cell fluorescence (CTCF) of 8–10 oocytes from three independent experiments was used for CTCF analysis. All parameters were kept constant, and for each oocyte the whole area was selected. Fluorescence intensity was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Catalase activity assay
The catalase activity in oocyte lysate was analyzed following our previous published protocol35 using catalase activity assay kit purchased from BioVision, Inc. The oocyte lysates were prepared as already described for the quantitative analysis of cAMP concentration. Lysates were immediately used for the estimation of catalase activity as per company manual protocol, and enzyme activity was calculated as the amount of H2O2 decomposed per minute per milliliter and is represented as micro-units per milligram of cell lysate protein. The optical density was determined using a microplate reader (Micro Scan MS5608A, ECIL). Three independent samples were run in triplicate to avoid interassay, and intra-assay variation was found to be 2.6%.
Effects of exogenous H2O2 supplementation on meiotic resumption
To find out in vitro effects of H2O2 on meiotic resumption, denuded oocytes were collected as already described. The diplotene-arrested oocytes (20–22) were transferred to a 3.5-mm petri dish containing 2 mL of sterile medium with various concentrations of H2O2 (0.0, 2.5, 5.0, and 10.0 μM). The culture flasks were maintained at 37°C in a humidified chamber for various amounts of time (1, 2, and 3 h). At the end of the incubation period, oocytes were removed, washed three times with culture medium, and transferred to a grooved slide with 100 μL of culture medium and then examined for morphological changes such as the presence or absence of germinal vesicle and nucleolus using a phase-contrast microscope (Nikon, Eclipse; E600) at × 400 magnification. Three independent experiments were conducted to confirm the observations.
Effects of BHA and db-cAMP on H2O2-induced meiotic resumption
To analyze in vitro effects of BHA and db-cAMP on H2O2-induced meiotic resumption from diplotene arrest, cumulus oocyte complexes collected from the ovary in medium and then denuded as described above for in vitro effects of H2O2. Oocytes (20–22 in each group) were cultured in a replicates of three in the 3.5-mm petri dish containing 2 mL of sterile medium with various concentrations of H2O2 (0.0, 2.5, 5.0, and 10.0 μM) and BHA or db-cAMP (1 mM) at 37°C in a humidified chamber for 3 h. At the end of incubation period, oocytes were removed, washed three times with medium, transferred onto a grooved slide with 100 μL of medium, and then examined for morphological changes such as the presence or absence of germinal vesicle and nucleolus using a phase-contrast microscope (Nikon, Eclipse; E600) at ×400 magnification. Three independent experiments were conducted to confirm the observations.
Detection of general and specific phosphorylation of CDK1 and cyclin B1 levels
To analyze the phosphorylation status of CDK1 and cyclin B1 levels, oocytes were separately exposed to anti- pThr-14/Tyr-15 CDK1, anti- pThr-161 CDK1, anti-CDK1, and anti-cyclin B1 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). For this purpose, a group of 20–22 oocytes arrested at diplotene stage or those that had resumed meiosis from diplotene arrest were prefixed with 3.7% buffered formaldehyde. Oocytes were permeabilized with Triton X-100 (0.01% in PBS) for 10 min at 37°C and then washed three times with prewarmed PBS. The polyclonal antibody raised against a short amino acid sequence containing phosphorylated Thr-14/Tyr-15, Thr-161 of CDK1, and anti-CDK1 (PSTAIRE) polyclonal antibody raised against a peptide mapping epitope with in the conserved PSTAIRE domain of CDK1 of human origin. Anti-cyclin B1 polyclonal antibody was raised against amino acids 1–433, representing full-length cyclin B1. The nonspecific sites were blocked using blocking buffer (2.5% bovine serum albumin–PBS solution) at 37°C for 30 min and then exposed to 100 μL of their respective primary antibodies (1:500 dilution in blocking buffer) at 37°C for 2 h. After five washes with prewarmed PBS, slides were exposed to either 100 μL of secondary antibody labelled with fluorescein isothiocyanate or tetramethyl rhodamine isothiocyanate (1:1000 dilutions in blocking buffer) for 1 h at 37°C in humidified chamber. After 1 h of incubation, slides were washed five times with prewarmed PBS, mounted with VECTASHIELD fluorescence mounting media (Vector Laboratories) for preventing photo bleaching, and then observed under fluorescence microscope (Model, Ni-U, Nikon Eclipse) at 488 and 520 nm, respectively, at×400 magnification. Three independent experiments were conducted to confirm the observations. A total of 36–42 oocytes from three independent experiments were used for the measurement of fluorescence intensity, and representative photographs are shown in the Results section. The CTCF of 8–10 oocytes from three independent experiments was used for CTCF analysis. All the parameters were kept constant, and for each oocyte, the whole area was selected. The fluorescence intensity was analyzed using ImageJ software.
Statistical analysis
Data are expressed as mean±standard error of mean (SEM) of three independent experiments. All percentage data were subjected to arcsine square-root transformation before statistical analysis. Data are analyzed either by Student's t-test or by two-way ANOVA followed by post hoc multiple test (i.e., Student-Newman-Keuls' test) using SPSS software, Version 17.0 (SPSS, Inc. Chicago, IL). A probability of p<0.05 was considered as statistically significant.
Results
A decrease of intra-oocyte cAMP and increase of H2O2 triggers meiotic resumption
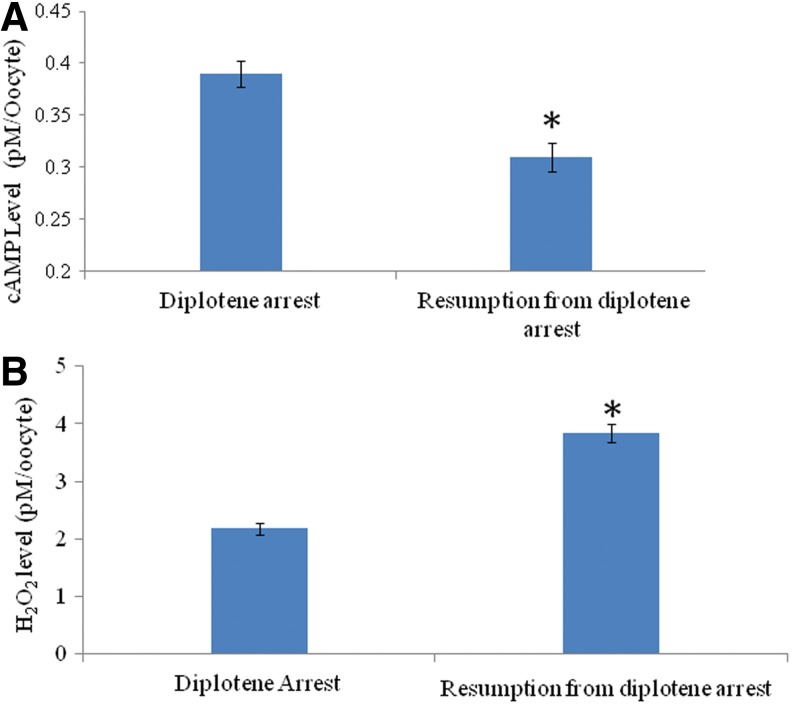
As shown in Figure 1A, a significant (p<0.05) decrease of intra-oocyte cAMP concentration (0.31±0.12 pM/oocyte) was noticed during spontaneous meiotic resumption from diplotene arrest as compare to diplotene-arrested oocytes (0.39±0.02 pM/oocyte). On the other hand, intra-oocyte H2O2 concentration was significantly increased (3.84±0.16 pM/oocyte) in oocytes that had undergone spontaneous resumption of meiosis from diplotene arrest as compared to diplotene stage oocytes (2.18±0.01 pM/oocyte: Fig. 1B). These results were further supported by fluorescence analysis of H2O2 using a specific dye (i.e., DCF). Results suggest that the increased level of H2O2 was associated with spontaneous meiotic resumption from diplotene arrest (Fig. 2B) as compared to diplotene-arrested oocytes (Fig. 2A). The diplotene arrest was morphologically identified by the presence of germinal vesicle and nucleolus in the center (Fig. 2C), and their absence was considered to indicate meiotic resumption from diplotene arrest (Fig. 2D). The CTCF analysis of fluorescence intensity of DCF using ImageJ software further supports these observations (Fig. 2E).
FIG. 1.
Quantitative analysis of intracellular cyclic 3′,5′-adenosine monophosphate (cAMP) and hydrogen peroxide (H2O2) in oocytes cultured in vitro. A decrease of intra-oocyte cAMP (A) and increase of H2O2 (B) are associated with spontaneous resumption of meiosis in diplotene-arrested oocytes cultured in vitro. Data are mean±SEM of three replicates and analyzed by Student's t-test. *Significant (p<0.001) difference as compared to diplotene arrested oocytes.
FIG. 2.
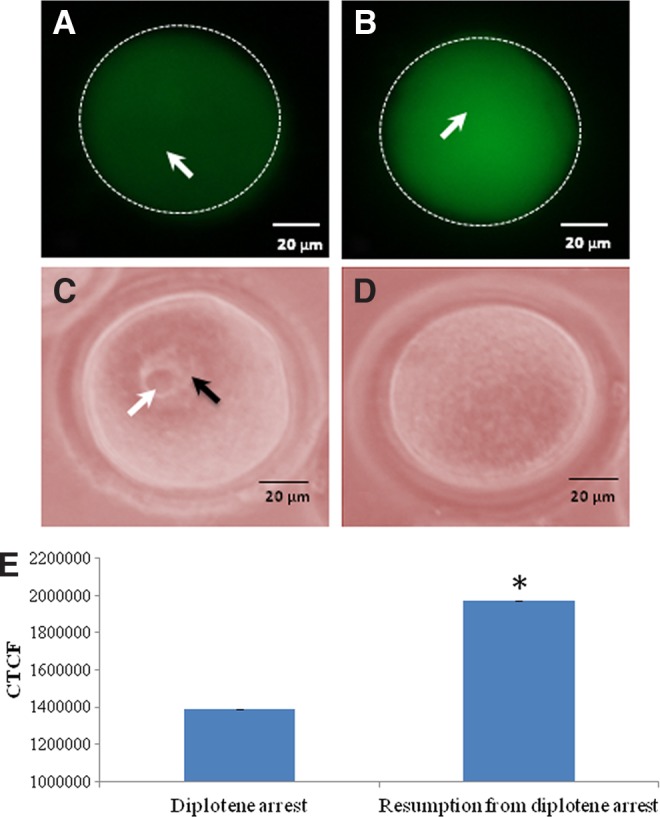
Representative photographs showing the fluorescence intensity of 2,7-dichlorodihydrofluorescein (DCF), a specific dye represents intra-oocyte H2O2 level. An increase in the level of intra-oocyte H2O2 (arrow) (B) is associated with spontaneous resumption of meiosis from diplotene arrest as compares to diplotene arrest (arrow) (A). The diplotene stage was identified by the presence of germinal vesicle (black arrow) and nucleolus (white arrow) (C), and their absence was treated as meiotic resumption from diplotene arrest (D). Bar=20 μM The corrected total cell fluorescence (CTCF) analysis of three independent oocytes further support above observations (E). Data are mean±SEM of three independent experiments and analyzed by Student's t-test. *Significant (p<0.001) difference as compared to diplotene-arrested oocytes.
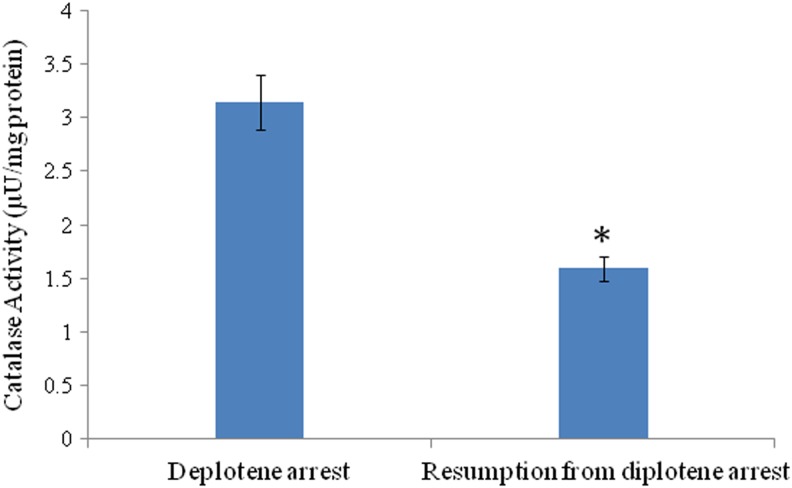
Inhibition of catalase activity results in spontaneous meiotic resumption
As shown in Figure 3, a significant (p<0.05) reduction of catalase activity was observed in oocytes (1.59±0.11 μU/mg protein) that underwent spontaneous meiotic resumption from diplotene arrest as compared to diplotene-arrested oocytes (3.14±0.25 μU/mg protein) that were arrested at the diplotene stage of the meiotic cell cycle.
FIG. 3.
Analysis of catalase activity in oocytes cultured in vitro. A decrease of catalase activity is associated with spontaneous resumption of meiosis in diplotene-arrested oocytes as compared to diplotene-arrested oocytes cultured in vitro. Data are mean±SEM of three replicates. *Significantly (p<0.05) higher as compared to diplotene-arrested oocytes.
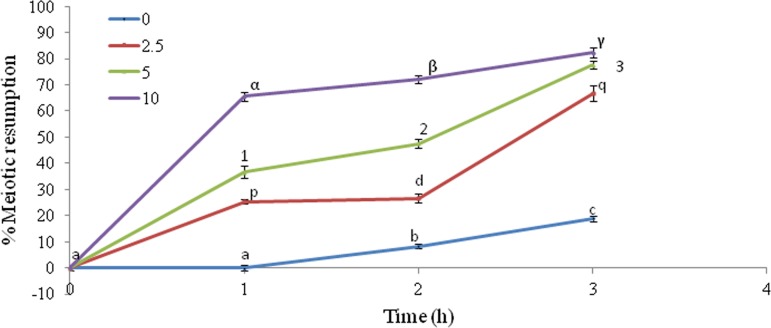
H2O2 induces meiotic resumption
The supplementation of exogenous H2O2 induced meiotic resumption from diplotene arrest in a concentration- and time-dependent manner (two-way ANOVA: FConcentration=793.28, p<0.001; FTime=346.619, p<0.001; and interaction of these two factors FTime×Conc.=24.90, p<0.001; Fig. 4). The Student-Newman-Keuls' test further revealed that 2.5 μM H2O2 significantly induced meiotic resumption (66.7±2.96%) and found maximum (82.33±1.76%) if the oocytes were treated with 10 μM H2O2 for 3 h in vitro. Three independent experiments were conducted to confirm these results.
FIG. 4.
Concentration- and time-dependent effects of exogenous H2O2 on induction of meiotic resumption from diplotene arrest in rat oocytes cultured in vitro. A group of 20–22 diplotene-arrested oocytes were exposed to various concentrations of H2O2 for various time periods. Data were expressed as mean±SEM and analyzed by two-way ANOVA (p<0.05) followed by Student-Newman-Keuls' test. Different letters show significant difference (p<0.001) from other groups.
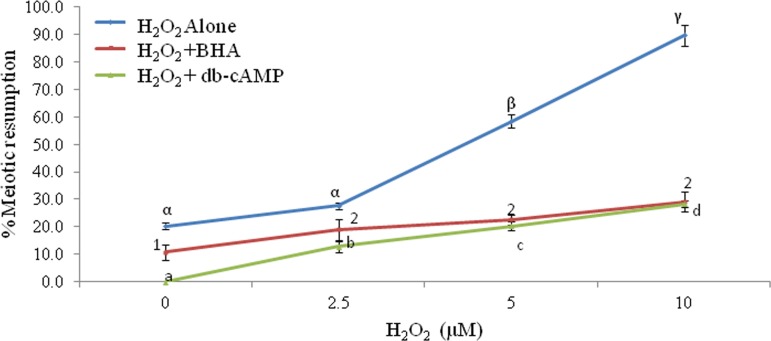
BHA and db-cAMP inhibit H2O2-induced meiotic resumption
As shown in Figure 5, the co-addition 100 mM BHA significantly inhibited H2O2-induced meiotic resumption from diplotene arrest at all concentrations (0.0, 2.5, 5.0, and 10.0 μM) used in the present study (two-way ANOVA: FH2O2=29.41, p<0.001; FBHA=20.327, p<0.001; and interaction of these two factors FBHA×H2O2=3.99, p<0.05; Fig. 5). Similarly co-addition of db-cAMP (1 mM) inhibited H2O2-induced meiotic resumption from diplotene arrest after 3 h of in vitro culture (two-way ANOVA: FH2O2=58.54, p<0.001; Fdb-cAMP=156.39, p<0.001; and interaction of these two factors FH2O2×db-cAMP=25.13, p<0.001; Fig. 5). A complete inhibition of spontaneous resumption was observed when diplotene-arrested oocytes were treated with 1 mM of db-cAMP for 3 h in vitro (Fig. 5). Three independent experiments were conducted to confirm these results.
FIG. 5.
Effects of 3-t-butyl-4-hydroxyanisole (BHA) and dibutyryl-cAMP (db-cAMP) on H2O2-induced meiotic resumption of meiosis in oocytes cultured in vitro. A group of 20–22 diplotene-arrested oocytes were exposed to various concentrations of H2O2 with or without BHA (1 mM) or db-cAMP (1 mM) for 3 h in vitro. Data were expressed as mean±SEM and analyzed by two-way ANOVA (p<0.05) followed by Student-Newman-Keuls' test. Different letters show significant difference (p<0.001) from other groups.
Increased level of Thr-14/Tyr-15 and reduced level of Thr-161 phosphorylated CDK1 and cyclin B1 levels trigger meiotic resumption
As shown in Figure 6, a significant increase in the level of Thr-14/Tyr-15 phosphorylated CDK1 was observed in oocytes that underwent spontaneous resumption of meiosis after 3 h of in vitro culture (Fig. 6A) as compared to diplotene-arrested oocytes (Fig. 6B). The CTCF analysis further supports these observations (Fig. 6C). As shown in Figure 7, a significant reduction in the level of Thr-161 phosphorylated CDK1 was observed in oocytes that underwent spontaneous resumption of meiosis after 3 h of in vitro culture (Fig. 7A) as compared to diplotene-arrested oocytes (Fig. 7B). The CTCF analysis further strengthens our observations (Fig. 7C). However, the total phosphorylation status of CDK1 remained unchanged during diplotene arrest (Fig. 8A) as well as during meiotic resumption from diplotene arrest (Fig. 8B). The CTCF analysis using Image J software further confirmed the already reported observations (Fig. 8C). Further, cyclin B1 level was significantly reduced in oocytes that underwent meiotic resumption (Fig. 9B) as compared to diplotene-arrested oocytes (Fig. 9A). These observations were further supported by the CTCF analysis (Fig. 9C).
FIG. 6.
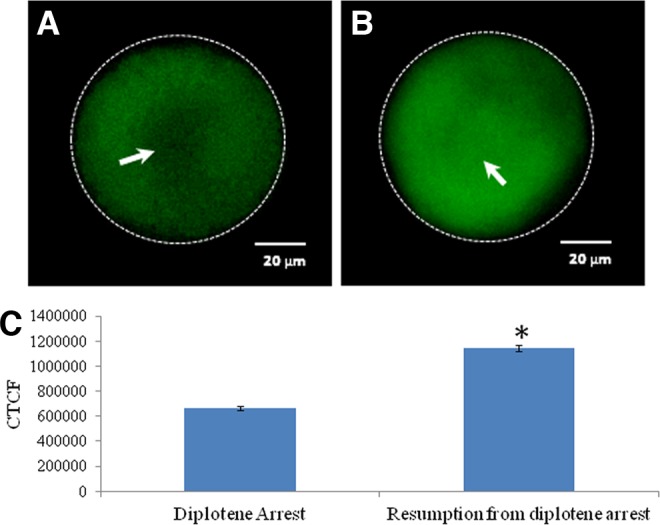
Representative photographs showing immunofluorescence intensity of Thr-14/Tyr-15 phosphorylated cyclin-dependent kinase 1 (CDK1) in oocytes. An increase of Thr-14/Tyr-15 phosphorylated CDK1 (arrow) (B) is associated with spontaneous resumption of meiosis from diplotene arrest as compare to diplotene-arrested oocyte (arrow) (A). (Bar=20 μM). (C) The CTCF analysis of three independent oocytes further support above observations. Data are mean±SEM of three independent experiments and analyzed by Student's t-test. *Significant (p<0.001) difference as compared to diplotene-arrested oocytes.
FIG. 7.
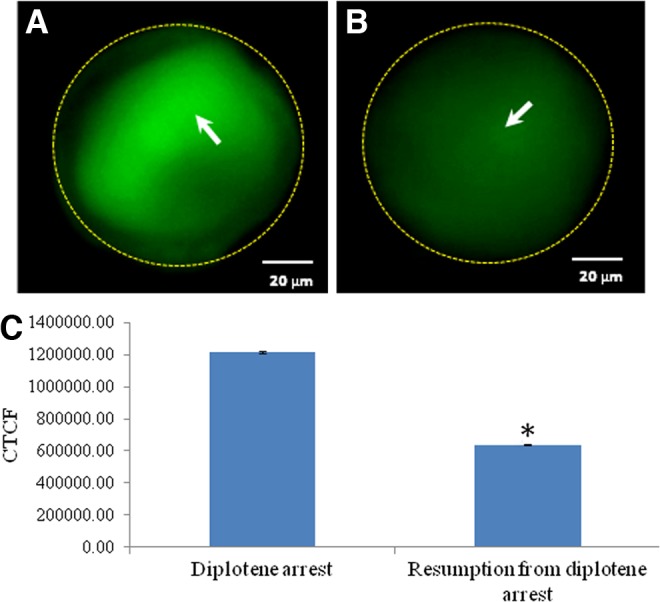
Representative photographs showing immunofluorescence intensity of Thr-161 phosphorylated CDK1 level in oocytes. Reduction of Thr-161 phosphorylated CDK1 (arrow) (B) is associated with spontaneous resumption of meiosis from diplotene arrest as compare to diplotene-arrested oocytes (arrow) (A). (Bar=20 μM). The CTCF analysis of three independent oocytes further support above observations (C). Data are mean±SEM of three independent experiments and analyzed by Student's t-test. *Significant (p<0.001) difference as compared to diplotene-arrested oocytes (Student's t-test).
FIG. 8.
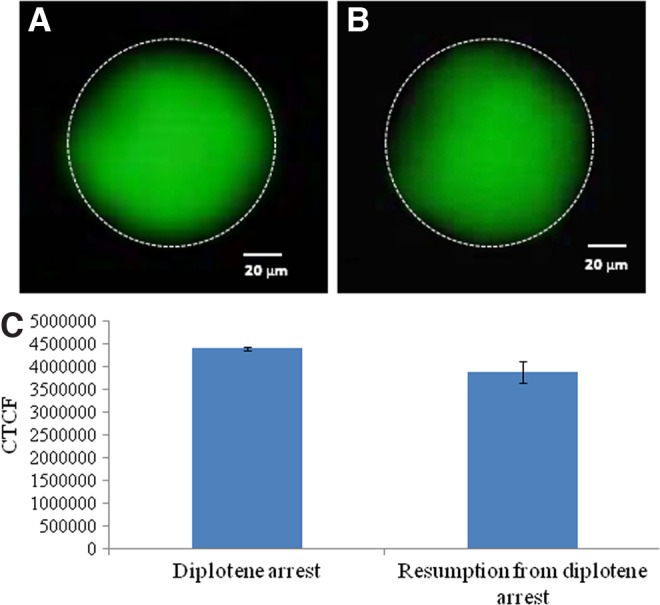
Representative photographs showing immunofluorescence intensity of phosphorylated CDK1 in oocytes. The total phosphorylation status of CDK1 remains unchanged during progression of meiotic cell cycle from diplotene arrest (arrow) (A) as compared to diplotene-arrested oocytes (B). (Bar=20 μM). (C) The CTCF analysis of three independent oocytes further support above observations. Data are mean±SEM of three independent experiments and analyzed by Student's t-test.
FIG. 9.
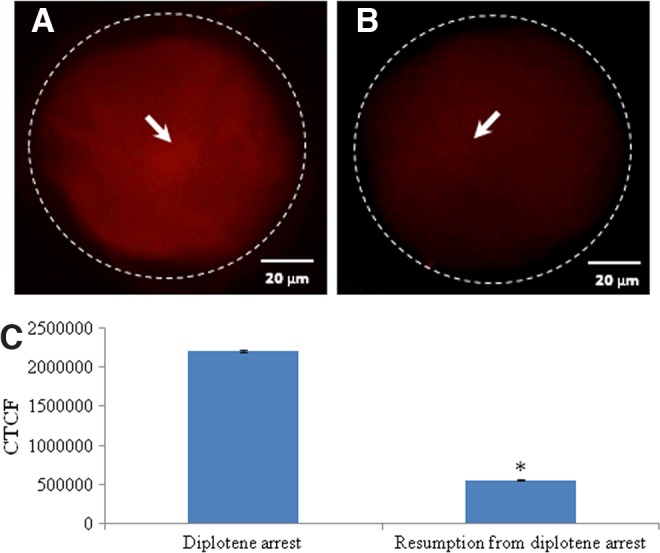
Representative photographs showing immunofluorescence intensity of cyclin B1 level in oocytes. Reduction in the levels of cyclin B1 (arrow) (B) are associated with spontaneous resumption of meiosis from diplotene arrest as compared to diplotene stage (A). (Bar=20 μM). The CTCF analysis of three independent oocytes further supports above observations (C). Data are mean±SEM of three independent experiments and analyzed by Student's t-test. *Significant (p<0.001) difference as compared to diplotene-arrested oocytes.
Discussion
It is well established that cAMP is one of the major intra-oocyte regulators of meiotic maturation in mammals.3,7 The continuous transfer of cAMP through gap junctions from encircling granulosa cells to the oocyte inside the follicle results in sustained high level of intra-oocyte cAMP level that maintains diplotene arrest for a long time inside the follicular microenvironment.5,7,9,10 Disruption in the gap junctions between cumulus cells and oocytes or removal of encircling cumulus cells from oocytes reduces the intra-oocyte cAMP level and leads to spontaneous resumption of meiosis from diplotene arrest in vitro.4,6,13 Our results revealed that the decrease of intra-oocyte cAMP was associated with spontaneous resumption of meiosis in denuded oocytes cultured in vitro, while a high level of cAMP is required for the maintenance of meiotic arrest at the diplotene stage.
A reduction of intra-oocyte cAMP level may induce the generation of ROS,16 particularly the production of intracellular H2O2.17,18 Hence, in the present study, we quantitated intra-oocyte level of H2O2 during spontaneous meiotic resumption from the diplotene stage in rat oocytes cultured in vitro. Our results suggest that the reduced level of intra-oocyte cAMP was associated with an increased level of H2O2 and spontaneous meiotic resumption from diplotene stage. The fluorescence analysis of DCF further strengthens our data that the increased level of intra-oocyte H2O2 triggers meiotic resumption from diplotene arrest. The beneficial role of the moderate level of H2O2 in inducing meiotic resumption20,36 and developmental potential have been reported for oocytes cultured in vitro.21,22 Although we have not studied the source of H2O2 generation in the present study, one recent study suggests the role of mitochondria in the generation of H2O2 in mammalian oocytes.8 In addition, catalase activity was significantly reduced in oocytes that underwent spontaneous meiotic resumption, further supporting our hypothesis that generation of ROS is beneficial for meiotic resumption from diplotene arrest.
A growing body of evidence suggests that the increase of a moderate level of H2O2 modulates the physiology of various cell types.8,37 Exogenous supplementation of 5 to 10 μM concentration of H2O2 triggers first polar body emission in rat oocytes cultured in vitro.20 Based on these findings, we hypothesized that exogenous supplementation of H2O2 may also trigger meiotic resumption from diplotene arrest. Data from the present study reveal that the exogenous supplementation of H2O2 induces meiotic resumption from diplotene arrest in a concentration- and time-dependent manner. On the other hand, cell-permeable antioxidants like BHA as well as db-cAMP inhibited H2O2-induced meiotic resumption in a concentration-dependent manner. These results corroborate previous observations that exogenous supplementation of H2O2 triggers meiotic resumption,20,38 and generation of ROS is beneficial for increasing the developmental potential of oocytes under in vitro culture conditions,38 while antioxidants reversibly inhibit meiotic resumption from diplotene arrest in vitro.19,22,23
A moderate increase of intracellular H2O2 can modulate the phosphorylation/dephosphorylation of certain amino acid sequences of CDK1.33 Recent studies have suggested that both the phosphorylation and dephosphorylation status of CDK1 as well as the dissociation and degradation of cyclin B1 are involved during exit from M-II arrest in mammalian oocytes.31,32 In somatic cells, phosphorylation at Thr-161 but not at Thr-14/Tyr-15 is required for maintenance of CDK1-cyclin B1 heterodimer.39 Based on these findings, we propose that the increased Thr-14/Tyr-15 phosphorylated CDK1 and reduced level of Thr-161 phosphorylated CDK1 may lead to dissociation and degradation of cyclin B1 and thereby spontaneous resumption of meiosis from diplotene arrest. Our results suggest that a significant increase of Thr-14/Tyr-15 phosphorylated CDK1 and decrease of Thr-161 phosphorylated CDK1 were associated with reduced cyclin B1 level during meiotic resumption from diplotene arrest. However, the total phosphorylation status of the CDK1 level remains unchanged, suggesting that increased Thr-14/Tyr-15 phosphorylation of CDK1 might have reduced the Thr-161 phosphorylation of CDK1. Changes in the phosphorylation status of CDK1 might have dissociated cyclin B1 from the MPF heterodimer and induced MPF destabilization. These data are in agreement with previous findings that high MPF activity is associated with the maintenance of meiotic arrest.29,31,32
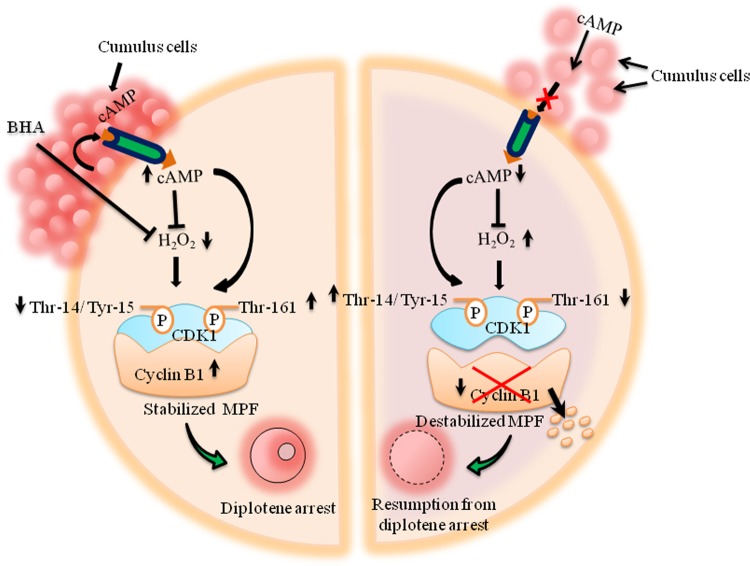
In summary, data from the present study suggest that the decrease of intra-oocyte cAMP induces the generation of H2O2, possibly by reducing catalase activity in oocytes. A moderate increase of H2O2 level induces phosphorylation of Thr-14/Tyr-15 but reduces Thr-161 phosphorylation of CDK1. Changes in the specific phosphorylation status of CDK1 triggered dissociation and degradation of cyclin B1 leading to MPF inactivation. The inactive MPF finally induces spontaneous meiotic resumption from diplotene arrest in rat oocytes cultured in vitro (Fig. 10). These data suggest that the increase of a moderate level of ROS under in vitro culture conditions could be one of the causative factors that trigger spontaneous resumption of meiosis during in vitro maturation or in vitro fertilization in several mammalian species including human.
FIG. 10.
Schematic hypothetical diagram showing possible involvement of cAMP and H2O2 during spontaneous meiotic resumption from diplotene arrest of rat oocyte. The decrease of intra-oocyte cAMP level results in the generation of H2O2. A rise of H2O2 induces Thr-14/Tyr-15 phosphorylated CDK1, and also reduces Thr-161 phosphorylated CDK1. Changes in the level of specific phosphorylation of CDK1 results in dissociation and degradation of cyclin B1, which finally triggers maturation promoting factor (MPF) destabilization. The destabilized MPFs lead to spontaneous meiotic resumption from diplotene arrest in rat eggs cultured in vitro.
Abbreviations Used
- BHA
3-t-butyl-4-hydroxyanisole
- cAMP
cyclic 3′,5′-adenosine monophosphate
- CDK1
cyclin-dependent kinase 1
- CTCF
corrected total cell fluorescence
- db-cAMP
dibutyryl-cAMP
- DCF-DA
2,7-dichlorodihydrofluorescein diacetate
- GV
germinal vesicle
- H2O2
hydrogen peroxide
- IBMX
3-isobutyl-1-methylxanthine
- MPF
maturation promoting factor
- NSB
nonspecific binding
- PBS
phosphate-buffered saline
- PMSG
pregnant mare's serum gonadotropin
- ROS
reactive oxygen species
- SEM
standard error of mean
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sirared MA. Resumption of meiosis: mechanism involved in meiotic progression and its relation with developmental competency. Theriogenology. 2001;55:1241–1254 [DOI] [PubMed] [Google Scholar]
- 2.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75 [DOI] [PubMed] [Google Scholar]
- 3.Mehlmann LM. Stops and starts in mammalian oocytes: Recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799 [DOI] [PubMed] [Google Scholar]
- 4.Rose RD, Gilchrist RB, Kelly JM, et al. Regulation of sheep oocyte maturation using cAMP modulators. Theriogenology. 2013;79:142–148 [DOI] [PubMed] [Google Scholar]
- 5.Chaube SK. Role of meiotic maturation regulatory factors in the meiotic competence of mammalian oocytes. Health and Population. 2001;24:218–231 [Google Scholar]
- 6.Chaube SK. Does cAMP act as a regulator for oocyte meiotic resumption in mammal? Health and Population. 2002;25:74–85 [Google Scholar]
- 7.Tripathi A, Premkumar KV, Chaube SK. Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol. 2010;223:592–600 [DOI] [PubMed] [Google Scholar]
- 8.Pandey AN, Tripathi A, Premkumar KV, et al. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem. 2010;111:521–528 [DOI] [PubMed] [Google Scholar]
- 9.Chaube SK, Chaki SP, Misro MM. Effects of pentoxifylline and caffeine on spontaneous maturation of rat oocytes. Health and Population. 2000;23:177–189 [Google Scholar]
- 10.Vaccari S, Weeks JL II, Hsieh M, et al. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Zhou B, Yan J, et al. Epidermal growth factor receptor activation by protein kinase C is necessary for FSH-induced meiotic resumption in porcine cumulus-oocyte complexes. J Endocrinol. 2008;197:409–419 [DOI] [PubMed] [Google Scholar]
- 12.Silvia Masciarelli S, Horner K, Liu C, et al. Cyclic nucleotide phosphodiesterase 3A–deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–1345 [DOI] [PubMed] [Google Scholar]
- 14.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805–810 [DOI] [PubMed] [Google Scholar]
- 15.Cetica PD, Pintos LN, Dalvit GC, et al. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51:57–64 [DOI] [PubMed] [Google Scholar]
- 16.Cheon YP, Kim SW, Kim SJ, et al. The role of RhoA in the germinal vesicle breakdown of mouse oocytes. Biochem Biophys Res Commun. 2000;273:997–1002 [DOI] [PubMed] [Google Scholar]
- 17.Piccoli C, Scacco S, Bellomo F, et al. cAMP controls oxygen metabolism in mammalian cells. FEBS Lett. 2006;580:4539–4543 [DOI] [PubMed] [Google Scholar]
- 18.Bellomo F, Piccoli C, Cocco T, et al. Regulation by the cAMP cascade of oxygen free radical balance in mammalian cells. Antioxid Redox Signal. 2006;8:495–502 [DOI] [PubMed] [Google Scholar]
- 19.Behrman HR, Kodaman PH, Preston SL, et al. Oxidative stress and ovary. J Soc Gynecol Investig. 2001;8:S40–S42 [DOI] [PubMed] [Google Scholar]
- 20.Chaube SK, Prasad PV, Thakur SC, et al. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis. 2005;10:863–874 [DOI] [PubMed] [Google Scholar]
- 21.Tripathi A, Khatun S, Pandey AN, et al. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res. 2009;43:287–294 [DOI] [PubMed] [Google Scholar]
- 22.Takami M, Preston SL, Toyloy VA, et al. Antioxidant reversibly inhibit the spontaneous resumption of meiosis. Am J Physiol Endocrinol Metab. 1999;276:E684–E688 [DOI] [PubMed] [Google Scholar]
- 23.Takami M, Preston SL, Behrman HR. Eicosatetraynoic and eicosatetraynoic acids, lipoxygenase inhibitors blockmeiosis via antioxidant action. Am J Physiol Cell Physiol. 2000;278:C646–C650 [DOI] [PubMed] [Google Scholar]
- 24.Forman HJ, Maiorino M, Ursin F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehennaut V, Lefebvre T, Sellier C, et al. O-linked N-acetylglucosaminyltransferase inhibition prevents G2/M transition in Xenopus laevis oocytes. J Biol Chem. 2007;282:12527–12536 [DOI] [PubMed] [Google Scholar]
- 26.Yamaura M, Mitsushita J, Furuta S, et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–2654 [DOI] [PubMed] [Google Scholar]
- 27.Verbon EH, Post JA, Boonstra J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene. 2012;511:1–6 [DOI] [PubMed] [Google Scholar]
- 28.Kubiak JZ, Ciemerych MA, Hupalowska A, et al. On the transition from the meiotic cell cycle during early mouse development. Int J Dev Biol. 2008;52:201–217 [DOI] [PubMed] [Google Scholar]
- 29.Madgwick S, Jones KT. How eggs arrest at metaphase-II: MPF stabilization plus APC/C inhibition equals cytostatic factor. Cell Div. 2007;2:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chesnel F, Bazile F, Pascal A, et al. Cyclin B dissociation from CDK1 precedes its degradation upon MPF inactivation in mitotic extracts of Xenopus laevis embryos. Cell Cycle. 2006;5:1687–1698 [DOI] [PubMed] [Google Scholar]
- 31.Oh JS, Susor A. Conti M. Protein tyrosine kinase Wee1B is essential for metaphase II exit in mouse oocytes. Science. 2011;332:462–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh JS, Susor A, Schindler K, et al. Cdc25A activity is required for the metaphase II arrest in mouse oocytes. J Cell Sci. 2013;126:1081–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aitken RJ, Harkiss D, Knox W, et al. A novel signal transduction cascade in capacitating human spermatozoa characterized by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci. 1998;111:645–656 [DOI] [PubMed] [Google Scholar]
- 34.Saller S, Merz-Lange J, Raffael S, et al. Norepinephrine, active norepinephrine transporter, and norepinephrine metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology. 2012;153:1472–1483 [DOI] [PubMed] [Google Scholar]
- 35.Tripathi A, Premkumar KV, Pandey AN, et al. Melatonin protects against clomiphene citrate-induced generation of hydrogen peroxide and morphological apoptotic changes in rat eggs. Eur J Pharmacol. 2011;667:419–424 [DOI] [PubMed] [Google Scholar]
- 36.Chaube SK, Tripathi A, Khatun S, et al. Extracellular calcium protects against verapamil-induced metaphase-II arrest and initiation of apoptosis in aged rat eggs. Cell Biol Int. 2009;33:337–343 [DOI] [PubMed] [Google Scholar]
- 37.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028 [DOI] [PubMed] [Google Scholar]
- 38.Blondin P, Coenen K, Sirard MA. The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation. J Androl. 1997;18:454–460 [PubMed] [Google Scholar]
- 39.Coulonval K, Kooken H, Roger PP. Coupling of T161 and T14 phosphorylations protects cyclin B–CDK1 from premature activation. Mol Biol Cell. 2011;22:3971–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]