Abstract
Objective
Microvascular dysfunction has been suggested to be a major pathogenic factor for the development of hypertension. We examined the association between retinal vascular caliber, a marker of systemic microvascular dysfunction, and incident hypertension on a meta-analysis of individual participant data.
Methods
We performed a systematic review with relevant studies identified through a search of electronic databases, a review of reference lists, and correspondence with experts. Studies were included if participants were selected from a general population, retinal vascular caliber was measured from photographs using computer-assisted methods at baseline, and individuals were followed up to ascertain the incidence of hypertension. Prespecified individual recorded data from six population-based prospective cohort studies were included. Discrete time proportional odds models were constructed for each study with adjustment for hypertension risk factors. Log odds ratios (ORs) per 20-μm difference were pooled using random-effects meta-analysis.
Results
Among 10 229 participants without prevalent hypertension, diabetes, or cardiovascular disease, 2599 developed new-onset hypertension during median follow-up periods ranging from 2.9 to 10 years. Both narrower retinal arterioles [pooled multivariate-adjusted OR per 20-μm difference 1.29, 95% confidence interval (CI) 1.20–1.39] and wider venules (OR per 20-μm difference 1.14, 95% CI 1.06–1.23) were associated with an increased risk of hypertension. Each 20 μm narrower arterioles at baseline were associated with a 1.12 mmHg (95% CI 0.25–1.99) greater increase in SBP over 5 years.
Conclusions
Retinal arteriolar narrowing and venular widening were independently associated with an increased risk of hypertension. These findings underscore the importance of microvascular remodeling in the pathogenesis of hypertension.
Keywords: hypertension, meta-analysis, microvascular dysfunction
Introduction
Hypertension is a major risk factor for cardiovascular diseases (CVDs) and premature mortality, affecting nearly 1 billion people [1]. The key mechanisms underlying the pathogenesis of hypertension remain a major focus of research. It has been hypothesized that microvascular dysfunction, characterized by structural and functional abnormalities of small vessels, or either of them, precedes and may be a causal component of hypertension, but the clinical evidence to support this has been sparse [2,3]. Elucidating the role of microvascular dysfunction in the initiation of hypertension is important given its potentially modifiable nature with appropriate pharmacological treatment [2].
The caliber of the retinal blood vessels measures 150– 300 μm, and provides a novel model to study correlates and consequences of generalized microvascular dysfunction [4–6]. Retinal vascular caliber can be assessed noninvasively from retinal photographs and computer-assisted approaches [7]. Several prospective, population-based cohort studies [8–15] have found that retinal vascular caliber changes, particularly narrower retinal arterioles, predict incident hypertension. However, there remain several important gaps in the literature. First, some previous studies [8,9] have used ratio of the caliber of arterioles to venules [arteriolar-to-venular diameter ratio (AVR)] as the sole index of the severity of generalized arteriolar narrowing. However, retinal arteriolar and venular calibers themselves carry different prognostic information [16], and use of a summary measure such as AVR may fail to differentiate specific pathophysiological changes in arterioles or venules and thus lead to incorrect inferences. For example, a smaller AVR could be either because of arteriolar narrowing or venular widening or both. When both arteriolar and venular vessel calibers are associated with hypertension but in opposite directions, AVR may substantially exaggerate or mask the apparent magnitude of the association with arteriolar caliber [16]. Second, some studies [12,17] suggest that in addition to arteriolar narrowing, retinal venular widening may be independently associated with risk of hypertension, but others [13,14] have found no association. Retinal venular widening has been shown to be related to systemic inflammation, measures of atherosclerosis, and metabolic abnormalities [18]. Thus far, there remains uncertainty over whether hypertension is associated exclusively with narrower retinal arterioles or is also associated with wider venules and the pattern and magnitude of these associations. Third, cross-sectional studies [19,20] suggest the relationship of retinal arteriolar narrowing and blood pressure (BP) is weaker in older people. However, it has not been demonstrated if such age interaction is present in prospective studies of retinal vascular caliber and incident hypertension.
To address these gaps, we performed a collaborative meta-analysis of individual participant data from published and unpublished studies to provide robust estimates of the associations of retinal vascular caliber changes with incident hypertension, incorporating adjustment for confounding caused by established conventional risk factors. We also examined whether these associations differ in younger and older persons.
Methods
Study selection and data extraction
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [21] guidelines throughout the design, implementation, analysis, and reporting. The criteria for study inclusion were that the study contained participants derived from a general population, retinal vascular caliber was measured at baseline from either digitized 35 mm photographic film or digital images using computer-assisted methods, and individuals were followed up to ascertain incident hypertension. A literature search (up to November 2012) was conducted of MEDLINE and EMBASE. Reference lists and conference proceedings also were searched to identify possible additional studies. The following search terms were used: ‘[exp retinal diseases/, retinopathy.tw., (retina or retinal).tw., microvessel.mp. or microvascular.tw., vessel.mp. or vascular.tw., arteriole.mp. or arteriolar.tw., venule.mp. or venular.tw.]’, ‘(exp hypertension/, blood pressure.tw.)’, and ‘(exp epidemiology/, exp epidemiologic studies/, incidence/, cohort$.tw,or cohort studies/)’. Further studies and unpublished data were sought by discussion between collaborators. Possible studies for inclusion were independently assessed for suitability by two authors (J.D. and T.Y.W.) and any lack of clarity or disagreement was resolved by discussion.
The principal investigators were invited to participate in this collaborative project and, following acceptance, were requested to provide original recorded data on individual demographic characteristics (e.g. age, sex, race/ethnicity, height, and weight), baseline and serial measurement on cardiovascular factors (e.g. SBP and DBP, serum total cholesterol, blood glucose, the presence of diabetes, and current smoking status), medication use and history of CVD, details of assessment of retinal vascular calibre, and hypertension. Because data on parental history of hypertension were available only in subsets of the participants and measures of physical activity were highly variable across studies, these variables were not included. The participants included in the present analysis had no prevalent hypertension or CVD, had values for retinal arteriolar and venular calibers recorded at baseline, and information of follow-up duration. We also excluded people with diabetes mellitus from this meta-analysis because lower cut-points of SBP and DBP define the target BP goal among this group [1] and diabetic changes might complicate the interpretation of the associations of retinal vascular caliber with hypertension [22]. For these purposes, diabetes was defined as a fasting serum glucose at least 7.0 mmol/l, 2-h plasma glucose at least 11.1mmol/l, hemoglobin A1c above 6.5%, use of diabetic medication, or a physician's diagnosis.
Retinal vascular caliber measurements
Retinal vascular caliber was measured in a similar manner in each study with some slight variations [23,24] (Table I in Data Supplement, http://links.lww.com/HJH/A284). Briefly, a prespecified eye (usually the right eye) was used for analysis of retinal vascular caliber. Trained graders, who were masked to participant characteristics, viewed the photographs centered on the optic and measured the diameters of all arterioles and venules that coursed through a zone surrounding the optic disc, one-half to one disc diameter away from the optic disc margin, by using a semiautomated computer program. Per cohort, we used the summarized measures of the central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE), respectively, calculated from individual mean retinal vascular calibers and representing ‘average’ diameters. All studies used Parr–Hubbard formula [25] to summarize the individual calibers except for one study [12], which applied Knudtson's revised formula [26]. Reproducibility statistics were high for CRAE and CRVE measurements, with intragrader and intergrader reliability correlation coefficients ranging from 0.69 to 0.99 [18].
Hypertension ascertainment
The sampling and hypertension identification procedures were all protocol-driven, although differences existed in age ranges (Table 1), period of data collection (Table 1), and BP assessment methods (Table II in Data Supplement, http://links.lww.com/HJH/A284). Most studies used the average of two consecutive, seated readings at a single visit to define BP, whereas two studies [11,14] measured BP once and recorded a single reading only. Incident hypertension was defined as first occurrence at any follow-up examination of SBP at least 140mmHg or DBP at least 90 mmHg or of the person initiating treatment with antihypertensive medications. Prehypertension was defined, for those who were free of hypertension at baseline, as an SBP of 120–139 mmHg or a DBP of 80–89 mmHg [1]. Mean arterial BP (MABP) at baseline was calculated as: 1/3 × SBP + 2/3 × DBP.
Table 1. Baseline characteristics of participants in the six cohort studies (n = 10229).
| No. (%) of participants by study | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| ARIC [8] | MESA [12] | BMES [10,11] | AusDiab [34] | Funagata [14] | BDES [9] | |
| No. of baseline populationa | 4884 | 2303 | 626 | 425 | 306 | 1685 |
|
| ||||||
| No. of incident cases of hypertension during follow-up | 679 | 445 | 404 | 70 | 101 | 900 |
|
| ||||||
| Follow-up durations after retinal measurement, median (IQR) (years) | 2.9 (2.8–3.0) | 3.1 (2.9–3.3) | 5.0 (5.0–10.0) | 5.0 (4.9–5.1) | 5.0 (5.0–5.0) | 10.0 (5.0–20.0) |
|
| ||||||
| Year of baseline data collection | 1993–1995 | 2002–2004 | 1992–1994 | 1999–2000 | 2000–2002 | 1988–1990 |
|
| ||||||
| Year of most recent follow-up | 1996–1998 | 2006–2008 | 2002–2004 | 2004–2005 | 2005–2007 | 2008–2010 |
|
| ||||||
| No. of follow-up visits | 1 | 2 | 2 | 1 | 1 | 4 |
|
| ||||||
| Men | 2086 (42.7) | 1098 (47.7) | 286 (45.7) | 155 (36.5) | 123 (40.2) | 734 (43.6) |
|
| ||||||
| Ethnicity | ||||||
| White | 4352 (89.1) | 1040 (45.2) | 626 (100) | 425 (100) | 0 | 1685 (100) |
| Black | 532 (10.9) | 437 (19.0) | 0 | 0 | 0 | 0 |
| Asian | 0 | 328 (14.2) | 0 | 0 | 306 (100) | 0 |
| Hispanic/others | 0 | 498 (21.6) | 0 | 0 | 0 | 0 |
|
| ||||||
| CRAE mean (SD) (μm) | 164.8 (16.6) | 146.5 (13.7) | 192.4 (16.9) | 181.9 (23.0) | 181.3 (20.6) | 152.9 (14.4) |
|
| ||||||
| CRVE mean (SD) (μm) | 192.3 (16.3) | 213.8 (20.9) | 227.1 (19.1) | 210.0 (22.5) | 214.6 (19.9) | 231.2 (22.2) |
|
| ||||||
| Age at baseline mean (SD) (years) | 58.8 (5.4) | 59.5 (9.2) | 60.9 (7.7) | 50.6 (11.6) | 54.8 (10.7) | 57.3 (9.7) |
|
| ||||||
| Age range (years) | 49–71 | 46–86 | 49–85 | 25–87 | 35–80 | 43–85 |
|
| ||||||
| Age group (years) | ||||||
| <60 | 3115 (63.8) | 1360 (59.1) | 325 (51.9) | 334 (78.6) | 206 (67.3) | 1090 (64.7) |
| 60–69 | 1648 (33.7) | 570 (24.8) | 216 (34.5) | 67 (15.8) | 71 (23.2) | 377 (22.4) |
| ≥70 | 121 (2.5) | 373 (16.2) | 85 (13.6) | 24 (5.6) | 29 (9.5) | 218 (13.0) |
|
| ||||||
| SBP mean (SD) (mmHg) | 114.3 (12.0) | 112.4 (12.8) | 125.0 (8.6) | 121.4 (10.9) | 116.9 (10.6) | 118.8 (11.1) |
|
| ||||||
| DBP mean (SD) (mmHg) | 68.5 (8.5) | 67.9 (8.7) | 76.8 (6.9) | 66.2 (9.7) | 71.3 (7.6) | 74.0 (8.1) |
|
| ||||||
| Prehypertension | 1610 (32.9) | 666 (28.9) | 422 (67.4) | 239 (56.2) | 115 (37.6) | 889 (52.8) |
|
| ||||||
| No. of blood pressure readings per visit | 3 | 3 | 1 | 3 | 1 | 3 |
|
| ||||||
| Serum total cholesterol mean (SD) (mmol/l) | 5.3 (0.9) | 5.0 (0.9) | 6.0 (1.1) | 5.5 (1.1) | 5.1 (0.9) | 5.9 (1.1) |
|
| ||||||
| BMI mean (SD) (kg/m2) | 27.0 (4.6) | 27.2 (5.0) | 25.3 (3.7) | 26.69 (4.9) | 23.3 (3.0) | 27.6 (4.8) |
|
| ||||||
| BMI category | ||||||
| <25.0 | 1783 (36.5) | 850 (36.9) | 314 (50.6) | 184 (43.7) | 225 (73.5) | 541 (32.2) |
| 25.0–29.0 | 1752 (35.9) | 771 (33.5) | 226 (36.5) | 122 (29.0) | 71 (23.2) | 591 (35.2) |
| ≥30.0 | 1349 (27.6) | 682 (29.6) | 80 (12.9) | 115 (27.3) | 10 (3.3) | 549 (32.7) |
|
| ||||||
| Current smoker | 886 (18.1) | 350 (15.2) | 89 (14.2) | 49 (11.5) | 58 (19.0) | 400 (23.7) |
ARIC, Atherosclerosis Risk in Communities; AusDiab, Australian Diabetes, Obesity and Lifestyle; BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; IQR, interquartile range; MESA, Multi-Ethnic Study of Atherosclerosis.
No history of hypertension, diabetes or cardiovascular disease, retinal vascular caliber available at baseline, and follow-up data available.
Statistical analyses
Both CRAE and CRVE were normally distributed and the pooled within-study SD was approximately 20 μm for CRAE and CRVE. For each study, we fitted the discrete time proportional odds models for interval-censored data for incident hypertension. Each model estimated the odds ratios (ORs) associated with CRAE and CRVE. Model 1 adjusted for age, sex, and ethnicity. Model 2 included variables from model 1 and also adjusted for cardiovascular risk factors including BMI, current smoking status, and total cholesterol level, and model 3 included variables from model 2 and baseline SBP. These risk factors were chosen to be included in the models mainly because they were the major components in the Framingham Hypertension Risk Score [27] and were also measured consistently across the studies. We included both CRAE and CRVE simultaneously in the same model in order to control for the shared variance between the fellow variables [16]. We pooled the log OR estimates of the different studies by random-effects meta-analysis [28] and displayed them in forest plots. The extent of heterogeneity between studies was evaluated with the inconsistency I statistic [29]. Sensitivity of the above two-stage analyses results (with estimates of association calculated separately within each study before data from different studies were pooled) was assessed by fitting a one-stage multilevel discrete time (logistic regression) model to pooled data with study as the random effect [30]. The one-stage approach was also used to investigate exposure–covariate interactions because this approach provides a flexible way of examining individual-level interactions [31].
To assess the pattern of associations, study-specific ORs calculated within overall quartiles of baseline CRAE and CRVE were pooled on a log scale by multivariate random-effects meta-analysis and plotted against mean CRAE and CRVE within each quartile, respectively. The 95% confidence intervals (CIs) were estimated from variances reflecting the amount of information within each group including the reference group. When associations were approximately log-linear, regression coefficients were calculated to estimate the ORs per 20 μm difference in CRAE and 20 μm difference in CRVE (as lower values for CRAE and higher values for CRVE).
To corroborate our analyses, we did several supplementary analyses. To account for differential effects of baseline level of risk factors, we stratified the multilevel effects logistic models for age group (<60, ≥60 years), sex, ethnicity (white, non-white), BMI category (<25, 25–29, ≥30 kg/m2), current smoking, and prehypertension status. Because most studies provided one follow-up measurement of BP, we examined the association of baseline CRAE and CRVE with change in SBP between the two occasions by fitting a linear mixed regression model for each study. The model contained a term measuring the association between retinal vascular caliber and baseline SBP, and a separate term measuring the association between retinal vascular caliber and the rate of change in SBP over time (i.e. interaction term between time interval and CRAE or CRVE). We pooled the coefficient estimates (β), respectively, using random-effects meta-analysis. To account for the influence of antihypertensive medication use on the SBP trajectory, a constant of 10 mmHg was added to SBP values of people with incident hypertension who were on antihypertensive treatment, assuming that treatment effects are the same across age, period, and cohort [32,33]. We indirectly corrected for regression dilution bias using follow-up measurements of CRAE and CRVE from one study [9], which provided a guide to the approximate mean ‘usual’ CRAE and CRVE levels over time that could be applied to other studies [34]. Finally, we used multiple multivariate imputation of variables with five imputed datasets per study to replace missing values, amounting to 1% of total values, for baseline risk factors (Stata ice/micombine procedures) [35]. These analyses were performed using Stata 10.1SE (Stata-Corp, College Station, Texas, USA), two-sided P values (<0.05 considered statistically significant) and 95% CI.
Results
The literature search yielded 3209 citations from which seven studies [8–14,36] fulfilled the inclusion criteria (Figure I in Data Supplement, http://links.lww.com/HJH/A284). Six of these studies [8–12,14,36] provided individual participant data and were included. The studies were based in the United States, Australia, and Japan, and comprised predominantly white populations, except for the Funagata study (Japanese) [14]. The populations in the Atherosclerosis Risk in Communities (ARIC) study [8] and the Multi-Ethnic Study of Atherosclerosis (MESA) [12] comprised 11 and 19% blacks, respectively. Additionally in the MESA, 22% were Hispanic and 14% Chinese. The characteristics of the participants in the studies at baseline when CRAE and CRVE were measured are shown in Table 1. A total of 10 229 participants without known hypertension, diabetes, or CVD at baseline were included. Of these, 44% were men and the mean age at baseline in the studies ranged from 50 to 60 years. In some contributing studies, the follow-up BP measurements occurred in a narrow time interval [12], but in others, the measurements spanned a long period [9,11,14]. During median follow-up periods ranging from 2.9 to 10 years across studies, 2599 developed hypertension. The mean (SD) CRAE at baseline ranged from 146.5 μm (13.7) to 192.4 μm (16.9), and CRVE from 192.3 μm (16.3) to 231.2 μm (22.2).
Central retinal arteriolar equivalent and risk of hypertension
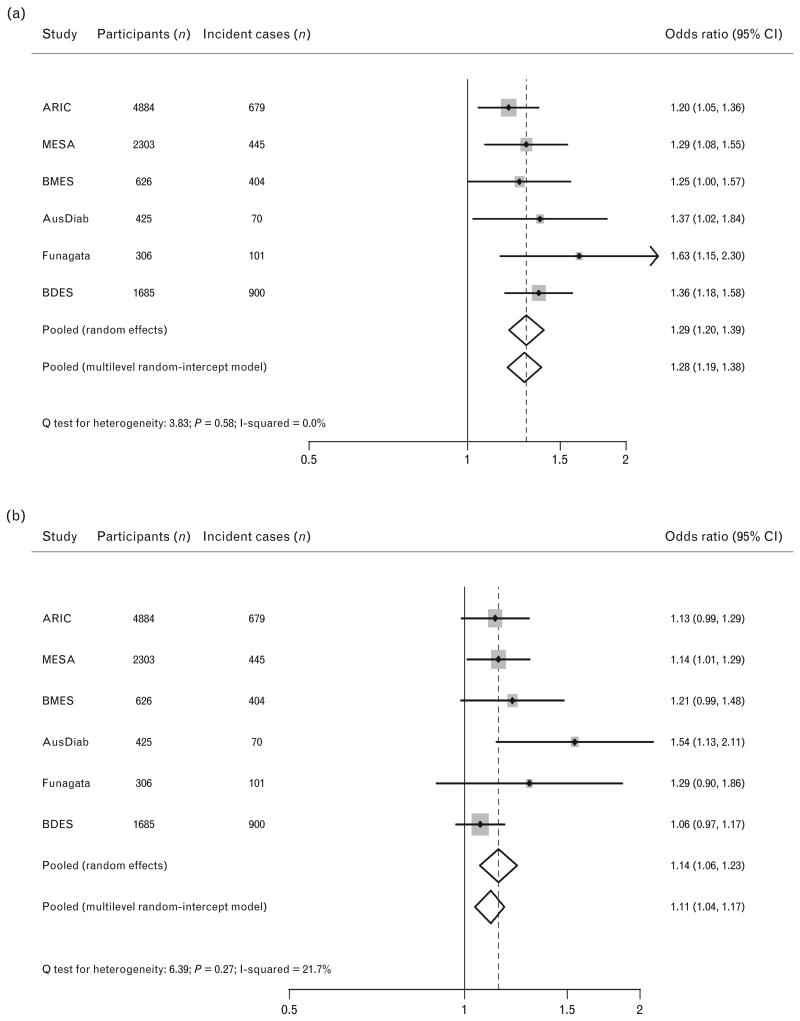
There were linear inverse associations between baseline CRAE and risk of hypertension across the range of values (Figure IIa in Data Supplement, http://links.lww.com/HJH/A284). The study-specific ORs for CRAE and incident hypertension in the fully adjusted model (model 3) are displayed in Fig. 1a. After adjustment for age, sex, and race (model 1), the overall estimated OR per 20-μm narrower CRAE was 1.65 (95% CI 1.53, 1.79) for hypertension (Table III in Data Supplement, http://links.lww.com/HJH/A284). The OR was not appreciably altered after additional adjustment for current smoking status, BMI, and total cholesterol level. When further adjusted for baseline SBP, the OR decreased to 1.29 (95% CI 1.20, 1.39). We observed no heterogeneity in the ORs between studies for CRAE (Q test of heterogeneity P value 0.58; I2 0.0%) in the fully adjusted model.
Figure 1.
Multivariate-adjusted odds ratios (ORs) (model 3) for risk of incident hypertension per 20 μm decrease in CRAE (a), per 20 μm increase in CRVE (b). The size of the data markers is proportional to weight of the study in the random effect meta-analysis. The error bars indicate 95% CIs. Pooled using random effects and one-stage multilevel random-intercept models. CI, confidence interval; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent.
Central retinal venular equivalent and risk of hypertension
Central retinal venular equivalent was positively and linearly associated with incident hypertension (Figure IIb in Data Supplement, http://links.lww.com/HJH/A284). Figure 1b and Table III (Data Supplement, http://links.lww.com/HJH/A284) show the same analyses for CRVE. The overall estimated OR per 20-mm larger CRVE for hypertension was 1.28 (95% CI 1.21, 1.36) in model 1. Adjustment for several vascular risk factors did not appreciably alter OR (1.23, 95% CI 1.16, 1.31) and the OR attenuated to 1.14 (95% CI 1.06, 1.23) when also adjusted for baseline SBP. We observed no significant heterogeneity in the ORs across studies for CRVE (Q test of heterogeneity P value 0.27; I2 21.7%) in model 3.
Supplementary analysis
Results were similar for both CRAE and CRVE when using combined individual record data from all studies and fitting a multilevel model with a random intercept to account for study-specific effect (Fig. 1). Similar results to those reported here were also observed in several supplementary analyses. Table IV (Data Supplement, http://links.lww.com/HJH/A284) shows the overall estimated ORs for CRAE, CRVE, and hypertension, respectively, by baseline characteristics after adjustment for vascular risk factors (model 3). We observed a significant interaction between CRAE and age (P=0.03) and the association of narrower CRAE with hypertension was strongest in participants younger than 60 years (OR per 20-μm difference 1.44, 95% CI 1.30, 1.60), but diminished and became nonsignificant in those aged 70 years and older (per 20-μm difference 0.99, 95% CI 0.80, 1.21). There was little evidence that the magnitude of the associations between retinal vascular caliber and hypertension was importantly influenced by sex, race, prehypertension status, current smoking status, and BMI categories. The adjustment using baseline MABP or the average of the baseline and prior SBP instead of baseline SBP did not qualitatively change the associations observed. When corrected concurrently for regression dilution in CRAE and CRVE, the hypertension associations were strengthened for both CRAE (OR per 20-μm difference 1.41, 95% CI 1.28, 1.56) and CRVE (per 20-μm difference 1.18, 95% CI 1.08, 1.31) in model 3. Analyses on the imputed datasets yielded results similar to those reported in the main analysis (results available on request).
Table V (Data Supplement, http://links.lww.com/HJH/A284) presents the relationships of CRAE and CRVE with baseline SBP and the change in SBP over time. Narrower CRAE was associated with higher SBP at baseline [per 20-μm difference: pooled estimated regression coefficient (β)=2.99 mmHg, 95% CI 1.56, 4.42] and with a 1.12 mmHg (95% CI 0.25, 1.99) greater 5-year increase in SBP. Larger CRVE was associated with higher baseline SBP (per 20-μm difference: pooled β=1.38 mmHg, 95% CI 0.73, 2.04), but not with increase in SBP over time.
Discussion
The present individual participant meta-analysis involving six prospective cohort studies among 10229 individuals provides the first reliable demonstration and confirmatory evidence that changes in retinal vascular caliber, specifically retinal arteriolar narrowing and venular widening, are associated with an increased risk of hypertension, developing over median follow-up periods of 2.9–10 years. The associations persist in those with optimal BP at baseline. On the basis of baseline and the follow-up SBP measurements, each 20-μm decrease on retinal arteriolar caliber at baseline is associated with a 1.12 mmHg greater increase in SBP over 5 years. These findings are consistent with the hypothesis that generalized microvascular dysfunction, seen in the retinal vasculature, precedes the onset and development of hypertension.
Our access to individual-level longitudinal data has enabled minimization of potential bias, standardization of statistical methods, analysis of clinically relevant subgroups, and consistent comparison through ‘harmonization’ of individual records across studies. Our findings therefore clarify a major gap in the pathophysiological understanding of hypertension and support previously reported evidence that diffuse retinal arteriolar narrowing, reflecting generalized arteriolar remodeling, antedates clinically manifest hypertension and is associated with future increases in BP levels. As a precursor of hypertension, increased peripheral vascular resistance resides primarily in small arteries (<350 μm of lumen diameter) and arterioles (10–150 μm of lumen diameter). Thus, it seems likely that narrowing of small arteries and arterioles (and greater peripheral resistance) might contribute to an elevation of BP, eventually leading to hypertension, and a dynamic ‘vicious cycle’ may exist in which the microcirculation maintains or even amplifies an initial increase in BP [37]. However, it has been proposed that in the absence of the abnormal renal function, increased peripheral resistance only temporarily raises BP, to be followed by an increase in renal sodium excretion restoring BP toward normal [3]. Importantly, retinal microvascular remodeling may resemble those in small resistance arteries of other end organs [38] and subtle renal arteriolar dysfunction [39] may reconcile the proposal with the putative role of arteriolar narrowing in the development of hypertension.
Our meta-analysis confirms findings in some studies [12,17] which have reported an association between retinal venular widening and incident hypertension after controlling for arteriolar caliber. Retinal venular widening has been hypothesized to be a general marker of retinal ischemia and hypoperfusion secondary to microvascular rarefaction [40–42]. The ensuing reduction in blood vessels can cause a significant increase in peripheral vascular resistance and can exacerbate an initial increase in blood flow or pressure. In addition, retinal venular widening may reflect endothelial dysfunction [43] and thereby increase leukocyte adhesion, which, in turn, is involved in microvascular remodeling [42,44]. However, our findings, together with other well established links with incident impaired fasting glucose [45], coronary heart disease [23], and stroke [24], suggest that retinal venular widening may have pleiotropic associations with cardiovascular risk factors and diseases, but not be a specific biomarker for hypertension.
Our analysis demonstrated an age interaction, specifically that the association of arteriolar narrowing with hypertension was more prominent in younger people (<60 years), and this association diminished with increasing age and became nonsignificant in those aged 70 years and older. This finding extends previous cross-sectional studies [19,20] that suggest the relationship of retinal arteriolar narrowing and BP is weaker in older people. The diminution of arteriolar narrowing–hypertension association with age could reflect increased retinal arteriosclerosis limiting the degree of vasoconstriction in older people, measurement of errors from poorer retinal image quality due to lens opacity, or selective survival. These findings may suggest that preventive and treatment strategies in hypertension that are targeted specially at improving microvascular structure (e.g. administration of agents with vasodilator effects) may be more useful in younger than older people.
The study has the limitations of observational research and there remains scope in these estimates for residual bias due to unmeasured (e.g. dietary factors and inflammation markers) or imprecisely measured confounding factors (e.g. long-term average SBP). The results of our analysis were based on single-occasion retinal measurements, and lack of information on serial measurements in most studies hampered the direct correction for regression dilution bias, which may have led to an underestimate of the true associations. All studies except MESA [12] used the Parr–Hubbard formula to summarize retinal vascular caliber. However, we do not have any reason to believe that a revised formula used in the study would affect the estimated associations [26]. Two studies [11,14] used a single reading to define BP, which exhibited significant terminal digit preference, may have resulted in nondifferential misclassification bias and our findings thus would also be an underestimate of the true associations. Excluding the two studies from our analyses did not essentially change the results. Whereas the definition of hypertension was the same among the studies, the clinical diagnosis of hypertension may have varied between the studies and over time. For a large proportion in each of the cohorts, the diagnosis was based on doctors' prescribed medication, which often was as a result of following different guidelines at the time they were prescribing the drugs. The possibility that confounding from drugs that may have affected retinal vascular caliber was not controlled for in the present analyses. Whereas people with higher BP at baseline may be more likely to develop hypertension, high baseline BP levels may be the consequence of more rapid increases in BP before the study's baseline measurement [46]. Furthermore, the changes in retinal vascular caliber at baseline could have incorporated influences or damages from various insults over lifetime, including BP levels even within ‘normal’ ranges. When participants were stratified according to prehypertension status, the associations were similar among those with optimal BP at baseline.
Narrower retinal arterioles have been found to be a predictor for cardiovascular mortality [47], and it is possible that people lost to follow-up may have died of CVD or developed hypertension before they participated in the follow-up examination(s), thereby leading to conservative estimates of associations. We compared the baseline characteristics between participants and nonparticipants in each study, and found there were no major differences in baseline retinal or BP measures. The heterogeneity of length of follow-up time across studies may have a potential impact on the associations. We did not test for this formally by performing a meta-regression because the small number of participating studies precluded a meaningful analysis. For a similar reason, we did not test for publication bias by assessing funnel plot asymmetry. However, we identified all known population studies to date that have measured retinal vascular caliber and BP outcome, and were also able to include unpublished data from some studies [Australian Diabetes Obesity and Lifestyle (AusDiab) study]. We could not obtain data from the Rotterdam study [13]; however, the principal analysis was repeated including published estimates from the Rotterdam study, which provided much the same overall results. We thus think that publication bias is unlikely to be present in the current study.
In conclusion, our meta-analysis showed that changes in retinal vascular caliber, specifically retinal arteriolar narrowing and venular widening, are independently and significantly associated with an increased risk of incident hypertension. Our findings provide robust confirmatory evidence that generalized microvascular dysfunction, seen by remodeling of the retinal vasculature, precedes the development of hypertension. These findings may lead to new preventive and treatment strategies in hypertension that are targeted specially at improving microvascular structure and tissue perfusion, for example administration of agents with vasodilator, antioxidative, and anti-inflammatory effects.
Supplementary Material
Acknowledgments
We used restricted access datasets of the Atherosclerosis Risk in Communities (ARIC) study and the Multiethnic Study of Atherosclerosis (MESA). The authors thank the staff and participants of the ARIC study for their important contributions. The MESA was supported by the National Heart, Lung and Blood Institute (Bethesda, Maryland, USA) and the National Eye Institute, USA. This article does not necessarily convey the opinions or views of the ARIC study, MESA, or the National Heart, Lung and Blood Institute. The authors also thank the staff and participants of all studies for their important contributions.
The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN26820 1100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The Beaver Dams Eye Study was supported by the National Eye Institute, National Institutes of Health Grant EY-06594, and by Research to Prevent Blindness New York, New York. The Blue Mountains Eye Study was supported by the Australian National Health & Medical Research Council. The Australian Diabetes, Obesity and Lifestyle (AusDiab) study was supported by National Health and Medical Research Council Grants 350448 and 233200, and a Sylvia and Charles Viertel Charitable Foundation grant. The Funagata study was supported by a grant-in-aid from the Global Century Center of Excellence (COE) program of the Japan Society for the Promotion of Science.
Sources of funding: The study was funded by grants from the National Medical Research Council, Singapore (STaR/0003/2008 and CG/SERI/2010), which had no role in study design, data analysis, or interpretation of results.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- AusDiab
Australian Diabetes Obesity and Lifestyle
- AVR
arteriolar-to-venular diameter ratio
- BDES
Beaver Dam Eye Study
- BMES
Blue Mountains Eye Study
- CRAE
central retinal arteriolar equivalent
- CRVE
central retinal venular equivalent
- CVD
cardiovascular disease
- IQR
interquartile range
- MABP
mean arterial blood pressure
- MESA
Multi-Ethnic Study of Atherosclerosis
- OR
odds ratio
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:736–741. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 3.Serné EH, de Jongh RT, Eringa EC, IJzerman RG, Stehouwer CD. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50:204–211. doi: 10.1161/HYPERTENSIONAHA.107.089680. [DOI] [PubMed] [Google Scholar]
- 4.Struijker-Boudier HA, Heijnen BF, Liu YP, Staessen JA. Phenotyping the microcirculation. Hypertension. 2012;60:523–527. doi: 10.1161/HYPERTENSIONAHA.111.188482. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann MV, Schmieder RE. Remodeling of retinal small arteries in hypertension. Am J Hypertens. 2011;24:1267–1273. doi: 10.1038/ajh.2011.166. [DOI] [PubMed] [Google Scholar]
- 6.Khavandi K, Arunakirinathan M, Greenstein AS, Heagerty AM. Retinal arterialhypertrophy:thenewLVH? Curr Hypertens Rep. 2013;15:244–252. doi: 10.1007/s11906-013-0347-2. [DOI] [PubMed] [Google Scholar]
- 7.Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging. 2008;1:156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Klein BE, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 9.Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329:79. doi: 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith W, Wang JJ, Wong TY, Rochtchina E, Klein R, Leeder SR, Mitchell P. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension. 2004;44:442–447. doi: 10.1161/01.HYP.0000140772.40322.ec. [DOI] [PubMed] [Google Scholar]
- 11.Wang JJ, Rochtchina E, Liew G, Tan AG, Wong TY, Leeder SR, et al. The long-term relation among retinal arteriolar narrowing, blood pressure, and incident severe hypertension. Am J Epidemiol. 2008;168:80–88. doi: 10.1093/aje/kwn100. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki R, Cheung N, Wang JJ, Klein R, Klein BE, Cotch MF, et al. Retinal vessel diameters and risk of hypertension: the Multiethnic Study of Atherosclerosis. J Hypertens. 2009;27:2386–2393. doi: 10.1097/HJH.0b013e3283310f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A, de Jong PT. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension. 2006;47:189–194. doi: 10.1161/01.HYP.0000199104.61945.33. [DOI] [PubMed] [Google Scholar]
- 14.Tanabe Y, Kawasaki R, Wang JJ, Wong TY, Mitchell P, Daimon M, et al. Retinal arteriolar narrowing predicts 5-year risk of hypertension in Japanese people: the Funagata study. Microcirculation. 2010;17:94–102. doi: 10.1111/j.1549-8719.2009.00006.x. [DOI] [PubMed] [Google Scholar]
- 15.Chew SK, Xie J, Wang JJ. Retinal arteriolar diameter and the prevalence and incidence of hypertension: a systematic review and meta-analysis of their association. Curr Hypertens Rep. 2012;14:144–151. doi: 10.1007/s11906-012-0252-0. [DOI] [PubMed] [Google Scholar]
- 16.Liew G, Sharrett AR, Kronmal R, Klein R, Wong TY, Mitchell P, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48:52–57. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- 17.Liew G, Wong TY, Mitchell P, Wang JJ. Are narrower or wider retinal venules associated with incident hypertension? Hypertension. 2006;48:e10. doi: 10.1161/01.HYP.0000231652.97173.4c. [DOI] [PubMed] [Google Scholar]
- 18.Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Kaushik S, Kifley A, Mitchell P, Wang JJ. Age, blood pressure, and retinal vessel diameter: separate effects and interaction of blood pressure and age. Invest Ophthalmol Vis Sci. 2007;48:557–561. doi: 10.1167/iovs.06-0893. [DOI] [PubMed] [Google Scholar]
- 20.Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 23.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151:404–413. doi: 10.7326/0003-4819-151-6-200909150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–1332. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 26.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 27.Muntner P, Woodward M, Mann DM, Shimbo D, Michos ED, Blumenthal RS, et al. Comparison of the Framingham Heart Study hypertension model with blood pressure alone in the prediction of risk of hypertension: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55:1339–1345. doi: 10.1161/HYPERTENSIONAHA.109.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. 2nd. College Station, Texas: Stata Press; 2008. [Google Scholar]
- 31.Thompson S, Kaptoge S, White I, Wood A, Perry P, Danesh J. Emerging Risk Factors Collaboration. Statistical methods for the time-to-event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010;39:1345–1359. doi: 10.1093/ije/dyq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8:e1000440. doi: 10.1371/journal.pmed.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 34.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1: prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 35.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen TT, Wang JJ, Islam FM, Mitchell P, Tapp RJ, Zimmet PZ, et al. Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes. 2008;57:536–539. doi: 10.2337/db07-1376. [DOI] [PubMed] [Google Scholar]
- 37.Pries AR, Secomb TW. Structural adaptation of microvascular networks and development of hypertension. Microcirculation. 2002;9:305–314. doi: 10.1038/sj.mn.7800144. [DOI] [PubMed] [Google Scholar]
- 38.Sabanayagam C, Shankar A, Klein BE, Lee KE, Muntner P, Nieto FJ, et al. Bidirectional association of retinal vessel diameters and estimated GFR decline: the Beaver Dam CKD Study. Am J Kidney Dis. 2011;57:682–691. doi: 10.1053/j.ajkd.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 40.Saldivar E, Cabrales P, Tsai AG, Intaglietta M. Microcirculatory changes during chronic adaptation to hypoxia. Am J Physiol Heart Circ Physiol. 2003;285:H2064–2071. doi: 10.1152/ajpheart.00349.2003. [DOI] [PubMed] [Google Scholar]
- 41.de Jong FJ, Schrijvers EM, Ikram MK, Koudstaal PJ, de Jong PT, Hofman A, et al. Retinal vascular caliber and risk of dementia: the Rotterdam study. Neurology. 2011;76:816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 43.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 44.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, de Jong PT. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 45.Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2012;32:3082–3094. doi: 10.1161/ATVBAHA.112.300291. [DOI] [PubMed] [Google Scholar]
- 46.Peto R. The horse-racing effect. Lancet. 1981;2:467–468. doi: 10.1016/s0140-6736(81)90791-1. [DOI] [PubMed] [Google Scholar]
- 47.Wang JJ, Liew G, Klein R, Rochtchina E, Knudtson MD, Klein BE, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28:1984–1992. doi: 10.1093/eurheartj/ehm221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.