Abstract
Determination of the respiratory tract deposition of airborne particles is critical for risk assessment of air pollution, inhaled drug delivery, and understanding of respiratory disease. With the advent of nanotechnology, there has been an increasing interest in the measurement of pulmonary deposition of nanoparticles because of their unique properties in inhalation toxicology and medicine. Over the last century, around 50 studies have presented experimental data on lung deposition of nanoparticles (typical diameter≤100 nm, but here≤300 nm). These data show a considerable variability, partly due to differences in the applied methodologies. In this study, we review the experimental techniques for measuring respiratory tract deposition of nano-sized particles, analyze critical experimental design aspects causing measurement uncertainties, and suggest methodologies for future studies. It is shown that, although particle detection techniques have developed with time, the overall methodology in respiratory tract deposition experiments has not seen similar progress. Available experience from previous research has often not been incorporated, and some methodological design aspects that were overlooked in 30–70% of all studies may have biased the experimental data. This has contributed to a significant uncertainty on the absolute value of the lung deposition fraction of nanoparticles. We estimate the impact of the design aspects on obtained data, discuss solutions to minimize errors, and highlight gaps in the available experimental set of data.
Key words: : aerosol, engineered nanoparticles, dosimetry, health, inhalation, NSAM, ultrafine particles, pulmonary, lung deposition
1. Introduction
Information about deposition of inhaled aerosols in the respiratory tract is important for risk assessment of occupational and environmental air pollution, drug delivery via inhalers, and diagnosis of lung diseases. Accurate knowledge of the lung deposited fraction of inhaled particles is the key to relate the more readily measured aerosol exposure concentration levels (particle number, surface area, or mass per volume of inhaled air) to the tissue-delivered dose, which is one of the main determinants of the particle-induced biological response.(1–3)
Several numerical (in silico) models of pulmonary particle deposition are available [e.g., the International Commission on Radiological Protection (ICRP) and multiple-path particle dosimetry (MPPD) models(4,5)], which, as good as possible at the time of release, represent the available experimental data (Fig. 1). The semiempirical ICRP model and all other (ab initio) models use a theoretical prediction of diffusional particle deposition to compensate for the limited amount of data for particles below 100 nm. However, lung deposition is governed by a complex set of parameters comprising the breathing pattern, particle characteristics, flow dynamics, and morphological structure of the lung. In addition, these parameters are subject-dependent and influenced by age, sex, and state of health. Because of the complexity of these issues, numerical calculations of the lung deposition of particles necessarily involve simplifications and need to be validated by experiments.
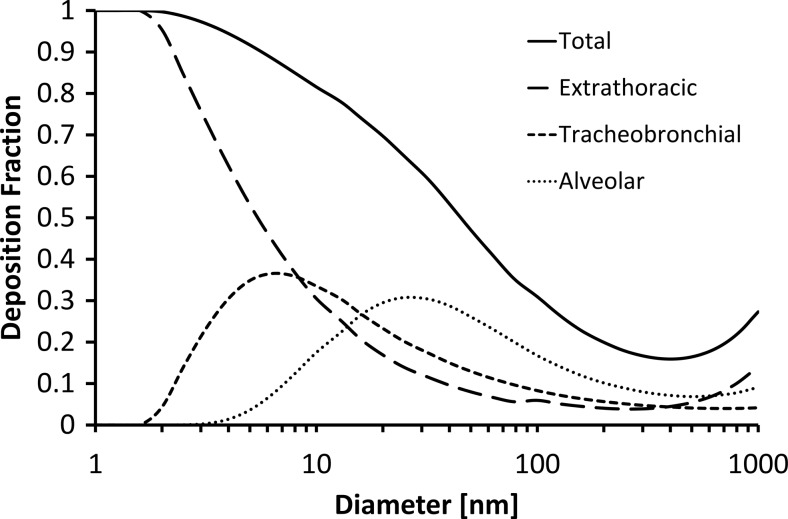
FIG. 1.
Total and regional deposition fractions (DFs) of aerosol particles in the range 1–1,000 nm according to the MPPD model. The values are averages for relaxed nose breathing in men and women (tidal volume, 0.75 L and 0.464 L; breathing frequency, 12 min–1 and 14 min–1).
More than a century has passed since the first observations of particle deposition in the respiratory tract.(6–10) The currently available database shows an emphasis on micrometer-sized rather than nano-sized particles mainly due to limitations in measurement technology and a paramount interest in traditional, medical/pharmaceutical applications and occupational hygiene where larger particles historically have been the main interest. More recently, there is a keen interest in predicting lung deposition of nano-sized particles (diameter<100 nm) due to the increasing awareness of the health effects of small particles (e.g., diesel exhaust) from ambient air pollution,(11,12) office devices such as printers,(13) and engineered nanoparticles,(14) as well as the recognition of the second, fourth, and ninth global risk factors for mortality to be tobacco smoke, household air pollution, and ambient particulate pollution, respectively.(15) Typically, such nanoparticles are coagulated into larger agglomerates. In addition, there are increasing efforts of utilizing inhaled nanoparticles for diagnostic and therapeutic purposes.(16)
As described below, only around 50 studies are reported that measure the respiratory tract deposition of particles smaller than 300 nm (see Table 2 in the Appendix for the complete list). The increasing interest in this issue is documented by the fact that more than a third of these studies have been performed over the last decade.
Table 2.
Summary of The Measurements Of Lung Deposition of Particles In The Diffusion-Dominated Regime (Here ≤300 nm) In The Human Respiratory Tract
| Subjects | Size distribution | ||||||
|---|---|---|---|---|---|---|---|
| M/F | Health | Size (nm) | Typea | Aerosol | Breathing | Particle detection | |
| Rissler et al., 2012(35) | 5 M/5 F | Healthy | 10–500 | Poly | Diesel exhaust | Mouth spontaneous | SMPS |
| Olvera et al., 2012(49) | 22 M | 17 children (9 asthma), 5 healthy adults | 10–200 | Poly | NaCl | Mouth spontaneous | SMPS |
| Löndahl et al., 2012(47) | 9 M/8 F | 10 healthy, 7 COPD | 10–500 | Poly | Diesel exhaust | Mouth spontaneous | SMPS |
| Goldoni et al., 2009(122) | 24 M/12 F | Healthy, workers | 300–5,000 | Poly | Cristobalite | Mouth controlled | Light scatter |
| Löndahl et al., 2009(72) | 5 M/4 F | Healthy | 12–580 | Poly | Busy street | Mouth spontaneous | SMPS |
| Möller et al., 2008(48) | 26 | 9 healthy, 7 COPD, 10 smokers | 100 | Poly | Technegas | Mouth, bolus | Gamma |
| Löndahl et al., 2008(43) | 4 M/6 F | Healthy | 15–680 | Poly | 2 types biomass smoke+DEHS | Mouth spontaneous | SMPS |
| Invernizzi et al., 2007(123) | 11 M/4 F | Healthy | 300–1,000 | Poly | Tobacco smoke | Mouth/nose | Light scatter |
| Löndahl et al., 2007(37) | 19 M/9 F | Healthy | 12–320 | Poly | NaCl and DEHS | Mouth spontaneous | SMPS |
| Invernizzi et al., 2006(124) | 7 M/3 F | Healthy | 300–1,000 | Poly | Ambient air | Mouth/nose | Light scatter |
| Löndahl et al., 2006(18) | 3 M | Healthy | 12–320 | Poly | NaCl and DEHS | Mouth spontaneous | SMPS |
| Wiebert et al., 2006(50) | 6 M/8 F | 10 healthy, 4 asthma | 37 | Poly | Technegas | Mouth | Gamma camera, SMPS |
| Wiebert et al., 2006(23) | 9 M/6 F | 6 healthy, 5 asthma, 4 smokers | 100 | Poly | Technegas | Mouth | Gamma camera |
| Morawska et al., 2005(20) | 14? | Healthy | 16–626 | Poly | Tobacco, diesel, petrol smoke | Nose spontaneous | SMPS |
| Kim and Jaques, 2005(125) | 5 M/2 F | Elderly | 40–100 | Mono | Oil (DEHS) | Mouth controlled | UCPC |
| Montoya et al., 2004(19) | 4 M/2 F | Healthy | 65–2,045 | Poly | Ambient Boston | Nose spontaneous | SMPS, Aerosizer |
| Chalupa et al., 2004(17) | 8 M/8 F | Asthma | 9–65 | Poly | Spark discharge carbon soot | Mouth spontaneous | SMPS, TEOM |
| Daigle et al., 2003(97) | 11 M/8 F | Healthy | 9–65 | Poly | Spark discharge carbon soot | Mouth spontaneous | SMPS, TEOM |
| Brown et al., 2002(46) | 3 M/6 F 7 M/3 F |
Healthy COPD |
33 | Poly | Spark discharge carbon soot+99mTc | Mouth natural breathing | NaI scintillator |
| Kim and Jaques, 2000(25) | 11 M/11 F | Healthy | 40–100 | Mono | Oil (DEHS) | Mouth bolus | UCPC |
| Jaques and Kim, 2000(24) | 11 M/11 F | Healthy | 40–100 | Mono | Oil (DEHS) | Mouth controlled | UCPC |
| Morawska et al., 1999(126) | 18 | 15 non-smokers, 3 smokers | 100–600 | Poly | Tobacco smoke | Mouth/nose spontaneous | SMPS |
| Cheng et al., 1996(127) | 4 M | Healthy | 5–100 | Mono | Ag, latex | Drawn through nose | UCPC |
| Roth et al., 1994(128) | 3 M | Healthy | 18 | Mono | In2O3 (111In) | Mouth breathing | Gamma camera, regional dep. |
| Anderson et al., 1990(39) | 3 M/5 F 8 M/2 F |
Diseased Healthy |
20–240 | Poly | Oil (DEHS) | Mouth controlled | EAA |
| Anderson et al., 1988(102) | 9 M | Healthy | 24–240 | Poly | Oil (DEHS) | Mouth controlled | EAA |
| Schiller et al., 1988(36) | 4 M | Healthy | 5–80 | Mono | Ag | Mouth/nose controlled | CNC |
| Hiller et al., 1987(103) | 5 M | Healthy | 75–420 | Poly | Tobacco smoke | Mouth controlled | EAA |
| Muir and Cena, 1987(129) | 3 M | Healthy | 9 | Mono | Ag | Mouth controlled | CNC |
| Schiller et al., 1986(81) | 4 M | Healthy | 5–80 | Mono | Ag | Mouth controlled | CNC |
| Prodi and Mularoni, 1985(130) | 4 | ? | 300–1,000 | Mono | Charged carnuba wax | Mouth controlled | Light scatter |
| Wilson et al., 1985(100) | 5 M | Healthy | 24–240 | Poly | Oil (DEHS) | Mouth controlled | EAA |
| Tu and Knutson, 1984(38) | 3 | ? | 30–400 | Mono | Kerosene, NaCl, aluminosilicate | Mouth/nose controlled | CNC |
| Blanchard and Willeke, 1984(69) | 4 M/1 F | Healthy | 26–190 | Mono | NaCl | Mouth controlled | Electrometer |
| Melandri et al., 1983(54) | 4 | Healthy | 300–1,000 | Mono | Carnuba wax | Mouth controlled | Light scatter |
| Chan and Lippmann, 1980(91) | 26 | Healthy | 200–7,000 | Mono | Fe2O3 | Mouth controlled | Radioactivity |
| Heyder et al., 1975(70) | 5 M | Healthy | 100–3200 | Mono | DEHS | Nose/mouth controlled | Light scatter |
| Heyder et al., 1973(71) | 4 M | Healthy | 200–2,000 | Mono | DEHS | Mouth controlled | Light scatter |
| Giacomelli-Maltoni et al., 1972(131) | 18 M/7 F | Healthy | 200–2,000 | Mono | Carnuba wax (paraffin) | Nose/mouth controlled | Light scatter |
| Hursh and Mercer, 1970(94) | 4 | ? | 20–200 | Room aerosol+212Pb | Mouth | Gamma camera | |
| George and Breslin, 1969(92) | 6 | ? | 5–80 | Poly | Radon daughters | Nose/mouth spontaneous | Filter, α-counter |
| Holleman et al., 1969(93) | 2 | ? | ∼3–10 | Poly | Radon daughters | Nose/mouth spontaneous | Filter, α-counter |
| Dautrebande et al., 1959(132) | 3 | “Normal” | 50–2,500 | Poly | Ink, Al, CaCO3, Fe2O3 | Nose controlled | Electron microscope |
| Morrow et al., 1958(133) | 7 M | “Normal” | ∼50 | Poly | NaCl | Spontaneous | Flame photo., titration |
| Altshuler et al., 1957(57) | 3 M | “Normal” | 140–3,200 | Mono | Triphenyl phosphate | Mouth controlled | Light scattering |
| Landahl et al., 1952(134) | 2 M | ? | 100–6,300 | Mono | Triphenyl phosphate | Mouth controlled | Impinger (+impactor, filter) |
| Landahl et al., 1951(61) | 21 M/3 F | ? | 100–6,300 | Mono | Triphenyl phosphate | Mouth controlled | Impingers (+impactor, filter) |
| Brown et al., 1950(60) | ? | ? | 240–5,000 | Mono | China clay | Nose | Electric precipitator |
| Wilson and Lamer, 1948(95) | 3 M/4 F | “Normal” | 200–2,600 | Mono | Glycerol mixed with 24Na (NaCl) | Mouth | Filter, β-counter |
| Landahl and Herrmann, 1948(9) | 4 | ? | 100–10,000 | Poly | Corn oil, bicarbonate, Ca3(PO4)2, methylene blue, glycerol | Mouth controlled | Impactors, impingers, filters |
| van Wijk and Patterson, 1940(10) | 3 | ? | <200–5,000 | Poly | Quartzite | Face mask | Thermal precipitator |
| Baumberger, 1923(7) | 9 | ? | ? | Poly | Tobacco smoke | Mouth | Electric precipitator/ gravimetric |
M, male; F, female; SMPS, scanning mobility particle sizer; TEOM, tapered element oscillating microbalance; UCPC, ultrafine condensation particle counter; EAA, electrical aerosol analyzer; CNC, condensation nucleus counter.
Question marks (“?”) indicate that information in the article is insufficient.
For monodisperse experiments, one particle size at a time has been produced and measured. For experiments with polydisperse aerosols, the complete size distribution has been measured at once and usually characterized with an instrument that provides a size distribution of the inhaled and exhaled particles.
In addition to the scarcity of data, there is a lack of coherence of the available data, i.e., there is considerable deviation among the reported values. In part, this may be due to intersubject differences in particle lung deposition, but it can at least partially also be attributed to the use of different measurement methods and biases due to measurement problems. When measuring particle lung deposition, a number of complex experimental challenges have to be handled ranging from aerosol generation, transport, and detection to physiological issues like dead-space volume in the lungs and respiratory conditions (controlled versus spontaneous breathing).
Recently, several devices have been introduced for measurements of lung deposition of inhaled particles in the nano-sized range. Chalupa et al., Löndahl et al., Montoya et al., Morawska et al., and Rosati et al.(17–21) independently constructed detection systems for nanoparticles down to about 10 nm utilizing size-resolved measurements of inhaled and exhaled aerosol with standard (mobility) particle sizers. Möller et al. and Wiebert et al.(22,23) developed a method for studies of the regional deposition of radiolabeled ultrafine particles using a gamma camera. Kim and co-workers(24–26) investigated the deposition of monodisperse nanoparticles breath by breath with a particle counter.
None of these systems for human studies of respiratory tract deposition is commercially available, but an increasing number of devices are emerging that report lung deposited surface area of particles smaller than about 300 nm such as Aerotrak 9000 (TSI Inc.), miniature diffusion size classifier (miniDISC, Matter Aerosol), NanoCheck (Grimm), NanoTracer (Philips), Nanoparticle Surface Area Monitor (NSAM; TSI model 3550, TSI Inc.), and Partector (Naneos Particle Solutions GmbH). None of these devices directly measures lung deposition of nanoparticles. Instead they have a size-dependent instrument response that has been shown to correlate with the surface area of the deposited particles in the lungs, if—and only if—the particles are spherical and nonhygroscopic and follow the lung deposition curves described by the ICRP model.(27) Hence, these devices do not actually measure particle lung deposition and are therefore not discussed in this review. The increasing number of measurement devices for lung deposited particle dose reflects the increasing interest in this topic.
One of the main challenges for calibration and comparison of instruments for lung deposition experiments is the lack of a realistic common “standard” or “reference” method. Previous approaches include filters, packed bead beds, or some type of physical model as reference, but these do not reproduce the dynamic structure of a breathing lung.(e.g., 28,29) In addition, the air also should be moistened to nearly saturated conditions of 99.5% relative humidity (RH) in order to simulate hygroscopic effects such as hygroscopic growth and particle restructuring. It is experimentally very difficult to maintain RH levels just below saturation. Using an actual human lung for comparison of instruments is also not possible, because of intersubject variability. Hence, currently the most favored option is to design and select the experimental methodology such that measurement errors are minimized.
The objective of this work is to identify the most critical parameters for particle lung deposition measurements with a particular focus on nano-sized particles (here diameter<300 nm) in order to provide guidance for measuring the lung deposition of inhaled nanoparticles, to facilitate comparison and harmonization of data, and to improve the quality of future particle lung deposition measurements. The critical design aspects of particle lung deposition measurement devices are identified based on a comprehensive review of the currently available experimental methods with a focus on the diffusion-dominated size regime (<300 nm), which is most relevant for nanoparticle deposition. However, most of the critical parameters for lung deposition measurements also apply to larger-sized particles. Finally, gaps in the currently available data pool are identified, and best practice recommendations for accurate particle lung deposition measurements are presented.
2. Background on Particle Lung Deposition Measurement
The simplest and most widely used measure of inhaled particle deposition is the total deposition faction (DF; a number between 0 and 1), i.e., the total fraction of inhaled aerosol that deposits in the respiratory tract including the extrathoracic region (nose or mouth). Some aspects of regional deposition (spatially resolved deposition) will be discussed below (see also Fig. 1 and Appendix), but the main focus of this work is total deposition.
If DF is known, the respiratory tract deposited particle dose rate, expressed as the total amount of particles (number, mass, etc.) deposited in the respiratory tract during a period of time (e.g., μg/min), can be inferred from
 |
where Cinhaled (e.g., μg/m3) is the particle concentration of the inhaled air (exposure level: amount of particle per volume air) and MV is minute ventilation (m3/min). Of these parameters, DF is the least accessible factor, because it depends on the subject-specific morphology of the lungs and respiratory parameters (rate, route, and volume), as well as on numerous other parameters including particle size, density, shape, and chemical composition as discussed below. Omission of DF from the dose-rate calculation, as is often done, results in a considerable uncertainty of up to a factor of 10, as DF may vary from less than 0.1 to almost 1, mainly depending on particle size.(5)
For inhalable aerosols (diameter<10 μm), the most important mechanisms of deposition in the respiratory system are inertial impaction, gravitational settling, and diffusion.(30,31) Both the relative contribution of these mechanisms to DF and the value of DF itself depend on particle size (see Fig. 1). Coarse particles (>3 μm) mainly deposit by impaction due to abrupt changes in the direction of the air flow that occur in the mouth (or nose) and the upper respiratory tract, including pharynx, larynx, trachea, and bronchial region. Gravitational settling is most efficient in the narrow, randomly oriented ducts and air spaces further down in the lungs (bronchiolar and alveolar region). Here, flow velocities are small and residence times are long, which facilitates gravitational settling of particles between about 0.3 and 3 μm. Deposition by diffusion is the principal deposition mechanism for particles with a diameter below 0.3 μm. Nanoparticles smaller than around 10 nm have high diffusion velocity and deposit mainly in the head airways and tracheobronchial region, whereas 30–300-nm particles primarily deposit in the alveolar region. For particles with diameters in the range 20–40 nm, the majority (up to about 50%) deposit in the alveolar region during exercise. No principal deposition mechanism is efficient for particles in the range 0.1–1 μm, which is the reason for the minimum in the total deposition curve over this size range (Fig. 1).
Figure 2 illustrates the diffusion-dominated size regime for particle deposition in the lungs, which is the relevant regime for nanoparticles. The characteristic diameter for diffusion-related transport and deposition is the mobility diameter, which is related to the geometric and aerodynamic diameter.(32–34) Diffusion is always the dominating deposition mechanism if the particles are small enough (<150–300 nm, depending on density). If DF is only due to diffusion, it is independent of particle density, because the diffusivity of particles is independent of particle mass.
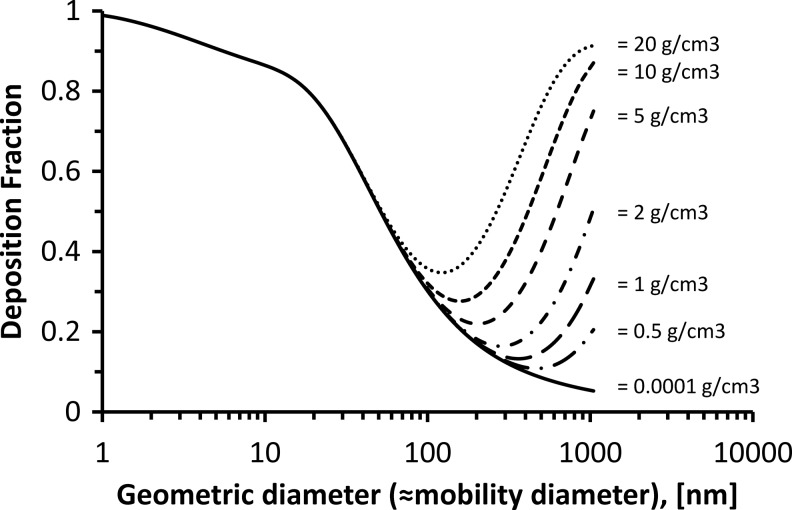
FIG. 2.
Size-resolved total DF as a function of particle density (0.0001–20 g/cm3). Data are calculated with the ICRP model for a sitting male adult (nose breathing; tidal volume, 0.75 L; breathing frequency, 12 min–1).(5) For small enough particles, DF is independent of particle density (diffusion-dominated regime). The upper limit of the diffusion-dominated regime is between 100 and 600 nm for densities between 20 and 0.5 g/cm3. For a density of 1–2 g/cm3 (typical for ambient particles), the upper size limit of the diffusion-dominated regime is about 300 nm, because at this size DF is twice the value of the zero density line (here: 0.0001 g/cm3), which represents DF due to diffusion only.
The deposition by diffusion is proportional to the diffusion coefficient D and the residence time, tr, in the general form (Dtr)1/2. The diffusion coefficient D of the particle is given by:
 |
where k is Boltzmann's constant, T is the absolute temperature, Cc the slip correction factor, η the dynamic viscosity, and dp the particle diameter.
The upper size limit of the diffusion-dominated regime can be defined as the size where the effect of diffusion on DF is matched by the combined effects of impaction and sedimentation. This size is found by comparing the size-dependent DF curve (obtained from, e.g., the ICRP model) of a spherical particle with a given density (DF due to diffusion, impaction, and sedimentation) with that of a hypothetical particle with zero density (DF due to diffusion only). The size of interest is that for which DF (actual density) is twice as high as DF (zero density). As seen from Figure 2, this size increases from about 150 to 700 nm for densities decreasing from 20 to 0.5 g/cm3. For a typical ambient particle, the effective density is less than 2 g/cm3, and hence the upper limit is about 300 nm (see Fig. 2). Hence, for the purpose of this study, we will focus on the diffusion-dominated size regime (diameter<300 nm for particle densities below 2 g/cm3), which includes the so-called ultrafine or nano-sized particles (diameter<100 nm). For simplicity, we will henceforth use the terms diffusion-dominated and nano-sized interchangeably.
It is important to note that the densities given in Figure 2 are effective densities. The effective density of agglomerated particles is less than the material density. As particle density and shape are not always known, it is recommended to measure (calculate) particle lung deposition in terms of the characteristic size, namely, mobility and aerodynamic diameter for the diffusion- and impaction/sedimentation-dominated regime, respectively. Using the mobility diameter eliminates the effect of shape and density on particle lung deposition, i.e., the calculations with the ICRP model (or other computer models of particle lung deposition) can be performed as if (1) the characteristic diameter is equal to the geometric diameter, (2) the density is unity, and (3) the shape factor is unity (spherical shape).(34,35) An exception is particles such as carbon nanotubes and rods with shapes that can not easily be fully described with a single diameter value.
Several factors influence particle lung deposition in the diffusion-dominated regime. The main factors are particle mobility size,(e.g., 36) hygroscopicity,(e.g., 37,38) breathing pattern,(e.g., 24) and lung morphology.(e.g., 39) As seen from Figure 1, DF is highly dependent on particle size. This requires information not only on the size of the particles prior to inhalation, but on the fate of the particle in the lungs where the particle size may be altered due to hygroscopic growth, evaporation, coagulation, or restructuring. In the lungs, where RH is close to 99.5%,(18,40,41) particles may grow by condensation of water vapor to a diameter of up to six times the original (dry) size depending on the hygroscopicity of the particle. As the growth times of nano-sized particles (<300 nm) are small compared with the residence time in the lungs,(42) the “wet” particle size is the relevant diameter for lung deposition. This was experimentally confirmed in studies showing that the size-resolved lung deposition for hygroscopic particles was shifted to larger size, when compared with nonhygroscopic particles, where the size shift was consistent with the hygroscopic growth factor.(37,43) In other words, a hygroscopic 50-nm NaCl particle has essentially the same DF as a hydrophobic 220-nm particle. This is important, because it implies that one can estimate the deposition of hygroscopic particles in the lungs, if the dry particle size and its hygroscopic growth factor (at RH=99.5%) are known.
In addition to hygroscopic growth, other factors may affect the particle size in the lungs. Evaporation is usually negligible, but it may have an effect for semivolatile particles inhaled from cold air and for fresh organic-rich combustion emissions (wood smoke, etc.). Also, coagulation is in most cases negligible, but should be considered when particle concentration is high (e.g., cigarette smoking) or breathing is slow (e.g., when holding the breath). Furthermore, agglomerated particles could shrink due to restructuring, if they contain some hygroscopic material. The surface tension caused by the absorbed water may lead to restructuring, resulting in a decrease in mobility size of up to about 20% depending on the stiffness of the structure and the hygroscopicity of the material.(44,45)
In addition to particle size, particle lung deposition is strongly affected by the respiratory conditions. Important parameters are primarily tidal volume (inhaled air volume per breath), breathing frequency, and flow rate. In the diffusion-dominated regime, the residence time of the particles in the lung primarily determines deposition; therefore, large tidal volume increases DF, whereas high breathing frequency lowers it. During exercise, both tidal volume and breathing frequency are increased compared with those under sitting conditions. It is remarkable that the net effect of this is that DF remains almost unchanged due to the compensating effects of increased tidal volume and increased breathing frequency on particle residence time.(2,37) However, as the total inhaled air volume is much higher during exercise, there is a sizeable increase in deposited dose rate (see Eq. 1).
Furthermore, lung morphology and airway geometry sometimes have a considerable effect on deposition. DF may be altered substantially in the diseased lung, where airway dimensions are sometimes abnormal. In the diffusion-dominated regime, DF has been shown to be altered for patients with asthma and chronic obstructive pulmonary disease (COPD).(17,39,46–50) It should be noted that in respiratory disease the dose rate is altered not only by DF, but also by changes in minute ventilation (see Eq. 1). In the diffusion-dominated regime, DF appears to be almost similar for nose and mouth breathing.(51)
A number of other factors may influence deposition, such as net charge on the particles, particle concentration (cloud motion, e.g., for cigarette smoke), interception, thermophoresis, and gas properties (e.g., changes in gas density or viscosity due to low pressure conditions). However, for most practical applications, these factors have a minor effect and are therefore discarded from the following discussion. As a caveat, we add that interception and electrostatic forces may become relevant under certain conditions.(52) The former is relevant for fiber-like particles (large aspect ratio and longer than about 3 μm), and the latter plays a role for highly charged particles (typically larger than 20 elementary charges), which is likely to occur only in the immediate vicinity of a particle source or if inhaled aerosol particles have been actively charged (unipolar) prior to inhalation.(53,54)
3. Measurement of Respiratory Tract Deposition: Main Device Components
The total deposition fraction, DF, is generally defined as
 |
where Cinhaled and Cexhaled are the inhaled and exhaled aerosol concentrations, respectively. Ideally, these should be directly measured. However, as will be discussed in detail in Section 4, some corrections to this equation are generally necessary depending on the experimental setup. For instance, volume changes, dead space in a breathing mask, and particle losses in the instrument have to be accounted for.
Although a variety of experimental setups have been used for measurement of respiratory tract deposition (see Table 2 in the Appendix for a list of studies in the diffusion-dominated regime), the main components are always (A) an aerosol source, (B) an aerosol conditioning and inhalation system, and (C) a method for particle detection.
A. Aerosol source
The aerosol source is selected depending on the experimental question to be answered and/or on the particle detection method at hand. For instance, if particles are detected with a gamma camera, they have to be radiolabeled.
B. Inhalation system
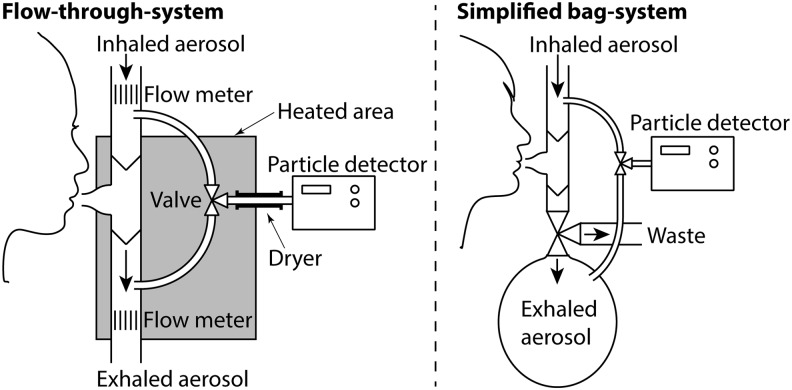
There is a considerable diversity in the design of inhalation systems, but most can be divided into either flow-through systems or bag systems (Fig. 3). Flow-through systems are generally open to the atmosphere and allow a continuous aerosol flow, whereas bag systems collect the exhaled aerosol in a bag. The inhalation system typically consists of an interface with the human respiratory tract (e.g., face mask, mouthpiece), connecting tubing, and containers for sampling of inhaled and exhaled aerosol. Most inhalation systems, but not all, have flow meters for logging the breathing pattern. Some systems allow spontaneous breathing, and others control breathing with automatic valves. In some systems, the aerosol is diluted, heated, or dried.
FIG. 3.
Schematic pictures of the two major types of inhalation systems used. The flow-through type (left) is the most common, but several groups have also used bag systems (right). Some critical parts usually needed are shown in the left part of the figure: heating of the exhaled aerosol, flow meter, and drying of the particles before the detector.
C. Particle detection
A wide range of detection devices have been used, such as radioactive techniques, gravimetric analysis of filters, electron microscopy, electric charge, thermal precipitator, impingers, condensation particle counters (CPC), and flame photometry (see Table 2 in the Appendix). There is a fundamental difference between detectors measuring total and size-resolved particle concentrations. The former often have the advantage of a short response time, but a sequence of measurements with monodisperse aerosols has to be performed in order to find the size-dependent DF. Size-resolving detectors require additional precautions as discussed below.
4. Critical Aspects for Particle Lung Deposition Measurements
There are a number of critical design parameters to consider when measuring respiratory tract deposition (Table 1). Most of these are relevant independent of the selected experimental setup, but some relate to specific methods (e.g., experiments with radiolabeled or polydisperse aerosols). Best accuracy of the measurements can be expected, if all (or most) of the critical design parameters are adequately incorporated in the experimental setup.
Table 1.
Recommended Procedures to Minimize Errors
| Design aspect | Procedures to minimize error |
|---|---|
| A. Aerosol source | |
| A1. Monodispersity | There are several ways of generating monodisperse particles with GSD<1.3: e.g., electrospray technology, nebulization of a liquid suspension containing monodisperse particles, or by selecting a narrow size fraction from a polydisperse aerosol generator by, e.g., DMAs. |
| A2. Multiple mechanical mobilities | If monodisperse particles are obtained from a polydisperse aerosol with a DMA, the aerosol should ideally contain few particles that are larger than the selected size. |
| A3. Radiolabeled particles | Particle size distribution needs to be provided. |
| A4. Concentration limits | Particle concentration could be reduced by dilution. |
| A5. High electrical charge | A bipolar charger (neutralizer) needs to be used for highly charged aerosols. |
| A6. Unstable concentration | The concentration may be stabilized by use of a mixing volume. |
| B. Inhalation system | |
| B1. Separation between samples | Not applicable. |
| B2. Particle losses | Particle losses may be minimized by use of short tubing to reduce deposition by diffusion and by conductive material to reduce electrostatic deposition. The remaining particle losses should be characterized and corrected for (details in Appendix). |
| B3. Leaks | Leaks may be tested by, e.g., inhalation of particle-free air. Use nose clip if breathing on mouthpiece. Special care must be taken when using nose or face masks. |
| B4. Change in temperature and RH | There are several options available. Usually inhaled and exhaled aerosols should be measured at similar temperature and RH. |
| B5. Pressure variations | Short tubing with large cross section decreases pressure drops. |
| B6. Dead space | If tidal volume of the breathing subject and mouth/nose piece dead space is known, a simple correction of data may be performed.(58) |
| B7. Discard first breaths | The first breaths may be wasted either by removing parts of the data or by a valve system that directs the flow. |
| B8. Varying exhaled concentration | The exhaled air may be mixed in a sufficiently large container or measured by fast time-dependent sampling combined with volume flow measurement. Long measurement times could also be used to smear the varying exhaled concentration. |
| B9. Condensation of exhaled aerosol | Condensation of exhaled aerosol is avoided by heating the inhalation system. |
| B10. Hygroscopic aerosol | The studied aerosol needs to be well characterized in terms of water uptake. |
| B11. Monitoring breathing pattern | Breathing pattern should be monitored during measurement and provided at BTPS. |
| B12. Defining breathing pattern | Calibration of flow meters is needed. |
| C. Particle detector, polydisperse aerosols, and radiolabeled aerosols | |
| Particle detector | |
| C1. Correct particle sizing | Calibration of the particle sizer with particles of known size is needed for accurate sizing. |
| C2. Detection efficiency | Preferably the same instrument should be used to measure both inhaled and exhaled samples during similar temperature, pressure, and RH. |
| C3. Size shift altering detection efficiency | Use stable particles or condition the particles to reduce size shifts. |
| C4. Low response time. | Most common solutions are a fast detector or a mixing volume. |
| C5. Proper particle diameter | Correct particle diameter should be reported for DF. |
| Polydisperse aerosols | |
| D1. Size shifts | The stability of the aerosol needs to be addressed (see Appendix). As outlined in the Appendix, several options to account for size shifts are available. |
| Radiolabeled aerosols | |
| E1. Attenuation and scattering | See Appendix. |
| E2. Leaching of tracer | See Appendix and section on radiolabeled aerosols. |
A more comprehensive description is found in the Appendix.
BTPS, body temperature and pressure, saturated; DMA, differential mobility analyzers.
We identified the critical design aspects by a review of the literature on particle lung deposition measurements including nano-sized particles (the studies are listed in Table 2 of the Appendix; see also Section 6 below). Below follows a short summary of these design aspects. For a more detailed description, we refer the reader to the Appendix. Suggestions or recommendations on how to address these design aspects when performing DF measurements are discussed in detail in the Appendix and are briefly summarized in Table 1 and Section 5.
Aerosol source
A1. Monodispersity
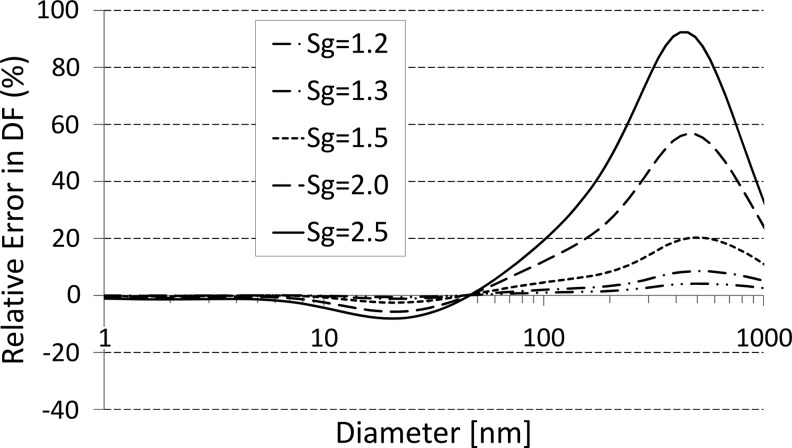
A common method to study the DF at a specific particle size is to measure total deposition of a monodisperse aerosol. A perfectly monodisperse aerosol (where all aerosol particles have exactly the same size) would have a geometric standard deviation of unity (GSD=1). Experimentally, a GSD of less than around 1.15 is generally considered as monodisperse. For comparison, the most widely used compressed-air (jet) nebulizers produce particle size distributions with GSD=1.8–3.4.(31) However, as DF is size-dependent, the measured DF changes with GSD. A bias arises if the DF of a polydisperse aerosol is utilized as a measure for the DF of a monodisperse aerosol or vice versa. Figure 4 illustrates the deviation between the measured quasi-monodisperse aerosol and true monodisperse DF depending on the extent of polydispersity. If we accept an error of 0.02 in DF (corresponding to a relative error of up to 8% depending on particle size), GSD should be less than 1.3. The absolute bias in DF for particles less than 1 μm is −0.009 to 0.016 for GSD=1.3 and −0.06 to 0.13 for GSD=2.5. A polydisperse aerosol could be used for size-dependent DF measurements if inhaled and exhaled size distributions are compared (see discussion below).
FIG. 4.
The error occurring if assuming that the DF of a polydisperse aerosol represented that of a monodisperse. Here the relative error in DF (i.e., ΔDF/DF) is depicted as a function of particle diameter for different GSD values (1.2–2.5). The calculations were performed based on DF data generated by the ICRP model (LUDEP v. 1.96; ICRP(5)) for a nose-breathing, sitting male (12 breaths/min; tidal volume, 0.75 L; constant air flow rate, 18 L/min) assuming spherical particles with unit density. It is evident that, for particle diameters below 50 nm, polydispersity does not have a major effect (<8% bias in the measured DF), whereas it introduces an almost 100% error near 500 nm for GSD=2.5. Maintaining less than 8% bias in DF over the entire submicrometer size range requires GSD<1.3.
A2. Multiple diameters
If monodisperse particles are separated from a polydisperse distribution in an electric field [e.g., with a differential mobility analyzer (DMA)], multiply charged particles need to be considered. These multiply charged particles will have larger (geometric/mobility) diameters and, hence, a lower lung deposition probability in the diffusion-dominated regime.(36,55)
A3. Radiolabeled particles
The major techniques for the generation of nano-sized radiolabeled aerosol particles are based on nucleation of vaporized material, either in a Technegas system or by spark discharge. These methods give size distributions with GSD>1.3 and particles that may contain some hygroscopic material. These factors have to be considered when providing data on DF.
A4. Concentration limits
The concentration has to be high enough to get sufficient counting statistics and to eliminate background noise. On the other hand, it has to be sufficiently low to avoid particle coagulation (<105–106 cm–3 depending on residence time of the aerosol in the apparatus). The latter is less critical, if particle mass rather than number is measured, because mass is conserved under coagulation. On the other hand, coagulation always introduces a bias in measured DF, because it leads to increase of the average particle diameter and decrease of particle concentration. The decreased concentration due to coagulation could be misinterpreted as deposition in the lung and, hence, an overestimate of DF.
A5. High electrical charge
If particles are highly charged, which may be the case for freshly generated particles, they have an increased DF due to electrostatic forces.(53,54)
A6. Unstable concentration
In most experimental setups, the inhaled and exhaled aerosol is not continuously monitored, but rather measured with a single instrument alternating between two containers. Thus, a varying source concentration is likely to alter the measured DF from the true value.
Inhalation system
B1. Separation between inhaled and exhaled samples
Most experiments separate inhaled and exhaled samples either (1) by a valve system directing the aerosol into reservoirs (exhalation filter in some cases) or (2) by using a particle detector close to a mouthpiece (or face mask) that continuously records both inhaled and exhaled air. Fast sampling directly at the mouth/nose is difficult in the diffusion-dominated regime because the currently available detectors for nanoparticles have a slow response time. One exception is a study by Kim and Jaques(25) where an ultrafine condensation particle counter (UCPC) was modified to decrease response time and thereby enabled exhaled concentration to be followed breath by breath.
B2. Particle losses in the inhalation system
Deposition of particles in the breathing apparatus may be interpreted as deposition in the lungs if not accounted for. Bag systems especially may have considerable particle losses that, in addition, depend on the fill ratio of the bags. For hygroscopic particles, losses are dependent on RH.(38) The reported values of particle losses in the various apparatuses described in the literature are usually around 6–15% for 20-nm particles and 1–2% for 300-nm particles. These losses, if neglected, will give rise to an absolute overestimation of DF with 0.015–0.04 at 20 nm (relative error 2–6%) and 0.01–0.02 at 300 nm (relative error 7–19%). It is essential to construct the inhalation system using conductive material. Natural rubber and silicon latex material are especially problematic, because they are prone to strong electrostatic charging and may give very high and nonreproducible losses in the system, as most aerosol particles do carry a net positive or negative charge.
B3. Leaks
The inhalation system has to be leak tight. Nose or face masks especially do not always fit perfectly. Inhaling through one mouthpiece and exhaling through another, as is done in some studies, obviously involves the risk of getting room air into the system. If particle concentration in the system is higher than in the surrounding air, leaks are likely to increase the measured DF compared with the true DF because the particle concentration is decreased by dilution.
B4. Change in temperature, RH, and volume
If inhaled air and exhaled air differ in temperature and humidity, the air volume will also be shifted. For example, there is about 12% increase in gas volume between an inhaled aerosol at 20°C with 50% RH and an exhaled aerosol almost saturated with water vapor at 37°C. If not accounted for, this may distort the concentration measurement as well as monitoring of the breathing pattern (see B11). An increasing gas volume dilutes the aerosol and may be misinterpreted as a decrease in particle concentration. If volume increases by 10%, a true DF of 0.13 may be mistaken to be 0.21 and a DF of 0.50 to be 0.55 (as could be derived from Eq. 3).There is also a small difference between the volume of absorbed O2 compared with the volume of exhaled CO2, leading to a decrease of the exhaled volume of about 0.5% (the ratio between produced CO2 and consumed O2—the respiratory exchange ratio—varies slightly depending on metabolism).
B5. Pressure variations
The varying breathing flow rate results in pressure differences for the aerosol. If equal volume flow rates are sampled from the inhaled and exhaled aerosols, a pressure difference gives rise to an increased sampling on the high-pressure side and vice versa. Thus, an increased pressure in exhaled air likely decreases the measured DF (Eq. 3). Some detectors, for instance, the tapered element oscillating microbalance (TEOM), are also very sensitive to rapid pressure variations.(56)
B6. Apparatus dead space
The inhaled aerosol concentration at the mouthpiece or face mask does, in most cases, differ from the measured concentration. The reason is that, at the beginning of each breath, the apparatus dead-space volume contains the aerosol from the end of the previous exhalation. Therefore, the inhaled aerosol will contain fewer particles than measured, and if not accounted for, this leads to an underestimation of DF—typically with 1–6% (or up to 0.07 in DF) as described in several articles.(57–59)
B7. Discard first breaths
At least a few breaths are needed before the studied aerosol has fully replaced the aerosol present in the lungs before the experiment. Typically, the surrounding air has a lower particle concentration than used in the inhalation system. Therefore, the initially exhaled aerosol will contain fewer particles, and if these data are not wasted, DF will be overestimated (see Eq. 3).
B8. Varying exhaled concentration
The particle concentration in the exhaled breath is not uniform. The air exhaled at the end of the breath contains fewer particles than the first part, because it has spent a longer time in the respiratory tract and reached deeper into the lung. If total DF is studied, it is therefore necessary to measure over the complete breath and, if not using a mixing volume, compensate for flow variations. On the other hand, the nonuniformity of the exhaled breath concentration is related to the depth of inhalation and offers the opportunity for a rough estimation of regional deposition (central or peripheral lung region) by measuring the particle concentration in a specific part of the exhaled breath (early exhaled breath: central lung; late exhaled breath: peripheral lung).(60,61)
B9. Condensation of water on exhaled aerosol
If the undiluted exhaled aerosol is cooled below approximately 35°C, it becomes supersaturated with water. Similar to the activation process of aerosol particles into cloud droplets, submicrometer-sized particles in the exhaled air may rapidly grow to droplets with diameters of several micrometers, which may dramatically increase deposition in the inhalation system because of gravitational settling or impaction (and consequently bring about an overestimate of DF).
B10. Hygroscopic aerosol
If the intention is to study the deposition of hydrophobic particles, it is important to ensure that the particles do not contain hygroscopic material. Even a small fraction of such material may alter DF as the RH in the lungs is close to saturation. In the diffusion-dominated regime (up to at least 200 nm), hygroscopic material on the particles increases particle size and, hence, decreases the diffusivity. A decreased diffusivity will decrease DF.(e.g., 37)
B11. Monitor breathing pattern
The breathing pattern needs to be monitored during the deposition measurement for comparison with other data and computer models. The international standard is to report breathing volumes at “body temperature and pressure, saturated” (BTPS), i.e., 37°C and 100% RH, which are the conditions in the lung. Conversion to BTPS could be made as
 |
where Vinitial, Pinitial, and Tinitial are the initial volume, pressure, and temperature and PW is the initial partial pressure of gaseous water. A 10% difference in breathing volume corresponds to approximately up to 0.02 in DF (or a relative difference of 3%) according to the ICRP model.
B12. Defining breathing pattern
Breathing pattern is one of the main determinants for lung deposition and has to be considered in study design. Either controlled or spontaneous breathing may be used. Controlled breathing conditions allow for easier comparison between laboratories and with computer models and may be useful for predicting the influence of physical particle transport mechanisms on lung deposition. Spontaneous breathing provides better data for the lung dose rate and, therefore, may be more relevant for the assessment of lung deposited particle dose rate and health effects. As breathing pattern is influenced by the measurement procedure, spontaneous breathing will be different from natural. An alternative approach is to perform an initial monitoring of the natural breathing pattern of the subjects with a noninvasive method such as inductance bands (e.g., Respitrace), and thereafter use this breathing pattern during the deposition measurements.(e.g., 62)
Particle detection
Most detection methods available for aerosol characterization can be applied to respiratory tract deposition measurements. A few crucial aspects have to be considered.
C1. Correct particle sizing
The characteristic particle size for diffusion-related effects is the mobility diameter. The most common techniques for measurement of this particle size are mobility particle sizers [differential, scanning, or fast (DMPS, SMPS, or FMPS, respectively)]. These instruments need to be calibrated, e.g., with particle standards, for correct sizing. For particles smaller than 300 nm, a size accuracy of ±4.2%,±6.9%, or±13.9% is sufficient for deposition measurements with an accuracy of±3%,±5%, or±10%, respectively.
C2. Detection efficiency
Provided the detection efficiency is relatively constant with concentration, it is of minor importance if a single instrument is used for measurement of both inhaled and exhaled air samples. If separate instruments are used, size-resolved experimental intercomparison of the detection efficiencies is crucial, and differences need to be accounted for in the data reduction algorithm. Detection efficiency may also be altered between inhaled and exhaled samples because of differences in temperature, humidity, or pressure (see B4 and B5).
C3. Size shift altering detection efficiency
A particle size shift between inhaled and exhaled samples could induce a bias in the deposition measurement. This needs to be considered only if particle detection efficiency is size-dependent, e.g., close to the cutoff of a particle counter.
C4. Low response time
The finite response times of detection instruments such as particle counters may delay and smear the signal.(63) Especially in studies where the exhaled concentration is monitored breath by breath, this is critical because the aerosol concentration at the end of the exhalation for nano-sized particles typically is 50–100% lower than at the beginning. A particle detector capable of following these rapid concentration variations is necessary together with correction for signal response time (see Eq. 5 in Brown et al.(64)). An easier alternative is to have a volume for smoothing and mixing the exhaled aerosol. Alternative solutions, such as sampling the exhaled aerosol in several containers, have also been presented.(e.g., 60)
C5. Proper particle diameter
As pointed out previously, the mobility diameter should be used to describe lung deposition of nanoparticles. If a mass-based detection method is used, the mass median diameter (MMD) should be presented rather than the count median diameter (CMD). For radiolabeled aerosols, the appropriate diameter is the activity median diameter (AMD), which is typically larger than CMD.(65) When DF is measured by mass, surface area, or activity, but reported by CMD, the value will underestimate the true DF in the diffusion-dominated regime. The error will increase with increasing GMD.
Polydisperse aerosols
It is sometimes advantageous to study respiratory tract deposition of a polydisperse aerosol, i.e., to use an aerosol with a broad size range and compare the inhaled and exhaled size distributions to get the size-dependent DFs. It is the easiest option for investigation of the deposition of ambient aerosols, it may reduce measurement time because a range of particle sizes is inhaled simultaneously, and it may improve size resolution. However, the use of a polydisperse aerosol also introduces some additional experimental difficulties. Most studies of this type have used an SMPS. The SMPS has superior size resolution and lower detection limit in terms of concentration compared with most other size spectrometers. The main disadvantage is the rather low time resolution, which puts high demands on the aerosol source stability as only a limited number of size scans in inhaled and exhaled air can be performed during an inhalation session.
D1. Size shifts
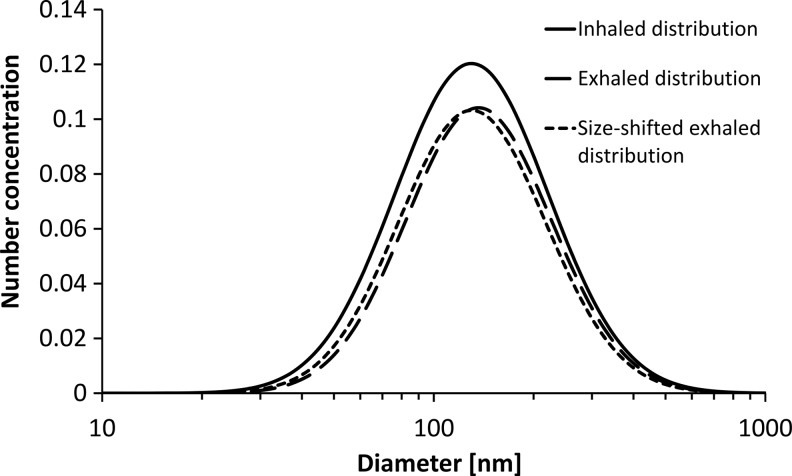
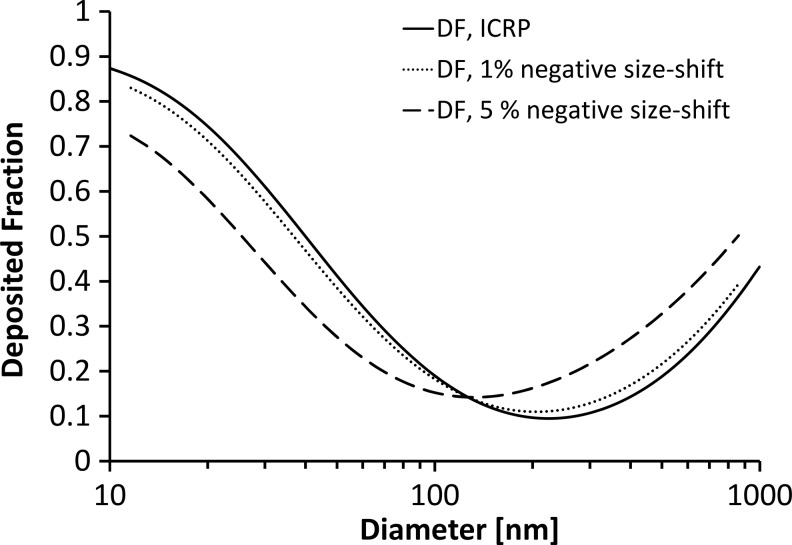
The major difficulty in studies of polydisperse aerosols is the effect of shifts in particle size between inhaled and exhaled samples (see Appendix). Such shifts may occur in the lungs because of restructuring, evaporation, coagulation, or condensation at 37°C and near 100% RH. For high but still realistic size shifts of 5%, a bias of more than 0.1 in DF may occur, which may be up to about 100% in relative difference, if DF is near its minimum value. The error increases for aerosols with narrow size distribution (low GSD).
Radiolabeled aerosols
E1. Attenuation and scattering
When DF is measured with a gamma camera (spatially resolved gamma ray counting), the tissue-specific attenuation and scattering of the gamma rays has to be taken into account. This attenuation factor is subject-specific and has to be assessed separately. A more comprehensive discussion on this issue is provided in the Appendix.
E2. Leaching of tracer
Leaching of the radiotracer will reduce the measured lung activity, which will result in an underestimate of the particle dose in the lungs. Leaching can be measured with particles on a filter probe or by collecting urine samples and investigating these for radioactive contamination (if the radiotracer is not metabolized in the body).(22,50) Very small particles (<10 nm) may to a significant extent penetrate the epithelium and urine.(66,67) However, only a minor fraction of these particles reaches the alveolar region, as most deposit in the upper airways, and a prerequisite of renal clearance is also an unchanged hydrodynamic size without formation of any protein corona—a rare feature.(68) Experiments with new types of radiolabeled aerosols may be disapproved by the ethical review board.
Recommended Experimental Setup and Procedures for Quality Assurance
To achieve high-quality measurements, all relevant design aspects discussed in the previous section have to be considered. There are a range of options to build such setups, and thus the experimental procedures reported in the literature differ substantially (see Fig. 3 and Appendix). The experimental design is largely determined by parameters such as aerosol type, particle detector, choice of breathing pattern, etc., and it is hence not possible to provide a description of the optimal setup. Nevertheless, some general guidelines and suggestions to minimize errors from the design aspects can be given (Table 1; see also Appendix for details on each design aspect).
Of the two types of inhalation systems—flow-through and bag systems (Fig. 3)—the flow-through systems are the most common and, in many respects, the preferable choice, although both have their benefits. Bag systems primarily have the advantage of efficient mixing of the exhaled aerosol (aspect B8) and smoothing of pressure variations (B5). For flow-through systems, the varying concentration of the exhaled particles can be handled, for instance, by a very fast detector combined with volume flow measurement(e.g., 25) or by leveling the concentration variations with a mixing volume.(e.g., 18) Difficulties with pressure variations can be managed by considering detector type and tubing dimensions. Bag systems have the major drawbacks of complex particle losses (B2) that vary with time and fill rate of the bag and the need of an exhaust valve to waste the first exhaled breaths (B7).
Many design aspects are controlled in similar ways irrespective of system type. Particle losses (B2) are minimized by the use of short tubing, conductive material to reduce electrostatic deposition, and correction for remaining system losses. Leaks (B3) may be tested by, e.g., inhalation of particle-free air. Changes in temperature and RH (B4) could, for instance, be handled by drying the exhaled air and measuring at constant temperature. A simple correction could be made to compensate for the effect of low particle concentration in the instrumental dead space (B6).(58) Condensation of water in the exhaled aerosol (B9, B10) is avoided by heating parts of the inhalation system (Fig. 3). Particle size shift of the aerosol during breathing is a substantial problem in polydisperse measurements (D1) and is discussed in detail in the Appendix.
Tests of the validity of the experimental setup are recommended and can be performed in a variety of ways. Precision of the instrumental setup may be determined by repeating identical deposition measurements (for instance, through a packed bead bed, parallel tubes, or some other device with well-defined geometry; if particle penetration is known, accuracy also may be validated(33)), and sensitivity canbe investigated by varying breathing patterns or aerosol properties.
For quality assurance during the exposures where exhaled aerosol is retained for sampling, it is suggested to include continuous monitoring of at least temperature in the container for exhaled air and RH before the inlet to the aerosol detector. Simultaneous measurements of air pressures, aerosol flow rates, temperature, and RH at other positions are also helpful. This does not fully apply to experiments with radiolabeled aerosols.
To help the volunteer adjust to the equipment and find a relaxed position, it may be advisable to perform a short test experiment before the full exposure. Especially if different aerosols are studied within the same experiment, it is preferable if the order of aerosols is randomized and that the study is single-blinded. Nonrandom order or subject knowledge of the exposure might affect the quality of DF measurements.
For comparison with lung deposition models and other experimental data, it is important to include a basic medical examination of the subjects and to provide data on at least the most common spirometric lung function values, as well as height, age, sex, smoking history, and relevant diseases. Additional measurements of total lung capacity, function residual capacity, and airway resistance may also be useful from a modeling standpoint. The participation of a physician is strongly recommended for correct interpretation of medical data. Historically, the vast majority of the studies carried out after 1980 provide at least basic information on health and smoking status of the subjects, whereas before this year it was omitted in almost all studies (see Table 2 in Appendix for a list of experiments).
Previous Experiments
Over the last century, around 50 empirical studies have been carried out to assess lung deposition of particles less than 300 nm in human subjects (see Table 2 in Appendix). In total, these studies have included close to 500 volunteers, most of them healthy men of European descent. Approximately 140 were women, 34 asthmatics and 32 patients with COPD. Predominantly hydrophobic, laboratory-generated particles have been examined.
The literature was reviewed to identify experimental difficulties in measurements of particle lung deposition, with a focus on nano-sized particles. Some studies on total deposition of cigarette smoke were omitted, as this aerosol includes a substantial fraction of larger particles (>300 nm). Thus, 40 relevant studies were obtained dating back into the 1940s (see Table 2 in Appendix).
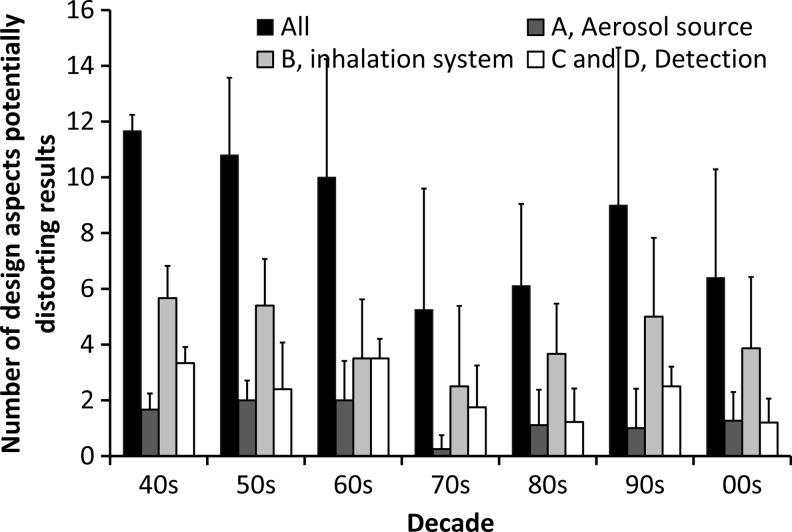
As a guide for evaluation of data quality, implementation of each of the critical design aspects was systematically assessed for the 40 found studies (Figs. 5 and 6). The criteria for considering a risk of bias due to insufficient consideration of a design aspect are listed in the Appendix. For instance, design aspect A1 (when a polydisperse aerosol is utilized as a measure for the DF of a monodisperse aerosol) was regarded as potentially distorting the results, if the GSD of the inhaled aerosol was not provided or exceeded 1.3. A few studies only present short and incomplete descriptions of their experimental systems, and may therefore be judged as neglecting many of the design aspects while, in reality, the methodology may have been appropriate. Some design aspects may have been addressed properly in the study, but not explicitly mentioned by the authors. Hence, it is impossible to provide a validated rating of the quality of individual studies with our approach. Therefore, only information on the average experimental quality related to the entire data pool or stratified according to the decade of publication is provided in this review article.
FIG. 5.
The number of critical design aspects that potentially may have led to a bias in the published experiments (A, B, C, and D refer to items in Table 1). The average for each decade is shown. In total, 40 studies measuring particles below 300 nm in diameter were included. It is important to note that this figure is based solely on information given by the authors of the reviewed studies. As it is likely that some design aspects were addressed properly, but not explicitly mentioned in the studies, this figure presents a “worst case” scenario. Criteria and explanations for each design aspect are found in the Appendix.
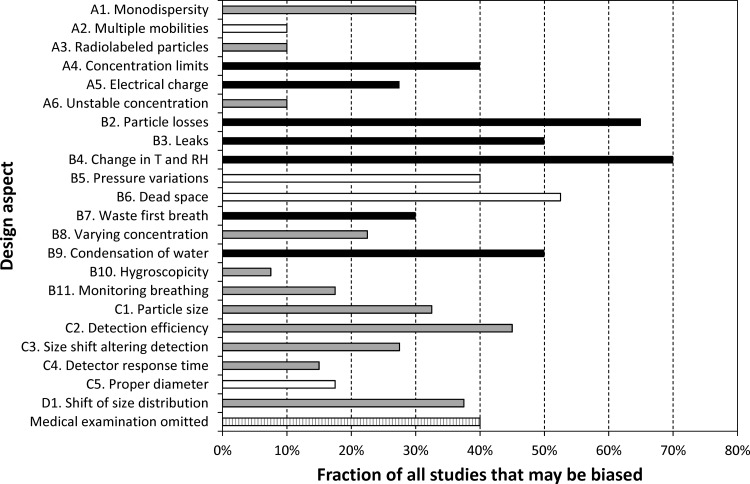
FIG. 6.
The fraction of all studies that may be biased because of not appropriately accommodating the critical design aspects introduced above (see Section 4; A, aerosol properties; B, inhalation system; C, particle detection; D, aerosol polydispersity). Black and white indicate design aspects that, if not considered, lead to an overestimate and underestimate of DF, respectively. Gray refers to design aspects that may cause an error in both directions depending on the specific operational conditions. It is important to note that this figure is based solely on information given by the authors of the reviewed studies. As it is likely that some design aspects were addressed properly, but not explicitly mentioned in the studies, this figure presents a “worst case” scenario. Criteria and explanations for each design aspect are found in the Appendix.
The number of design aspects potentially distorting the results, summarized in Figures 5 and 6, show some clear trends: (1) improvement in methodology has been limited over time; (2) almost no studies incorporate all design aspects; and (3) frequent not-mentioning of some important design aspects, for instance, correction for apparatus dead space and particle losses, suggests that substantial biases in parts of the reviewed experimental data exist.
It is noteworthy that the development of particle detection and sizing techniques, in general, has not been accompanied by progress in methodology (Fig. 5). Some of the early studies of lung deposition were well designed and carefully prepared, whereas there are more recent studies with serious flaws. Thus, the quality of data has not necessarily improved with time. A few studies consider almost all the design aspects.(e.g., 36,37,69–72) For instance, it was the express purpose of Heyder's studies to reduce experimental errors and to accurately characterize deposition.(70,71) However, it is noteworthy that the majority of these were carried out during the 1970s and 1980s with only a few subjects (typically four male volunteers with unknown lung function) and limited data for particles below 100 nm. In 14 of the 40 studies (i.e., 35%), there is a risk of bias due to 10 or more design aspects. Detailed descriptions of the previous assessments of the various design aspects are provided in the Appendix.
Some design aspects may have biased 30–70% of all experiments and thus have a potential to distort the collection of measured data. As seen from Figure 6, the top five design aspects not considered appropriately were: (1) changes in temperature and RH, 70% may be biased; (2) particle losses in the inhalation system, 65%; (3) apparatus dead space, 53%; (4) leaks, 50%; and (5) condensation of exhaled aerosol, 50%. Apart from correction for apparatus dead space, omission of these design aspects is likely to lead to an overestimation of the measured DF compared with the true value.
Conclusion, Outlook, and Identification of Critical Research Needs
Accurate knowledge on pulmonary deposition of nanoparticles is essential for various reasons. In light of the rapid economic growth of nanotechnology and the ongoing anthropogenic emission of ultrafine particles (from, e.g., combustion emissions), validated predictions of the nanoparticle lung burden are needed for regulatory measures on acceptable exposure levels. Moreover, inhalation of nanoparticles provides a noninvasive means of drug delivery that may play an important role in the emerging field of nanomedicine. For instance, in spite of the large therapeutic potential of peptides and proteins, they are very difficult to use in therapy due to their poor stability in physiological media and difficulties in delivering them across biological barriers. Nanoparticle drug-delivery systems are considered one of the most promising technologies to overcome these limitations, because they are known to cross biological barriers and to enter cells in high yields, thus improving cellular delivery of macromolecules.(73) In addition, nanoparticles play a significant role in diagnostics as imaging agents.(74) Moreover, it is even conceivable that careful measurement of particle lung deposition may serve directly as a sensitive diagnostic tool for lung disease (e.g., COPD, emphysema), as suggested by Heyder and co-workers.(47,75)
Experimental data on respiratory tract deposition of particles in the diffusion-dominated regime are very limited. Within the 50 experimental studies reviewed here, women are generally underrepresented and few studies used nose breathing. Data for children, diseased, and elderly are extremely scarce. In light of the higher vulnerability of these segments of the population and the need for more efficient drug-delivery techniques for treating diseases via inhalation (noninvasive application option), there is an urgent need for more particle lung deposition measurements stratified for age and health state.
Furthermore, more information is needed on the deposition of specific particle types. The predominantly used hydrophobic particles may be relevant for freshly generated aerosols from, for instance, traffic exhaust. However, environmental (nonsoluble) particles typically become moderately hygroscopic and polydisperse due to atmospheric processing.(76) As hygroscopic particles grow in the humid environment of the respiratory tract due to water uptake, attention also needs to be directed to the effect of hygroscopicity on particle lung deposition. A group of particles that recently has attracted much attention is carbon nanotubes. By analogy with asbestos fibers, it has been suggested that these could have substantial adverse health effects because of their shape and insolubility.(77) However, no experimental data on respiratory tract deposition of carbon nanotubes are available. In addition, information on the deposition of nano-sized particles with high electrical charge is largely missing.
Ultimately, accurate data on nanoparticle lung deposition enables validation and improvement of already existing and new computational models of particle lung deposition. Currently, both semiempirical (e.g., ICRP model) and ab initio models (e.g., MPPD model) use theoretical predictions of diffusional particle deposition in ducts mimicking the respiratory tract to compensate for the limited amount of data for particles below 100 nm. The models are, however, in reasonable agreement (typically<0.1 difference in DF) with much of the available, more recent, data.(e.g., 25,37)
To facilitate comparison of measured and modeled lung deposition values, it is important to include sufficient information on breathing pattern and subjects: (1) respiratory parameters—respiratory frequency, tidal volume, minute ventilation, breath holding, mouth or nose breathing; (2) anthropometric parameters—age, sex, weight, height, cigarette smoking history (pack years); (3) lung function parameters (usually given as %predicted)—FEV1 (forced expiratory volume in 1 sec), FVC (forced vital capacity, maximum tidal volume), FEV1/FVC (reduced in obstructive lung diseases, such as asthma, COPD), RV (reserve volume, elevated in patients with emphysema); and (4) information about lung disease state and medical treatment—disease stage (e.g., gold stage for COPD), exacerbation, use of drugs (like steroids).
As shown in this review, reliable measurement of the respiratory tract deposition of nanoparticles is less straightforward than the task initially may appear. Several studies have accounted for most of the discussed critical design aspects, but almost none has reportedly included all. This likely contributes to the considerable variation found in the experimental lung deposition data reported in the literature. One of the main objectives of this study is to ensure best possible data quality for future nanoparticle lung deposition measurements. The list of design aspects presented here may serve as a guideline for designing accurate measurement systems.
Biases in DF introduced by not adequately considering one or more critical design aspects have most likely had an impact on current lung deposition models. For instance, the study by Schiller et al.(36) on four male subjects has been highly influential. This study is among the most carefully designed in the literature, and its data can be considered of the highest quality. Nevertheless, particle loss correction in the inhalation system appears to be a formidable task due to a flexible mixing volume, and some design aspects were not recognized or may have had an impact, such as mouthpiece dead space.
In the end, uncertainties in the deposition of nanoparticles in the lung will remain due to considerable intersubject variability in lung morphology, breathing pattern, and possibly even circadian rhythms affecting the respiratory tract. This is particularly relevant for vulnerable subgroups of the population. Thus, it is likely that accurate nanoparticle lung deposition for a specific individual will always require direct measurement of the particle lung deposition for the individual of interest. Hence, accurate, compact, and easy-to-handle devices for particle lung deposition measurement are expected to be used even if perfectly validated deposition models for the general population are available.
Finally, it is important to note that the ultimate goal of respiratory tract deposition measurements would be to identify the site of deposition of each single aerosol particle in the lung. Information on regional deposition is crucial, because different areas of the respiratory tract have different anatomy, physiological function, and clearance mechanisms. This might possibly be achievable in the future with a combination of vastly improved lung imaging techniques and intricate means of particle labeling. Currently, only very little information on regional particle deposition is available based on gamma camera data and/or on bolus inhalation techniques, where the inhaled aerosol is only provided during part of the inhalation period, which allows targeting of the lower airways and the alveoli, respectively. However, the precision of the bolus method is limited by factors such as turbulence in the upper airways, a relatively small volume in the conducting airways, and left-to-right asymmetry of the lung.(78,79) Computational lung deposition models provide a rough estimate of regional deposition, but in light of the complexity of the morphology of the lungs, any of these models requires reliable experimental data for validation.
Appendix
Detailed Description of Critical Aspects for Particle Lung Deposition Measurements
Aerosol source
A1. Monodispersity of the challenge aerosol
There are several ways of generating monodisperse particles with GSD<1.3. Narrow nanometer-sized aerosol can be directly generated by the electrospray technology.(80) Alternatively, one can generate monodisperse particles (GSD<1.1) by nebulization of a liquid suspension containing monodisperse particles. These suspensions are available from various vendors covering a size range of 0.02–100 μm. However, both electrospray and nebulization also generate a background of particles from dried droplets that contain other material than the fabricated particles. This background can be reduced by a DMA. Polydisperse aerosol generators or ambient particles can be used to produce monodisperse aerosol with GSD<1.1 by selecting a narrow size fraction, for instance, by DMAs, which typically operate in the size range of 1–1,000 nm.(31) However, care must be taken to avoid multiply charged particles with different mobility diameter: see A2.
Previous experiments
In 70% (28/40) of the reviewed studies, the particle distribution had a GSD<1.3 or was presumably measured with correspondingly high size resolution from a polydisperse aerosol. In 30%, GSD was>1.3 or unclear.
A2. Multiple mechanical mobility diameters obtained while separating a single electrical mobility
If monodisperse particles are separated from a polydisperse distribution in an electric field (e.g., with a DMA), they will have similar electrical mobility but multiple mechanical mobilities because of varying electric charge. For particles with equal electrical mobility, those with multiple charges have larger mechanical mobility (i.e., larger diameter) than those that are singly charged. In the diffusion-dominated regime, it is (primarily) the mechanical mobility that determines deposition. In this size range, DF is decreasing with size, and thus the multiply charged particles have lower deposition; DF will be underestimated. This has probably only caused a small deviation from the true DF in a few studies during the 1980s. Based on the size distributions in these studies and on an assumption of equilibrium bipolar charge distribution, the maximum error was estimated to be −0.03 in DF (relative error −6%).
There are different means to minimize errors from multiply charged particles with single electrical mobility but varying mechanical mobility. If monodisperse particles are selected from a polydisperse aerosol with an electric field, the selected particle size should be large compared with the peak size in the original polydisperse aerosol to ensure that the number concentrations of multiply charged particles are considerably smaller than for singly charged particles.
Previous experiments
In 90% of the studies, aerosol particles were generated in ways where this was no difficulty. In 10% (four of the reviewed 40 studies), monodisperse particles were obtained from a polydisperse distribution by selecting a single electrical mobility.(36,38,69,81) It seems the original size distribution in some of these cases had a CMD below the size selected by the electrostatic classifier, and thus the fraction of multiply charged particles could be presumed to be low.
A3. Radiolabeled particles
The two most prominent techniques for the generation of nano-sized radiolabeled aerosol particles are based on nucleation and condensation of supersaturated material (e.g., carbon, gold, silver, or indium) vaporized either in a Technegas system or by spark discharge.
Because of the high number of very small primary particles formed in the supersaturated atmosphere, they immediately coagulate and form particle clusters, where primary particles usually can be in the size range of a few nanometers. These clusters then have a much larger mobility diameter compared with the volume equivalent diameter. The cluster particles can further be compacted or sintered in another furnace, resulting in a complete change of morphology. By using this technique, radiolabeled Au, Ag, and In particles have been produced.(82–84) For studies of lung ventilation in nuclear medicine, a specific technique has been developed (Technegas, Media Cybernetics, Canberra, Australia) where carbon particles are formed after a carbon crucible is heated to 2,600°C.(85) Before heating, the crucible is loaded with [99mTc]pertechnetate. As technetium has a lower melting temperature compared with carbon, it condenses first, forming a radiolabeled technetium core, which is covered by a carbon shell, making the technetium-carbon particles insoluble.(22) The Technegas particles usually have diameters above 100 nm and are hygroscopic because they contain saline from the technetium elution. By using ion exchange, the saline can be removed from the eluate and nonhygroscopic particles can be prepared in the mobility diameter range between 30 and 200 nm.(22)
Nano-sized aerosol generation by spark ignition between two electrodes is, meanwhile, a well-established method to produce particles between 10 and 100 nm diameter, and commercial devices are available.(86,87) As spark ignition also ablates atoms from the electrode, producing a supersaturated atmosphere, there are many similarities in structure and properties to the condensation aerosol, namely, the particles are clusters of smaller primary particles. Radiolabeling can be achieved if one or both electrodes are made of radiolabeled material, as has been done using iridium, gold, silver, and TiO2.(46,88,89) When both electrodes are of different materials, multicomponent nano-sized particles can be formed, such as carbon particles labeled with iridium. The most common spark-generated particles are soot particles, and they have been widely used in inhalation and toxicology studies.(90)
These generation methods for radiolabeled particles give size distributions with GMD>1.3 (typical values for GMD are 1.5–1.8), and the particles may carry some hygroscopic material. The hygroscopic material lowers the deposition probability in the diffusion-dominated size regime. These factors have to be considered when providing data on DF.
Previous studies
In 22.5% (9/40) of the studies, radiolabeled particles were used.(23,46,48,50,91–95) In four of these, size distribution is unclear (all nine studies have GMD>1.3).
A4. Lower and upper concentration limits
The aerosol concentration needs to be high enough to give useful counting statistics, but sufficiently low to avoid coagulation. Concentration limits may also be determined by the instruments used for particle detection. A bias due to particles generated during respiratory activity (typically<10 cm–3(96)) is unlikely, but if the concentration in the inhalation system is much lower than in the surrounding environment, measurements become more sensitive to small leaks at, for instance, the breathing mask. For very high concentrations, coagulation may distort the measurements. Experimental setups with long residence times of the aerosol, for example, by storing it in bags before measurement, are more sensitive to high concentrations. Concentrations up to 2×106 have been used for deposition studies in the diffusion-dominated regime.(17,97) The maximum amount of particles lost between the inhaled and exhaled sample due to coagulation, as estimated with equations provided by Baron and Willeke,(98) was around 2.5%. This may have caused an overestimation of DF with 0.01 to 0.02 (relative error 2–3%), but probably it was partly compensated for in the loss correction.
Previous experiments
Sixty percent (24/40) of the studies were considered to use acceptable aerosol concentrations; 23% (nine studies) had concentrations high enough to suspect significant coagulation; and 17% (seven studies) did not provide concentration of the aerosol.
A5. High electrical charge on the particles
Freshly generated particles may carry high levels of charge, and this may influence particle deposition in the lungs.(53,54) Typically, the naturally present radiation generates enough ions for gradual neutralization of the aerosol. However, in the vicinity of aerosol sources (e.g., certain occupational settings, combustion sources, nebulizers, dry particle disperses, or even electrospray generators), highly charged particles may be available for inhalation.
Melendri et al.(54) provide an equation for the limiting number of charges (depending on particle size) characterizing the onset of additional lung deposition due to image charges. If the purpose of the experiment is to study deposition of neutral aerosol, potential biases due to charge effects should be eliminated by neutralizing the aerosol with standard neutralizers (e.g., irradiation with β-radiation or soft X-ray).
Previous experiments
Seventy-two percent (29/40) of the studies were unlikely to be affected by excessive particle charge. High electrical charging is primarily assumed to be a problem in studies where the aerosol is generated with a nebulizer without particle neutralization before inhalation.
A6. Stability of the aerosol concentration
The aerosol concentration should be sufficiently stable during exposure. In most experimental setups, the inhaled and exhaled aerosol is not continuously monitored, but rather measured with a single instrument alternating between the two. The error in the measured DF depends on the time delays in these alterations compared with the time scale of the variations in aerosol concentration.
Previous experiments
Ninety percent are unlikely to be affected by variations in concentration of the inhaled aerosol—in most cases because mixing volumes were used.
Inhalation system
B1. Separation between inhaled and exhaled samples
Two main methods have been used for separating inhaled and exhaled samples: (1) a counter close to the mouthpiece (or face mask) that continuously records inhaled and exhaled air, or (2) a valve system that directs the air into separate reservoirs (including exhalation filter sampling).
Previous experiments
The vast majority of experiments use valve systems, but there are exceptions. Landahl et al.(61) used two separate mouthpieces, with inhalation from one and exhalation into the other. However, this method introduces several experimental difficulties, such as leak problems and limitations of the breathing pattern. Kim and Jaques(25) counted the particles at the mouthpiece with a modified UCPC for high time resolution.
B2. Particle losses in the inhalation system
Particle losses in the inhalation system may be interpreted as deposition in the lungs. If the magnitude of the particle losses is known, they could to some extent be corrected for. But efforts have to be made to minimize losses, because any correction will be associated with uncertainties. Losses are dependent on particle size, particle electrical charge, aerosol flow rates and their variability, RH (for hygroscopic aerosols), and temperature. For some bag systems, there is an additional difficulty because losses also depend on the fill ratio of the bags and the surface properties of the inside of the flexible bag.
Particle losses could be decreased by use of electrically conducting material, by avoiding sharp bends in the tubing (for large particles), and by keeping residence time of the aerosol in the system low. Sampling close to the mouthpiece may be preferable. However, some losses in the inhalation system are usually unavoidable, and to correct DF for these, a modified version of Equation 1 can be used (DFequipment is the deposition fraction of particles in the inhalation system)(18):
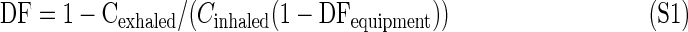 |
DFequipment has been measured for a number of different systems.(18,38,99,100) It is important to note that losses may be RH-dependent.(38)
There are special concerns in measurements of regional deposition with radiolabeled aerosol using radionuclide imaging devices because of attenuation and scattering of the radiation in the body. This is discussed separately further on.
Previous experiments
In 35% (14/40) of the studies, losses are either corrected for or likely to be negligible. In the other studies, particle losses were not fully considered or bags were used in a way that makes loss correction difficult.
B3. Leaks
The system has to be leak tight. If breathing by mouthpiece, it is necessary to use a nose clip. Dry lips have also been noted to be a potential problem.(72) Errors caused by leaks are decreased if the aerosol concentration surrounding the inhalation system is comparable to the concentration inside. Leaks could be tested, for instance, by inhalation of particle-free air.
Previous experiments
Fifty percent (20/40) of the studies could be assumed to have leak tight systems. The experiments where leaks were considered plausible had one or more of the following features: face mask without leak test, mouthpiece without mentioning nose clip, or inhalation and exhalation through different mouthpieces.
B4. Change in temperature and RH between inhaled and exhaled samples
If inhaling dry or room temperature aerosol, the volume of the air is larger in the lung due to body temperature and humidity saturation and, in addition, the exhaled air is larger than the inhaled air because of increased temperature and humidity (see B11). This may distort both the particle concentration measurement and the monitoring of the breathing pattern. Therefore, all lung function measurement devices predict a BTPS correction based on environmental conditions (temperature, pressure, RH).
To avoid this problem, inhaled and exhaled aerosols should be measured at similar temperature and RH. This does not necessarily mean that the inhaled aerosol needs to be heated and humidified. The exhaled aerosol could also be dried and cooled before measurement. Alternatively, if all exhaled particles are sampled, for instance, on a filter, the change in volume makes no difference.
Previous experiments
Thirty percent (12/40) were assumed to account fully for change in volume due to heating and humidification.
B5. Pressure variations
Pressure variations caused by breathing distort the aerosol flow and may give errors in particle sizing and counting. If subjects are breathing spontaneously, a large pressure drop will also make the breathing pattern less natural. If equal volume flows (and not mass flows) are sampled from the inhaled and exhaled aerosols, a pressure difference gives rise to an oversampling on the high-pressure side and vice versa. The maximum difference in pressure between inhaled and exhaled air that could be exerted by a healthy woman is around 10 kPa.(101) It is unlikely that the pressure difference in an inhalation system exceeds 10% of this. A pressure that is 1 kPa higher in the sampling point for exhaled aerosol may cause an underestimation of up to 0.01 in DF, or 7% relative error. Some detectors are also sensitive to pressure variations because of flow distortions.
Previous experiments
Sixty percent (24/40) use systems where pressure variations are unlikely to cause difficulties. Several of the remaining systems were difficult to evaluate regarding pressure disturbances.
B6. Apparatus dead space
The air trapped in the dead space in the mouthpiece or face mask after exhalation is inhaled again. Thus, the measured inhaled and exhaled aerosol concentrations may differ from the concentrations that are breathed at the mouthpiece.
Previous experiments
Forty-seven percent (19/40) correct for mouthpiece dead space. Thus, this design aspect has probably resulted in a systematic underestimation of much of the available experimental data, including those used for the ICRP evaluation. Few studies provide information on the dead-space volume and could be corrected afterwards. It is notable that many of the early studies took dead space into account,(e.g., 38,57,92,95,100,102,103) whereas it tended to be forgotten over the last two decades.
B7. Discard first breaths
The data from the first breaths contain room aerosol and need to be discarded. If bags or containers are used for collection of the aerosol, the inhalation system must include a valve for waste of the initially exhaled air. In the flow-through systems (Fig. 3, left), the data from the first breaths need to be discarded.
Previous experiments
In 70% (28/40) of the studies, the first breaths are discarded or the exposure times are long enough (hours) to make the effect negligible.
B8. Varying exhaled concentration
The particle concentration in the exhaled breath is not uniform. The air exhaled at the end of the breath contains fewer particles than initially. If total deposition is measured, it is therefore necessary to measure over the complete breath. This could be achieved by mixing the exhaled aerosol in a container or by fast time-dependent sampling combined with volume flow measurement. In the latter, concentration multiplied by flow rate is integrated over time—thus a high precision is needed in both time-resolved concentration and flow rate to obtain reliable data. Long measurement times have also been used to smear over the varying exhaled concentration.
Previous experiments
Seventy-eight percent (31/40) sampled the complete breath or had sufficient mixing.
B9. Condensation of water on exhaled aerosol
If the undiluted exhaled aerosol is cooled below approximately 35°C, it becomes supersaturated with water, which may induce a strong particle growth followed by substantially altered particle losses in the system. An increase in RH from dry to above 70% is likely to alter the size of hygroscopic particles substantially and thereby shift the particle losses. See also the discussion on polydisperse aerosols below.
Previous experiments
In 50% (20/40) of the inhalation systems, heating or drying was used to avoid condensation of water.
B10. Hygroscopic aerosol
Hygroscopicity may alter deposition. This is a problem only if it is not a property that is studied. For example, iron oxide particles have sometimes been used in experiments where they, probably falsely, have been assumed to be hydrophobic.(5)
Previous experiments
Ninety-three percent (37/40) are not biased by unwanted size shifts because of hygroscopicity. One study used iron oxide(91); another compared ambient aerosol deposition with ICRP data and found a deviation that may very well be explained by hygroscopicity(93); and a third study considered the slightly hygroscopic glycerol particles to have negligible growth.(95)
B11. Monitoring of breathing pattern
Not all inhalation systems include monitoring of the breathing pattern. This is necessary for comparison with models and previous experiments.
Previous experiments
Eighty-two percent (33/40) of the reviewed studies provide breathing pattern, but only one explicitly reports BTPS.(104)
B12. Defining breathing pattern
A controlled and predetermined breathing pattern may be achieved either by having subjects breathing according to a displayed signal or by a valve system regulating the inhaled and exhaled flow and volumes; the former is by far the most common. The breathing pattern is most likely disturbed by the inhalation system, and therefore the spontaneous breathing of the subjects will deviate from their natural behavior.(105)
Previous experiments
Most experiments use a controlled breathing pattern where subjects breathe according to a signal.
Particle detector
C1. Particle sizing accuracy
The error in particle diameter measured with a mobility particle sizer is determined by a number of factors, such as DMA design and accuracy in flow rate, voltage, and particle charge distribution. Calibration with, for instance, polystyrene particles of known size, is needed for accurate sizing. The precision of a calibrated instrument is typically within a few percent of the true diameter.
Previous experiments
Sixty-seven percent (27/40) of the studies provide reasonable particle size data. Correct sizing in the diffusion-dominated regime was a major difficulty in many of the studies until the 1980s. These studies used varying methods, such as diffusion batteries, light scattering, electron microscopy (in some cases, on semivolatile particles), or impactors.
C2. Detection efficiency
Usually a single instrument is used for measurement of both inhaled and exhaled particle concentrations. In these cases, detection efficiency has a minor influence on the measured respiratory tract deposition—provided that the detection is linearly proportional to concentration. As long as the fractional error in the concentration measurement is constant, the obtained DF will not be affected. If separate instruments are used for inhaled/exhaled samples, a deviation of only a few percent may cause a major error, especially when the DF is low.
Previous experiments
Fifty-five percent (22/40) of the studies used one detector measuring at constant temperature and RH; 13% (5/40) used separate instruments(10,17,60,94,97); and 33% (13/40) measured at varying temperature or humidity between inhaled and exhaled samples.
C3. Size shift that alters detection efficiency
A particle size shift between inhaled and exhaled samples could induce a bias in the deposition measurement if it leads to altered detection efficiency.
Previous experiments
Twenty-eight percent (11/40) of the reviewed studies were at risk of having altered detection efficiency due to size shifts, but in most of these the effect could be assumed to be of minor importance. This design aspect is primarily influencing a number of older studies where particle growth due to condensation was likely to change detection.
C4. Low response time
The finite response times of detection instruments such as particle counters may delay and smear the signal.(63) This difficulty could be minimized either by use of a fast detector or by a mixing volume for the exhaled air. In the mixing volume, concentration variations are smoothed out.
Previous experiments
Eighty-five percent (34/40) of the studies are unlikely to be affected by finite response time of particle detectors; most of these use mixing volumes to level out the varying exhaled particle concentration.
C5. Proper particle diameter
In some experiments, parameters proportional to total particle surface area or mass are measured. This is most notable for studies with radiolabeled aerosols, but other mass measurement methods have also been used, such as filter collection, flame photometry, impingers, or thermal precipitators. If particle mass is measured, the MMD needs to be provided and not only the CMD.
Previous experiments
In 83% (33/40) of the studies, the proper particle size metric appears to be reported; 17% use techniques measuring particle mass or activities, but provide number size distributions.
Additional design aspects for particle lung deposition measurements with polydisperse aerosols
When a polydisperse measurement is performed, an on-line particle size spectrometer is typically used to determine the complete size distribution of particles in inhaled and exhaled air samples. The main disadvantage is the rather low time resolution, which puts high demands on the aerosol source stability as only a limited number of size scans in inhaled and exhaled air can be performed during an inhalation session.
More recently, electrical mobility spectrometers have been developed based on parallel detection of the complete size distribution with a set of electrometers. Using such instruments, a full-size spectrum (typically 5–500 nm) is achieved with a sampling frequency of 0.1–1 Hz, which can be beneficial when determining respiratory deposition of particles from combustion systems or at work places. However, both the size resolution and the sensitivity are significantly reduced compared with the SMPS. Particularly, the lower sensitivity is a major limitation when performing ambient measurements. Examples are the FMPS (TSI Inc.) and the DMS (Cambustion). An Electrical Low Pressure impactor(106) has similar performance as the FMPS, with the exception that particles are classified according to their aerodynamic diameter, which is not optimal for describing deposition of particles smaller than about 200 nm.
To investigate respiratory tract deposition as a function of chemical composition and size, it may in the future be possible to sample with an aerosol mass spectrometer (AMS)(107) in inhaled and exhaled air. Components that can be quantified with the AMS include ammonia, nitrates, and sulfates, as well as individual organic fragments. The AMS would have sensitivity enough for ambient respiratory deposition measurements of size-integrated chemically resolved data. At elevated concentrations, size-resolved deposition (vacuum aerodynamic diameter) data may be possible.
D1. Size shifts
The most important, but often overlooked, difficulty with polydisperse methods is small size shifts of the particle diameter between inhaled and exhaled samples (Fig. 7). A few percent shift in size is usually insignificant for techniques where the total concentration of a monodisperse aerosol is studied. However, for polydisperse aerosols, even a small shift of the exhaled aerosol may distort the size distribution sufficiently to bring about major errors when it is compared with the inhaled distribution. For many aerosols, the size distribution can be altered in the lungs due to evaporation, agglomerate restructuring, coagulation, or condensation.
FIG. 7.
Inhaled size distribution and the exhaled distribution as calculated by the ICRP model (tidal volume, 0.750 L; breathing frequency, 12 min–1). A presumed 5% negative size shift of the exhaled distribution due to, for instance, evaporation or agglomerate restructuring is also shown. If the size-shifted exhaled distribution is used to calculate the DF, an error will occur as shown in Figure 8.
Figures 7 and 8 illustrate how a small size shift may give rise to a substantial measurement error in polydisperse experiments. Figure 7 shows a typical size distribution with a CMD of 130 nm and a GSD of 1.7. The exhaled size distribution is estimated by the ICRP model. A negative shift of 5% is introduced (for instance, because of particle evaporation in the lungs). When calculating the DF of the unaltered size distributions at 300 nm with Equation 3, the value becomes:
 |
FIG. 8.
Error caused by 1% and 5% size shifts of the exhaled size distribution from Figure 7. The erroneous deposition curves are calculated by using size-shifted exhaled distributions (ICRP model; tidal volume, 0.75 L; breathing frequency, 12 min–1).
However, if there is a 5% negative size shift of exhaled distribution due to evaporation, the exhaled concentration at 300 nm appears to decrease by 11% (for this particular size distribution; the decrease would be larger for a more narrow distribution and smaller for a broad distribution). Thus, the calculated DF instead turns out to be:
 |
In other words, for a measured DF that is a factor two higher than the true DF, a small shift in size of the exhaled particles could cause a major error in the measured concentration because the concentration varies with size. As demonstrated in Figure 8, even a size shift as minor as 1% may introduce a significant error in the measured DF. Errors caused by size shifts increase with decreasing GSD of the distribution.
To investigate potential size shifts, a single particle size can be selected in a DMA, and these particles can then either be artificially conditioned to respiratory conditions or preferentially be inhaled by a human subject. The altered size can thereafter be measured with an SMPS with a precision potentially better than 1%.
There are several approaches to account for size shift in polydisperse experiments: (A) use stable particles; (B) precondition the particles before inhalation(43); (C) process the particles measured from the inhaled air(72); or (D) adjust the measured DF for the size shift.(37) In addition, it is necessary to dry the aerosol before measurement to avoid particle growth by water uptake (see B4).
Previous experiments
Sixty-two percent of the studies are unlikely to be affected by size shifts. Of the 40 reviewed studies, 23 were using polydisperse aerosols. Of these, 15 may be biased due this design aspect, four took measures, and four only reported total DF.
Additional design aspects for measurements with radiolabeled aerosols
The dose of inhaled radiolabeled aerosols is usually assessed using the count rate of radionuclide imaging devices, such as a shielded whole-body scintillation counter, a planar gamma camera, single-photon emission CT (SPECT), or positron emission tomography.(108–110) Besides the deposited dose, the distribution of deposited activity is of great interest in order to assess deposition in the different anatomical compartments of the respiratory tract, such as the extrathoracic (upper) airways, the thoracic (lower) airways, and the alveoli. This is of importance because many lung diseases are often, at least in an early stage, limited to one region of the respiratory tract. For example, asthma is primarily a thoracic airway disease.
The most frequently used technique of imaging deposited radiolabeled particles in the lung is planar gamma camera imaging.(111) A planar gamma camera consists of a large NaI crystal (can be up to 40 cm×60 cm) coupled to multiple photomultiplier tubes distributed over the area of the NaI crystal. Gamma rays hitting the crystal induce light flashes that are detected by the photomultipliers and converted into voltage impulses, the height of which depends on the gamma ray energy. A collimator in front of the NaI crystal allows only gamma rays penetrating perpendicular to the collimator surface to pass. Together with mathematical algorithms, this allows a localization of the gamma source.
Attenuation and scattering in the body lowers the detection sensitivity of deposited radiotracers.(112,113) In order to estimate attenuation correction factors (ACF) of radioactivity distributed in the lung, all tissues have to be considered. A first-step approximation method to calculate the ACF was proposed by Fleming and Pitcairn et al.,(110,114) where it was assumed that the activity is homogeneously distributed in the lung with thickness L and covered by the chest wall with thickness a (anterior) and b (posterior). The ACF of a posterior planar gamma camera measurement (ACFP) is then given by:
 |
where μST and μLT are the linear gamma ray attenuation coefficients of soft tissue (μST=0.151 cm–1) and of lung tissue (μLT=0.038 cm–1).(115) Using standard anatomic dimensions (a=b=2 cm and L=21 cm), Equation 1 yields ACFP ∼ 2.
Recent computational analysis based on realistic lung and anatomical structures predicted differences in attenuation between radiolabeled aerosols deposited in the trachea and central airways versus lung periphery.(113) For example, the high spinal or sternal attenuation in the central region separates between the left and right lungs in almost all thorax scintigrams, although both lungs are next to each other. This was confirmed by attenuation measurements after radiolabeled aerosol bolus inhalation,(116) where ACF can double for central airway aerosol deposition.
Approaches to assess patient individual ACFs was developed using density mapping of X-ray computed tomography (CT) scans,(112,113,117) lung uptake of 99mTc-macroaggregated albumin after intravenous injection,(118) r total chest gamma ray transmission analysis.(119,120)
Valuable ACFs are important regarding the dose assessment of radiolabeled drug inhalation. Most drug formulations contain larger-sized aerosols, which have preferred central airway deposition, and incorrect ACF determination implies an error of up to 100% in estimation of the deposited dose.(116) In addition, patients with obstructive lung diseases (COPD, asthma) show higher deposition rates at central airway bifurcations due to impaction, caused by higher inhalation flow rates and airway narrowing. Clinical studies require planar gamma camera imaging in assessing the deposition and distribution of inhaled radioaerosols.
Analysis of particle distribution in the lung from planar gamma camera imaging
The pattern of activity distribution between central (C) and peripheral (P) lung regions was analyzed after defining total lung and central airway regions of interest (ROIs). Counts in peripheral lung regions were calculated as the difference in count rate between the total lung and the central ROI of each lung lobe. Normalized C/P ratios of the aerosol inhalations have to be obtained by dividing each aerosol C/P ratio by the Kr-gas ventilation C/P ratio,(121) which can be considered as normalization to the volume distribution of the lung. Normalized aerosol C/P was 1.04±0.07, 0.83±0.12, and 1.91±0.56 after full breath, after deep, and after shallow aerosol bolus inhalations, respectively.(116) Deep bolus C/P was significantly lower compared with full breath C/P (p<0.01), whereas shallow bolus C/P was significantly higher (p<0.01), confirming the local targeting of the aerosol bolus technique.
Measurement of Regional Particle Lung Deposition
Clearance mechanisms, and hence the retention of particles, depend on lung region. For example, the alveolar regime is particularly vulnerable due to the absence of mucociliary clearance and a very thin air–blood barrier, which may facilitate particle translocation into secondary organs. The distribution of the deposited particles may also be altered in the diseased lung. For these, and other, reasons, it is often of interest to measure not only total, but also regional lung deposition.
There are three main methods to assess regional lung deposition: (1) aerosol bolus inhalation or analysis of the nonuniform exhaled concentration; (2) clearance kinetics (fast clearance—upper respiratory tract; slow clearance—alveolar region); and (3) spatially resolved analysis of gamma-camera pictures and SPECT scans, preferably in combination with a lung imaging technique such as CT.
Acknowledgments
The following organizations are gratefully acknowledged for financial support: The Swedish Research Council (project 621-2011-3560); The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS, project 216-2009-606); and the Crafoord Foundation. J.L., E.S., and O.S. designed the project; all authors contributed to identification of critical design aspects; J.L. reviewed design aspects in previous experiments; J.L. and O.S. carried out the model calculations; W.M. and O.S. reviewed measurements with radiolabeled aerosols; and all authors contributed to writing of the manuscript and have read and approved its final version.
Author Disclosure Statement
The authors declare that there are no conflicts of interests.
References
- 1.Paur HR, Cassee FR, Teeguarden J, Fissan H, Diabate S, Aufderheide M, Kreyling WG, Hanninen O, Kasper G, Riediker M, Rothen-Rutishauser B, and Schmid O: In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung—a dialog between aerosol science and biology. J Aerosol Sci. 2011;42:668–692 [Google Scholar]
- 2.Schmid O, Moller W, Semmler-Behnke M, Ferron GA, Karg E, Lipka J, Schulz H, Kreyling WG, and Stoeger T: Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers. 2009;14:67–73 [DOI] [PubMed] [Google Scholar]
- 3.Stoeger T, Reinhard C, Takenaka S, Schroeppel A, Karg E, Ritter B, Heyder J, and Schulz H: Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice. Environ Health Perspect. 2006;114:328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Winter-Sorkina R, and Cassee FR: From concentration to dose: factors influencing airborne particulate matter deposition in humans and rats. National Institute of Public Health and the Environment. RIVM report 650010031. Bilthoven, The Netherlands; pp. 1–36, 2002 [Google Scholar]
- 5.ICRP: Human respiratory tract model for radiological protection. ICRP Publication 66. Ann ICRP. 1994;24(1–3) [PubMed] [Google Scholar]
- 6.Tyndall J: Essays on the Floating-Matter of the Air in Relation to Putrefaction and Infection. Longmans, Green, and Co., London; 1881 [Google Scholar]
- 7.Baumberger JP: The amount of smoke produced from tobacco and its absorption in smoking as determined by electrical precipitation. J Pharmacol Exp Ther. 1923;21:47–57 [Google Scholar]
- 8.Drinker P, Thomson RM, and Finn JL: Quantitative measurements of the inhalation, retention, and exhalation of dusts and fumes by man: 1. Concentrations of 50 to 450 mg per cubic meter. J Ind Hyg Toxicol. 1928;10:13–25 [Google Scholar]
- 9.Landahl HD, and Herrmann RG: On the retention of air-borne particulates in the human lung. J Ind Hyg Toxicol. 1948;30:181–188 [PubMed] [Google Scholar]
- 10.van Wijk AM, and Patterson HS: The percentage of particles of different sizes removed from dust-laden air by breathing. J Ind Hyg Toxicol. 1940;22:31–35 [Google Scholar]
- 11.Krewski D, Burnett R, Jerrett M, Pope CA, Rainham D, Calle E, Thurston G, and Thun M: Mortality and long-term exposure to ambient air pollution: ongoing analyses based on the American Cancer Society cohort. J Toxicol Environ Health A. 2005;68:1093–1109 [DOI] [PubMed] [Google Scholar]
- 12.Peters A, Wichmann HE, Tuch T, Heinrich J, and Heyder J: Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med. 1997;155:1376–1383 [DOI] [PubMed] [Google Scholar]
- 13.Hänninen O, Brüske-Hohlfeld I, Loh M, Stoeger T, Kreyling W, Schmid O, and Peters A: Occupational and consumer risk estimates for nanoparticles emitted by laser printers. J Nanopart Res. 2010;12:91–99 [Google Scholar]
- 14.Oberdörster G, Oberdörster E, and Oberdörster J: Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen HL, Chen JS, Cheng ATA, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan HD, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CDH, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi PL, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJC, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJL, and Ezzati M: A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W, Goldberg EP, Karamanos N, and Zarogoulidis K: Inhaled chemotherapy in lung cancer: future concept of nanomedicine. Int J Nanomed. 2012;7:1551–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalupa DC, Morrow PE, Oberdorster G, Utell MJ, and Frampton MW: Ultrafine particle deposition in subjects with asthma. Environ Health Perspect. 2004;112:879–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Löndahl J, Pagels J, Swietlicki E, Zhou JC, Ketzel M, Massling A, and Bohgard M: A set-up for field studies of respiratory tract deposition of fine and ultrafine particles in humans. J Aerosol Sci. 2006;37:1152–1163 [Google Scholar]
- 19.Montoya LD, Lawrence J, Murthy GGK, Sarnat JA, Godleski JJ, and Koutrakis P: Continuous measurements of ambient particle deposition in human subjects. Aerosol Sci Technol. 2004;38:980–990 [Google Scholar]
- 20.Morawska L, Hofmann W, Hitchins-Loveday J, Swanson C, and Mengersen K: Experimental study of the deposition of combustion aerosols in the human respiratory tract. J Aerosol Sci. 2005;36:939–957 [Google Scholar]
- 21.Rosati JA, Brown JS, Peters TM, Leith D, and Kim CS: A polydisperse aerosol inhalation system designed for human studies. J Aerosol Sci. 2002;33:1433–1446 [Google Scholar]
- 22.Möller W, Felten K, Seitz J, Sornmerer K, Takenaka S, Wiebert P, Philipson K, Svartengren M, and Kreyling WG: A generator for the production of radiolabelled ultrafine carbonaceous particles for deposition and clearance studies in the respiratory tract. J Aerosol Sci. 2006;37:631–644 [Google Scholar]
- 23.Wiebert P, Sanchez-Crespo A, Seitz J, Falk R, Philipson K, Kreyling WG, Moller W, Sommerer K, Larsson S, and Svartengren M: Negligible clearance of ultrafine particles retained in healthy and affected human lungs. Eur Respir J. 2006;28:286–290 [DOI] [PubMed] [Google Scholar]
- 24.Jaques PA, and Kim CS: Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women. Inhal Toxicol. 2000;12:715–731 [DOI] [PubMed] [Google Scholar]
- 25.Kim CS, and Jaques PA: Respiratory dose of inhaled ultrafine particles in healthy adults. Philos Trans R Soc Lond A Math Phys Eng Sci. 2000;358:2693–2705 [Google Scholar]
- 26.Kim CS, and Jaques PA: Analysis of total respiratory deposition of inhaled ultrafine particles in adult subjects at various breathing patterns. Aerosol Sci Technol. 2004;38:525–540 [Google Scholar]
- 27.Fissan H, Neumann S, Trampe A, Pui DYH, and Shin WG: Rationale and principle of an instrument measuring lung deposited nanoparticle surface area. J Nanopart Res. 2007;9:53–59 [Google Scholar]
- 28.Rosati JA, Leith D, and Kim CS: Monodisperse and polydisperse aerosol deposition in a packed bed. Aerosol Sci Technol. 2003;37:528–535 [Google Scholar]
- 29.Scheckman JH, and Mcmurry PH: Deposition of silica agglomerates in cast of human lung airways: enhancement relative to spheres of equal mobility and aerodynamic diameter. J Aerosol Sci. 2011;42:508–516 [Google Scholar]
- 30.Brain JD, and Valberg PA: Deposition of aerosol in the respiratory-tract. Am Rev Respir Dis. 1979;120:1325–1373 [DOI] [PubMed] [Google Scholar]
- 31.Hinds W: Aerosol Technology, 2nd ed. John Wiley & Sons, Inc., New York; 1999 [Google Scholar]
- 32.Gebhart J: To the relevant diameter of aerosol particles in the 0.1 to 1 μM transition range. J Aerosol Sci. 1992;23 (Suppl 1):305–308 [Google Scholar]
- 33.Heyder J, Gebhart J, and Scheuch G: Interaction of diffusional and gravitational particle-transport in aerosols. Aerosol Sci Technol. 1985;4:315–326 [Google Scholar]
- 34.Schmid O, Karg E, Hagen DE, Whitefield PD, and Ferron GA: On the effective density of non-spherical particles as derived from combined measurements of aerodynamic and mobility equivalent size. J Aerosol Sci. 2007;38:431–443 [Google Scholar]
- 35.Rissler J, Swietlicki E, Bengtsson A, Boman C, Pagels J, Sandström T, Blomberg A, and Löndahl J: Experimental determination of deposition of diesel exhaust particles in the human respiratory tract. J Aerosol Sci. 2012;48:18–33 [Google Scholar]
- 36.Schiller CF, Gebhart J, Heyder J, Rudolf G, and Stahlhofen W: Deposition of monodisperse insoluble aerosol particles in the 0.005 to 0.2 μm size range within the human respiratory tract. Ann Occup Hyg. 1988;32 (Suppl 1):41–49 [Google Scholar]
- 37.Löndahl J, Massling A, Pagels J, Swietlicki E, Vaclavik E, and Loft S: Size-resolved respiratory-tract deposition of fine and ultrafine hydrophobic and hygroscopic aerosol particles during rest and exercise. Inhal Toxicol. 2007;19:109–116 [DOI] [PubMed] [Google Scholar]
- 38.Tu KW, and Knutson EO: Total deposition of ultrafine hydrophobic and hygroscopic aerosols in the human respiratory system. Aerosol Sci Technol. 1984;3:453–465 [Google Scholar]
- 39.Anderson PJ, Wilson JD, and Hiller FC: Respiratory-tract deposition of ultrafine particles in subjects with obstructive or restrictive lung-disease. Chest. 1990;97:1115–1120 [DOI] [PubMed] [Google Scholar]
- 40.Anselm A, Heibel T, Gebhart J, and Ferron G: “In vivo”-studies of growth factors of sodium chloride particles in the human respiratory tract. J Aerosol Sci. 1990;21:S427–S430 [Google Scholar]
- 41.Ferron GA: The size of soluble aerosol particles as a function of the humidity of the air. Application to the human respiratory tract. J Aerosol Sci. 1977;8:251–267 [Google Scholar]
- 42.Ferron GA, Kreyling WG, and Haider B: Inhalation of salt aerosol-particles. 2. Growth and deposition in the human respiratory-tract. J Aerosol Sci. 1988;19:611–631 [Google Scholar]
- 43.Löndahl J, Pagels J, Boman C, Swietlicki E, Massling A, Rissler J, Blomberg A, Bohgard M, and Sandstrom T: Deposition of biomass combustion aerosol particles in the human respiratory tract. Inhal Toxicol. 2008;20:923–933 [DOI] [PubMed] [Google Scholar]
- 44.Pagels J, Khalizov AF, Mcmurry PH, and Zhang RY: Processing of soot by controlled sulphuric acid and water condensation—mass and mobility relationship. Aerosol Sci Technol. 2009;43:629–640 [Google Scholar]
- 45.Weingartner E, Burtscher H, and Baltensperger U: Hygroscopic properties of carbon and diesel soot particles. Atmos Environ. 1997;31:2311–2327 [Google Scholar]
- 46.Brown JS, Zeman KL, and Bennett WD: Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med. 2002;166:1240–1247 [DOI] [PubMed] [Google Scholar]
- 47.Löndahl J, Swietlicki E, Rissler J, Bengtsson A, Boman C, Blomberg A, and Sandström T: Experimental determination of the respiratory tract deposition of diesel combustion particles in patients with chronic obstructive pulmonary disease. Part Fibre Toxicol. 2012;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Haussinger K, and Kreyling WG: Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008;177:426–432 [DOI] [PubMed] [Google Scholar]
- 49.Olvera HA, Perez D, Clague JW, Cheng YS, Li WW, Amaya MA, Burchiel SW, Berwick M, and Pingitore NE: The effect of ventilation, age, and asthmatic condition on ultrafine particle deposition in children. Pulm Med. 2012;2012:736290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiebert P, Sanchez-Crespo A, Falk R, Philipson K, Lundin A, Larsson S, Moller W, Kreyling WG, and Svartengren M: No significant translocation of inhaled 35-nm carbon particles to the circulation in humans. Inhal Toxicol. 2006;18:741–747 [DOI] [PubMed] [Google Scholar]
- 51.Cheng YS, Yamada Y, Yeh HC, and Swift DL: Deposition of ultrafine aerosols in a human oral cast. Aerosol Sci Technol. 1990;12:1075–1081 [Google Scholar]
- 52.Yu CP: Theories of electrostatic lung deposition of inhaled aerosols. Ann Occup Hyg. 1985;29:219–227 [DOI] [PubMed] [Google Scholar]
- 53.Cohen BS, Xiong JQ, Fang CP, and Li W: Deposition of charged particles on lung airways. Health Phys. 1998;74:554–560 [DOI] [PubMed] [Google Scholar]
- 54.Melandri C, Tarroni G, Prodi V, Dezaiacomo T, Formignani M, and Lombardi CC: Deposition of charged-particles in the human airways. J Aerosol Sci. 1983;14:657–669 [Google Scholar]
- 55.Scheibel HG, and Porstendörfer J: Generation of monodisperse Ag- and NaCl-aerosol with particle diameters between 2 and 300 nm. J Aerosol Sci. 1983;14:113–126 [Google Scholar]
- 56.Lee CK, Ashtekar S, Gladden LF, and Barrie PJ: Adsorption and desorption kinetics of hydrocarbons in FCC catalysts studied using a tapered element oscillating microbalance (TEOM). Part 1: Experimental measurements. Chem Eng Sci. 2004;59:1131–1138 [Google Scholar]
- 57.Altshuler B, Yarmus L, Palmes ED, and Nelson N: Aerosol deposition in the human respiratory tract. Arch Ind Health. 1957;15:293–303 [PubMed] [Google Scholar]
- 58.Gebhart J, Schiller CF, Egan MJ, and Nixon W: On the relationship between experimental-data for total deposition and model-calculations. 1. Effect of instrumental dead space. J Aerosol Sci. 1989;20:141–147 [Google Scholar]
- 59.Thomas JW: Correction for dead space in face masks in inhalation studies. Am Ind Hyg Assoc J. 1967;28:39–42 [DOI] [PubMed] [Google Scholar]
- 60.Brown JH, Cook KM, Ney FG, and Hatch T: Influence of particle size upon the retention of particulate matter in the human lung. Am J Public Health. 1950;40:450–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landahl HD, Tracewell TN, and Lassen WH: On the retention of air-borne particulates in the human lung: II. AMA Arch Ind Hyg Occup Med. 1951;3:359–366 [PubMed] [Google Scholar]
- 62.Bennett WD, and Zeman KL: Effect of body size on breathing pattern and fine-particle deposition in children. J Appl Physiol. 2004;97:821–826 [DOI] [PubMed] [Google Scholar]
- 63.Wang J, McNeill VF, Collins DR, and Flagan RC: Fast mixing condensation nucleus counter: application to rapid scanning differential mobility analyzer measurements. Aerosol Sci Technol. 2002;36:678–689 [Google Scholar]
- 64.Brown JS, Gerrity TR, Bennett WD, Kim CS, and House DE: Dispersion of aerosol boluses in the human lung—dependence on lung-volume, bolus volume, and gender. J Appl Physiol. 1995;79:1787–1795 [DOI] [PubMed] [Google Scholar]
- 65.Brown JS, Kim CS, Reist PC, Zeman KL, and Bennett WD: Generation of radiolabeled “soot-like” ultrafine aerosols suitable for use in human inhalation studies. Aerosol Sci Technol. 2000;32:325–337 [Google Scholar]
- 66.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, and Frangioni JV: Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, and Oberdorster G: Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;21 (Suppl 1):55–60 [DOI] [PubMed] [Google Scholar]
- 68.Jackson AM, Myerson JW, and Stellacci F: Spontaneous assembly of subnanometre-ordered domains in the ligand shell of monolayer-protected nanoparticles. Nat Mater. 2004;3:330–336 [DOI] [PubMed] [Google Scholar]
- 69.Blanchard JD, and Willeke K: Total deposition of ultrafine sodium-chloride particles in human lungs. J Appl Physiol. 1984;57:1850–1856 [DOI] [PubMed] [Google Scholar]
- 70.Heyder J, Armbruster L, Gebhart J, Grein E, and Stahlhofen W: Total deposition of aerosol particles in the human respiratory tract for nose and mouth breathing. J Aerosol Sci. 1975;6:311–328 [Google Scholar]
- 71.Heyder J, Gebhart J, Heigwer G, Roth C, and Stahlhofen W: Experimental studies of the total deposition of aerosol particles in the human respiratory tract. J Aerosol Sci. 1973;4:191–208 [Google Scholar]
- 72.Löndahl J, Massling A, Swietlicki E, Brauner EV, Ketzel M, Pagels J, and Loft S: Experimentally determined human respiratory tract deposition of airborne particles at a busy street. Environ Sci Technol. 2009;43:4659–4664 [DOI] [PubMed] [Google Scholar]
- 73.Dombu CY, and Betbeder D: Airway delivery of peptides and proteins using nanoparticles. Biomaterials. 2013;34:516–525 [DOI] [PubMed] [Google Scholar]
- 74.Buxton D: The promise of nanotechnology for heart, lung and blood diseases. Expert Opin Drug Deliv. 2006;3:173–175 [DOI] [PubMed] [Google Scholar]
- 75.Lehnigk B, Schleiss M, Brand P, Heyder J, Magnussen H, and Jorres RA: Aerosol-derived airway morphometry (ADAM) in patients with lung emphysema diagnosed by computed tomography—reproducibility, diagnostic information and modelling. Eur J Med Res. 2007;12:74–83 [PubMed] [Google Scholar]
- 76.Swietlicki E, Hansson HC, Hameri K, Svenningsson B, Massling A, McFiggans G, Mcmurry PH, Petaja T, Tunved P, Gysel M, Topping D, Weingartner E, Baltensperger U, Rissler J, Wiedensohler A, and Kulmala M: Hygroscopic properties of submicrometer atmospheric aerosol particles measured with H-TDMA instruments in various environments—a review. Tellus B Chem Phys Meteorol. 2008;60:432–469 [Google Scholar]
- 77.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, and Donaldson K: Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428 [DOI] [PubMed] [Google Scholar]
- 78.Möller W, Meyer G, Scheuch G, Kreyling WG, and Bennett WD: Left-to-right asymmetry of aerosol deposition after shallow bolus inhalation depends on lung ventilation. J Aerosol Med Pulm Drug Deliv. 2009;22:333–339 [DOI] [PubMed] [Google Scholar]
- 79.Bennett WD, Brown JS, Zeman KL, Hu SC, Scheuch G, and Sommerer K: Targeting delivery of aerosols to different lung regions. J Aerosol Med. 2002;15:179–188 [DOI] [PubMed] [Google Scholar]
- 80.Chen DR, Pui DYH, and Kaufman SL: Electrospraying of conducting liquids for monodisperse aerosol generation in the 4 nm to 1.8 μm diameter range. J Aerosol Sci. 1995;26:963–977 [Google Scholar]
- 81.Schiller CF, Gebhart J, Heyder J, Rudolf G, and Stahlhofen W: Factors influencing total deposition of ultrafine aerosol-particles in the human respiratory-tract. J Aerosol Sci. 1986;17:328–332 [Google Scholar]
- 82.Ku BK, and Maynard AD: Generation and investigation of airborne silver nanoparticles with specific size and morphology by homogeneous nucleation, coagulation and sintering. J Aerosol Sci. 2006;37:452–470 [Google Scholar]
- 83.Roth C: Generation of ultrafine gold aerosols. J Aerosol Sci. 1986;17:477–480 [Google Scholar]
- 84.Roth C, and Stahlhofen W: Radioactively labeled ultrafine particles for clearance measurements. J Aerosol Sci. 1990;21:S443–S446 [Google Scholar]
- 85.Burch WM, Sullivan PJ, and McLaren CJ: Technegas—a new ventilation agent for lung-scanning. Nucl Med Commun. 1986;7:865–871 [DOI] [PubMed] [Google Scholar]
- 86.Roth C, Ferron GA, Karg E, Lentner B, Schumann G, Takenaka S, and Heyder J: Generation of ultrafine particles by spark discharging. Aerosol Sci Technol. 2004;38:228–235 [Google Scholar]
- 87.Schwyn S, Garwin E, and Schmidtott A: Aerosol generation by spark discharge. J Aerosol Sci. 1988;19:639–642 [Google Scholar]
- 88.Kreyling WG, Biswas P, Messing ME, Gibson N, Geiser M, Wenk A, Sahu M, Deppert K, Cydzik I, Wigge C, Schmid O, and Semmler-Behnke M: Generation and characterization of stable, highly concentrated titanium dioxide nanoparticle aerosols for rodent inhalation studies. J Nanopart Res. 2011;13:511–524 [Google Scholar]
- 89.Szymczak W, Menzel N, Kreyling WG, and Wittmaack K: TOF-SIMS characterisation of spark-generated nanoparticles made from pairs of Ir-Ir and Ir-C electrodes. Int J Mass Spectrom. 2006;254:70–84 [Google Scholar]
- 90.Alessandrini F, Schulz H, Takenaka S, Lentner B, Karg E, Behrendt H, and Jakob T: Effects of ultrafine carbon particle inhalation on allergic inflammation of the lung. J Allergy Clin Immunol. 2006;117:824–830 [DOI] [PubMed] [Google Scholar]
- 91.Chan TL, and Lippmann M: Experimental measurements and empirical modeling of the regional deposition of inhaled particles in humans. Am Ind Hyg Assoc J. 1980;41:399–408 [DOI] [PubMed] [Google Scholar]
- 92.George A, and Breslin AJ: Deposition of radon daughters in humans exposed to uranium mine atmospheres. Health Phys. 1969;17:115–124 [DOI] [PubMed] [Google Scholar]
- 93.Holleman DF, Martz DE, and Schiager KJ: Total respiratory deposition of radon daughters from inhalation of uranium mine atmospheres. Health Phys. 1969;17:187–192 [DOI] [PubMed] [Google Scholar]
- 94.Hursh JB, and Mercer TT: Measurement of Pb-212 loss rate from human lungs. J Appl Physiol. 1970;28:268–274 [DOI] [PubMed] [Google Scholar]
- 95.Wilson IB, and Lamer VK: The retention of aerosol particles in the human respiratory tract as a function of particle radius. J Ind Hyg Toxicol. 1948;30:265–280 [PubMed] [Google Scholar]
- 96.Haslbeck K, Schwarz K, Hohlfeld JM, Seume JR, and Koch W: Submicron droplet formation in the human lung. J Aerosol Sci. 2010;41:429–438 [Google Scholar]
- 97.Daigle CC, Chalupa DC, Gibb FR, Morrow PE, Oberdorster G, Utell MJ, and Frampton MW: Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol. 2003;15:539–552 [DOI] [PubMed] [Google Scholar]
- 98.Baron A, and Willeke K: Aerosol Measurement: Principles, Techniques and Applications, Vol. 2 John Wiley & Sons, Inc., New York; 2005 [Google Scholar]
- 99.Chalupa DC, Gibb FR, Morrow PE, Oberdorster G, Riesenfeld E, Gelain R, Utell MJ, and Frampton MW: A facility for controlled human exposures to ultrafine particles. In: Heinrich U. and Mohr U, (eds). Crucial Issues in Inhalation Research—Mechanistic, Clinical and Epidemiologic. ILSI Press, Washington, DC; pp. 241–253, 2002 [Google Scholar]
- 100.Wilson FJ, Hiller FC, Wilson JD, and Bone RC: Quantitative deposition of ultrafine stable particles in the human respiratory-tract. J Appl Physiol. 1985;58:223–229 [DOI] [PubMed] [Google Scholar]
- 101.Lausted CG, Johnson AT, Scott WH, Johnson MM, Coyne KM, and Coursey DC: Maximum static inspiratory and expiratory pressures with different lung volumes. Biomed Eng Online. 2006;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson PJ, Hiller FC, and Mazumder MK: Deposition of 0.02–0.2 μm particles in the human respiratory tract: effects of variations in respiratory pattern. Ann Occup Hyg. 1988;32(Suppl 1):91–99 [Google Scholar]
- 103.Hiller FC, Anderson PJ, and Mazumder MK: Deposition of sidestream cigarette-smoke in the human respiratory-tract. 2. Deposition of ultrafine smoke particles. Toxicol Lett. 1987;35:95–99 [DOI] [PubMed] [Google Scholar]
- 104.Blanchard JD, and Willeke K: An inhalation system for characterizing total lung deposition of ultrafine particles. Am Ind Hyg Assoc J. 1983;44:846–856 [Google Scholar]
- 105.Gilbert R, Auchincloss JH, Jr, Brodsky J, and Boden W: Changes in tidal volume, frequency, and ventilation induced by their measurement. J Appl Physiol. 1972;33:252–254 [DOI] [PubMed] [Google Scholar]
- 106.Keskinen J, Pietarinen K, and Lehtimaki M: Electrical low-pressure impactor. J Aerosol Sci. 1992;23:353–360 [Google Scholar]
- 107.DeCarlo PF, Kimmel JR, Trimborn A, Northway MJ, Jayne JT, Aiken AC, Gonin M, Fuhrer K, Horvath T, Docherty KS, Worsnop DR, and Jimenez JL: Field-deployable, high-resolution, time-of-flight aerosol mass spectrometer. Anal Chem. 2006;78:8281–8289 [DOI] [PubMed] [Google Scholar]
- 108.Fleming JS, Conway JH, Holgate ST, Moore EA, Hashish AH, Bailey AG, and Martonen TB: Evaluation of the accuracy and precision of lung aerosol deposition measurements from planar radionuclide imaging using simulation. Phys Med Biol. 1998;43:2423–2429 [DOI] [PubMed] [Google Scholar]
- 109.Lee ZH, Berridge MS, Nelson AD, and Heald DL: The effect of scatter and attenuation on aerosol deposition as determined by gamma scintigraphy. J Aerosol Med. 2001;14:167–183 [DOI] [PubMed] [Google Scholar]
- 110.Pitcairn GR, and Newman SP: Tissue attenuation corrections in gamma scintigraphy. J Aerosol Med. 1997;10:187–198 [Google Scholar]
- 111.Wagner HN, Buchanan JW, and Szabo Z. Principles of Nuclear Medicine, 2nd ed. Elsevier Health Sciences, Philadelphia, PA; 1995 [Google Scholar]
- 112.Fleming JS: A technique for using CT images in attenuation correction and quantification in SPECT. Nucl Med Commun. 1989;10:83–97 [DOI] [PubMed] [Google Scholar]
- 113.Lee ZH, Ljungberg M, Muzic RF, and Berridge MS: Usefulness and pitfalls of planar gamma-scintigraphy for measuring aerosol deposition in the lungs: a Monte Carlo investigation. J Nucl Med. 2001;42:1077–1083 [PubMed] [Google Scholar]
- 114.Fleming JS: Technique for the absolute measurement of activity using a gamma-camera and computer. Phys Med Biol. 1979;24:176–180 [DOI] [PubMed] [Google Scholar]
- 115.Parker RP, Smith PH, and Taylor DM: Basic Science of Nuclear Medicine, 2nd ed. Churchill Livingstone, London; 2012 [Google Scholar]
- 116.Möller W, Felten K, Meyer G, Meyer P, Seitz J, and Kreyling WG: Corrections in dose assessment of Tc-99m radiolabeled aerosol particles targeted to central human airways using planar gamma camera imaging. J Aerosol Med Pulm Drug Deliv. 2009;22:45–54 [DOI] [PubMed] [Google Scholar]
- 117.Eberl S, Chan HK, and Daviskas E: SPECT imaging for radioaerosol deposition and clearance studies. J Aerosol Med. 2006;19:8–20 [DOI] [PubMed] [Google Scholar]
- 118.Forge NI, Mountford PJ, and Odoherty MJ: Quantification of Tc-99m lung radioactivity from planar images. Eur J Nucl Med. 1993;20:10–15 [DOI] [PubMed] [Google Scholar]
- 119.Fleming JS, Conway JH, Bolt L, and Holgate ST: A comparison of planar scintigraphy and SPECT measurement of total lung deposition of inhaled aerosol. J Aerosol Med. 2003;16:9–19 [DOI] [PubMed] [Google Scholar]
- 120.Macey DJ, and Marshall R: Absolute quantitation of radiotracer uptake in the lungs using a gamma-camera. J Nucl Med. 1982;23:731–734 [PubMed] [Google Scholar]
- 121.Ilowite JS, Smaldone GC, Perry RJ, Bennett WD, and Foster WM: Relationship between tracheo-bronchial particle clearance rates and sites of initial deposition in man. Arch Environ Health. 1989;44:267–273 [DOI] [PubMed] [Google Scholar]
- 122.Goldoni M, Caglieri A, De Palma G, Longo S, Acampa O, Poli D, Manini P, Apostoli P, Franchini I, Corradi M, and Mutti A: Development and set-up of a portable device to monitor airway exhalation and deposition of particulate matter. Biomarkers. 2009;14:326–339 [DOI] [PubMed] [Google Scholar]
- 123.Invernizzi G, Ruprecht A, De Marco C, Paredi P, and Boffi R: Residual tobacco smoke: measurement of its washout time in the lung and of its contribution to environmental tobacco smoke. Tob Control. 2007;16:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Invernizzi G, Boffi R, Ruprecht AA, Barnes PJ, Kharitonov SA, and Paredi P: Real-time measurement of particulate matter deposition in the lung. Biomarkers. 2006;11:221–232 [DOI] [PubMed] [Google Scholar]
- 125.Kim CS, and Jaques PA: Total lung deposition of ultrafine particles in elderly subjects during controlled breathing. Inhal Toxicol. 2005;17:387–399 [DOI] [PubMed] [Google Scholar]
- 126.Morawska L, Barron W, and Hitchins J: Experimental deposition of environmental tobacco smoke submicrometer particulate matter in the human respiratory tract. Am Ind Hyg Assoc J. 1999;60:334–339 [DOI] [PubMed] [Google Scholar]
- 127.Cheng YS, Yeh HC, Guilmette RA, Simpson SQ, Cheng KH, and Swift DL: Nasal deposition of ultrafine particles in human volunteers and its relationship to airway geometry. Aerosol Sci Technol. 1996;25:274–291 [Google Scholar]
- 128.Roth C, Scheuch G, and Stahlhofen W. Clearance measurements with radioactively labelled ultrafine particles. Inhal Part VII. 1994;38(Suppl 1):101–106 [Google Scholar]
- 129.Muir DCF, and Cena K: Deposition of ultrafine aerosols in the human respiratory-tract. Aerosol Sci Technol. 1987;6:183–190 [Google Scholar]
- 130.Prodi V, and Mularoni A: Electrostatic lung deposition experiments with humans and animals. Ann Occup Hyg. 1985;29:229–240 [DOI] [PubMed] [Google Scholar]
- 131.Giacomelli-Maltoni G, Melandri C, Prodi V, and Tarroni G: Deposition efficiency of monodisperse particles in human respiratory tract. Am Ind Hyg Assoc J. 1972;33:603–610 [DOI] [PubMed] [Google Scholar]
- 132.Dautrebande L, Beckmann H, and Walkenhorst W: Studies on deposition of submicronic dust particles in the respiratory tract. Arch Ind Health. 1959;19:383–391 [PubMed] [Google Scholar]
- 133.Morrow PE, Mehrhof E, Casarett LJ, and Morken DA: An experimental study of aerosol deposition in human subjects. Arch Ind Health. 1958;18:292–298 [PubMed] [Google Scholar]
- 134.Landahl HD, Tracewell TN, and Lassen WH: Retention of air-borne particulates in the human lung. III. AMA Arch Ind Hyg Occup Med. 1952;6:508–511 [PubMed] [Google Scholar]