Abstract
Background: Childhood obesity and overweight affect approximately 30% of US children. Many of these children have obesity-related comorbidities, such as hypertension, dyslipidemia, fatty liver disease, diabetes, polycystic ovary syndrome (PCOS), sleep apnea, psychosocial problems, and others. These children need routine screening and, in many cases, treatment for these conditions. However, because primary care pediatric providers (PCPs) often are underequipped to deal with these comorbidities, they frequently refer these patients to subspecialists. However, as a result of the US pediatric subspecialist shortage and considering that 12.5 million children are obese, access to care by subspecialists is limited. The aim of this article is to provide accessible, user-friendly clinical consensus statements to facilitate the screening, interpretation of results, and early treatment for some of the most common childhood obesity comorbidities.
Methods: Members of the Children's Hospital Association (formerly NACHRI) FOCUS on a Fitter Future II (FFFII), a collaboration of 25 US pediatric obesity centers, used a combination of the best available evidence and collective clinical experience to develop consensus statements for pediatric obesity-related comorbidities. FFFII also surveyed the participating pediatric obesity centers regarding their current practices.
Results: The work group developed consensus statements for use in the evaluation and treatment of lipids, liver enzymes, and blood pressure abnormalities and PCOS in the child with overweight and obesity. The results of the FFFII survey illustrated the variability in the approach for initial evaluation and treatment as well as pattern of referrals to subspecialists among programs.
Conclusions: The consensus statements presented in this article can be a useful tool for PCPs in the management and overall care of children with overweight and obesity.
Introduction
Childhood overweight and obesity affect approximately one third of US children.1 Many of these children have one or more obesity-related comorbidities, such as abnormal blood pressure, dyslipidemia, fatty liver disease, prediabetes, diabetes, polycystic ovary syndrome (PCOS), obstructive sleep apnea, psychosocial problems, and others. Children who are overweight or obese need to be routinely screened and, in many cases, treated for these obesity-associated conditions. Unfortunately, access to care for these comorbidities is inadequate because primary care pediatric providers (PCPs) often are underequipped to deal with these complications. Therefore, these children are frequently referred to multiple pediatric subspecialists to address their comorbidities.2–5 Furthermore, many regions across the United States are experiencing significant shortages of pediatric subspecialists, resulting in limited access to timely evaluation and management by subspecialists.5–7 Considering that 12.5 million US children are obese,8 new methods must be developed to evaluate and manage the care of such a large number of patients.
In 2009, the National Association of Children's Hospitals and Related Institutions (now known as the Children's Hospital Association) FOCUS on a Fitter Future II (FFFII) assembled leaders from 25 leading pediatric obesity centers from across the United States. From this collaboration, an expert treatment committee work group was formed that proceeded to meet every 4 months for the following 3 years. As leaders of tertiary care childhood obesity clinics and centers, the authors are frequently asked by primary care colleagues what evaluation should be done for children with childhood obesity and, beyond that, what then should be done with the results. Various sources recommend initial laboratory evaluations for all children, but few recommendations address how to manage and treat the results of abnormal screening tests for obesity-related comorbidities in the pediatric population. Furthermore, current screening recommendations9,10 are primarily for children without overweight or obesity and no literature covers how to specifically evaluate children with obesity who are at greater risk for many medical conditions, compared to their healthy weight peers. No consensus statements or guidelines exist to provide a framework for PCPs regarding what to do with abnormal laboratory results in children who already are obese.
Given the lack of tools for the PCP that are easily accessible and user friendly and the shortage of subspecialty care, the FFFII expert work group embarked on the development of the clinical consensus statements presented in this article. The clinical statements include a summary of the screening, evaluation and initial treatment for obesity-associated abnormalities of lipids, blood pressure, liver enzymes, and PCOS. Consensus statements for other conditions (e.g., sleep apnea) are also needed; however, they are not presented in this article. The presented consensus statements were based on the best available evidence at the time of writing, including recommended guidelines from national associations9–17 and advice from subspecialists from the participating FFFII centers as well as nationally recognized experts in the pertinent fields. The use of these consensus statements can improve the care provided by PCPs to children with overweight and obesity by facilitating appropriate evaluation, treatment, and potential thresholds for referral to subspecialty care. Earlier identification of comorbidities and resultant prompt treatment will decrease future health complications, lead to healthcare cost savings, and increase quality of life for these children.
Methods
FFFII surveyed the participating pediatric obesity centers regarding their current evidence-informed approaches regarding the initial evaluation and treatment of obesity and its comorbidities. The survey was designed by the treatment committee work group of FFFII and completed by the leader of each participating obesity center. In centers with some clinical variation between different providers, the center's leader who completed the survey was asked to include only those tests obtained by the majority of their providers. The results of this survey illustrated the variability in the approach used for initial evaluation and pattern of referrals to subspecialists among the participating obesity centers and prompted the work group to develop user-friendly consensus statements for use in the evaluation and treatment of children with overweight and obesity.
These consensus statements were developed through:
• Committee discussions among the pediatric obesity experts from 25 pediatric obesity centers. All of these centers met the criteria for stage III weight management programs (comprehensive multidisciplinary teams, including a dietician, physician, behavioralist, and exercise physiologist or physical therapist), as described by Barlow and colleagues.11
• A targeted review of the literature and review of the existing guidelines from national associations9–17
• Consultation with subspecialists in nephrology, hepatology, cardiology, endocrinology, pulmonology, gastroenterology, and gynecology for review of the consensus statements and questions pertinent to their specific areas of expertise. Nationally recognized experts were also approached by members of the FFFII, as needed.
Each section of this article concludes with a flow diagram developed by this group that summarizes their consensus recommendations based on best available literature and practice experience.
Extracts from the results of the survey administered to the 25 pediatric obesity centers participating in FFFII are shared in Figure 1 and throughout the article. These data are presented to illustrate common practice and practice variability, even among expert pediatric obesity centers, but not as recommended best practice guidelines.
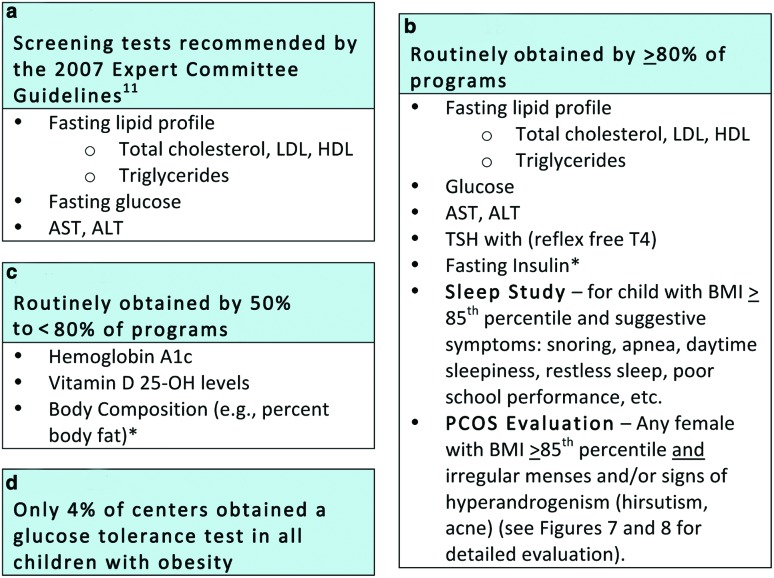
Figure 1.
Initial evaluation of the obese child: Current recommendation and practice variability among stage III pediatric obesity centers participating in FFFII. *Obtained generally for research purposes or to monitor progress in specialized weight management programs. LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine transaminase; TSH, thyroid-stimulating hormone; free T4, free thyroxine. Color image is available online at www.liebertpub.com/chi
No institutional review board approval was required for this study.
Results
Initial Assessment of the Child with Overweight or Obesity
Multidisciplinary weight management programs evaluate patients' baseline metabolic measures and body composition for three primary purposes: (1) to evaluate possible underlying diagnoses that may have contributed to the development of obesity; (2) to identify physiological sequelae of obesity; and (3) to provide data for monitoring progress and response to treatment. Ultimately, this information helps providers adjust treatment plans and also serves to enhance the motivation of patients and families in view of present or impending serious health issues.
The 2007 American Medical Association (AMA) Expert Committee recommendations on the prevention, assessment, and treatment of child and adolescent overweight and obesity include recommended laboratory testing that should be performed when evaluating children with obesity (Fig. 1a).11 Almost all members of FFFII obtain these recommended tests along with additional select tests obtained to monitor patients' progress (Fig. 1).
The results of the FFFII survey demonstrated significant practice variability in the initial evaluation across the participating pediatric weight management centers. Figure 1b describes the screening tests obtained by ≥80% of the FFFII hospitals. Interestingly, despite the lack of clear evidence for utility and that obtaining insulin is not supported by international recommendations,18 many programs continue to order fasting insulin levels. The reason for obtaining insulin was not collected in the survey, but anecdotally, FFFII members reported monitoring intervention outcomes as part of a research protocol or motivating behavior change in families as reasons for obtaining the test.
Other specific tests were ordered in response to history or physical exam findings. Evaluations for PCOS or sleep apnea, for example, were performed when relevant signs and symptoms were noted (detailed PCOS evaluation is described later in the article).
As a result of the high-risk population observed in such referral centers and the need to monitor progress during intensive treatment, most (50–<80%) of the FFFII participating centers also obtained additional tests recommended and supported by varying degrees of available evidence (Fig. 1c). Screening for vitamin D deficiency is an example of this variability and lack of consensus. Fifty-six percent of centers routinely obtained vitamin D levels, whereas another 9% obtained it only for high-risk and/or minority patients. All hospitals followed the American Dietetic Association (now the Academy of Nutrition and Dietetics) recommendations for supplementation, though the exact treatment protocols also varied by program.19 Percentage body fat was obtained by a majority of sites as a means of monitoring progress, although participants acknowledged that this is not a recommended practice for the general practitioner. While acknowledging the inaccuracies often noted with bioelectrical impedance analysis (BIA), most centers measured percentage body fat using BIA. Seventy-five percent of FFFII pediatric obesity centers reported obtaining hemoglobin A1c (HbA1c). Although some debate remains about the accuracy of HbA1c as a diabetes screening test in the pediatric population,12,20 HbA1c was adopted by the American Diabetes Association (ADA) as an accepted tool to screen for glucose abnormalities in adults in 2010.21 Only 4% of centers obtain oral glucose tolerance tests (OGTTs) on all children referred to their centers (Fig. 1d), but many centers considered OGTTs in children with severe obesity or obesity with several risk factors for diabetes. Details on the OGTT protocol, such as number of glucose or insulin measurements performed, were not collected in the survey. The recommended methodology for OGTTs was published in 1998 by the World Health Organization publication,22 which is also the methodology currently supported by the ADA.23
Lipid Abnormalities
The Expert Committee recommendations for pediatric obesity recommend including a fasting lipid panel for assessment of youth with BMI ≥85th percentile, recognizing that disordered lipid metabolism is one of the most common early indicators of cardiovascular (CV) risk (CVR) in children with overweight and obesity.11 These guidelines focus on low-density lipoprotein cholesterol (LDL-c) and total cholesterol only. However, as many as 42.9% of obese children will have combined dyslipidemia, which manifests as elevated triglycerides and low high-density lipoprotein cholesterol (HDL-c) with or without accompanying elevation of LDL-c.24 Insulin resistance (IR) contributes to this combined pattern, in which an increased burden of small LDL particles may be masked by an unremarkable LDL-c level.25
Selective lipid screening with a fasting lipid profile for children over 2 years of age has been recommended since 2008 for children with BMI ≥95th percentile.10,26 BMI ≥95th and 97th percentiles constitute moderate and high-level risk factors that guide management in the 2011 Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents.10 These guidelines also introduce non-HDL-cholesterol (calculated by subtracting HDL-c from total cholesterol) as an acceptable nonfasting screen for disordered lipid metabolism that is followed, if abnormal, by a fasting sample to delineate the specific nature of the dyslipidemia. All subsequent follow-up labs for abnormal lipids should be fasting.
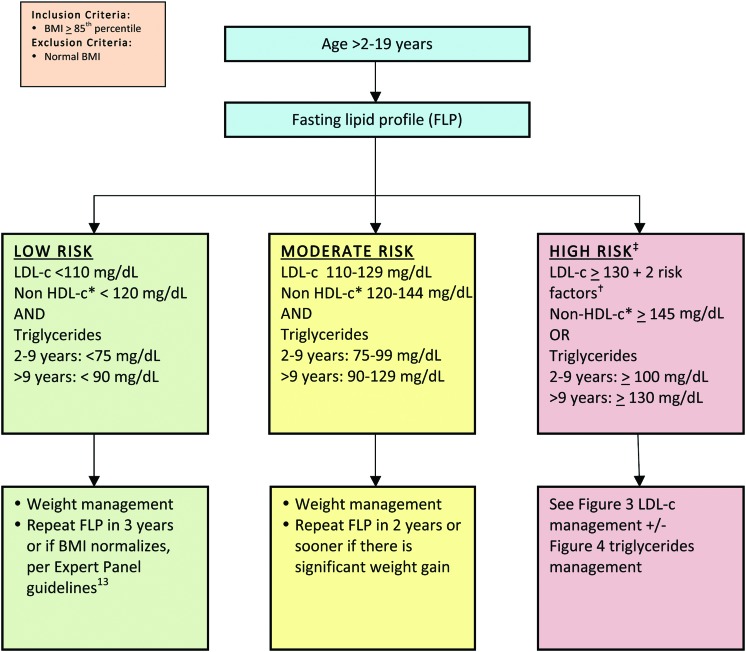
The management consensus statement presented in this article is consistent with the most current CVR management guidelines, with an emphasis on recognition and management of the combined dyslipidemia associated with obesity.10 In this consensus statement, obese/overweight children are classified according to age and by risk based on their lipid profile (Fig. 213). It is essential to note that weight management is the foundation of treatment for dyslipidemia for children with overweight/obesity of all ages. Moderate and high-risk thresholds, despite optimized lifestyle management that may justify adjunct nutraceutical and pharmacological management, are detailed in Figures 3 and 427–29 and Tables 130,31 and 2. The LDL-c elevation that accompanies obesity rarely exceeds 160 mg/dL, above which primary genetic lipid disorders are more common. Obesity is so prevalent, however, that a primary hypercholesterolemia certainly can overlap with, and be exacerbated by, excessive weight. If LDL-c exceeds 250 mg/dL, the likelihood of concomitant familial hypercholesterolemia warrants consultation with a lipid specialist. Full CVR management guidelines are found in the Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, 2011.13
Figure 2.
Lipid management in children and adolescents with overweight or obesity (for nonobese children, refer to the American Academy of Pediatrics guidelines).13 *Non-HDL-c=total cholesterol−HDL-c. †Risk factors described in Table 1. ‡Children with LDL-c ≥130 PLUS 0–1 risk factors fall between moderate and high risk. LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol. Color image is available online at www.liebertpub.com/chi
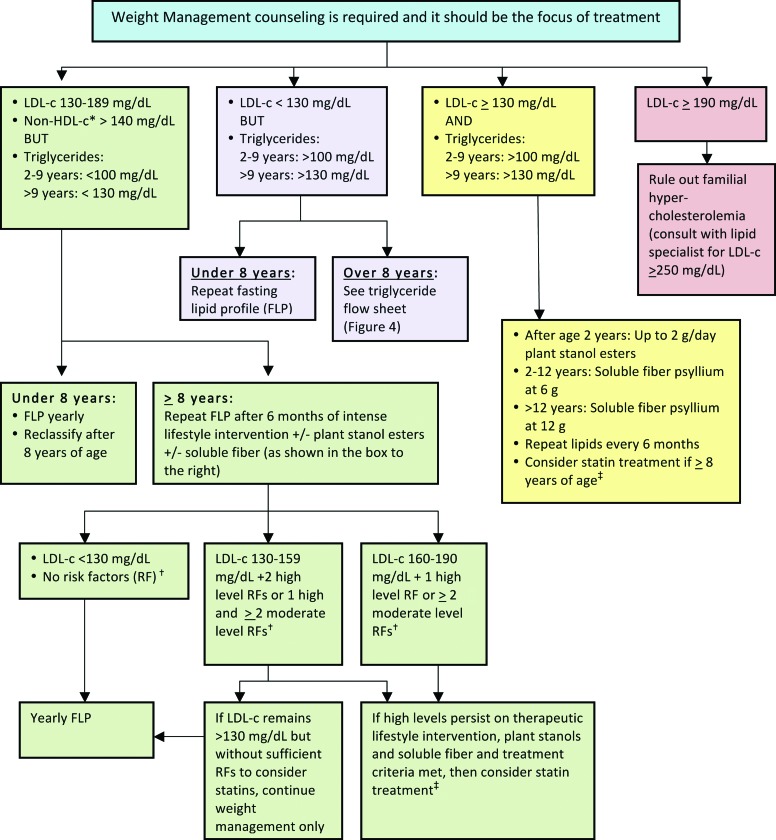
Figure 3.
LDL-c management in children and adolescents with overweight or obesity with “high-risk” lipid profile (see Fig. 2). *Non-HDL-c=total cholesterol−HDL-c. †Risk factors (see Table 1). ‡Statin therapy (see Table 2). LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol. Color image is available online at www.liebertpub.com/chi
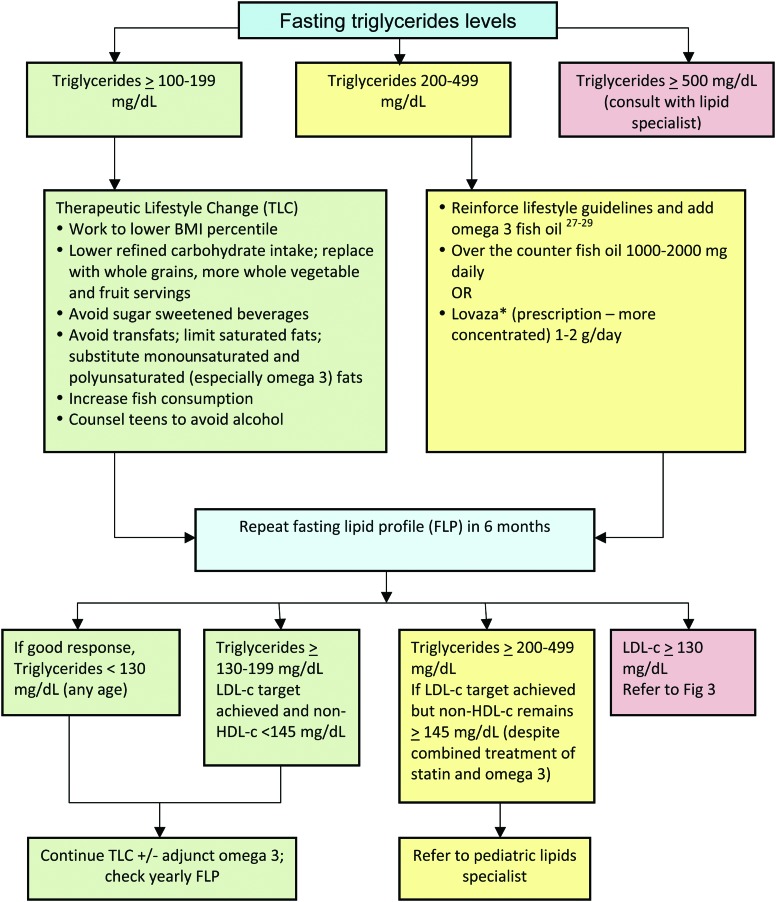
Figure 4.
Triglyceride treatment (children ≥8 years) based on fasting triglyceride levels. *Before treatment with Lovaza: rule out alcohol use by patient or family history of bleeding disorders; discontinue aspirin-containing products; and note that comparably concentrated preparations are increasingly available without prescription. LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol. Color image is available online at www.liebertpub.com/chi
Table 1.
Risk Factors for Cardiovascular Disease
| High-level risk factors and conditions |
| • Parent/grandparent/aunt/uncle with history of premature cardiovascular disease (age at diagnosis <55 years in males and <65 years in females), coronary atherosclerosis, peripheral vascular disease, cerebrovascular disease, or early deaths before 50 years of age of unknown cause in relatives |
| • BMI ≥97th percentile |
| • Diabetes mellitus, type 1 or 2 |
| • Hypertension (blood pressure ≥99th percentile+5 mm Hg, requiring therapy) |
| • Current smoker |
| • Chronic renal disease |
| • History of solid organ kidney or heart transplant |
| • History of Kawasaki disease with current coronary aneurysms |
| Moderate-level risk factors and conditions |
| • BMI 95th–97th percentile |
| • Hypertension not requiring therapy |
| • HDL-c <40 mg/dL |
| • Chronic inflammatory disease (lupus or juvenile rheumatoid arthritis) |
| • HIV infection |
| • Nephrotic syndrome |
| • History of Kawasaki disease with regressed coronary aneurysms |
| Consider as potential risk factors |
| • Prediabetes |
| • History of cancer30,31 or congenital heart disease |
| • Passive smoker |
| • Unknown family history |
HDL-c, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus.
Table 2.
Statin Therapy
| • Document normal hepatic panel before initiating therapy. |
| • If a patient meeting eligibility for statins is a young woman of reproductive age, it is essential to counsel to avoid pregnancy. Statins should be withheld during pregnancy because of the potential for teratogenicity for a developing fetus. |
| • Dosing: |
| – Start at lowest dose, usually at bedtime. |
| – If target LDL-c levels are not achieved, double the dose and repeat lipid profile, creatine kinase (CK), and liver enzymes at 4 weeks. Continue stepped titration up to the maximum recommended dose until the target LDL-c levels are achieved (<130, optimally <110 mg/dL) or there is evidence of toxicity. Repeat blood work at 4 weeks, 3 months, and then every 3–4 months for first year and every 6 months thereafter. |
| – Suggested titration: |
| • Pravastatin (Pravachol) 10→20 mg |
| • Rosuvastatin (Crestor) 5→10→20 mg |
| • Atorvastatin (Lipitor) 10→20 mg |
| – Note: Simvastatin (Zocor) is the least expensive, but most lipophilic, and therefore passes readily across the blood–brain barrier (BBB) and suppresses brain cholesterol synthesis. Plasma cholesterol does not cross the BBB. Therefore, to avoid interference with cholesterol needs for adolescent brain development, more hydrophilic statins are favored. |
| – If LDL-c remains >130 mg/dL on the maximum statin dose, can add a bile acid sequestrant (Cholestyramine or Colestipol, 8 g per day). Do not go higher in statin dose without consulting a lipid specialist. |
| • Side effects: |
| – Instruct patient to immediately report side effects suggestive of myopathy. If myopathy is present, the medication should be stopped, and CK level and relation of symptoms to physical activity should be assessed in 4–6 weeks. The patient should be monitored for resolution of the myopathy and any associated increases in CK. Consideration can be given to either an alternate statin or restarting the same statin medication at half the previous dose once symptoms and laboratory abnormalities have resolved. |
| – Advise female patients about contraindication during pregnancy and contraception if warranted. Advise about drug interactions (cyclosporine, fibric acid derivatives, niacin, erythromycin, azole antifungals, nefazodone, and HIV protease inhibitors). |
LDL-c, low-density lipoprotein cholesterol; HIV, human immunodeficiency virus.
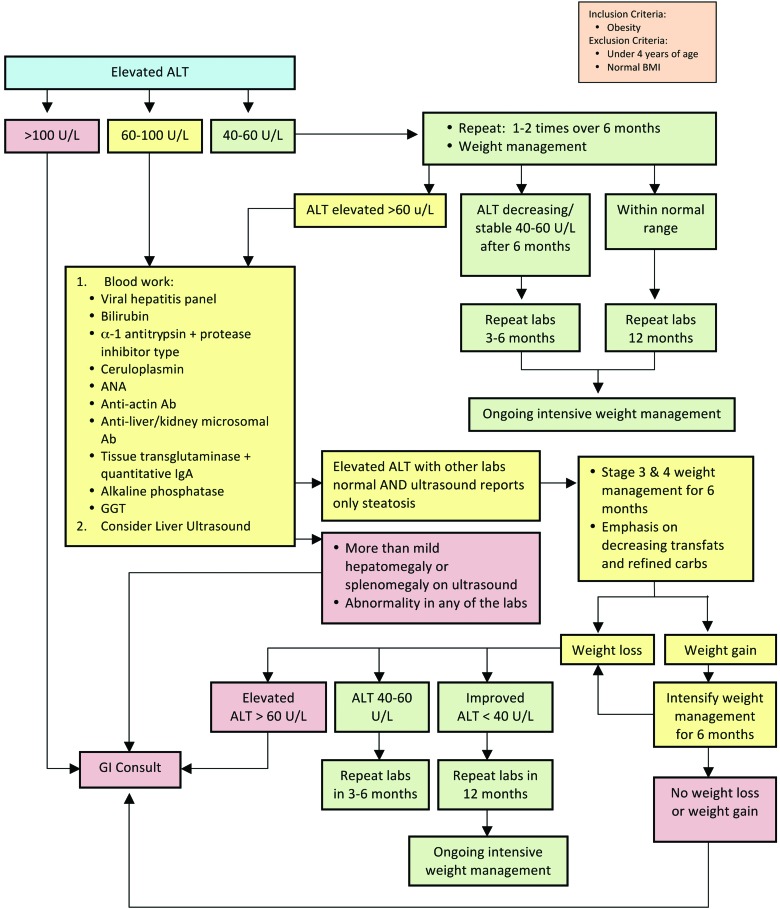
Abnormal Liver Enzymes
As the prevalence of childhood obesity continues to increase, so has the incidence of nonalcoholic fatty liver disease (NAFLD). Several studies have confirmed that male gender and Asian and Mexican ethnicity are risk factors for NAFLD, whereas African Americans seem to be protected.32 NAFLD includes a range of disease severity from simple steatosis to nonalcoholic steatohepatitis, which may progress to cirrhosis. Clinically, most NAFLD is silent, although some patients may present with abdominal pain. Metabolic syndrome is strongly correlated with development and severity of NAFLD,33 and NAFLD is also a risk factor independent of obesity in the development of CV disease (CVD).34 Chronic overnutrition that results in obesity creates an inflammatory cycle that promotes IR and hepatic lipid deposition. Components of the current American diet most responsible for the development of NAFLD are saturated fats, particularly transfats, fructose, and animal protein sources, and specifically branched-chain amino acids, and alcohol.35
An Expert Committee (AMA, Health Resources and Services Administration, and CDC) in 200711 recommended assessment of liver enzymes in those children 10 years of age with BMI ≥85th–94th percentile with risk factors and in children 10 years of age with BMI ≥95th percentile. Though alanine transaminase (ALT) and aspartate aminotransferase (AST) levels are to be tested every 2 years and coincide with diabetes screening recommendations, they have low sensitivity and specificity for NAFLD. Normal levels occur in patients found to have NAFLD on liver biopsy and elevated levels are found in individuals with normal liver histology. The majority of the surveyed FFFII programs checked liver enzymes at baseline starting at age 2 years. NAFLD can be strongly suspected based on clinical parameters, liver enzymes (AST and ALT), and ultrasound (US), but staging and grading currently require biopsy. With worsening liver enzyme values, and before biopsy, other forms of liver disease should be ruled out. Current treatment for NAFLD is healthy diet and exercise. Recommendations to limit or eliminate transfats, fructose, and alcohol exposure and decrease animal protein intake are also suggested.35
The consensus statement presented for assessment of NAFLD (Fig. 5) starts with the recommendation to measure ALT in all children with obesity. The exact cut points for referral and further evaluation are controversial because insufficient evidence exists to guide decisions.36 This consensus statement leans conservatively to assure that virtually all those children with detectable NAFLD are followed closely and referred early while also balancing access to gastrointestinal specialists. Further, upper limits of normal (ULNs) vary in laboratories, and at times, these ULNs are very high. For these reasons, the authors chose to use a number to guide decisions in the consensus statement while acknowledging that much of the evidence uses 2 times the ULN for the typical cut point.11 Further evaluation to assess the presence of other conditions, such as viral hepatitis and autoimmune or metabolic abnormalities causing liver enzyme elevation, is recommended (suggested tests are listed in Fig. 5).
Figure 5.
Evaluation of abnormal liver enzymes in children with obesity. ALT, alanine transaminase; ANA, antinuclear antibody; Ab, antibody; IgA, immunoglobulin A; GGT, gamma-glutamyl transpeptidase; GI, gastrointestinal. Color image is available online at www.liebertpub.com/chi
In addition to the steps described in the consensus statement (Fig. 5), the authors recommend talking with the receiving subspecialist about preferences and additional potential laboratory work that they might desire before the referral.
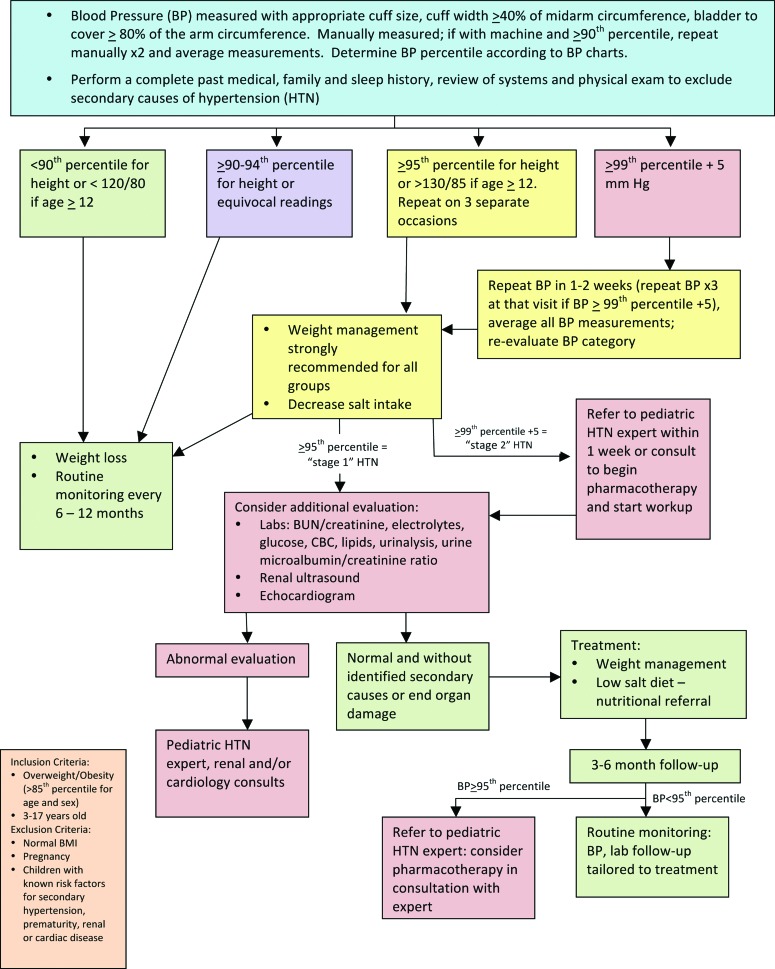
Hypertension
Normal blood pressure (BP) is defined as systolic and diastolic BP (SBP/DBP) at less than the 90th percentile for sex, age, and height,9 hypertension (HTN) as average SBP or DBP ≥95th percentile on at least three separate occasions, and pre-HTN is an SBP or DBP between the 90th and <95th percentile or BP exceeding 120/80 mm Hg in adolescents.9 Obesity has been identified as a primary risk factor for pre-HTN and HTN in childhood.9 As many as 13% of youth with obesity have elevated SBP and 9% have elevated DBP.37 The presence of obesity increases the likelihood that a hypertensive child will become a hypertensive adult, thus compounding the risk for CVD these children already face. Therefore, early identification and timely treatment of HTN in youth with obesity is of paramount importance.
To develop the HTN consensus statement, the FFFII members primarily used the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents, which reviewed the best available evidence for BP measurement and management through 2003,9 and the Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report, which reviewed this evidence through mid-200813 and stratified recommendations by age. The resulting consensus statement is shown in Figure 6. Cuff size and placement are critical to the accurate measurement of BP, and cuff placement can be more difficult in children and adolescents with obesity because larger cuff sizes are often required. In this population, weight loss/management and dietary intervention will play a role in all pathways of the HTN consensus statement with additional assessment, treatment, and consultation depending on the diagnosis of HTN and its severity.
Figure 6.
Evaluation and treatment of hypertension in children with overweight or obesity. BUN, blood urea nitrogen; CBC, complete blood count. Color image is available online at www.liebertpub.com/chi
Polycystic Ovary Syndrome
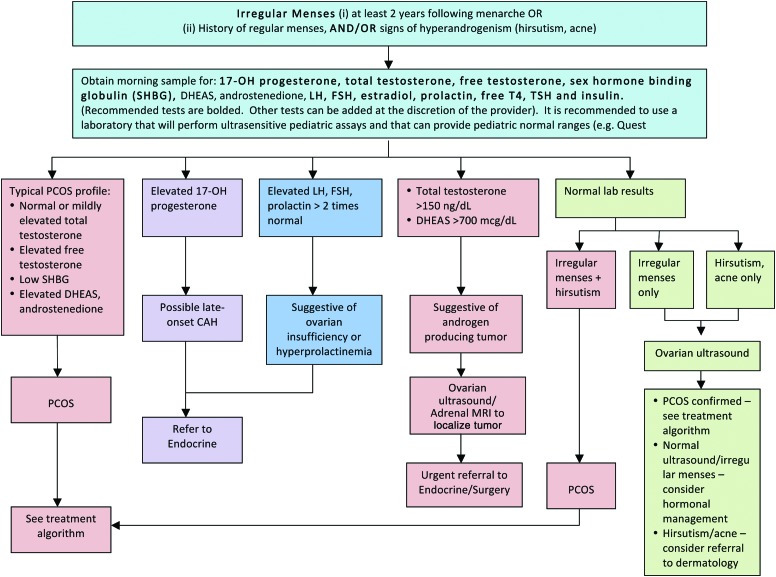
PCOS is often described as the most common endocrinopathy in women of childbearing age, with reported prevalence rates ranging from 5% to 10%.38–41 In 1990, the National Institutes of Health consensus conference defined PCOS as the combination of chronic anovulation or oligomenorrhea and clinical or biochemical signs of hyperandrogenism.42 Since then, two consensus groups have released revised criteria that have incorporated polycystic ovaries into the diagnostic criteria for PCOS.14,43 With the most recent criteria proposed by the Androgen Excess Society, nine different PCOS phenotypes can currently be identified, characterized by hyperandrogenism (either clinical with hirsutism and/or biochemical hyperandrogenemia) and ovarian dysfunction (either oligoanovulation and/or polycystic ovaries).44 In addition, the diagnosis of PCOS must exclude other disorders of adrenal excess, such as nonclassic or late-onset congenital adrenal hyperplasia, or disorders affecting menstrual function, such as hyperprolactinemia.14
For adolescents, existing criteria for PCOS pose some problems for diagnosis and management because some of the symptoms or signs may be difficult to evaluate in this age group.44,45 For instance, in the first 2 years following menarche, oligomenorrhea is not uncommon because menstrual cycles are frequently anovular.45 Therefore, both adult PCOS guidelines14–16,46 and recommendations from the Expert Committee on the prevention, assessment, and treatment of child and adolescent overweight and obesity11 were used to inform the proposed management consensus statements presented here for adolescent girls with obesity (Figs. 7 and 8). In screening for PCOS, a primary goal is to exclude and appropriately manage other medical conditions that may cause irregular menstrual cycles and/or androgen excess, such as hyperprolactinemia, thyroid dysfunction, or nonclassic or late-onset congenital adrenal hyperplasia. When performing laboratory tests, it is recommended to use a laboratory that will perform ultrasensitive pediatric assays with pediatric reference ranges. Pelvic US is typically only needed when either ovarian dysfunction (i.e., irregular menses) or clinical hyperandrogenism (e.g., hirsutism) presents alone with normal laboratory tests. In such cases, a diagnosis of PCOS can still be made if polycystic ovaries are present on US.
Figure 7.
Management of polycystic ovary syndrome (PCOS) in adolescents with obesity. DHEAS, dehydroepiandrosterone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; TSH, thyroid-stimulating hormone; free T4, free thyroxine; CAH, congenital adrenal hyperplasia; MRI, magnetic resonance imaging. Color image is available online at www.liebertpub.com/chi
Figure 8.
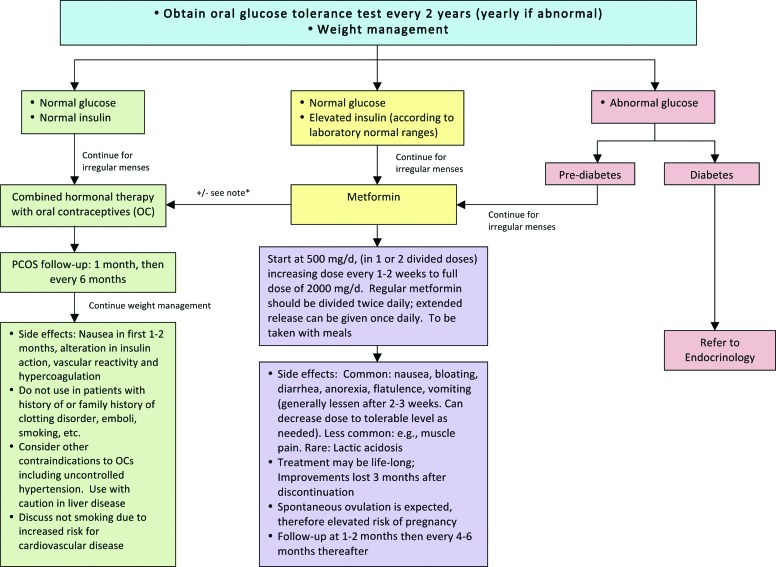
Treatment of polycystic ovary syndrome (PCOS) in adolescents with obesity. *Consider combination therapy of oral contraceptives and metformin. Consider referral to endocrine, adolescent medicine, gynecology, or dermatology according to your institutional resources and policies. Color image is available online at www.liebertpub.com/chi
Pharmacological management of irregular menses in adolescent girls with PCOS is guided by the status of IR and glucose tolerance.46–48 However, all obese adolescent girls with PCOS will reduce their risk of CV complications through intensive lifestyle modification and weight management as a complement to pharmacological therapy.15,17 Girls with normal glucose and normal insulin levels can be treated initially with hormonal contraceptive pills. Estrogen suppresses luteinizing hormone (LH), and thus ovarian androgen production, while also increasing sex hormone-binding globulin production in the liver, and thus reducing free testosterone. Some progestins, such as norgestrel and levonorgestrel, may have higher androgenic activity and should be avoided. In counseling families about hormone treatment options, particularly in the nonsexually active female, it may be important to refer to the medication as “hormonal therapy” marketed as oral contraceptives. This approach may decrease resistance to the treatment among families, who may otherwise have concerns about placing an adolescent on oral contraceptives. Adolescent girls with overt IR (e.g., elevated insulin levels) and/or impaired glucose homeostasis can be managed initially with metformin. Patients have a high likelihood of ovulatory cycles while taking metformin, and girls who are sexually active need to be appropriately counseled. Data on reproductive outcomes in adolescents are limited; however, modest weight loss of 5–10% of total body weight has also been shown to restore ovulatory menstrual periods in obese adult women with PCOS.49 Typically, treatment is continued for a minimum of 1 year followed by re-evaluation for spontaneous menses.
Hirsutism is a common complication of PCOS.50 For adolescents with significant hirsutism, nonpharmacological treatments may include shaving, waxing, and use of chemical depilatories and/or bleaching cream. Spironolactone, 50–100 mg twice-daily, is an effective primary therapy for hirsutism. However, because of its teratogenicity, adolescent patients using this medication should also be placed on hormonal contraceptive therapy. Newer agents, such as eflornithine, are also available and dermatology consultation may also be considered.
Discussion
Because of the absence of guidelines for management of pediatric obesity-related comorbidities specifically in obese children, the FFFII participants generated a set of practical consensus statements developed by reviewing best evidence and guidelines from the literature as well as by consensus from the participating pediatric obesity centers. These statements focus on the clinical and laboratory screening, interpretation of results, and treatment of the most common comorbidities found in children and adolescents who are overweight or obese.
These are recommended consensus statements for clinicians to consider when developing treatment plans for their pediatric patients with obesity. The unique circumstances of each patient must be considered. Practice variability is expected as a result of demographics, population served, location, and resources available, among other factors. This variability was clearly illustrated in the results from the FFFII survey. The intention for the development of the consensus statements is to improve the care of children affected by obesity and its related comorbidities and reduce the need for referrals to subspecialists, who are scarce in many parts of the country. The availability of these consensus statements should facilitate not only the screening and diagnosis of the most common comorbidities, but also lead to early initial management of these conditions. By reducing referrals to subspecialists, earlier diagnosis and treatment, these consensus statements can improve the patient experience and can make care for pediatric patients more cost-effective. Practice patterns and access to subspecialists vary greatly by geography, so these factors also need to be considered when creating a treatment plan for a patient.
More research is needed to continue to improve the treatment of childhood obesity-related comorbidities. These consensus statements present a possible framework from which the evaluations can be further studied and adjusted based on the rapidly evolving field of pediatric and adolescent obesity medicine.
Conclusions
The combination of a high number of children at risk for obesity-related comorbidities, PCPs that have not enough training or resources to deal with these problems, and the shortage of pediatric subspecialists in the United States creates a hazardous situation for these children. Obesity is a relatively new disease, and comorbidities are just beginning to be identified; therefore, one can only expect this situation to deteriorate over time.
The consensus statements presented in this article may help keep the management of these children in their medical home and provide guidance to those sites that may not have subspecialists available.
Further studies and large-scale clinical trials to assess childhood obesity comorbidities are required to provide more firm guidelines that are evidence based.
Acknowledgments
Elizabeth Estrada and Stephen Pont wrote the first draft of this article.
The authors thank Gloria Lukasiewicz (Children's Hospital Association) for her assistance and tireless coordination support with this project. The authors thank the subspecialists in nephrology, hepatology, cardiology, endocrinology, pulmonology, gastroenterology, and gynecology at their own institutions, as well as all other nationally recognized experts that contributed to the development of these consensus statements. Dr. Rhodes is supported by the New Balance Foundation Obesity Prevention Center Boston Children's Hospital.
We thank Dell Children's Medical Center's Texas Center for the Prevention and Treatment of Childhood Obesity and the Obesity Center at Connecticut Children's Medical Center for funding immediate open access to this publication.
Author Disclosure Statement
The Children's Hospital Association was the project sponsor. The sponsor facilitated collection of data and hosted study group meetings, but was not involved in the data analysis or the decision to submit the article for publication. The views expressed are those of the authors and do not necessarily reflect those of the Children's Hospital Association.
Dr. Rhodes reports receiving research funding from Merck and her spouse owns stock in Bristol-Myers Squibb. The remaining authors have no disclosures.
References
- 1.Odgen C, Carroll M. Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2007–2008. CDC, National Center for Health Statistics: Atlanta, GA, 2010 [Google Scholar]
- 2.Rubin CM. Management of pediatric overweight/obesity: A survey of primary care providers. Is it time for a clinical alternative? Child Obes 2011;7:400–408 [Google Scholar]
- 3.Zamosky L. The obesity epidemic. While America swallows $147 billion in obesity-related healthcare costs, physicians called on to confront the crisis. Med Econ 2013;90:14–17 [PubMed] [Google Scholar]
- 4.Mazur A, Matusik P, Revert K, et al. Childhood obesity: Knowledge, attitudes, and practices of European pediatric care providers. Pediatrics 2013;132:e100–e108 [DOI] [PubMed] [Google Scholar]
- 5.Walsh CO, Milliren CE, Feldman HA, et al. Factors affecting subspecialty referrals by pediatric primary care providers for children with obesity-related comorbidities. Clin Pediatr (Phila) 2013;52:777–785 [DOI] [PubMed] [Google Scholar]
- 6.National Association of Children's Hospitals and Related Institutions (NACHRI). Pediatric subspecialist physician shortages affect access to care. 2009. Available at www.childrenshospitals.net/Content/ContentFolders34/BenchmarkingData/AnnualSurvey/pediatric_subspecialty_poster.pdf Last accessed November15, 2013
- 7.Jelalian E, Boergers J, Alday CS, et al. Survey of physician attitudes and practices related to pediatric obesity. Clin Pediatr (Phila) 2003;42:235–245 [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114(2 Suppl 4th Report):555–576 [PubMed] [Google Scholar]
- 10.National Institutes of Health (NIH) Lipids and lipoproteins. In: Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. NIH Publication No. 12-7486. National Institutes of Health: Bethesda, MD, 2012 [Google Scholar]
- 11.Barlow SE; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007;120(Suppl. 4):S164–S192 [DOI] [PubMed] [Google Scholar]
- 12.Kapadia C, Zeitler P; and Drugs and Therapeutics Committee of the Pediatric Endocrine Society. Hemoglobin A1c measurement for the diagnosis of Type 2 diabetes in children. Int J Pediatr Endocrinol 2012;2012:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011;128(Suppl. 5):S213–S256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azziz R, Carmina E, Dewailly D, et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–4245 [DOI] [PubMed] [Google Scholar]
- 15.Moran L, Pasquali R, Teede H, et al. Treatment of obesity in polycystic ovary syndrome: A position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 2009;92:1966–1982 [DOI] [PubMed] [Google Scholar]
- 16.American Association of Clinical Endocrinologists Polycystic Ovary Syndrome Writing Committee. American Association of Clinical Endocrinologists Position Statement on Metabolic and Cardiovascular Consequences of Polycystic Ovary Syndrome. Endocr Pract 2005;11:126–134 [DOI] [PubMed] [Google Scholar]
- 17.Wild R, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 2010;95:2038–2049 [DOI] [PubMed] [Google Scholar]
- 18.Levy-Marchal C, Arslanian S, Cutfield W, et al. Insulin resistance in children: Consensus, perspective, and future directions. J Clin Endocrinol Metab 2010;95:5189–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross AC, Manson JE, Abrams SA, et al. The 2011 dietary reference intakes for calcium and vitamin D: What dietetics practitioners need to know. J Am Diet Assoc 2011;111:524–527 [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Wu EL, Tarini B, et al. Diagnosis of diabetes using hemoglobin A1c: Should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36(Suppl. 1):S67–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook S, Kavey R. Dyslipidemia and pediatric obesity. Pediatric Clinics N Am 2011;58:1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavey RE, Mietus-Snyder M. Beyond cholesterol: The atherogenic consequences of combined dyslipidemia. J Pediatr 2012;161:977–979 [DOI] [PubMed] [Google Scholar]
- 26.Daniels S, Greer F. Lipid screening and cardiovascular health in childhood. Pediatrics 2008;122:198–208 [DOI] [PubMed] [Google Scholar]
- 27.Garaiova I, Muchova J, Nagyova Z, et al. Effect of a plant sterol, fish oil and B vitamin combination on cardiovascular risk factors in hypercholesterolemic children and adolescents: A pilot study. Nutr J 2013;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantyselka P, Niskanen L, Kautiainen H, et al. Cross-sectional and longitudinal associations of circulating omega-3 and omega-6 fatty acids with lipoprotein particle concentrations and sizes: Population-based cohort study with 6-year follow-up. Lipids Health Dis 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engler MM, Engler MB, Malloy MJ, et al. Effect of docosahexaenoic acid on lipoprotein subclasses in hyperlipidemic children (the EARLY study). Am J Cardiol 2005;95:869–871 [DOI] [PubMed] [Google Scholar]
- 30.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2328–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dengel DR, Kelly AS, Zhang L, et al. Signs of early sub-clinical atherosclerosis in childhood cancer survivors. Pediatr Blood Cancer 2014;61:532–537 [DOI] [PubMed] [Google Scholar]
- 32.Mencin A, Lavine J. Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care 2011;14:151–157 [DOI] [PubMed] [Google Scholar]
- 33.Mencin AA, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Pediatr Clin North Am 2011;58:1375–1392 [DOI] [PubMed] [Google Scholar]
- 34.Schwimmer JB, Pardee PE, Lavine JE, et al. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 2008;118:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bremer A, Mietus-Snyder M, Lustig R. Toward a unifying hypothesis of metabolic syndrome. Pediatrics 2012;129:557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwimmer JB, Dunn W, Norman GJ, et al. SAFETY study: Alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 2010;138:1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedman D, Khan L, Dietz W, et al. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: The Bogalusa Heart Study. Pediatrics 2001;108:712–718 [DOI] [PubMed] [Google Scholar]
- 38.Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333:853–861 [DOI] [PubMed] [Google Scholar]
- 39.Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond) 2008;32:1035–1041 [DOI] [PubMed] [Google Scholar]
- 40.Azziz R, Woods K, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–2749 [DOI] [PubMed] [Google Scholar]
- 41.Ehrmann D. Polycystic ovary syndrome. N Engl J Med 2005;352:1223–1236 [DOI] [PubMed] [Google Scholar]
- 42.Zawadski J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, Givens J, Haseltine F. (eds), Polycystic Ovary Syndrome. Blackwell Scientific: Boston, MA, 1992, pp. 377–384 [Google Scholar]
- 43.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–47 [DOI] [PubMed] [Google Scholar]
- 44.Krebs N, Himes J, Jacobson D, et al. Assessment of child and adolescent overweight and obesity. Pediatrics 2007;120:s193–s228 [DOI] [PubMed] [Google Scholar]
- 45.Diaz A, Laufer M, Breech L. Menstruation in girls and adolescents: Using the menstrual cycle as a vital sign. Pediatrics 2006;118:2245–2250 [DOI] [PubMed] [Google Scholar]
- 46.Salley K, Wickham E, Cheang K, et al. Glucose intolerance in polycystic ovary syndrome: A position statement of the Androgen Excess Society. J Clin Endocrinol Metab 2007;92:4546–4556 [DOI] [PubMed] [Google Scholar]
- 47.Palmert M, Gordon C, Kartashov A, et al. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab 2002;87:1017–1023 [DOI] [PubMed] [Google Scholar]
- 48.Warren-Ulanch J, Arslanian S. Treatment of PCOS in adolescence. Best Pract Res Clin Endocrinol Metab 2006;20:311–330 [DOI] [PubMed] [Google Scholar]
- 49.Hoeger K. Role of lifestyle modification in the management of polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab 2006;20:293–310 [DOI] [PubMed] [Google Scholar]
- 50.Escobar-Morreale H, Carmina E, Dewailly D, et al. Epidemiology, diagnosis and management of hirsutism: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update 2012;18:146–170 [DOI] [PubMed] [Google Scholar]