Abstract
Background and Purpose
Early blood brain barrier (BBB) damage after acute ischemic stroke (AIS) has previously been qualitatively linked to subsequent intracranial hemorrhage (ICH). In this quantitative study, it was investigated whether the amount of BBB damage evident on pre-tPA MRI scans was related to the degree of post-tPA ICH in patients with AIS.
Methods
Analysis was performed on a database of patients with AIS provided by the STIR and VISTA Imaging Investigators. Patients with perfusion-weighted imaging (PWI) lesions >10mL and negative gradient-recalled echo (GRE) imaging prior to IV tPA were included. Post processing of the PWI source images was performed to estimate changes in BBB permeability within the perfusion deficit relative to the unaffected hemisphere. Follow-up GRE images were reviewed for evidence of ICH and divided into three groups according to ECASS criteria: no hemorrhage (NH), hemorrhagic infarction (HI), and parenchymal hematoma (PH).
Results
75 patients from the database met the inclusion criteria, 28 of whom experienced ICH, of which 19 were classified as HI, and nine were classified as PH. The mean permeability (±standard deviations), expressed as an index of contrast leakage, was 17.0%±8.8 in the NH group, 19.4%±4.0 in the HI group, and 24.6%±4.5 in the PH group. Permeability was significantly correlated with ICH grade in univariate (p=0.007) and multivariate (p=0.008) linear regression modeling.
Conclusions
A PWI-derived index of BBB damage measured prior to IV tPA is associated with the severity of ICH after treatment in patients with AIS.
Keywords: Blood Brain Barrier, Hemorrhagic Transformation, MRI, IV tPA
Background
Thrombolytic therapy for acute ischemic stroke (AIS) has been the standard of care for over a decade.1 Intravenous (IV) tissue plasminogen activator (tPA) is approved for use if given within three hours of AIS onset, and can also be given within 4.5 hours in a subset of patients.2 Intracranial hemorrhage (ICH) is the most serious complication of IV tPA. The development of the most severe form of ICH, parenchymal hematoma (PH), can result in clinical deterioration and death. In the ECASS criteria grading system3 for ICH, PH is separated from hemorrhagic infarction (HI), as the former is much more likely to be symptomatic than the latter. Approximately 6% of patients treated with IV tPA will develop symptomatic ICH even when administered in an approved time window.4 There is currently no method in use that predicts who will develop PH with IV tPA prior to its administration.
One approach to detect who is at risk of ICH with IV tPA is to look for evidence of damage to the blood brain barrier (BBB) prior to tPA administration. It has been shown in animals and in humans that accumulation of exogenous contrast agents in the extravascular space is associated with an increased risk of ICH.5–8 Gadolinium is often given to patients with AIS in order to perform perfusion weighted imaging (PWI).9 Gadolinium will remain in the intravascular space unless there is damage to the BBB, in which case it can extravasate into the brain parenchyma and the cerebrospinal fluid (CSF) serving as a marker for an increased risk of ICH and poor outcome.10–12 However, it has not been determined whether the amount of gadolinium leaking through the BBB is related to the magnitude of the ICH. Using a novel postprocessing technique on standard MRI scans, we aimed to examine this relationship. We hypothesized that increasing BBB damage would be associated with increasing severity of ICH in the setting of IV tPA.
Methods
Patients
This study is a retrospective analysis of a database of patients with AIS that was collected as part of a natural history MRI study of patients receiving IV tPA with local institutional review board approval. The anonymized database was made available for the current study by an application to the STIR/VISTA consortium through a process that is publically accessible. The database contained 185 patients with MRI scans obtained at multiple time points which variably included acute (prior to treatment), two hours post treatment, 24 hours post treatment, five days (or discharge), 30 days, and 90 days. From this dataset, patients were identified that met the inclusion criteria for this study: (1) Patients needed to have a complete PWI acquisition and a gradient-recall echo (GRE) sequence prior to IV tPA administration; and (2) they must have had follow-up GRE imaging at two hours, 24 hours, or five days/discharge. Patients who did not have a PWI deficit greater than 10 mL on their pretreatment scan, defined by a time-to-peak (TTP) delay of six seconds, were excluded to ensure that all patients had active ischemia at the time of BBB permeability measurement. Follow-up GRE images at the specified time points were reviewed for ICH and graded as no hemorrhage (NH), hemorrhagic infarction (HI), or parenchymal hematoma (PH), first independently and then by consensus of two authors (RL, SSJ) according to ECASS criteria.3 For comparison with previously published definitions of ICH risk in patients receiving IV tPA, DWI lesion volumes were calculated using an ADC threshold of 600 ×10−6 mm2/sec and PWI lesion volumes were calculated using a TTP threshold of eight seconds delay.13
MRI Scans
Imaging was performed using a 1.5-T (Twinspeed; General Electric) or 3-T (Achieva; Philips) clinical MRI scanner. Typical imaging parameters for DWI spin-echo echo-planar series included either 40 3.5-mm-thick or 20 7-mm-thick contiguous axial oblique slices, with b of 0 and b of 1000 s/mm2, were trace-weighted or isotropically weighted, had repetition time/echo time of 6000 to 7000/72 to 90 ms, an acquisition matrix of 96×96 or 128×128, and a 22 cm field of view. Typical imaging parameters for PWI gradient echoplanar series included 20 contiguous axial oblique slices with single-dose gadolinium contrast injection of 0.1 mmol/kg through a power injector using 25 to 40 phase measurements, repetition time/echo time=2000 to 2200/45 ms, an acquisition matrix of 64×64 to 128×128, a 7-m slice thickness, and a 22-cm field of view. Typical imaging parameters for gradient-echo series were field of view 24 cm, repetition time (TR) 800 ms, echo time (TE) 20 ms, flip angle 30°, and acquisition matrix 256×192.
Permeability Analysis
Permeability analysis was performed on the acute pre-tPA PWI scans. The derivation and application of this technique has been described in detail elsewhere.14, 15 In brief, the source images of the PWI acquisition were analyzed on a voxel-by-voxel basis for evidence of contrast leakage T1 signal change. PWI source images are T2* weighted images; however, when contrast accumulates in the brain parenchyma due to damage to the BBB, a T1 signal component, which is usually negligible, becomes detectable. After applying an arrival-time correction to remove the effects of hypoperfusion and tracer dispersion, the change in signal over time was modeled and compared to tissue with an intact BBB.16 This generates a measure for T1 signal change that is an index of the amount of gadolinium that has leaked into that voxel. This index is then expressed as a percent of cerebral blood volume which is a measure of the amount of gadolinium that flowed through the voxel during the PWI acquisition. Thus for each voxel, a relative measure of the percentage of gadolinium that leaked through the BBB can be obtained.
A user-independent automated process calculated the mean permeability derangement for each patient from the voxels within the perfusion deficit that had elevated permeability based on the per-voxel permeability analysis (Figure 1). Elevated permeability was defined as two standard deviations above the rest of the brain. The Matlab software package (http://www.mathworks.com/) was used for all image processing.
Figure 1.
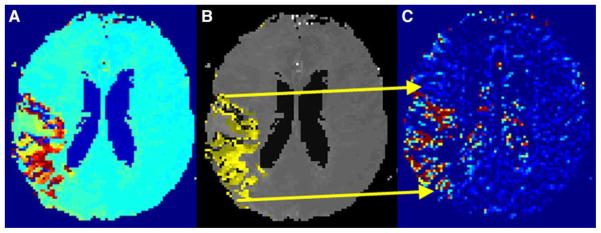
Example of mean permeability ROI: The TTP map (A) is thresholded (B) and overlain on the permeability map (C).
Statistics
The ECASS criteria ICH grade for each patient was considered to be a dependent variable reflecting the severity of ICH, with NH representing no ICH, HI representing minor ICH, and PH representing major ICH. This degree of ICH was treated as a dependent variable for each of the clinical parameters [independent variables] listed in Table 1 for the univariate analysis. Categorical variables were analyzed using logistic regression. Continuous variables were analyzed using linear regression. A p-value of less than 0.05 was considered significant. To assess for interactions between variables, a multivariate linear regression was also performed which included all variables with a p-value less than 0.1 from the univariate analysis. Statistical analysis was done using the STATA software package (http://www.stata.com/).
Table 1.
| All Subjects (n=75) | No Hemorrhage (n=47) | Hemorrhagic Infarction (n=19) | Parenchymal Hematoma (n=9) | Univariate p-value | Multivariate p-value | |
|---|---|---|---|---|---|---|
| Mean Permeability Derangement (% leak) | 18.6±7.8 | 17.0±8.8 | 19.4±4.0 | 24.6±4.5 | 0.007* | 0.008* |
| DWI volume (mL) | 22.3±40.9 | 20.2±44.2 | 27.2±41.0 | 23.5±19.8 | 0.653 | |
| PWI volume (mL) | 40.8±42.3 | 38.0±46.1 | 44.4±33.4 | 48.2±40.4 | 0.441 | |
| Age | 69.5±17.2 | 68.8±18.5 | 69.7±13.8 | 72.4±18.2 | 0.593 | |
| Baseline NIHSS | 11.6±8.7 | 10.5±8.8 | 10.6±6.1 | 19.7±9.5 | 0.021* | 0.34 |
| Time to MRI (minutes) | 97.5±41.0 | 97.0±47.4 | 88.6±25.5 | 199.2±21.6 | 0.454 | |
| Time to tPA (minutes) | 147.2±30.3 | 148.2±32.2 | 137.3±27.0 | 163.2±19.2 | 0.595 | |
| Glucose (mg/dL) | 128±30.8 | 121±21.8 | 132±28.7 | 155±55.1 | 0.002* | 0.013* |
| Systolic Blood Pressure (mm Hg) | 153±24.2 | 152±22.0 | 158±26.4 | 148±33.6 | 0.858 | |
| Diastolic Blood Pressure (mm Hg) | 83.9±14.1 | 83.4±12.7 | 86.1±16.3 | 79.5±16.7 | 0.95 | |
| Sex (female) | 44 (59%) | 31 (66%) | 7 (38%) | 6 (67%) | 0.099† | 0.849 |
| Hypertension | 49 (65%) | 30 (64%) | 13 (68%) | 6 (67%) | 0.723 | |
| Diabetes Mellitus | 14 (19%) | 6 (13%) | 7 (37%) | 1 (11%) | 0.097† | 0.515 |
| Coronary Artery Disease | 20 (27%) | 8 (17%) | 11 (58%) | 1 (11%) | 0.017* | 0.344 |
| Hyperlipidemia | 30 (40%) | 19 (47%) | 8 (42%) | 3 (33%) | 0.922 | |
| Tobacco Abuse | 22 (29%) | 15 (32%) | 5 (26%) | 2 (22%) | 0.526 | |
| Atrial Fibrillation | 8 (11%) | 5 (11%) | 3 (16%) | 0 (0%) | 0.992 |
Continuous variables are shown as mean±standard deviation and categorical variables are shown as total (percent).
indicates p-values less than 0.05.
indicates p-value less than 0.1 (for entry into the multivariate analysis).
Results
The baseline characteristics of the population are shown in Table 1. Out of a database of 185 patients, 75 patients were identified that met the inclusion criteria. More than half (44) of the patients were women, and the mean ± standard deviation of the age of the cohort was 70±17. The mean NIHSS was 12±9. The mean time from stroke onset to tPA administration was 147±30 minutes. The majority (68) of patients received tPA in the three-hour window and all patients were treated within the 4.5-hour window.
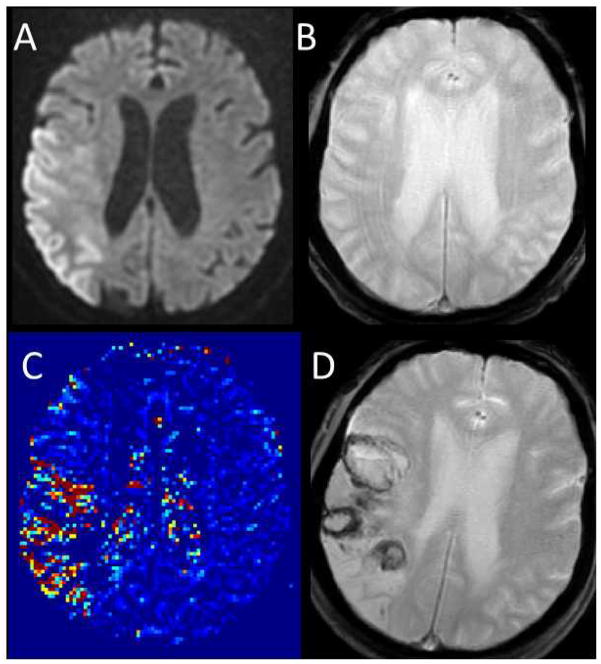
Of the 75 patients included in the study, 47 had NH, 19 had HI, and nine had PH. Figure 2 shows an example of a patient who subsequently developed PH after tPA in the setting of BBB damage detected with permeability imaging [Panel C]. The average mean permeability derangement for each group is shown graphically in Figure 3A with 95% confidence intervals. P-values listed in Table 1 demonstrate that mean permeability derangement (p=0.007), baseline NIHSS (p=0.021), glucose level on admission (p=0.002), and a history of coronary artery disease (p=0.017) were significantly associated with the degree of ICH according to ECASS criteria grading. After multivariate linear regression, the only independent predictors of ICH severity were mean permeability derangement (p=0.008) and glucose level on admission (p=0.013) [Table 1].
Figure 2.
Example images for a patient who suffered parenchymal hematoma after tPA are shown. Panel A: pre-treatment DWI. Panel B: pre-treatment GRE. Panel C: pre-treatment permeability image. Panel D: post-treatment GRE demonstrating ICH.
Figure 3.
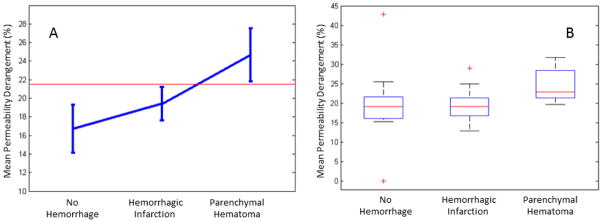
Panel A shows the mean permeability derangement for each group with 95% confidence intervals. The red line demonstrates a threshold that separates parenchymal hematoma from all other patients with 95% accuracy. Panel B shows box plots for the mean permeability derangement of each group; the central mark is the median, the edges of the box are the 25th and 75th percentiles, the bars extend to the most extreme data points not considered outliers, and outliers are plotted individually.
Bivariate linear regression of mean permeability derangement with ICH grade demonstrated a statistically significant relationship (p=0.007). The strength of this relationship is reflected in the r-squared term of 0.095 (adjusted r-squared 0.083). This indicates that approximately 10% of the ICH grade is explained by the mean permeability derangement. The effect size is reflected in the beta score of 0.31 indicating a moderate effect. Thus, BBB damage, as detected with mean permeability derangement, has a significant but moderate effect on the severity of subsequent ICH of acute stroke patients treated with IV tPA in this study.
Discussion
The blood-brain barrier (BBB) refers to the protection of the neuronal microenvironment of the brain from the circulating systemic blood by a complex interaction of cells,17 commonly referred to as the neurovascular unit (NVU). Soon after onset, ischemia is thought to cause an initial early reversible opening of the BBB due to activation of matrix metalloproteinases (MMPs), which is distinct from a delayed secondary opening caused by a neuroinflamatory response days later.18 When the NVU is compromised, tPA, which would usually remain in the intra-vascular space, can cross into the brain where it plays a role in activating the cell signaling pathways of endogenous tPA associated with hemorrhagic transformation.19 In addition to the direct effects of tPA on the ischemic brain, reperfusion of damaged vasculature due to tPA-induced clot lysis is also thought to contribute to hemorrhagic transformation.20
This is the first study to correlate the degree of BBB damage with the degree of ICH in humans treated with IV tPA. While this relationship may be suspected intuitively, the dose-dependent interaction identified by this study provides the strongest support to date. BBB integrity was assessed within 4.5 hours of ischemia onset, which falls into the time frame of MMP-mediated BBB opening. Leakage of gadolinium, a substance that generally does not penetrate an intact BBB, into the brain parenchyma was used as a measure of BBB damage. Larger amounts of gadolinium accumulation were a marker for more severe ICH when exposed to tPA. Whether this was a direct effect of the tPA on an exposed neuronal environment, or instead due to restoration of blood flow to significantly damaged tissue, is not established by this study. But it leads to the question: Is BBB damage just a marker for severe ischemia, or does BBB damage itself contribute to ICH? One way to differentiate between these two causes would be to identify the reperfusion status on follow-up imaging. Unfortunately, ICH creates an artifact on PWI source images that prevents assessment of blood flow to areas with hemosiderin deposition. Thus, such an analysis was not possible.
Another way to assess what role tissue damage may have played in hemorrhagic transformation of ischemic tissue is to examine diffusion weighted imaging. Restricted diffusion on DWI is a marker for cell injury. Prior studies have found that the volume of tissue below an ADC threshold of 600 ×10−6 mm2/sec is a marker for subsequent development of parenchymal hematoma when exposed to tPA in an extended time window.13 However, in our study of patients in an early time window, DWI volumes defined in the same manner did not correlate with ICH. Similarly PWI volumes also did not correlate with ICH. This may suggest that it is the exposure of an unprotected brain to tPA, rather than the volume of tissue affected, that contributes to subsequent ICH. However, this study was not designed to determine the role of DWI volume or PWI volume in this early tPA time window, thus further investigation is needed.
Several prior studies have demonstrated that BBB damage detected with MRI is associated with subsequent ICH. Extravasation of contrast through a damaged BBB and into CSF can be detected with FLAIR imaging, a phenomenon called hyperintense reperfusion marker (HARM).10, 21 Studies of patients treated in a more extended time window have found that changes in the slope of the gadolinium concentration curve can be a marker for ICH.12 Several other measures extracted from the PWI source images have also been associated with ICH.22–24 T1 post-contrast enhancement can be very specific for subsequent ICH, however it is not very sensitive.5, 6
These prior studies have not been quantitative in a manner that is able to relate the degree of ICH with the magnitude of BBB damage detected on MRI. However, in our study, a novel algorithm, which uses an arrival time correction, was employed in a quantitative manner.16 The advantage to this approach is that it removes the effects of blood flow and dispersion from the recorded signal allowing an index related to BBB permeability to be estimated. An even more quantitative measure of BBB damage can be obtained using steady-state dynamic contrast enhanced MRI;25 however the time constraints of that technique prevent it from being usable in AIS.
Hemorrhagic transformation of post-tPA ischemic tissue is a complex pathophysiologic state that involves many factors, not just BBB damage. Regression analysis in our study indicated that BBB damage only accounted for 10% of the effect. The overlap of the 95% confidence intervals in Figure 3A demonstrates that differentiating between those who are likely to experience minor bleeding vs. those who are likely to experience no bleeding is not possible just using BBB damage measured with our approach. However, in this dataset, a threshold can be set that separates major bleeding from all other patients with 95% confidence as shown by the red line in Figure 3A. This is an intriguing finding since major bleeding, and not minor bleeding, is the complication of tPA that is most feared. This study is not powered to establish such a threshold as predictive, but a subsequent study in which this threshold is tested on a unique population may clarify the clinical utility of this finding.
Another interesting finding in this study was the heterogeneity of BBB damage in the population. Some degree of BBB disruption was detected in all patients, even those who did not develop any ICH, which supports the notion that ischemia itself results in the opening of the BBB. This leads to the question of why early, severe BBB damage is more prominent in a subset of ischemic stroke patients. Multivariate analysis revealed that glucose level was associated with ICH in accordance with previous studies.26 Future studies should expand the range of clinical markers evaluated. Animal data have supported the use of therapies directed at BBB protection that could be given in combination with tPA.27 Thus BBB imaging techniques may be useful in identifying a patient population who would benefit from such a therapy. Additionally, although MRI-guided selection of patients for intra-arterial (IA) treatments has been controversial, the use of BBB imaging may be useful in such a setting, particularly when combined IV/IA treatments are being considered.
There were several assumptions involved in the design of this study. First, ICH was graded according to ECASS criteria, not volume. ECASS criteria, which were originally designed for use on HCT, were used on MRI in this study and thus may carry a different meaning. Although there are subtypes in the ECASS grading system that are related to the volume of blood, the main distinction is between hemorrhagic transformation and hematoma formation. The former is generally asymptomatic while the latter is often not, which is why the ECASS grading is clinically relevant. However, in using such a classification system in this study, it is assumed that as BBB permeability increases, the likelihood of hematoma formation increases. The method of calculating mean permeability derangement in this study is weighted towards areas of focal, high BBB damage rather than areas of diffuse, less-severe BBB damage. Additionally, the use of a linear regression model further assumes that the transition from one classification to another is linear, which it likely is not. Figure 3A suggests that the relationship may be exponential, however log transformation prior to linear regression did not strengthen the relationship (p=0.10). Thus, further investigation is needed to work out the nature of the relationship detected in this study.
There are also several limitations to our study. The protocol for enrolling patients in the provided data set is not known and may have changed over time. The use of MRI to exclude stroke mimics and to grade hemorrhage may account for the higher-than-usual rate of ICH. Conversely, patients with MRI contra indications to tPA, such as microbleeds, may have been excluded. The data set is relatively small and may not be representative of a larger, more diverse population. Additionally, the MRI parameters were not standardized and magnet strengths varied. Lastly, this was a retrospective analysis and cannot be used to determine if the thresholds identified would apply to a prospectively acquired cohort.
Summary/Conclusions
When analyzed with an algorithm that uses an arrival time correction, the amount of BBB damage after acute ischemic stroke as detected by gadolinium leakage on MRI is correlated with the degree of subsequent ICH when exposed to IV tPA. Lesser degrees of BBB damage were less likely to result in major intracranial bleeding than more severe degrees of BBB damage in patients who received standard FDA approved acute ischemic stroke treatment. Further studies should be performed to determine how these findings might improve the treatment of acute ischemic stroke patients.
Acknowledgments
Sources of Funding:
Richard Leigh and Argye E Hillis are supported in part by NIH R01DC05375 and RO1NS47691 for imaging studies of recovery of function in stroke. This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) and the Seton Healthcare Family.
Appendix
*STIR/VISTA Imaging steering committee members: Gregory W. Albers, Stephen M. Davis, Geoffrey A. Donnan, Marc Fisher, Anthony J. Furlan, James C. Grotta, Werner Hacke, Dong-Wha Kang, Chelsea Kidwell, Walter J. Koroshetz, Kennedy R. Lees, Michael H. Lev, David S. Liebeskind, A. Gregory Sorensen, Vincent N. Thijs, Götz Thomalla, Steven J. Warach, Joanna M. Wardlaw, Max Wintermark
Footnotes
Conflicts-of-interest/Disclosures:
Peter B. Barker has served as a consultant to Olea Medical.
Contributor Information
Richard Leigh, Departments of Neurology & Radiology, Johns Hopkins University, Baltimore, MD.
Shyian S. Jen, Department of Radiology, Emory University, Atlanta, GA.
Argye E. Hillis, Departments of Neurology, Physical Medicine & Rehabilitation & Cognitive Science, Johns Hopkins University, Baltimore, MD.
John W. Krakauer, Department of Neurology, Johns Hopkins University, Baltimore, MD.
Peter B. Barker, Department of Radiology, Johns Hopkins University, Baltimore, MD.
References
- 1.The national institute of neurological disorders and stroke rt-pa stroke study group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von KR, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The european cooperative acute stroke study (ecass) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 4.The ninds t-pa stroke study group. Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 5.Hjort N, Wu O, Ashkanian M, Solling C, Mouridsen K, Christensen S, et al. Mri detection of early blood-brain barrier disruption: Parenchymal enhancement predicts focal hemorrhagic transformation after thrombolysis. Stroke. 2008;39:1025–1028. doi: 10.1161/STROKEAHA.107.497719. [DOI] [PubMed] [Google Scholar]
- 6.Kim EY, Na DG, Kim SS, Lee KH, Ryoo JW, Kim HK. Prediction of hemorrhagic transformation in acute ischemic stroke: Role of diffusion-weighted imaging and early parenchymal enhancement. AJNR Am J Neuroradiol. 2005;26:1050–1055. [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Q, Zhang RL, Zhang ZG, Knight RA, Ewing JR, Ding G, et al. Magnetic resonance imaging characterization of hemorrhagic transformation of embolic stroke in the rat. J Cereb Blood Flow Metab. 2002;22:559–568. doi: 10.1097/00004647-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Knight RA, Barker PB, Fagan SC, Li Y, Jacobs MA, Welch KM. Prediction of impending hemorrhagic transformation in ischemic stroke using magnetic resonance imaging in rats. Stroke. 1998;29:144–151. doi: 10.1161/01.str.29.1.144. [DOI] [PubMed] [Google Scholar]
- 9.Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, et al. Acute stroke imaging research roadmap. Stroke. 2008;39:1621–1628. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PubMed] [Google Scholar]
- 10.Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- 11.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 12.Bang OY, Buck BH, Saver JL, Alger JR, Yoon SR, Starkman S, et al. Prediction of hemorrhagic transformation after recanalization therapy using t2*-permeability magnetic resonance imaging. Ann Neurol. 2007;62:170–176. doi: 10.1002/ana.21174. [DOI] [PubMed] [Google Scholar]
- 13.Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, et al. Refining the definition of the malignant profile: Insights from the defuse-epithet pooled data set. Stroke. 2011;42:1270–1275. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaharchuk G. Theoretical basis of hemodynamic mr imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. AJNR Am J Neuroradiol. 2007;28:1850–1858. doi: 10.3174/ajnr.A0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 16.Leigh R, Jen SS, Varma DD, Hillis AE, Barker PB. Arrival time correction for dynamic susceptibility contrast mr permeability imaging in stroke patients. PLoS One. 2012;7:e52656. doi: 10.1371/journal.pone.0052656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nature neuroscience. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 20.Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–99. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidwell CS, Burgess R, Menon R, Warach S, Latour LL. Hyperacute injury marker (harm) in primary hemorrhage: A distinct form of cns barrier disruption. Neurology. 2011;77:1725–1728. doi: 10.1212/WNL.0b013e318236ef46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornhill RE, Chen S, Rammo W, Mikulis DJ, Kassner A. Contrast-enhanced mr imaging in acute ischemic stroke: T2* measures of blood-brain barrier permeability and their relationship to t1 estimates and hemorrhagic transformation. AJNR Am J Neuroradiol. 2010;31:1015–1022. doi: 10.3174/ajnr.A2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Thornhill RE, Chen S, Rammo W, Mikulis DJ, Kassner A. Relative recirculation: A fast, model-free surrogate for the measurement of blood-brain barrier permeability and the prediction of hemorrhagic transformation in acute ischemic stroke. Invest Radiol. 2009;44:662–668. doi: 10.1097/RLI.0b013e3181ae9c40. [DOI] [PubMed] [Google Scholar]
- 24.Scalzo F, Alger JR, Hu X, Saver JL, Dani KA, Muir KW, et al. Multi-center prediction of hemorrhagic transformation in acute ischemic stroke using permeability imaging features. Magnetic resonance imaging. 2013;31:961–969. doi: 10.1016/j.mri.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewing JR, Knight RA, Nagaraja TN, Yee JS, Nagesh V, Whitton PA, et al. Patlak plots of gd-dtpa mri data yield blood-brain transfer constants concordant with those of 14c-sucrose in areas of blood-brain opening. Magn Reson Med. 2003;50:283–292. doi: 10.1002/mrm.10524. [DOI] [PubMed] [Google Scholar]
- 26.Leigh R, Zaidat OO, Suri MF, Lynch G, Sundararajan S, Sunshine JL, et al. Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy. Stroke. 2004;35:1903–1907. doi: 10.1161/01.STR.0000132571.77987.4c. [DOI] [PubMed] [Google Scholar]
- 27.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]